CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9-based genome editing has revolutionized functional genomics in many biological research fields. The specificity and potency of CRISPR-Cas9 genome editing make it ideal for investigating the function of genes in vivo (Hsu et al., 2014). Gene duplication is a key driver of evolutionary novelty (Taylor and Raes, 2004). However, duplicated genes with near-identical sequences and functional redundancy have posed challenges for genetic analysis (Woollard, 2005). The function of duplicated genes can be assessed by simultaneous knockdown using homology-based methods such as RNA interference (RNAi) (Tischler et al., 2006). Generation of double or triple mutants is an alternative way to assess functional redundancy of duplicated genes. However, generation of such comopound mutants by forward or reverse genetic methods is time consuming. Since CRISPR-Cas9 genome editing can mutate multiple genes simultaneously with combined sgRNAs (Cong et al., 2013; Wang et al., 2013), and a single sgRNA can have multiple targets (Shen et al., 2014; Singh et al., 2015), we reasoned that if duplicated genes share sufficient sequence similarity in their sgRNA target region, it should be possible to simultaneously mutate two or more gene duplicates using a single sgRNA. Here we report that such compound mutants are readily generated in C. elegans using CRISPR-Cas9.
As proof of principle, we first chose the three miro (mitochondrial RHO GTPase) genes, located on different chromosomes, for investigation. MIRO is a key component of a motor/adaptor complex that mediates mitochondrial transport in neurons (Schwarz, 2013). The C. elegans miro genes, miro-1, miro-2, and miro-3, have high sequence similarity at the nucleotide and amino acid levels (Fig. S1, S2). miro-2 is duplicated from miro-1; miro-3 closely resembles miro-2 but is a pseudogene (Takao Inoue, Personal communication). Here, we designed a sgRNA to target a region of 100% sequence identity between exon 4 of miro-1 and exon 3 of miro-2 and miro-3 (Fig. 1A). To increase the efficiency of CRISPR-Cas9-mediated mutagenesis and mutant detection, we combined both the 3′ GG-guide RNA design and co-CRISPR methods (Farboud and Meyer, 2015; Kim et al., 2014). dpy-10 sgRNA (Arribere et al., 2014) was used for co-CRISPR selection to facilitate later outcrossing, as dpy-10 is unlinked to the miro genes.
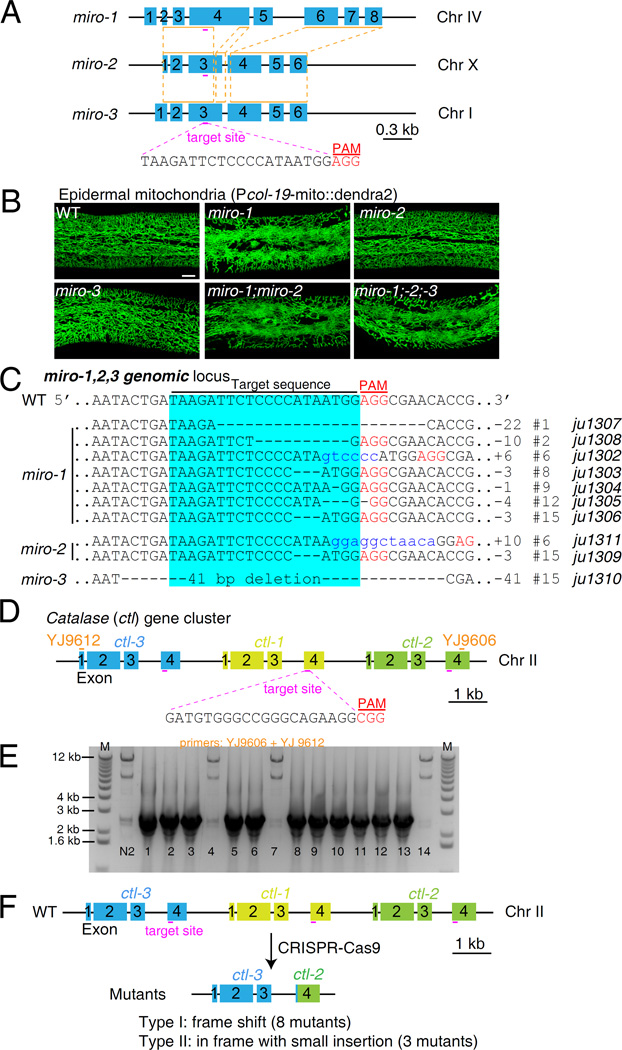
Figure 1. Targeted mutagenesis of duplicated genes via CRISPR-Cas9 genome editing in C. elegans.
A: Schematic of genomic loci of three miro genes. Orange lines and red dashed lines indicate the most homologous regions of the three miros. The protospacer adjacent motif (PAM) sequence is highlighted in red. B: Representative confocal images of mitochondria in C. elegans epidermis. Epidermal mitochondria were labeled with mito::dendra2 under the control of col-19 promoter (juSi271). Wild type animals show a network of tubular mitochondria distributed throughout the epidermal syncytium, while mutant animals with miro-1(ju1306) mutation generated by CRISPR-Cas9 show thinner thread-like mitochondrial morphology. miro-2(ju1309) and miro-3(1310) deletion mutants show normal mitochondrial morphology. miro-1(ju1302); miro-2(ju1311) double and miro-1(ju1306); miro-2(ju1309); miro-3(ju1310) triple mutants show similar phenotype as miro-1(ju1306) single mutant. Bar = 10 µm. C: Genomic DNA sequences of miro genes in the wild type and CRISPR-Cas9-generated mutants. Sequences with light blue background are the Cas9 target sites. PAM sequences are highlighted in red. Dashed lines and blue lowercases indicate deletions and small insertions, respectively. Numbers on the right indicate independent lines generated in one experiment. D: Schematic of the catalase gene cluster (ctl-1, ctl-2, and ctl-3). Short red lines are the sgRNA target sites. PAM sequences are highlighted in red. YJ9606 and YJ9612 are the primers used for genotyping. E: PCR genotyping of the ctl locus in the F2 twitching offspring from 14 F1 roller animals, using primer pairs YJ9606 and YJ9612. N2 wild-type animals were used as a control. The wild-type PCR amplicon should be 10.7 kb. Each smaller band (2–3 kb) indicates a deletion. F: ctl mutations generated in one experiment by CRISPR-Cas9. All mutations are deletions resulting in fusion of exon 4 of ctl-3 to exon 4 of ctl-2. Two types of mutations were found. Eight are predicted to cause a frame shift and introduce a premature stop codon in the CTL-2 part of the fusion protein. Three mutations result in an in-frame fusion and insert a small number of nucleotides.
Deletion (predicted loss of function) alleles of all three miro genes were previously isolated by reverse genetic screening in the Mitani lab (Japan), however the function of the miro genes has not been reported in C. elegans. In wild-type worms, epidermal mitochondria form a complex tubular network (Xu and Chisholm, 2014). In miro-1(tm1966) deletion mutants, epidermal mitochondria were thinner and ‘thread-like’ in morphology, and altered in distribution within the epidermal syncytium (Fig. S3A). miro-2(tm2933) and miro-3(tm3150) mutants were superficially wild type with no detectable aberrations in epidermal mitochondrial morphology (Fig. S3A). Since miro-1 mutants displayed a specific mitochondrial defect, we predicted that miro-1 loss-of-function mutants generated via Cas9 should resemble miro-1(tm1966). Thus, to reduce hands-on work, we injected Cas9 and sgRNAs into animals containing the mitochondrial marker (Fig. S3B).
We injected 12 P0 animals, selected individual F1 Dpy (dumpy body shape) worms potentially due to somatic or germline mutations of dpy-10, and then examined their F2 progeny for Dpy offspring (Fig. S3B). Twenty-four out of seventy F1 animals produced Dpy offspring (Fig. S3C), suggesting Cas9 had been active in the germline of parental animals. We then screened these 24 F2 broods for animals with thread-like mitochondria. Seven plates contained animals with mitochondrial morphology similar to miro-1(tm1966) (Fig. 1B), and thus were candidate miro-1 alleles (Fig. S3C). We genotyped miro-1, miro-2, and miro-3 by PCR and DNA sequencing, and found that seven plates contained miro-1 mutations with various indels (Fig. 1C); of these plates, two (#6 and #15) also contained miro-2 mutations, one of which (#15) contained an additional miro-3 mutation (Fig. 1C; Supplementary Data). These data suggested that a single sgRNA can generate double-stranded DNA breaks in three different genomic regions in the same parental germline. The double (miro-1;miro-2) and triple (miro-1;miro-2;miro-3) mutants resemble the miro-1 single mutant in epidermal mitochondrial morphology, and miro-2 or miro-3 single mutants, isolated after outcrossing, displayed normal epidermal mitochondrial morphology (Fig. 1B), suggesting miro-1 plays the major and non-redundant role in C. elegans epidermal mitochondrial morphology. Because we selected animals with miro-1-like mitochondrial morphology defects, our screening may underestimate the rate of mutagenesis at these loci.
We next attempted to mutate clusters of duplicated genes. The cluster of three catalase enzymes, ctl-1, ctl-2, and ctl-3, was chosen (Fig. 1D). Catalases function in vivo as antioxidants that protect cells from reactive oxygen species (ROS), and play important roles in regulating longevity in C. elegans (Murphy et al., 2003). The three C. elegans ctl genes are 70%–90% identical (Fig. S4C, Fig. S5) and encode proteins with 75%–96% identity (Fig. S4A and B). It is not known whether ctl-1, ctl-2, and ctl-3 function redundantly. The three genes are arranged in a tandem cluster (in order: 5′ ctl-3 ctl-1 ctl-2 3′) spanning ~ 11 kb (Fig. 1D), making it impractical to generate double or triple mutants by genetic recombination.
We designed a 3′ GG-guide RNA to target a region of 100% sequence identity among three ctl genes. As the ctl genes are located on Chr II, we used unc-22 as an unlinked co-CRISPR marker (Kim et al., 2014). Cas9 and sgRNA constructs were co-injected into wild-type animals, and pRF4 (rol-6) was also included as a co-injection marker to facilitate the identification of F2 twitchers from F1 roller animals, since the unc-22 twitcher phenotype is recessive (Kim et al., 2014) (Fig. S6A). After injecting 12 P0 animals, we picked 52 F1 roller animals, 14 of which generated F2 twitching progeny due to the presence of unc-22 mutations. We then genotyped the 14 F2 broods containing twitching worms by PCR using primers flanking the ctl cluster, and observed that 11 out of 14 plates of F2 animals displayed smaller amplicons indicative of a deletion of most of the ctl cluster (Fig. 1D and E). Since ctl sgRNA can target three different sites within the 11-kb genomic locus, we reasoned that these might be large deletions, similar to our previous experience using dual sgRNAs to generate large deletions (Xu and Chisholm, 2014). DNA sequencing further revealed that ctl-1 was completely deleted in these 11 mutations (Fig. 1F; Supplementary Data). Moreover, of these mutations, eight result in a frame shift after residue V340 of CTL-3, affecting the C-terminal 70 residues of its core catalase domain and possibly causing loss of function in all three ctl genes (Figs. 1F and S7); three are in-frame mutations but with small nucleotide insertions. The ctl-1;ctl-2;ctl-3 triple deletion mutants are viable and fertile and will be described in detail elsewhere. None of the mutations was a partial deletion with a breakpoint in ctl-1. Our observations suggest that a cluster of duplicated genes can be disrupted with a single sgRNA targeted to a conserved region.
It has been estimated that about 1/3 of genes in the C. elegans genome are either duplicated or members of closely related gene families (Cavalcanti et al., 2003; Rubin et al., 2000). At least 130 gene pairs display very high sequence similarity and are thought to have been recently duplicated (Katju, 2013). Our results suggest CRISPR-Cas9 allows rapid mutation of two or more members from the same gene family. Although we have not extensively explored sequence requirements, based on other studies we expect the duplicated genes must share at least 23 bp of identical nucleotide sequence ending in the 3′ NGG PAM sequence. Together, our study shows that targeted mutagenesis in duplicated genes can be generated with a single sgRNA using CRISPR-Cas9. Similar approaches might also be used to mutate highly repetitive regions of the genome, such as transposons, telomeres or subtelomeric repeats, or pericentric heterochromatin.
Supplementary Material
Acknowledgments
We thank members of the Jin and Chisholm labs for discussions on CRISPR-Cas9. We thank Shohei Mitani lab for providing the tm alleles of miro genes. Y. Jin is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health (NIH grant R01 GM054657) to A.D.C.
Footnotes
Note Added in Proof: While this work was in review, highly multiplexed mutation of families of endogenous retroviruses using CRISPR/Cas9 has been reported in mammalian cell lines (Yang et al., 2015, Science 350: 1101–1104).
References
- Arribere JA, Bell RT, Fu BX, Artiles KL, Hartman PS, Fire AZ. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics. 2014;198:837–846. doi: 10.1534/genetics.114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti AR, Ferreira R, Gu Z, Li WH. Patterns of gene duplication in Saccharomyces cerevisiae and Caenorhabditis elegans. J Mol Evol. 2003;56:28–37. doi: 10.1007/s00239-002-2377-2. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farboud B, Meyer BJ. Dramatic Enhancement of Genome Editing by CRISPR/Cas9 Through Improved Guide RNA Design. Genetics. 2015;199:959–971. doi: 10.1534/genetics.115.175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katju V. To the beat of a different drum: determinants implicated in the asymmetric sequence divergence of Caenorhabditis elegans paralogs. BMC Evol Biol. 2013;13:73. doi: 10.1186/1471-2148-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ishidate T, Ghanta KS, Seth M, Conte D, Jr, Shirayama M, Mello CC. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics. 2014;197:1069–1080. doi: 10.1534/genetics.114.166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, Cherry JM, Henikoff S, Skupski MP, Misra S, Ashburner M, Birney E, Boguski MS, Brody T, Brokstein P, Celniker SE, Chervitz SA, Coates D, Cravchik A, Gabrielian A, Galle RF, Gelbart WM, George RA, Goldstein LS, Gong F, Guan P, Harris NL, Hay BA, Hoskins RA, Li J, Li Z, Hynes RO, Jones SJ, Kuehl PM, Lemaitre B, Littleton JT, Morrison DK, Mungall C, O'Farrell PH, Pickeral OK, Shue C, Vosshall LB, Zhang J, Zhao Q, Zheng XH, Lewis S. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Zhang X, Chai Y, Zhu Z, Yi P, Feng G, Li W, Ou G. Conditional knockouts generated by engineered CRISPR-Cas9 endonuclease reveal the roles of coronin in C. elegans neural development. Dev Cell. 2014;30:625–636. doi: 10.1016/j.devcel.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist's practical guide to CRISPR applications. Genetics. 2015;199:1–15. doi: 10.1534/genetics.114.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. [DOI] [PubMed] [Google Scholar]
- Tischler J, Lehner B, Chen N, Fraser AG. Combinatorial RNA interference in Caenorhabditis elegans reveals that redundancy between gene duplicates can be maintained for more than 80 million years of evolution. Genome Biol. 2006;7:R69. doi: 10.1186/gb-2006-7-8-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard A. Gene duplications and genetic redundancy in C. elegans. WormBook. 2005:1–6. doi: 10.1895/wormbook.1.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.