Abstract
Fibronectin binding protein F1 (Sfb1) of Streptococcus pyogenes (group A streptococcus [GAS]) is a well-characterized adhesin that has been shown to induce protection in mice against a lethal intranasal GAS challenge after intranasal immunization with cholera toxin B subunit (CTB) as adjuvant. With a murine skin infection model, we have shown that Sfb1/CTB vaccination neither elicits opsonizing antibodies nor prevents systemic bacterial growth and dissemination to internal organs after a subcutaneous GAS challenge. These results indicate that an Sfb1-based vaccine should be complemented with additional protective antigens in order to be used in areas such as the tropical north of Australia, where the skin is the primary route of entry for invasive streptococcal diseases.
Among the aboriginal population living in the Northern Territory of Australia (NT), the incidence and prevalence of streptococcal infection and streptococcal diseases are high. Unlike in Europe and the United States, where the throat is often the primary tissue reservoir, the skin is the major site of infection for the aboriginal population (12). Rates of group A streptococcal (GAS) skin infection are extremely high, owing in part to infection of scabies lesions, with pyoderma prevalence rates in children of up to 70% (13). The rate of streptococcal invasive diseases among the aboriginal population is five times that of the general population, with skin infections underlying most cases (5). The incidence of poststreptococcal sequelae is also high, with rates of acute rheumatic fever in aboriginal populations among the highest reported and acute glomerulonephritis being endemic in many regions (4). Genetic typing of strains causing GAS infection in aboriginal communities has demonstrated that the diversity and turnover rate of these strains are much higher than those reported in other regions and has revealed no evidence of a dominant clone that has been a common cause of GAS invasive infections elsewhere in the world (3, 6). Vaccination might constitute the most suitable strategy to control GAS infections in these communities.
Bacterial adhesins have been proposed as potential vaccine targets for the prevention of infectious diseases (36). In this regard, Streptococcus pyogenes produces a number of MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that are believed to mediate adhesion of the pathogen to host tissue, a critical step in the initial stages of infection (27). A number of GAS MSCRAMMs capable of binding fibronectin have been identified, and they include protein F1/Sfb1 (16, 31), 28-kDa antigen (9), FBP54 (10), serum opacity factor (21, 28), protein F2 (18), PFBP (29), FbaA (33), FbaB (34), and SfbX (19). This diversity of fibronectin binding proteins suggests both the importance of fibronectin binding in the pathogenesis of GAS infection and the possibility that these proteins are differentially expressed at different stages of the infection process (17).
GAS fibronectin binding proteins have been suggested as potential vaccine targets for preventing GAS infections (7). Antibodies directed against such adhesins may prevent bacterial attachment and inhibit colonization (36). Sfb1 is a well-characterized fibronectin binding protein of GAS and is believed to mediate bacterial attachment to host cells and internalization of GAS into nonphagocytic cells (26, 31). Sfb1 has also been shown to interfere with host macrophage-mediated clearance mechanisms by binding to the Fc fragment of human immunoglobulins (24). When adjuvanted with cholera toxin B subunit (CTB), intranasal immunization with Sfb1 induces protection against an intranasal challenge with a lethal dose of GAS (15). Vaccinated mice produce a strong immunoglobulin G (IgG) serum antibody response in a Th2-like pattern (30). However, critical to this level of protection is the elicitation of mucosal immunity in the lungs of vaccinated mice. It is believed that this mucosal immune response may prevent S. pyogenes from binding to the upper respiratory epithelium, thereby preventing colonization and establishment of infection (36). Other characteristics that make Sfb1 an attractive vaccine candidate are the presence of highly conserved epitopes and the fact that Sfb1 is expressed on the surface of 70% of clinical GAS isolates belonging to different serotypes and strains, independent of geographic origin (14, 32, 35). Anti-Sfb1 antibodies do not cross-react with heart proteins and therefore may not trigger autoimmune reactions that might be responsible for poststreptococcal sequelae (35). Although vaccination with Sfb1 conferred protection against mucosal infection with S. pyogenes, the question remains whether it is also protective against systemic spread subsequent to skin infections. The objective of this study was therefore to determine whether the immune response generated against Sfb1 was able to confer protection against a subcutaneous challenge with S. pyogenes.
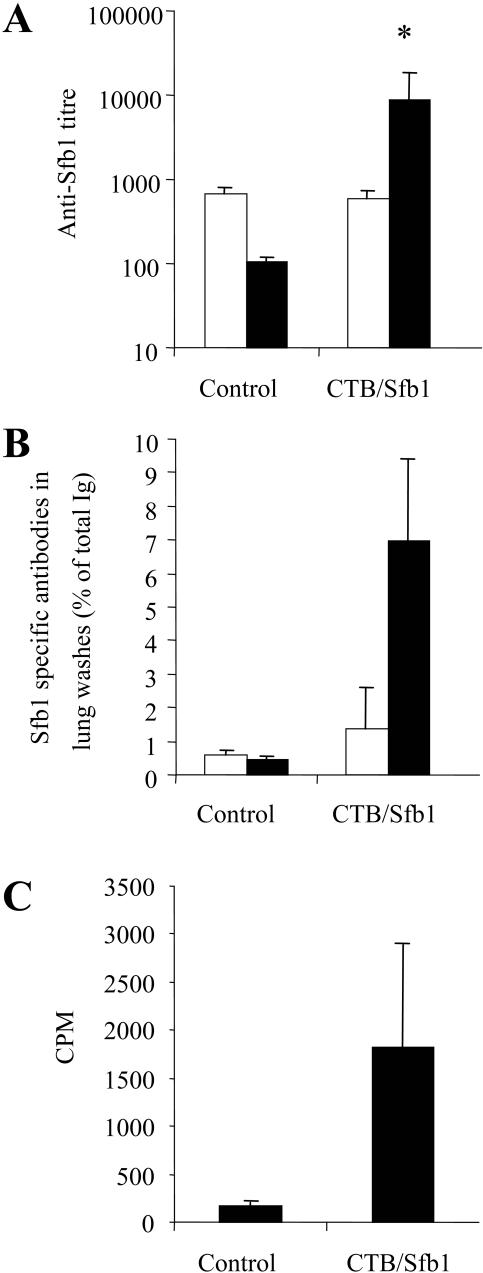
BALB/c mice were immunized by intranasal inoculation (10 μl/nare) with a mixture containing 30 μg of Sfb1 and 10 μg of CTB on days 1, 3, 5, and 15 as previously described (15). For characterization of the immune response, serum samples, lung washes, and spleen samples were collected 14 days after the last booster immunization. Lung washes were obtained by tracheal cannulation and gentle washing with 0.7 ml of cold phosphate-buffered saline (PBS) containing 2 mM phenylmethylsulfonyl fluoride. To assess the generation of antigen-specific effector cells by vaccination, lymphocytes were isolated from the spleens of immunized mice and restimulated in vitro for 3 days in the presence of Sfb1 as previously described (25). Results in Fig. 1A show that intranasal immunization with Sfb1 elicited a significant serum IgG antibody response to the vaccine antigen compared to that of nonvaccinated control mice (P = 0.029). As shown in Fig. 1B, immunization with Sfb1 also elicited elevated antigen-specific IgA and IgG antibody responses in the lung on day 14 postvaccination. Spleen cells isolated from mice vaccinated with Sfb1, compared with controls, displayed elevated proliferative responses on day 14 postvaccination (Fig. 1C).
FIG. 1.
The immune response directed against Sfb1 at 14 days postimmunization. (A) Specific IgG (solid bar) and IgA (unfilled bar) present in the serum of control and vaccinated mice. Results are expressed as the geometric means of five mice per group. The standard error of the mean is indicated by vertical bars. (B) Specific antibodies in lung washes of control and vaccinated mice. Results are expressed as the percentage of Sfb1-specific antibodies with respect to the total immunoglobulin isotype. The standard error of the mean is indicated by vertical lines. (C) Sfb1-specific proliferative responses of spleen cells. Results are expressed as the mean counts per minute of triplicate samples for three or four mice per group. The standard error of the mean is indicated by vertical lines. The results are statistically significantly different from the values for control mice (*, P < 0.05; Student's t test).
A previously described mouse skin infection model (23) was then used to examine the capacity of the immune response generated after vaccination with Sfb1 to restrain bacterial dissemination from the local infection foci at the skin. For this purpose, groups of Sfb1-vaccinated and nonvaccinated control mice were challenged with a subcutaneous injection containing of S. pyogenes strain A20 (M type 23) (22) obtained from the German Culture Collection (DSM 2071) at 106 CFU/100 μl administered into the back on day 14 postvaccination. At various times after infection, mice were sacrificed by CO2 asphyxiation, their livers and spleens were removed, and bacterial loads were determined in homogenates of these organs after serial dilutions were plated in blood agar.
The results presented in Fig. 2 show that both vaccinated and nonvaccinated mice had comparable rates of systemic bacterial growth and dissemination. Vaccination with Sfb1 had no effect on the growth of S. pyogenes in the blood of challenged mice (Fig. 2A) and also failed to limit bacterial dissemination and growth in both the spleen (Fig. 2B) and the liver (Fig. 2C) in infected animals. By contrast, GAS extracts containing other antigens were found to protect against systemic infection in this animal model (E. Medina, personal communication), thus validating this model system for such vaccination studies.
FIG. 2.
Clearance of S. pyogenes A20 from the blood, spleen, and liver of mice after a subcutaneous challenge with 106 CFU. Results are expressed as the mean CFU count determined for five mice per Sfb1-vaccinated mouse group (solid line) and PBS-vaccinated control mouse group (dashed line). The standard error of the mean is indicated by vertical lines.
These results suggest that serum antibodies generated against Sfb1 might be devoid of bactericidal activity against S. pyogenes. To further demonstrate this assumption, a bactericidal assay was performed with serum from mice vaccinated with either PBS, CTB, or CTB/Sfb1. Serum samples obtained from mice immunized with pepsin-extracted M protein (PepM) were used as a positive control for the opsonizing assay. Serum samples were mixed with an inoculum of S. pyogenes A20 containing approximately 30 CFU and added to heparinized fresh human blood obtained from a donor known to be nonopsonic for this streptococcal strain. The mixture was rotated for 3 h at 37°C, and the mean CFU count was determined by plating dilutions on blood agar plates. As shown in Fig. 3, serum samples from mice vaccinated with PBS, CTB, or CTB/Sfb1 failed to inhibit bacterial growth while serum samples from mice immunized with the PepM extract totally inhibited the growth of S. pyogenes A20 (Fig. 3). The bactericidal effect of rabbit polyclonal anti-Sfb1 serum samples was also tested, and they failed to inhibit bacterial growth. However, this antiserum was able to detect Sfb1 in Western blot assays performed to confirm the expression of Sfb1 by S. pyogenes A20 under the growth conditions used for opsonization (data not shown).
FIG. 3.
Bactericidal effect of systemic antibodies against DSM2071. Serum from mice intranasally vaccinated with PBS, CTB, or CTB/Sfb1 was mixed with an inoculum of DSM2071 containing approximately 30 CFU, added to heparinized human blood, and rotated for 3 h at 37°C. The mean CFU count was determined by plating dilutions on blood agar plates. Serum samples from mice immunized with pepsin-extracted M protein from DSM2071 (PepM) was used as a positive control for the assay. The opsonic effect of rabbit polyclonal anti-Sfb1 serum was also assessed.
As GAS produces a number of MSCRAMMs capable of binding to a variety of extracellular matrix components, including fibronectin, it is likely that the expression of these proteins is regulated in a coordinated manner during the infection process. It is not known if Sfb1 is expressed during colonization of or invasion through the skin. Protective immunity against GAS infection is believed to be via two major mechanisms (11). (i) Bacteria can be prevented from entering the host by blocking attachment and colonization at mucosal surfaces, and (ii) once GAS has entered host tissues, infection can be eliminated by opsonization with specific antibody and complement, followed by phagocytosis. It has been reported that immune responses against peptides based on the conserved region of streptococcal M protein confer protective mucosal immunity against colonization but do not reduce the rate of mortality due to systemic streptococcal infection and are also nonopsonic (2). Similarly, while an IgA response against Sfb1 will protect mice from an intranasal GAS challenge, this study for the first time has shown that serum IgG against Sfb1 is not opsonic and does not reduce the growth or dissemination of GAS in a murine skin infection model. Other GAS fibronectin binding MSCRAMMs, SOF and FBP54, have been shown to elicit opsonizing antibodies and protect against a systemic intraperitoneal GAS challenge (8, 20). This suggests that GAS fibronectin binding MSCRAMMs, and other potential GAS vaccine candidates, should be assessed with both intranasal and systemic GAS infection models. Furthermore, it remains to be determined whether the apparent lack of efficacy against a skin challenge found in this study is also seen for other vaccine candidates that have looked promising in oral and nasal challenge studies (1).
Despite the presence of high anti-Sfb1 titers in the serum of vaccinated mice, GAS strain A20 was still able to grow and disseminate at a rate comparable to that in naive mice. In the NT, the most common focus of invasive infection is the skin (5). In this population, high serum anti-Sfb1 titers are also seen in both aboriginal controls and aboriginal patients with defined streptococcal infections (14), and yet this immune response does not offer protection against systemic GAS infection. Together these observations suggest that high anti-Sfb1 titers in serum do not prevent dissemination of GAS into deeper tissues from the skin. The results of this study appear to reflect epidemiological observations in the NT, where skin infection predisposes to severe GAS infection despite high IgG antibody titers against Sfb1 in the population (14).
In summary, while an anti-Sfb1 immune response protects against pharyngeal colonization (15, 30), this response is inadequate for protection against systemic infections as a consequence of skin colonization. Thus, while Sfb1 may be a useful vaccine candidate in regions where the throat is the primary site of infection, this antigen may not fulfil such a role in regions where the skin is the primary infection site.
Acknowledgments
This work was supported, in part, by the National Health and Medical Research Council of Australia.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Batzloff, M. R., W. A. Hayman, M. R. Davies, M. Zeng, S. Pruksakorn, E. R. Brandt, and M. F. Good. 2003. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187:1598-1608. [DOI] [PubMed] [Google Scholar]
- 2.Bessen, D., and V. A. Fischetti. 1988. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect. Immun. 56:2666-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, E. R., W. A. Hayman, B. Currie, J. Carapetis, D. C. Jackson, K. A. Do, and M. F. Good. 1999. Functional analysis of IgA antibodies specific for a conserved epitope within the M protein of group A streptococci from Australian aboriginal endemic communities. Int. Immunol. 11:569-576. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis, J. R., and B. J. Currie. 1998. Preventing rheumatic heart disease in Australia. Med. J. Aust. 168:428-429. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis, J. R., A. M. Walker, M. Hibble, K. S. Sriprakash, and B. J. Currie. 1999. Clinical and epidemiological features of group A streptococcal bacteraemia in a region with hyperendemic superficial streptococcal infection. Epidemiol. Infect. 122:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. [DOI] [PubMed] [Google Scholar]
- 7.Courtney, H. S., J. B. Dale, and D. L. Hasty. 2000. Strategies for preventing group A streptococcal adhesion and infection, p. 553-579. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods and applications, vol. 35. Humana Press Inc., Totowa, N.J.
- 8.Courtney, H. S., D. L. Hasty, and J. B. Dale. 2003. Serum opacity factor (SOF) of Streptococcus pyogenes evokes antibodies that opsonize homologous and heterologous SOF-positive serotypes of group A streptococci. Infect. Immun. 71:5097-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney, H. S., D. L. Hasty, J. B. Dale, and T. P. Poirier. 1992. A 28-kilodalton fibronectin-binding protein of group A streptococci. Curr. Microbiol. 25:245-250. [DOI] [PubMed] [Google Scholar]
- 10.Courtney, H. S., Y. Li, J. B. Dale, and D. L. Hasty. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 62:3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie, B. J., and J. R. Carapetis. 2000. Skin infections and infestations in aboriginal communities in northern Australia. Australas. J. Dermatol 41:139-145. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner, D. L., and K. S. Sriprakash. 1996. Molecular epidemiology of impetiginous group A streptococcal infections in aboriginal communities of northern Australia. J. Clin. Microbiol. 34:1448-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodfellow, A. M., M. Hibble, S. R. Talay, B. Kreikemeyer, B. J. Currie, K. S. Sriprakash, and G. S. Chhatwal. 2000. Distribution and antigenicity of fibronectin binding proteins (SfbI and SfbII) of Streptococcus pyogenes clinical isolates from the Northern Territory, Australia. J. Clin. Microbiol. 38:389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman, C. A., S. R. Talay, G. Molinari, E. Medina, and G. S. Chhatwal. 1999. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect. Dis. 179:901-906. [DOI] [PubMed] [Google Scholar]
- 16.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus, Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasty, D. L., and H. S. Courtney. 1996. Group A streptococcal adhesion. All of the theories are correct. Adv. Exp. Med. Biol. 408:81-94. [PubMed] [Google Scholar]
- 18.Jaffe, J., S. Natanson-Yaron, M. G. Caparon, and E. Hanski. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21:373-384. [DOI] [PubMed] [Google Scholar]
- 19.Jeng, A., V. Sakota, Z. Li, V. Datta, B. Beall, and V. Nizet. 2003. Molecular genetic analysis of a group A streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 185:1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawabata, S., E. Kunitomo, Y. Terao, I. Nakagawa, K. Kikuchi, K. Totsuka, and S. Hamada. 2001. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect. Immun. 69:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreikemeyer, B., S. R. Talay, and G. S. Chhatwal. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17:137-145. [DOI] [PubMed] [Google Scholar]
- 22.Medina, E., O. Goldmann, M. Rohde, A. Lengeling, and G. S. Chhatwal. 2001. Genetic control of susceptibility to group A streptococcal infection in mice. J. Infect. Dis. 184:846-852. [DOI] [PubMed] [Google Scholar]
- 23.Medina, E., O. Goldmann, A. W. Toppel, and G. S. Chhatwal. 2003. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187:597-603. [DOI] [PubMed] [Google Scholar]
- 24.Medina, E., G. Molinari, M. Rohde, B. Haase, G. S. Chhatwal, and C. A. Guzman. 1999. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J. Immunol. 163:3396-3402. [PubMed] [Google Scholar]
- 25.Medina, E., S. R. Talay, G. S. Chhatwal, and C. A. Guzman. 1998. Fibronectin-binding protein I of Streptococcus pyogenes promotes T cell-independent proliferation of murine B lymphocytes and enhances the expression of MHC class II molecules on antigen-presenting cells. Int. Immunol. 10:1657-1664. [DOI] [PubMed] [Google Scholar]
- 26.Molinari, G., S. R. Talay, P. Valentin-Weigand, M. Rohde, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 28.Rakonjac, J. V., J. C. Robbins, and V. A. Fischetti. 1995. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect. Immun. 63:622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha, C. L., and V. A. Fischetti. 1999. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect. Immun. 67:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze, K., E. Medina, S. R. Talay, R. J. Towers, G. S. Chhatwal, and C. A. Guzman. 2001. Characterization of the domain of fibronectin-binding protein I of Streptococcus pyogenes responsible for elicitation of a protective immune response. Infect. Immun. 69:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talay, S. R., P. Valentin-Weigand, P. G. Jerlstrom, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talay, S. R., P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1994. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 13:531-539. [DOI] [PubMed] [Google Scholar]
- 33.Terao, Y., S. Kawabata, E. Kunitomo, J. Murakami, I. Nakagawa, and S. Hamada. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 42:75-86. [DOI] [PubMed] [Google Scholar]
- 34.Terao, Y., S. Kawabata, M. Nakata, I. Nakagawa, and S. Hamada. 2002. Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J. Biol. Chem. 277:47428-47435. [DOI] [PubMed] [Google Scholar]
- 35.Valentin-Weigand, P., S. R. Talay, A. Kaufhold, K. N. Timmis, and G. S. Chhatwal. 1994. The fibronectin binding domain of the Sfb protein adhesin of Streptococcus pyogenes occurs in many group A streptococci and does not cross-react with heart myosin. Microb. Pathog. 17:111-120. [DOI] [PubMed] [Google Scholar]
- 36.Wizemann, T. M., J. E. Adamou, and S. Langermann. 1999. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 5:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]