Abstract
Surfactant proteins A (SP-A) and D (SP-D) play an important role in the innate immune defenses of the respiratory tract. SP-A binds to the lipid A region of lipopolysaccharide (LPS), and SP-D binds to the core oligosaccharide region. Both proteins induce aggregation, act as opsonins for neutrophils and macrophages, and have direct antimicrobial activity. Bordetella pertussis LPS has a branched core structure and a nonrepeating terminal trisaccharide. Bordetella bronchiseptica LPS has the same structure, but lipid A is palmitoylated and there is a repeating O-antigen polysaccharide. The ability of SP-A and SP-D to agglutinate and permeabilize wild-type and LPS mutants of B. pertussis and B. bronchiseptica was examined. Previously, wild-type B. pertussis was shown to resist the effects of SP-A; however, LPS mutants lacking the terminal trisaccharide were susceptible to SP-A. In this study, SP-A was found to aggregate and permeabilize a B. bronchiseptica mutant lacking the terminal trisaccharide, while wild-type B. bronchiseptica and mutants lacking only the palmitoyl transferase or O antigen were resistant to SP-A. Wild-type B. pertussis and B. bronchiseptica were both resistant to SP-D; however, LPS mutants of either strain lacking the terminal trisaccharide were aggregated and permeabilized by SP-D. We conclude that the terminal trisaccharide protects Bordetella species from the bactericidal functions of SP-A and SP-D. The O antigen and palmitoylated lipid A of B. bronchiseptica play no role in this resistance.
Pulmonary surfactant is a lipid, carbohydrate, and protein mixture that serves to lower the surface tension at the air-liquid interface of the lungs. Two protein components of surfactant, surfactant protein A (SP-A) and surfactant protein D (SP-D), play a role in resistance to microbial infection. SP-A and SP-D are members of the collectin (collagen-like lectin) family of proteins. Collectins have a globular carbohydrate recognition domain (CRD) head region, an amphipathic helical neck region, an extended collagen-like domain, and a disulfide-bonded tail (for reviews see references 14 and 22). Three monomers of SP-A or SP-D form a triple helix via the neck and collagen-like domain (14). In the case of SP-A, six trimers assemble into a bouquet-of-tulips structure, similar to complement protein C1q, while four SP-D trimers associate tail to tail into a dodecameric cruciform structure (15).
SP-A and SP-D knockout mice were observed to have increased susceptibility to infection by a number of viruses, bacteria, and fungi (6, 17, 19, 20), and SP-A and SP-D have been shown to have a number of antimicrobial functions. The CRD heads of SP-A and SP-D mediate calcium-dependent binding to a variety of carbohydrates present on microorganisms, and the presence of multiple binding domains on a single molecule can promote aggregation (4, 7, 12, 23, 41). SP-A and SP-D also enhance uptake and killing by phagocytic cells (11, 16, 20, 23, 31). Recently, binding of SP-A and SP-D has been shown to permeabilize susceptible gram-negative bacteria, leading to microbial death (40).
Both SP-A and SP-D bind to the lipopolysaccharide (LPS) of gram-negative bacteria (18, 35). The LPS of most gram-negative bacteria consists of a lipid A domain, a relatively conserved core oligosaccharide, and a highly variable O-antigen polysaccharide. SP-A has been shown to bind to the lipid A portion of purified LPS (35), while SP-D has been shown to bind to the core oligosaccharide (18). The respiratory pathogen Bordetella pertussis, the causative agent of human whooping cough (32), has a typical lipid A region linked to a branched core oligosaccharide by a single ketodeoxyoctulosonic acid (Kdo) residue (Fig. 1). It lacks a polymerized O-side chain but has a nonrepeating trisaccharide consisting of α-N-acetylglucosamine (GlcNAc), β-2-acetamido-3-acetamido-2,3-dideoxy-mannuronic acid (diNAcManA), and β-l-2-acetamido-4-methylamino-fucose (FucNAcMe) (8, 26). Expression of the trisaccharide is controlled by a 12-gene operon, known as the wlb locus (1, 3). The wlbG gene is necessary for the synthesis of FucNAcMe, and WlbG mutants fail to express the entire terminal trisaccharide (1, 3). The WaaF protein adds a heptose residue to the Kdo, and WaaF mutants produce a truncated core and also fail to express the terminal trisaccharide (2). Previous studies demonstrated that SP-A does not bind to wild-type B. pertussis (32). However, SP-A was able to bind to strains with mutations in the wlb operon, which is responsible for the addition of the terminal trisaccharide, and these mutants were susceptible to the SP-A-mediated antimicrobial functions (32).
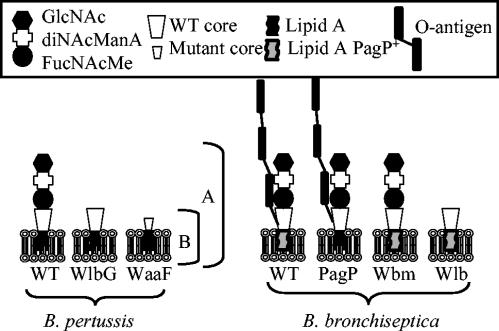
FIG. 1.
Schematic of the LPS of wild-type and mutant B. pertussis and B. bronchiseptica. B. pertussis LPS has lipid A, a branched-core oligosaccharide, and a nonrepeating trisaccharide composed of GlcNAc, diNAcManA, and FucNAcMe. Full-length band A LPS (A) and truncated band B LPS (B), lacking the trisaccharide, are indicated. The WlbG mutant of B. pertussis fails to add the trisaccharide and only produces band B LPS. The WaaF mutant lacks the terminal trisaccharide, has only a single heptose in the core, and fails to react with the band B antibody. B. bronchiseptica LPS is similar to that of B. pertussis but has an additional repeating O-antigen homopolymer of 2,3-di-NAcGalA and palmitoylated lipid A. The PagP mutant fails to palmitoylate lipid A. The Wbm mutant of B. bronchiseptica has a deletion of the wbmB to wbmE genes, fails to add the O antigen, and produces band A LPS similar to that of wild-type B. pertussis. The Wlb mutant of B. bronchiseptica has a deletion in the operon required for the addition of the trisaccharide. Failure to add the trisaccharide appears to prevent addition of the O antigen, and the LPS produced by the Wlb mutant of B. bronchiseptica is similar to the band B LPS produced by the WlbG mutant of B. pertussis.
Bordetella bronchiseptica is the causative agent of a number of important veterinary diseases, including kennel cough in dogs and atrophic rhinitis in pigs. B. bronchiseptica is evolutionarily closely related to B. pertussis, and it has been suggested to be the recent ancestor of B. pertussis (25). The LPS of B. bronchiseptica is similar to that of B. pertussis (Fig. 1), but it has two major differences. B. bronchiseptica LPS contains a repeating O-antigen structure comprised of a homopolymer of2,3-dideoxy-2,3-di-N-acetylgalactosaminuronic acid (2,3-di-NAcGalA). The Wbm operon is responsible for synthesis of O antigen, and mutants in this operon lack O-antigen expression (28). The Wlb operon of B. bronchiseptica directs the synthesis of the terminal trisaccharide, and a B. bronchiseptica strain deleted for the Wlb operon, like the WlbG mutant of B. pertussis, fails to express terminal trisaccharide. Interestingly, however, the Wlb mutant also lacks expression of the O antigen (3). B. bronchiseptica also possesses a functional copy of the pagP gene. PagP adds a palmitate to lipid A (29). The pagP gene in B. pertussis has an insertion sequence element in the promoter region, and PagP palmitoylation has not been observed in B. pertussis (29). In this study we examined the interaction of SP-A and SP-D with wild-type and LPS mutant strains of B. pertussis and B. bronchiseptica. The unique LPS structure of the bordetellae provides an opportunity to examine the structural basis of the interaction between collectins and LPS.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are described in Table 1. For the phagocytosis studies, we used B. pertussis derivatives containing plasmid pGB5P1, pCW505, or pCW382-6 encoding green fluorescent protein (GFP), as described in previous studies (36). B. pertussis and B. bronchiseptica strains were grown with the appropriate selective antibiotics on Bordet Gengou (BG) agar (Becton Dickinson, Sparks, Md.) supplemented with 15% defibrinated sheep's blood. Antibiotics were used at the following concentrations: gentamicin sulfate, 30 μg/ml; nalidixic acid, 30 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 15 μg/ml; streptomycin, 100 μg/ml; erythromycin, 5 μg/ml. A human fecal isolate of Klebsiella pneumoniae was grown on Luria-Bertani (LB) agar (Becton Dickinson), and B. bronchiseptica was grown in LB broth.
TABLE 1.
Bacterial strains used in this study
| Strain | GFP plasmid | Phenotypea | Reference |
|---|---|---|---|
| B. pertussis | |||
| BP338 | pGB5P1 | B. pertussis Tohama I, wild-type LPS, Nalr, Kanr | 39 |
| WlbG | pCW382-6 | BP536, ΔwlbG::Kan; Nalr, Kanr, Strepr, Cmr | 1, 3 |
| WaaF | pCW382-6 | BP338, waaF::pLMS023, Nalr, Kanr, Gentr, Cmr | 32 |
| B. bronchiseptica | |||
| RB50 | B. bronchiseptica wild type, isolated from rabbit | 9 | |
| RB54 | RB50, deletion in bvgS, avirulent | 9 | |
| Wbm | RB50, ΔwbmB-E::Cm; Strepr, Cmr | 28 | |
| Wlb | RB50, Δwlb::Cm; Strepr, Cmr | 3 | |
| PagP | RB50, Δpag::Eryc; Strepr, Erycr | 29 | |
| K. pneumoniae | |||
| K.pn. | Human fecal isolate, susceptible to SP-A and SP-D mediated killing | 40 |
Gentr, gentamicin resistant; Cmr, chloramphenicol resistant; Kanr, kanamycin resistant; Nalr, nalidixic acid resistant; Strepr, streptomycin resistant; Erycr, erythromycin resistant.
Isolation and characterization of LPS.
Mutant and wild-type LPS was isolated by incubating bacteria with 0.1 mg of proteinase K (Sigma, St. Louis, Mo.)/ml for 1.5 h at 56°C, followed by acetone precipitation (32). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), silver staining (34), and immunoblotting were performed as previously described (32). Following SDS-PAGE, gels were transferred to Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Mass.) and probed with primary antibodies at a 1:1,000 dilution. Primary antibodies were the following: band A LPS, monoclonal BL-2 (5); band B LPS, monoclonal antibody BL-8 (21).
SP-A and SP-D.
Human SP-A was purified from lung washings of patients with the lung disease alveolar proteinosis as previously described (40). Rat SP-D was purified from the bronchoalveolar lavage (BAL) of silica-pretreated Sprague-Dawley rats as previously described (40). Recombinant mouse SP-D was purified from insect cells as previously described (40).
Aggregation by SP-A or SP-D.
The ability of SP-A or SP-D to aggregate strains of B. pertussis and B. bronchiseptica was determined as previously described (32). Briefly, bacteria were grown overnight on BG agar and suspended to an optical density at 600 nm (OD600) of approximately 0.6 in Stainer-Schulte (SS) salts containing 0.135 mM CaCl2, which is sufficient to support the calcium-dependent activities of the surfactant proteins. Purified SP-D was added at 5 or 10 μg/ml as indicated in the figure legends, and SP-A was added at 10 μg/ml. Samples were incubated at 37°C with shaking at 150 rpm, and the OD600 was determined after 3 h.
Assessment of membrane integrity.
Bacterial membrane integrity was assessed using the membrane-impermeant fluorescent probe propidium iodide (Molecular Probes, Eugene, Oreg.) as previously described (32). Briefly, bacteria were grown on agar media for 16 h and suspended in SS salts prewarmed to 37°C to an OD600 of approximately 0.07. Bacterial controls or bacteria with SP-A (100 μg/ml) or SP-D (10 μg/ml) in a final volume of 250 μl in a 5-ml polypropylene tube were incubated for 2 h at 37°C with shaking. An equal volume of propidium iodide in SS salts was added to a final concentration of 30 μM, and samples were incubated for 15 min at room temperature, protected from light. Bacteria were pelleted and suspended in fresh SS salts to one-tenth the original volume, and 7 μl was mounted on a glass slide. Samples were observed by phase-contrast microscopy to identify the bacterial cells and under a Texas Red filter (excitation, 560 nm; emission, 630 nm) to assess propidium iodide staining with a Zeiss Axioplan 2 microscope. Images were photographed with a Hamamatsu CCD digital camera. At least three fields were photographed for each slide, and three independent experiments were performed. Representative images from each group are shown.
Assessment of viability.
Viability following treatment with SP-A and SP-D was by monitoring incorporation of [3H]uridine as previously described (40). Briefly, wild-type B. bronchiseptica strain RB50 and the Wlb mutant were grown overnight in SS salts at 37°C with shaking and were centrifuged, washed, and suspended at an OD600 of approximately 0.04 in 50% SS salts and 50% HBSS (Hank's balanced salt solution; BioWhittaker, Rockland, Md.) containing 40 mM Tris (pH 7.4), 0.1% Casamino Acids, 0.9% NaCl, and 0.4 μCi of [3H]uridine (Amersham Pharmacia Biotech, Piscataway, N.J.)/ml in the presence or absence of 50 μg of SP-A/ml or 20 μg of recombinant mouse SP-D/ml. The low-molecular-weight filtrate (<10,000 Da), which was not retained in the dialysis tubing from the dialysis step in the purification of SP-A, served as a negative control. The bacteria were incubated at 37°C with shaking for 3 h. The reaction was stopped by addition of ice-cold 10% trichloroacetic acid. The precipitated cell products were captured on filter membranes and counted in a scintillation counter.
Phagocytosis by rat alveolar macrophages.
Alveolar macrophages were harvested from adult Harlan rats by three serial lung lavages with 10 ml of phosphate-buffered saline (PBS) each, pelleted, and suspended to 6 × 106 per ml in HBSS with 10 mM HEPES (Sigma) and 10% fetal bovine serum (Gibco). One milliliter of cells was added to the wells of a sterile 24-well tissue culture dish containing glass coverslips, and the cells were incubated for 1 h at 37°C and 5% CO2 to allow for adherence of the macrophages. For SP-D opsonization, SP-D was added to bacteria at 10 μg/ml and was incubated for 15 min at 37°C, while control bacteria were incubated without SP-D. The samples were diluted to 400 μl and were added to wells containing adherent macrophages. GFP-expressing bacteria were added at a multiplicity of infection (MOI) of approximately 5, the plates were centrifuged for 5 min at 640 × g to facilitate contact between the bacteria and the macrophages, and the solution was incubated for 1 h at 37°C and 5% CO2 to allow phagocytosis to occur. Following phagocytosis, wells were washed three times with HBSS containing 10 mM HEPES to remove nonadherent bacterial cells, and the coverslips were stained with ethidium bromide and processed as previously described (36-38). Slides were observed by fluorescence microscopy under a GFP filter (excitation, 470; emission, 535). Adherent B. pertussis will take up the ethidium bromide and appear orange under fluorescence microscopy, while the internalized B. pertussis cells resist ethidium bromide staining and remain green fluorescent (36-38). The numbers of adherent and internalized bacteria were counted for 100 macrophages per coverslip, performed in triplicate, with at least three independent repetitions. Statistical analysis was performed using Student's t test. B. bronchiseptica resists staining with ethidium bromide, and phagocytosis was not characterized for these strains.
RESULTS AND DISCUSSION
Characterization of LPS.
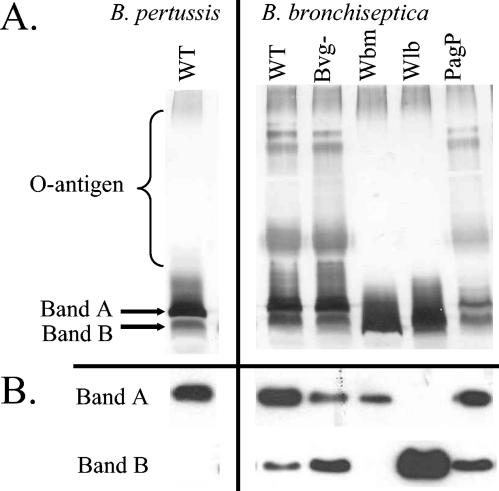
The LPS phenotypes of the strains used in this study are summarized in Fig. 1. LPS and especially O-antigen expression (29) can be influenced by growth conditions, and LPS expression under the growth conditions used in this study was characterized. B. pertussis LPS often resolves as two bands on silver-stained polyacrylamide gels, referred to as band A and band B (26). Band A represents the full-length LPS molecule, including lipid A, core oligosaccharide, and the terminal trisaccharide. Band B is a biosynthetic intermediate for band A, and band B represents incomplete LPS lacking the terminal trisaccharide (Fig. 1). Band A was the predominant form observed in silver-stained gels of LPS from wild-type B. pertussis (Fig. 2A) and on Western blots (Fig. 2B). The lack of reactivity of the wild type with the monoclonal antibody to band B suggests that this intermediate fails to accumulate in the wild-type strain, and the faster-migrating bands may represent other LPS intermediates.
FIG. 2.
LPS production by wild-type and mutant B. pertussis and B. bronchiseptica. (A) LPS was separated by SDS-PAGE gels and silver stained. O antigen and bands A and B are indicated. (B) Bacterial cells were separated by SDS-PAGE and transferred to PVDF membranes. Band A, immunoblot probed with monoclonal antibody to band A; band B, immunoblot probed with monoclonal antibody to band B. B. pertussis WT, wild-type strain BP338; B. bronchiseptica WT, wild-type strain RB50; Bvg−, strain RB54.
The LPS from the B. bronchiseptica Bvg-minus mutant was characterized. The Bvg (Bordetella virulence gene) regulatory locus controls transcription of numerous genes known to play a role in virulence, and previous studies have shown that Bvg mutants also fail to express PagP (39). On silver-stained gels all three strains expressed O antigen (Fig. 2A). Both the Bvg mutant and the PagP mutant are unable to modify lipid A (29); however, modified and unmodified lipid A are not resolved by standard electrophoresis conditions. Band A and band B expression is most clearly observed on Western blots; the wild type, Bvg-minus mutant, and PagP mutant all expressed both band A and band B LPS (Fig. 2B).
The Wbm and Wlb mutants of B. bronchiseptica failed to express the O antigen (Fig. 2A). The Wbm mutant of B. bronchiseptica has a deletion in the genes needed for O-antigen expression and expressed predominantly band A LPS (Fig. 2B), a structure that is similar to that of wild-type B. pertussis. The B. bronchiseptica Wlb mutant has an insertion in the genes required for the addition of the terminal trisaccharide to the core oligosaccharide (13). Wlb only expressed band B LPS (Fig. 2B). As previously shown (13), the Wlb mutant failed to express O antigen (Fig. 2A). The O antigen of B. bronchiseptica LPS is attached to the core, not the trisaccharide, and it is not known why deletion of the wlb locus prevents expression of the O antigen (13). It is possible that the Wbm enzyme that attaches the O antigen to the core only when full-length band A LPS is present.
Aggregation of B. bronchiseptica by SP-A.
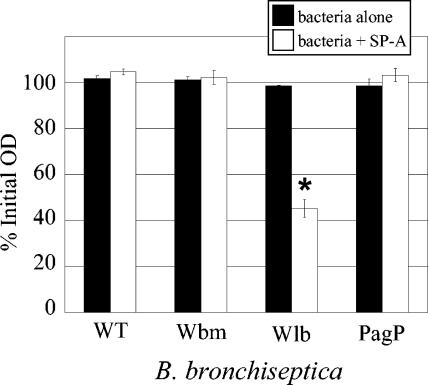
The ability of SP-A to promote aggregation of B. bronchiseptica was examined. Bacterial suspensions were incubated with or without SP-A and the optical density was monitored. In the absence of SP-A, none of the strains demonstrated a significant drop in optical density (Fig. 3) over 3 h, indicating that the bacteria remained in suspension. When SP-A was added, only the optical density of the Wlb strain decreased significantly (P < 0.5), suggesting that this mutant was aggregated by SP-A. These results are similar to those observed with B. pertussis, where SP-A was only able to aggregate mutants lacking one or more of the sugars of the terminal trisaccharide (32).
FIG. 3.
Aggregation of B. bronchiseptica by SP-A. Bacteria were suspended to an OD600 of 0.6 in SS salts. Bacteria alone or bacteria with purified human SP-A (10 μg/ml) were incubated at 37°C, shaking for 3 h. Values are reported as a percentage of the starting OD600 and bars represent means ± standard errors of the means. Only the Wlb strain showed a statistically significant (P ≤ 0.05) difference by Student's t test from the no SP-A control.
Bactericidal activity of SP-A.
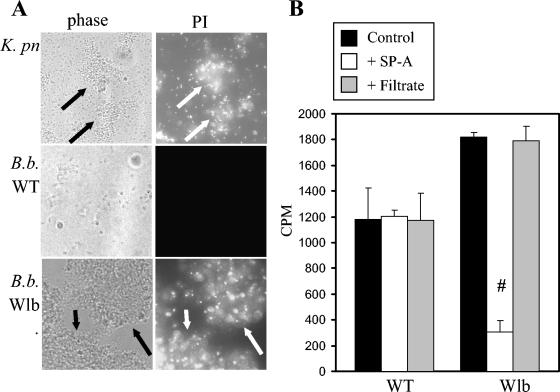
Both SP-A and SP-D are able to directly inhibit the growth of some gram-negative bacteria by permeabilizing the bacterial membrane via a CRD-mediated mechanism (40). To test for SP-A-mediated membrane permeability, the bacteria were incubated with SP-A for 2 h and the membrane-impermeant DNA-specific fluorescent dye propidium iodide was used as an indicator of membrane permeability. A K. pneumoniae strain, known to be susceptible to SP-A and SP-D (40), was used as a positive control. Bacterial aggregation was not seen in the absence of SP-A when the organisms were viewed by phase-contrast microscopy (data not shown). In the presence of SP-A, the wild-type strain (Fig. 4A) and the Wbm and PagP mutants (data not shown) were not aggregated or permeabilized by SP-A. However, the K. pneumoniae strain and the B. bronchiseptica Wlb mutant were aggregated and permeabilized by SP-A as evidenced by propidium iodide staining of the bacterial clusters (Fig. 4A).
FIG. 4.
Viability of SP-A-treated B. bronchiseptica. Bacteria incubated with SP-A were assessed for membrane permeability (A) or incorporation of [3H]uridine (B). (A) Membrane permeability was assessed by propidium iodide. The same field was photographed (630× magnification) with phase-contrast microscopy (phase) to show the location of all bacteria present in the field and with fluorescence microscopy with a Texas Red filter to show organisms stained by propidium iodide (PI). Arrows indicate aggregates. K. pn, K. pneumoniae; B.b. WT, B. bronchiseptica RB50. (B) Incorporation of [3H]uridine (in counts per minute [cpm]) was assessed for bacteria grown in LB broth (control), LB broth with 50 μg of SP-A/ml (+SP-A), or LB broth in the presence of the low-molecular-weight filtrate (<10,000 Da) from dialysis of SP-A (+Filtrate) lacking SP-A. Bars represent means ± standard errors of the means for three independent trials. #, significantly different cpm compared to that of the control (P < 0.002) by Student's t test.
To confirm that membrane permeability was associated with bacterial death, we monitored uptake of [3H]uridine in the presence or absence of SP-A (Fig. 4B). Wild-type strain RB50 incorporated similar amounts of uridine under all growth conditions tested. In contrast, the ability of the Wlb mutant to incorporate uridine was significantly inhibited by SP-A but not by the filtrate from the SP-A purification, which lacks SP-A. These results corroborate previous studies that demonstrated that SP-A-mediated permeability is associated with loss of viability (40).
Aggregation of B. pertussis and B. bronchiseptica by SP-D.
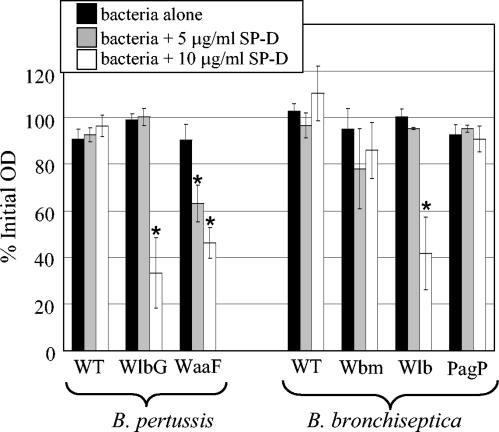
The ability of SP-D to promote aggregation was also examined. In the absence of SP-D, none of the strains demonstrated a significant drop in optical density (Fig. 5, black bars). In the presence of 5 μg of SP-D/ml, only the B. pertussis WaaF mutant lacking the full-length core was aggregated (Fig. 5, gray bars). When 10 μg of SP-D was added/ml, the strains lacking the terminal trisaccharide, the B. pertussis WlbG and B. bronchiseptica Wlb mutants, were also aggregated (Fig. 5, white bars), indicating that the terminal trisaccharide and, to a lesser extent, the full-length branched core, confer resistance to SP-D binding.
FIG. 5.
Aggregation of B. pertussis and B. bronchiseptica in the presence of SP-D. Bacteria were suspended to an OD600 of 0.6 in SS salts. Bacteria alone or bacteria with purified rat SP-D added at the indicated concentrations were incubated at 37°C with shaking for 3 h. Values are reported as a percentage of the starting OD600, and bars represent means ± standard errors of the means. An asterisk indicates a statistically significant (P ≤ 0.05) difference from the no SP-D control. B. pertussis WT, strain BP338; B. bronchiseptica WT, strain RB50.
Membrane permeabilization by SP-D.
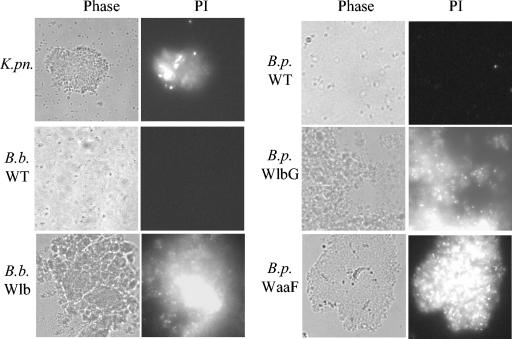
The ability of SP-D to permeabilize wild-type and mutant B. pertussis and B. bronchiseptica was also examined. Bacteria incubated with SP-D were stained with propidium iodide and examined by phase-contrast and fluorescence microscopy. The wild-type B. pertussis or B. bronchiseptica (Fig. 6), as well as the B. bronchiseptica PagP and Wbm mutants (data not shown), were not aggregated by SP-D or stained by propidium iodide in the presence or absence of SP-D. In contrast, the SP-D susceptible K. pneumoniae strain, the B. bronchiseptica Wlb mutant, and the B. pertussis WlbG and WaaF mutants were aggregated and permeabilized by SP-D (Fig. 6).
FIG. 6.
Membrane permeability of SP-D-treated B. pertussis and B. bronchiseptica. Bacteria were incubated with 10 μg of SP-D/ml for 2 h and were assessed for membrane permeability by using propidium iodide. The same field was photographed (630× magnification) with phase-contrast microscopy (Phase) to show the location of all bacteria present in the field and with fluorescence microscopy with a Texas Red filter to show organisms stained by propidium iodide (PI). K. pn, K. pneumoniae; B. b. WT, B. bronchiseptica RB50; B.p. WT, B. pertussis BP338.
The ability of SP-D to prevent incorporation of [3H]uridine was also examined. In the presence of 20 μg of mouse recombinant SP-D/ml, an 80% reduction in [3H]uridine incorporation was observed for the B. bronchiseptica Wlb mutant; however, no effect was seen for the wild-type B. bronchiseptica strain (date not shown). These data suggest that band A LPS containing the terminal trisaccharide confers protection from the bactericidal activities of SP-D.
Effects of SP-D on phagocytosis of B. pertussis by rat alveolar macrophages.
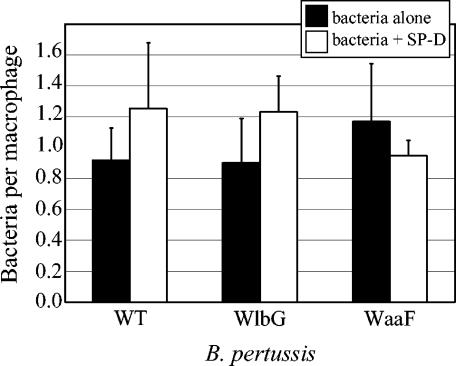
Previous studies have demonstrated a role for SP-D in enhancing the uptake of a variety of opsonized particles by monocytes and macrophages (20, 24, 27, 30, 31). Studies of Pseudomonas aeruginosa (31) and Mycobacterium tuberculosis (10) have shown that SP-D can affect phagocytosis without mediating aggregation, indicating that these two functions are separate. We examined the effects of SP-D opsonization on phagocytosis of wild-type and LPS mutant strains of B. pertussis by rat alveolar macrophages (Fig. 7). As observed in previous studies with human peripheral blood monocytes (33), phagocytosis of unopsonized B. pertussis by rat alveolar macrophages was relatively inefficient, with less than one internalized bacterium per monocyte when experiments were conducted at an MOI of 5 (Fig. 7). Internalization of wild-type and LPS mutant strains by rat alveolar macrophages was similar in the presence or absence of SP-D (Fig. 7). Phagocytosis of B. pertussis by monocytes (33) and macrophages (Fig. 3) appears to be inherently inefficient, and resistance to phagocytosis does not correlate with expression of wild-type LPS or protein virulence factors (33).
FIG. 7.
Effects of SP-D on internalization of B. pertussis by rat alveolar macrophages. Bacteria alone or bacteria incubated with SP-D (10 μg/ml) were added at an MOI of 5 to alveolar macrophages from adult Harlan rats, and phagocytosis was allowed to occur for 1 h. The number of internalized bacteria was determined by fluorescence microscopy. Samples were prepared in triplicate for each experiment, and at least three independent experiments were performed. Data represent means ± standard errors of the means.
It was previously reported that wild-type LPS of B. pertussis is resistant to binding, aggregation, opsonization, and permeabilization by SP-A (32). In this study, we have shown that Bordetella strains expressing the terminal trisaccharide, a structure equivalent to wild-type B. pertussis LPS, are resistant to aggregation by both SP-A and SP-D. Membrane permeabilization was observed only in cases where SP-A or SP-D was able to aggregate the bacteria.
Expression of the intact, branched core appears to confer additional resistance to SP-D, because the WaaF mutant, which produces a core consisting of only a single heptose (2), was aggregated at lower doses of SP-D than the Wlb trisaccharide mutants, which produce intact core. In studies of SP-D binding to a variety of Salmonella minnesota LPS mutant strains, SP-D bound to Rd mutant strains which express a terminal heptose molecule, but not to LPS mutants lacking this residue, strongly implicating heptose residues as a binding site for SP-D on gram-negative bacteria (18). Furthermore, SP-D bound less efficiently to S. minnesota mutants with less extensive truncations of their LPS than the Rd mutant (18). The increased susceptibility of the WaaF mutant compared to that of the Wlb mutants is consistent with these findings.
B. bronchiseptica produces LPS with an O antigen and palmitoylated lipid A. Neither of these modifications altered susceptibility to SP-A or SP-D. Previous studies have shown that the PagP mutants of B. bronchiseptica are cleared more rapidly than wild-type strains in a mouse model of infection (29). Our studies suggest that this difference is not due to susceptibility to SP-A or SP-D.
The pulmonary surfactant proteins represent an important first line of defense in the respiratory tract. The inability of SP-A and SP-D to recognize the LPS expressed by the Bordetella species to may contribute to their success as respiratory pathogens.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 AI45715 (A.A.W.) and HL61612 (F.X.M.). L.M.S. was the recipient of a University Research Council Summer Graduate Student Research Fellowship at the University of Cincinnati.
We wish to acknowledge the technical assistance of Alexander Kuzmenko, Sijue Wan, and Christopher Harrison.
Editor: J. T. Barbieri
REFERENCES
- 1.Allen, A., and D. Maskell. 1996. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 19:37-52. [DOI] [PubMed] [Google Scholar]
- 2.Allen, A. G., T. Isobe, and D. J. Maskell. 1998. Identification and cloning of waaF (rfaF) from Bordetella pertussis and use to generate mutants of Bordetella spp. with deep rough lipopolysaccharide. J. Bacteriol. 180:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, A. G., R. M. Thomas, J. T. Cadisch, and D. J. Maskell. 1998. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol. Microbiol. 29:27-38. [DOI] [PubMed] [Google Scholar]
- 4.Allen, M. J., D. R. Voelker, and R. J. Mason. 2001. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect. Immun. 69:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archambault, D., P. Rondeau, D. Martin, and B. R. Brodeur. 1991. Characterization and comparative bactericidal activity of monoclonal antibodies to Bordetella pertussis lipo-oligosaccharide A. J. Gen. Microbiol. 137:905-911. [DOI] [PubMed] [Google Scholar]
- 6.Brinker, K. G., E. Martin, P. Borron, E. Mostaghel, C. Doyle, C. V. Harding, and J. R. Wright. 2001. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L1453-L1463. [DOI] [PubMed] [Google Scholar]
- 7.Bufler, P., B. Schmidt, D. Schikor, A. Bauernfeind, E. C. Crouch, and M. Griese. 2003. Surfactant Protein A and D differently regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am. J. Respir. Cell. Mol. Biol. 28:249-256. [DOI] [PubMed] [Google Scholar]
- 8.Caroff, M., J. Brisson, A. Martin, and D. Karibian. 2000. Structure of the Bordetella pertussis 1414 endotoxin. FEBS Lett. 477:8-14. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson, J. S., D. R. Voelker, J. A. Ufnar, A. J. Dawson, and L. S. Schlesinger. 2002. Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is independent of bacterial agglutination. J. Immunol. 168:1309-1314. [DOI] [PubMed] [Google Scholar]
- 11.Hartshorn, K. L., E. Crouch, M. R. White, M. L. Colamussi, A. Kakkanatt, B. Tauber, V. Shepherd, and K. N. Sastry. 1998. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am. J. Physiol. 274:L958-L969. [DOI] [PubMed] [Google Scholar]
- 12.Hartshorn, K. L., M. R. White, and E. C. Crouch. 2002. Contributions of the N- and C-terminal domains of surfactant protein D to the binding, aggregation, and phagocytic uptake of bacteria. Infect. Immun. 70:6129-6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmskov, U., S. Thiel, and J. C. Jensenius. 2003. Collectins and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547-578. [DOI] [PubMed] [Google Scholar]
- 15.Holmskov, U. L. 2000. Collectins and collectin receptors in innate immunity. APMIS Suppl. 100:1-59. [PubMed] [Google Scholar]
- 16.Kabha, K., J. Schmegner, Y. Keisari, H. Parolis, J. Schlepper-Schaeffer, and I. Ofek. 1997. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am. J. Physiol. 272:L344-L352. [DOI] [PubMed] [Google Scholar]
- 17.Korfhagen, T. R., A. M. LeVine, and J. A. Whitsett. 1998. Surfactant protein A (SP-A) gene targeted mice. Biochim. Biophys. Acta 1408:296-302. [DOI] [PubMed] [Google Scholar]
- 18.Kuan, S. F., K. Rust, and E. Crouch. 1992. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J. Clin. Investig. 90:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeVine, A. M., J. A. Whitsett, J. A. Gwozdz, T. R. Richardson, J. H. Fisher, M. S. Burhans, and T. R. Korfhagen. 2000. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165:3934-3940. [DOI] [PubMed] [Google Scholar]
- 20.LeVine, A. M., J. A. Whitsett, K. L. Hartshorn, E. C. Crouch, and T. R. Korfhagen. 2001. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 167:5868-5873. [DOI] [PubMed] [Google Scholar]
- 21.Martin, D., M. S. Peppler, and B. R. Brodeur. 1992. Immunological characterization of the lipooligosaccharide B band of Bordetella pertussis. Infect. Immun. 60:2718-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack, F. X., and J. A. Whitsett. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Investig. 109:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeely, T. B., and J. D. Coonrod. 1994. Aggregation and opsonization of type A but not type B Hemophilus influenzae by surfactant protein A. Am. J. Respir. Cell. Mol. Biol. 11:114-122. [DOI] [PubMed] [Google Scholar]
- 24.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. C. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J. Clin. Investig. 95:2699-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 26.Peppler, M. S. 1984. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect. Immun. 43:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pikaar, J. C., W. F. Voorhout, L. M. van Golde, J. Verhoef, J. A. Van Strijp, and J. F. van Iwaarden. 1995. Opsonic activities of surfactant proteins A and D in phagocytosis of gram-negative bacteria by alveolar macrophages. J. Infect. Dis. 172:481-489. [DOI] [PubMed] [Google Scholar]
- 28.Preston, A., A. G. Allen, J. Cadisch, R. Thomas, K. Stevens, C. M. Churcher, K. L. Badcock, J. Parkhill, B. Barrell, and D. J. Maskell. 1999. Genetic basis for lipopolysaccharide O-antigen biosynthesis in Bordetellae. Infect. Immun. 67:3763-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preston, A., E. Maxim, E. Toland, E. J. Pishko, E. T. Harvill, M. Caroff, and D. J. Maskell. 2003. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 48:725-736. [DOI] [PubMed] [Google Scholar]
- 30.Reid, K. B. 1998. Functional roles of the lung surfactant proteins SP-A and SP-D in innate immunity. Immunobiology 199:200-207. [DOI] [PubMed] [Google Scholar]
- 31.Restrepo, C. I., Q. Dong, J. Savov, W. I. Mariencheck, and J. R. Wright. 1999. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell. Mol. Biol. 21:576-585. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer, L. M., F. X. McCormack, H. Wu, and A. A. Weiss. 2004. Bordetella pertussis lipopolysaccharide resists the bactericidal effects of pulmonary surfactant protein A. J. Immunol. 173:1959-1965. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 69:7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 35.Van Iwaarden, J. F., J. C. Pikaar, J. Storm, E. Brouwer, J. Verhoef, R. S. Oosting, L. M. van Golde, and J. A. van Strijp. 1994. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem. J. 303:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weingart, C. L., G. Broitman-Maduro, G. Dean, S. Newman, M. Peppler, and A. A. Weiss. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weingart, C. L., P. S. Mobberley-Schuman, E. L. Hewlett, M. C. Gray, and A. A. Weiss. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 68:7152-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weingart, C. L., and A. A. Weiss. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect. Immun. 68:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, H., A. Kuzmenko, S. Wan, L. Schaffer, A. Weiss, J. H. Fisher, K. S. Kim, and F. X. McCormack. 2003. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 111:1589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yong, S. J., Z. Vuk-Pavlovic, J. E. Standing, E. C. Crouch, and A. H. Limper. 2003. Surfactant protein D-mediated aggregation of Pneumocystis carinii impairs phagocytosis by alveolar macrophages. Infect. Immun. 71:1662-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]