Abstract
Recent studies from mammalian, fish, and in vitro models have identified bone and cartilage development as sensitive targets for dioxins and other aryl hydrocarbon receptor ligands. In this study, we assess how embryonic 2,3,7,8-tetrachlorochlorodibenzo-p-dioxin (TCDD) exposure impacts axial osteogenesis in Japanese medaka (Oryzias latipes), a vertebrate model of human bone development. Embryos from inbred wild-type Orange-red Hd-dR and 3 transgenic medaka lines (twist:EGFP, osx/sp7:mCherry, col10a1:nlGFP) were exposed to 0.15 nM and 0.3 nM TCDD and reared until 20 dpf. Individuals were stained for mineralized bone and imaged using confocal microscopy to assess skeletal alterations in medial vertebrae in combination with a qualitative spatial analysis of osteoblast and osteoblast progenitor cell populations. Exposure to TCDD resulted in an overall attenuation of vertebral ossification characterized by truncated centra, and reduced neural and hemal arch lengths. Effects on mineralization were consistent with modifications in cell number and cell localization of transgene-labeled osteoblast and osteoblast progenitor cells. Endogenous expression of osteogenic regulators runt-related transcription factor 2 (runx2) and osterix (osx/sp7), and extracellular matrix genes osteopontin (spp1), collagen type I alpha I (col1), collagen type X alpha I (col10a1), and osteocalcin (bglap/osc) was significantly diminished at 20 dpf following TCDD exposure as compared with controls. Through global transcriptomic analysis more than 590 differentially expressed genes were identified and mapped to select pathological states including inflammatory disease, connective tissue disorders, and skeletal and muscular disorders. Taken together, results from this study suggest that TCDD exposure inhibits axial bone formation through dysregulation of osteoblast differentiation. This approach highlights the advantages and sensitivity of using small fish models to investigate how xenobiotic exposure may impact skeletal development.
Keywords: receptor, aryl hydrocarbon, gene expression/regulation, bone, transgenic models, methods, developmental/teratology, reproductive and developmental toxicology.
With more than 35 defined disorders, skeletal dysplasias represent a diverse class of congenital defects that occur approximately once in every 5000 human births (Orioli et al., 1986). Affected individuals display a range of functional deficits resulting from defects in early skeletal pattern formation or subsequent defects in growth and development of cartilage and bone (Krakow and Rimoin, 2010). In past decades, clinical and molecular studies have improved our understanding of mechanisms underlying skeletal dysplasias through identification of loss-of-heterozygosity (LOH) mutations in key osteogenic mediators (Hermanns and Lee, 2002).
Normal bone development requires strict temporal and spatial coordination of early gene regulatory networks that govern commitment of mesenchymal stem cells (MSCs) to an osteogenic lineage, and subsequent differentiation to become mature, matrix-secreting osteoblasts (Lefebvre and Bhattaram, 2010). These events integrate stimuli from canonical developmental pathways (Wnt, Hedgehog, Notch, Fibroblast growth factor, Bone morphogenetic protein signaling [Kneissel and Baron, 2013; Marcellini et al., 2012]) and other endocrine and paracrine mediators (Imai et al., 2013) to regulate osteoblast differentiation (for review see Karsenty et al., 2009; Sinha and Zhou, 2013). In mammals and teleosts, mature osteoblasts secrete extracellular bone matrix to form ossified, or mineralized bone comprising the appendicular skeleton and the vertebral bodies and craniofacial elements of the axial skeleton (Apschner et al., 2011). In mammals, vertebrae form via endochondral ossification where cartilage is gradually replaced by bone matrix. To contrast, teleosts undergo perichordal ossification after the initial mineralization and segmental patterning of the notochordal sheath, a cartilage-like structure (Fleming et al., 2015; Grotmol et al., 2005; Nordvik et al., 2005). Despite these distinctions in how vertebrae are ossified, the molecular events driving bone formation remain conserved among most vertebrates (Lefebvre and Bhattaram, 2010; Zhang, 2009).
Within these conserved gene regulatory networks, the runt-related transcription factor 2 (runx2) and the zinc-finger transcription factor osterix/sp7 (osx/sp7) transcription factors are considered the master regulators of osteoblast differentiation. Runx2 drives MSC commitment toward an osteoblast fate via binding of its runt DNA-binding domain to gene promoters containing runx consensus sequences (Karsenty et al., 2009; Komori, 2010). In sclerotome-derived MSCs Runx2 activity is inhibited by the basic helix-loop-helix transcription factor Twist1 (Bialek et al., 2004). Osx/Sp7 drives differentiation of pre-osteoblasts into immature osteoblasts, a critical step in the formation of mature osteoblasts (Nakashima et al., 2002). As osteoblast maturation progresses, Runx2 and Osx/Sp7 regulate the expression of extracellular matrix proteins that support adhesion, proliferation, differentiation, and migration of osteoblasts in the bone microenvironment. Matrix molecules consisting of collagenous (eg, Collagen Type X alpha 1, Collagen Type I alpha 1) as well as non-collagenous proteins (eg, Osteocalcin, Osteonectin, Osteopontin) also serve as structural support for bone (Kirkham and Cartmell, 2007).
There is increasing concern, however, that xenobiotic exposure during embryonic development may perturb the complex transcriptional landscape required for osteoblast differentiation and ossification. Assessments of chemical toxicity in mammalian species (mouse, rat, rabbit) suggest that alterations to skeletal development are a commonly observed phenotype (Sipes et al., 2011). Over the past decade small aquarium fish such as zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes) have seen increasing use as vertebrate models of human bone development due to their high fecundity, transparent embryonic development, genetic tractability, and ease of creating transgenic models (Hammond and Moro, 2012). By screening 1060 chemicals in a zebrafish developmental assay, Truong et al. (2014) identified numerous compounds that elicit craniofacial and axial deficits. Specifically, alterations in axial skeletal development in fish were identified as a sensitive endpoint in screening assays with dissimilar classes of chemical agents (McCollum et al., 2011; Tyl et al., 2007), suggesting that there may be multiple mechanisms associated with chemically induced skeletal deficits.
The toxicity of the environmental contaminant, 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD, or dioxin) has been extensively studied as a potent ligand to the aryl hydrocarbon receptor (AhR). The AhR is a well characterized transcription factor in medaka and has been identified as early as in the neurula stage of development (medaka stage 17, approximately 24 h post fertilization (hpf) [Iwamatsu, 2004]) (Hanno et al., 2010). Conversely, functional assays demonstrate AhR transactivation as early as 6 hpf in tg(cyp1a:gfp) medaka (Ng and Gong, 2013), where cyp1a induction serves as a sensitive marker of AhR activation. Numerous studies have reported the teratogenic effects of TCDD in multiple organ systems in medaka and zebrafish following developmental exposure (King-Heiden et al., 2012). Specifically in terms of skeletal toxicity, TCDD exposure in teleosts disrupts cartilage formation in craniofacial (Burns et al., 2015; Planchart and Mattingly, 2010; Teraoka et al., 2006; Xiong et al., 2008) and axial regions (Baker et al., 2014a,b; Dong et al., 2012). Additionally, TCDD has been shown to alter dermal bone formation within the craniofacial skeleton (Burns et al., 2015), however the effects of TCDD on axial bone formation remain unknown.
In this study, Japanese medaka were exposed to low concentrations (pico- to nanomolar) of TCDD during early embryonic development to investigate whether TCDD inhibits formation of the vertebral bodies in larvae. We hypothesized that TCDD would inhibit expression of key regulators of osteoblast differentiation, most notably osx/sp7. To address this, we performed high-resolution confocal microscopy of tg(twist:EGFP, osx/sp7:mCherry, col10a1:nlGFP) medaka, and assessed gene expression of osteogenic regulators and ECM genes. Results of this study demonstrate that 0.15–0.3 nM TCDD elicits a teratogenic effect on axial skeletal development likely through attenuation of osx/sp7 expression.
METHODS
Medaka care and culture
All Japanese medaka (O. latipes) in this study were used in compliance with protocols approved by the North Carolina State University Institutional Animal Care and Use Committee. Brood stocks were housed in an enclosed, recirculating aquaculture system under a 14:10 light:dark cycle. Temperature, pH, and conductivity were monitored daily and maintained at 24 ±2 °C, 7.2 ± 0.2, and 300 ± 50 μS, respectively. Under these conditions, medaka spawned daily and offspring were reared to adulthood to replace aging cohorts in the breeding colony. For the following experiments, the inbred Orange-red Hd-dR (OR) and the previously described transgenic strains tg(osx/sp7:mCherry) (Renn and Winkler, 2009), tg(col10a1:nlGFP) (Renn et al., 2013), tg(twist:EGFP) (Yasutake et al., 2004) were used.
TCDD exposure
Embryos were collected from breeding tanks, cleaned, and staged according to previously established methods (Iwamatsu, 2004). Fertilized embryos were distributed to 6-well tissue culture plates containing 5 ml of 1× embryo rearing medium (ERM) (17.1 mM NaCl, 272 μM CaCl2•2H2O, 402 μM KCl, and 661 μM MgSO4•7H2O, pH 7.4) at a density of 12–15 embryos/well. At approximately 4 hpf, stages 8–9 embryos were exposed for 1 h to 0.1% DMSO (Sigma Aldrich, St Louis, Missouri) or 2,3,7,8-tetrachlorochlorodibenzo-p-dioxin (TCDD, or dioxin) (Cambridge Isotopes Laboratory, Andover, Massachusetts) in DMSO. Each experiment consisted of 3 or more replicates. Following TCDD exposure, embryos were washed 4 times with 1× ERM and cultured at 26–27 °C with static media renewal every other day until hatch or until dechorionation at 9 days postfertilization (dpf). Briefly, embryos were dechorionated with 20-mg/ml pronase (EMD Millipore, Darmstadt, Germany) followed by incubation in hatching enzyme (Kinoshita et al., 2009). Hatched individuals were transferred to plastic containers containing 100 ml of 1X ERM, were fed 1–2 mg of ground Otohime B1 larval diet (Reed Mariculture, Campbell, California) daily, and media was renewed every other day until the termination of the experiment at 20 dpf.
Whole-mount histological staining for bone
Larvae were euthanized at 20 dpf with 0.125% Tricaine methanesulfonate (MS-222) (Sigma Aldrich, St Louis, Missouri, USA) and fixed overnight in 4% paraformaldehyde/0.1% PBS-Tween (PBST). Following fixation, larvae were washed 3 times with PBST and dehydrated with 50 and 70% ethanol prior to staining. A subset of 10–12 individuals was stained for mineralized bone with 0.05% alizarin Red S (Sigma Aldrich, St Louis, Missouri, USA) in 70% ethanol overnight. Poststaining, individuals were washed with ddH2O, followed by a 60-min incubation in 2% hydrogen peroxide in 0.5% KOH, and washed twice in 0.25% KOH. Samples were digested in 0.05% trypsin (Difco BD Biosciences, San Jose, California, USA) dissolved in 30% saturated sodium borate for 20 min at room temperature. Following tissue digestion, samples were washed with 0.25% KOH, and cleared in a graded series of 25, 50, and 75% glycerol in 0.1% KOH, and stored at 4°C. Representative samples were imaged under light microscopy (Nikon SMZ1500).
In vivo staining for bone mineralization and confocal microscopy
Tg(osx/sp7:mCherry), tg(twist:EGFP) and tg(col10a1:nlGFP) medaka were stained and imaged at 20 dpf to assess localization of key cell populations during osteoblastogenesis in relation to mineralized bone. Approximately 12 h prior to imaging, DMSO- and TCDD-treated 20 dpf col10a1:nlGFP and twist:EGFP medaka were counterstained with 0.1% alizarin-3-methylimino-diacetic acid (alizarin complexone or ALC) (Sigma Aldrich, St Louis, Missouri, USA) in ERM for 2 h. Similarly, osx/sp7:mCherry medaka were counter-stained with 0.01% Calcein (Sigma Aldrich, St Louis, Missouri, USA) in ERM (pH 9.0) for 2 h. For both staining procedures, larvae were briefly washed twice with 1× ERM, and remained in 1× ERM overnight to allow equilibration of ALC and Calcein stains.
A representative sample (n=4–6) of stained individuals from each treatment were anesthetized in 0.03% (w/v) tricaine in 1× ERM, immobilized in 35 mm glass-bottom petri dishes (MatTek Corporation, Ashland, Massachusetts, USA) in 1.3% low-melting agarose (Apex BioResearch Products, Genesee Scientific, San Diego, California, USA) containing 0.03% tricaine/ERM. Fish were oriented laterally to permit imaging of the axial vertebrae (vertebrae 17–19) and were imaged in vivo using a Zeiss LSM laser scanning confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with ZEN 2009 version 5.5 SP1 Image Acquisition Software (Carl Zeiss). Vertebrae 17–19 were imaged due to the presence of well mineralized centra and neural and hemal arch structures at 20 dpf, and due to the fact that this region of vertebrae is located at a sufficient distance to minimize signal:noise ratio caused by robust ALC/calcein staining in the gastrointestinal tract.
Morphological assessment
Maximum intensity projections of confocal serial z-stack images (20× magnification) of 20 dpf-stained medaka [tg(twist:EGFP)/ALC, tg(osx/sp7:mCherry)/Calcein, and tg(col10a1:nlGFP)/ALC)] were generated using ZEN 2009 Imaging Software to enable a 2D assessment of axial vertebrae morphology. Vertebrae 17–19 from 3 to 4 individuals from each treatment were assayed using ImageJ software (NIH) to quantify the following morphological phenotypes: area of mineralized centra (pixels2), area of intervertebral ligament (IVL) (pixels2), and lengths of mineralized neural and hemal arches (pixels), where mineralized bone matrix was identified via the presence of positive ALC or Calcein staining. For each phenotype, the mean of 3 measurements for each individual vertebrae was obtained, and subsequently pooled to obtain the mean for vertebrae 17–19. DMSO-treated medaka were set to 1, and TCDD-treated medaka were reported as a proportion relative to DMSO controls.
Axial dissection and RNA isolation
Larval medaka were euthanized at 20 dpf in 0.125% tricaine/ERM solution and replicates were pooled to ensure 15–25 individuals per RNA sample. Specimens were transferred to Leibowitz’s media (L-15) (Corning, Mediatech, Inc., Manassas, Virginia, USA) containing 10% Fetal Bovine Serum (Corning, Mediatech). Dechorionated embryos or hatched larvae were passed through a 20–22 gauge hypodermic needle (Becton, Dickinson, and Company, Franklin Lakes, New Jersey, USA) and 3-ml syringe (Becton, Dickinson, and Company) several times to isolate the axial skeleton from craniofacial and abdominal viscera. Sheared tissue was collected on 105-μm nylon mesh (Component Supply Co., Fort Meade, Florida, USA) and transferred to fresh L-15/10% FBS media. Under a dissecting light microscope (Nikon SMZ1500, Nikon Instruments, Inc., Melville, New York, USA) axial tissue was inspected and trimmed of any remaining craniofacial or abdominal tissue (Supplementary Figure 1). Samples were immediately flash frozen in liquid nitrogen. Tissues were homogenized in TRI Reagent (Ambion, Life Technologies, Carlsbad, California, USA) using a handheld BioVortexer (Thomas Scientific, Swedesboro, New Jersey, USA). Total RNA was isolated according to the TRI Reagent manufacturer’s protocol. Finally, total RNA was quantified using Agilent 2100 Bioanalyzer and 2100 Expert Software package (Agilent Technologies, Santa Clara, California, USA). RNAs with RNA Integrity numbers (RINs) > 9 were used for downstream gene expression applications.
qPCR
The following qPCR methods were conducted and reported in accordance with MIQE guidelines (Bustin et al., 2009). cDNA was synthesized from 2 μg of total RNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California) containing random primers, MultiScribe Reverse Transcriptase, RNase inhibitor, deoxynucleotide triphosphate mix, and 10× reverse transcription buffer in a 20 μl reaction. Medaka-specific real time PCR primers were designed in Primer3 and PrimerQuest (Integrated DNA Technologies [IDT], Coralville, Iowa, USA) and procured from IDT. Primer sets were tested for target accuracy by blasting sequences to the medaka genome in Ensembl and NCBI. All primer sequences were validated and are specific for intended targets. Primer efficiencies were conducted for each gene-specific primer set across a range of cDNA dilutions (1/10–1/1000 of original/template cDNA) to ensure efficient amplification of the target exon sequence (Supplementary Table S1). To quantify relative gene expression, cDNA from DMSO- and TCDD-treated samples (n = 3–5) were PCR amplified in triplicate on clear, 96-well PCR plates (Olympus Plastics, Genesee, San Diego, California, USA) using an Applied Biosystems 7300 real time PCR System and normalized to gapdh, a previously described reference gene in medaka (Zhang and Hu, 2007). Briefly, the 25 μl qPCR reaction was comprised of 12.5 μl of SYBR Green real time PCR Master Mix (Life Technologies), 8.5 μl UltraPure Distilled H2O (Life Technologies), 1 μl of 10 μM forward primer, 1 μl of 10 μM reverse primer, and 2 μl of cDNA. The conditions for each reaction were as follows: (1) 50 °C for 2 min, (2) 95°C for 10 min, (3) 95°C for 15 seconds (s) followed by 60 °C for 60s, repeated 40×, and (4) 95°C for 15 s, 60°C for 60 s, 95°C for 15 s, and 60°C for 15 s to derive dissociation or melt curves and ensure specificity for each primer set used. Threshold cycle (Ct) values for each reaction were determined by the ABI 7300 software package and relative gene expression was quantified according to the ΔΔCt method (Livak and Schmittgen, 2001).
RNA-Seq
To assess global changes in gene expression, axial tissue from DMSO- and 0.3 nM TCDD-treated (n=3/treatment) samples was dissected, homogenized, and RNA was isolated as previously described. Next, total RNA was quantified and RIN values were determined using the Agilent 2100 Bioanalyzer and manufacturer’s instructions therein. Samples with RINs greater than 9.9 were used. Samples used for RNA-Seq were validated through qPCR assessment as described earlier.
Whole transcriptome libraries were prepared using Ion Total RNA-Seq Kit v2 (Ion Torrent, Life Technologies, Carlsbad, California) for the Ion PGM sequencing platform as described by the manufacturer. Briefly, polyA mRNA was purified for each sample from 2 μg of denatured total RNA with oligo(dT)25 Dynabeads. Following elution from the beads, poly(A) mRNA yield was analyzed using Agilent RNA 6000 Pico Kit. Poly(A) mRNA was then fragmented with RNaseIII and subsequently purified with Nucleic Acid Binding Beads. Next, adaptors from Ion Adaptor Mix v2 were ligated to poly(A) RNA, and each sample was reverse transcribed to form cDNA (at 42 °C in a 20 μl reaction containing 4 μl 10× Superscript III Enzyme Mix, 2 μl of 10× RT Buffer, 2.5 μl of 2.5 mM dNTP Mix, 8 μl Ion RT Primer v2, and 2 μl of nuclease-free water) and stored overnight at −20 °C. The following day, the cDNA was purified with Nucleic Acid Binding Beads, and then amplified using Platinum PCR Supermix High Fidelity, Ion Xpress RNA 3′ Barcode Primers, and a different Ion Xpress RNA-Seq BC Primer (BC #11-16) for each sample to form a barcoded library to aid in downstream analysis. The amplified cDNA was purified once more with Nucleic Acid Binding Beads, a 1:20 dilution was made, and the 1:20 cDNA diluent yield and fragment size distribution was assessed using the Agilent High Sensitivity DNA Kit. The library was then pooled with equimolar amounts from each of the 6 barcoded samples, and analyzed once more using the Agilent High Sensitivity DNA Kit. Finally, the pooled library was diluted to 20 ρM for subsequent template preparation.
Amplification of the pooled library was performed on the Ion OneTouch 200 using Ion PGM Template OT2 200 Kit. The amplification solution consisting of the 20 ρM pooled library, nuclease-free water, Ion PGM OT2 200 Reagent Mix, Ion PGM OT2 200 PCR Reagent B, Ion PGM OT2 200 Enzyme, and Ion PGM OT2 200 ion sphere particles (ISPs) was prepared and run overnight according to manufacturer instructions. The following day, the solution containing template-positive ISPs was enriched on the Ion OneTouch ES following the manufacturer’s protocol. Next, the Ion PGM System was cleaned, initialized, and calibrated for the Ion 318 chip. Sequencing primers were annealed to the template-positive ISPs, Ion PGM Sequencing 200 v2 Polymerase was added to the ISP solution, and incubated at room temperature for 5 min. Finally, the template-positive ISPs + Sequencing Polymerase mixture were loaded into the Ion 318 chip and sequenced overnight on the Ion PGM System.
The resulting reads were trimmed to remove adaptor sequences and low quality base-calls, and were aligned to the O. latipes (Medaka) genome (Version 76; www.ensembl.org) using Bowtie 2.2.6. Binary files generated by Bowtie were visualized using the Integrated Genome Viewer. Differentially expressed genes were identified using Cuffdiff2, whereas principal component analysis was performed with DESeq2 in R. Pathway analysis was conducted using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, www.ingenuity.com). All RNA-Seq data have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE87168.
Statistical analysis
For morphometry and qPCR data, a 1-way ANOVA with a Tukey post hoc test were performed using GraphPad Prism Software (La Jolla, California, USA). P-values < .05 were deemed significant. For RNA-Seq, genes with corrected P-values (q-values) < .05 were deemed significant and used for downstream analysis in IPA.
RESULTS
TCDD Exposure Elicits Gross Skeletal Deficits at 20 dpf
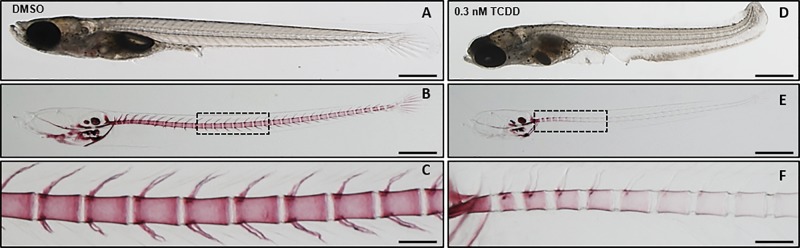
From a preliminary range-finding experiment in Orange-red medaka, we chose concentrations of 0.15 and 0.3 nM TCDD due to a high rate of survivorship to 20 dpf (larval stage) of 85 ± 11 and 49 ± 24%, respectively. These concentrations were also chosen due to the low frequency of adverse cardiovascular effects typically associated with dioxin exposure in medaka and other teleost models at higher TCDD concentrations. Despite no cardiovascular defects, medaka exposed to 0.3 nM TCDD demonstrated gross skeletal morphological deficits including dorsalization of the tail, a truncated lower jaw structure, and an overall reduction in total body length (Figs. 1A and D). Alizarin Red S staining was performed on fixed 20 dph larvae to investigate whether TCDD affects bone mineralization in the axial skeleton. DMSO-treated individuals displayed positive staining of the craniofacial elements, and axial vertebral bodies (Figure 1B). Neural and hemal arches were well defined and mineralized in DMSO-treated fish (Figure 1C). Individuals exposed to 0.15 nM TCDD also displayed positive staining of the vertebral bodies and hemal arches in the abdominal and medial regions; however, staining of caudal fin rays and caudal vertebrae was reduced when compared with DMSO-treated medaka (Supplementary Figure S2). The most dramatic effect was observed at 0.3 nM TCDD where a marked attenuation in alizarin red staining was observed throughout the axial skeleton (Figure 1E), with positive staining observed only in the vertebrae that comprise the Weberian apparatus and abdominal vertebrae (Figure 1F).
FIG. 1.
Whole-mount alizarin red S staining for mineralized bone in medaka larvae at 20 dpf. Representative individuals from DMSO (A–C) and 0.3 nM TCDD-exposed (D–F) groups are shown. Panels C and F are magnified regions boxed in B and E, respectively. Scale bars represent 500 μm in A, B, D, E, 2× magnification; 100 μm in C and F, 10× magnification.
TCDD Exposure Results in Morphological Deficits in Vertebral Bodies by Targeting Differentiating Osteoblasts
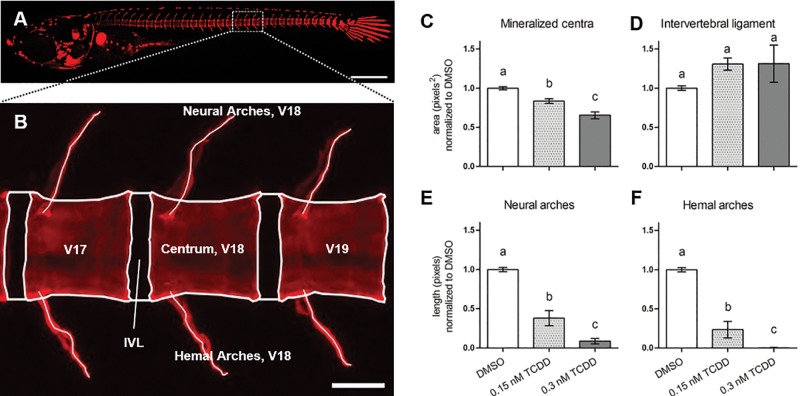
Medaka transgenic lines including tg(twist:EGFP), tg(osx/sp7:mCherry), and tg(col10a1:nlGFP) were utilized to assess how different cell populations within the osteogenic lineage were impacted following TCDD exposure. Each transgene consists of a fluorescent reporter whose expression is coupled to the promoter of cell-specific genes of interest (Supplementary Table S2). Prior to confocal imaging, transgenic individuals were stained in vivo with ALC or calcein to further assess osteoprogenitor [tg(twist:EGFP)] or osteoblast [tg(osx/sp7:mCherry) and tg(col10a1:nlGFP)] populations within the context of mineralized osteoid tissue in vertebrae 17 through 19 (V17–19) at 20 dpf (Figure 2A). At this larval stage, centra from DMSO-treated individuals begin to resemble the hourglass-like morphology observed in adult medaka, while the neural and hemal arches can be observed extending from the rostral nodes of the centra in a dorsal and ventral fashion, respectively (Figure 2B). In contrast to the control fish, the centra of TCDD-treated transgenic medaka exhibited reduced growth and mineralization. A morphometric analysis of the centra area revealed a concentration-dependent and significant decrease in centra area of 16 and 35% in response to 0.15 and 0.3 nM TCDD (DMSO vs 0.15 nM TCDD, P ≤ .001; DMSO vs 0.3 nM TCDD, P ≤ .001; 0.15 nM TCDD vs 0.3 nM TCDD, P ≤ .001), respectively (Figure 2C), thus providing a more sensitive assay than gross light microscopy to detect deficits in bone formation. Though not significant, the IVL area appeared qualitatively larger with TCDD treatment (Figure 2D). TCDD treatment also resulted in significant and dose-dependent reductions in neural arch length (DMSO vs 0.15 nM TCDD, P ≤ .001; DMSO vs 0.3 nM TCDD, P ≤ .001; 0.15 nM TCDD vs 0.3 nM TCDD, P ≤ .01) (Figure 2E) and a complete loss of hemal arch formation in the 0.3 nM TCDD treated fish (DMSO vs 0.15 nM TCDD, P ≤ .001; DMSO vs 0.3 nM TCDD, P ≤ .001; 0.15 nM TCDD vs 0.3 nM TCDD, P < .05) (Figure 2F).
FIG. 2.
Morphological assessment of axial vertebral elements from V17 to 19) of DMSO-treated medaka at 20 dpf. Depicted in (A) is a representative whole-mount individual stained with ALC, 6× magnification. Panel B shows boxed region in A) with morphological atlas labeling V17–19, and centrum, IVL, and hemal and neural arches of vertebra 18 as a representative example, 20× magnification. Centra and IVL areas are shown in (C) and (D), respectively; lengths of neural and hemal arches are shown in (E) and (F), respectively. Values represent the mean from 3 separate measurements taken from V17–19. Letters denote statistical significance between groups analyzed by 1-way ANOVA with Tukey’s post hoc analysis. Scale bars in (A) and (B) denote 500 and 50 µM, respectively.
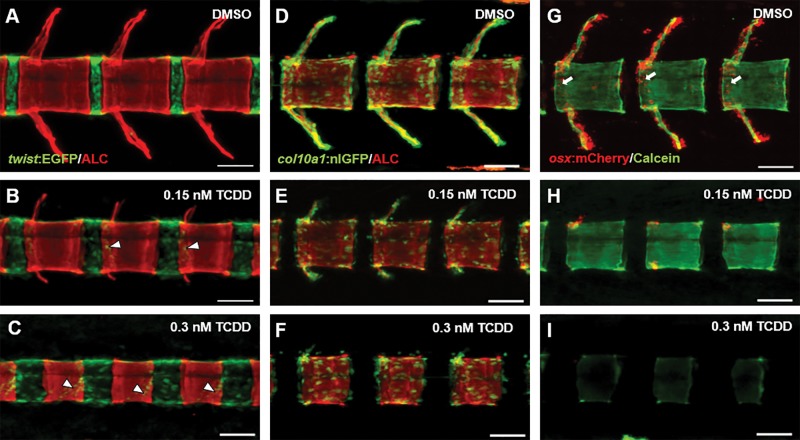
In control animals, twist:EGFP expression is observed within sclerotome-derived MSCs densely localized to the IVL. Moreover, EGFP+ cells appear confined within the boundary between the IVL and centra, and these observations were consistent among all centra/IVL imaged. With TCDD treatment, EGFP+ cells display a more dispersed pattern and appear less confined to the IVL-centrum border. In fact, a subset of EGFP+ cells appear localized on the mineralized centra, likely in the process of migrating and differentiating into osteoblasts. This effect was more pronounced in 0.3 nM TCDD-treated fish (Figs. 3A–C).
FIG. 3.
Confocal microscopy of representative DMSO- and TCDD-exposed tg(twist:EGFP) (A–C), tg(col10a1:nlGFP) (D–F), tg(osx/sp7:mCherry) (G–I) medaka at 20 dpf. Individuals were stained with ALC or calcein to identify mineralized bone matrix within the context of osteoblasts and osteoblast precursors. Arrowheads in (B) and (C) highlight twist:EGFP+ cells on the mineralized chordacentra. Arrows in (G) denote osx/sp7:mCherry+ cells localized to the periphery of centra undergoing perichordal ossification. Images of V17–19 were captured using the Zeiss LSM 710 confocal system, 20× magnification (scale bars = 50 µM).
The tg(col10a1:nlGFP) transgenic line labels a heterogeneous population of immature to mature osteoblasts. In control fish, nlGFP+ cells were observed in areas of active mineralization and growth along the centra periphery and the elongating neural and hemal arches. Less abundant, though still present, were nlGFP+ cells within the center of centra. Following TCDD exposure, nlGFP+ cells were distributed along the centra periphery and to a lesser extent within primitive neural and hemal arch structures. Qualitatively, however, the TCDD-treated fish demonstrated an overall attenuation in distribution and intensity of nlGFP+ signal (Figs. 3D–F), consistent with gene expression data (see below).
Differentiating osteoblasts are labeled with mCherry in tg(osx/sp7:mCherry) medaka. In DMSO-controls at 20 dpf, mCherry+ cells are localized along the neural and hemal arches, and lining the rostral and caudal centra periphery. Following exposure to 0.15 nM TCDD, 20 dpf larvae exhibited significant attenuation in mCherry+ expression with an apparent qualitative reduction in number of mCherry+ cell number consistent with a decrease previously described in Dong et al. (2012). At 0.3 nM TCDD osx/sp7:mCherry+ expression was nearly absent, consistent with the absence of neural and hemal arches where mCherry+ cells are normally observed (Figs. 3G–I). Taken together, despite the presence of twist:EGFP+ and col10a1:nlGFP+ cells in TCDD-treated animals, the fact that osx/sp7:mCherry+ cells were clearly diminished in a concentration-dependent manner suggests that TCDD may alter differentiation following the initial commitment of mesenchymal cells to an osteoblastic fate.
TCDD Significantly Downregulates Transcriptional Regulators of Osteogenesis and Downstream Targets
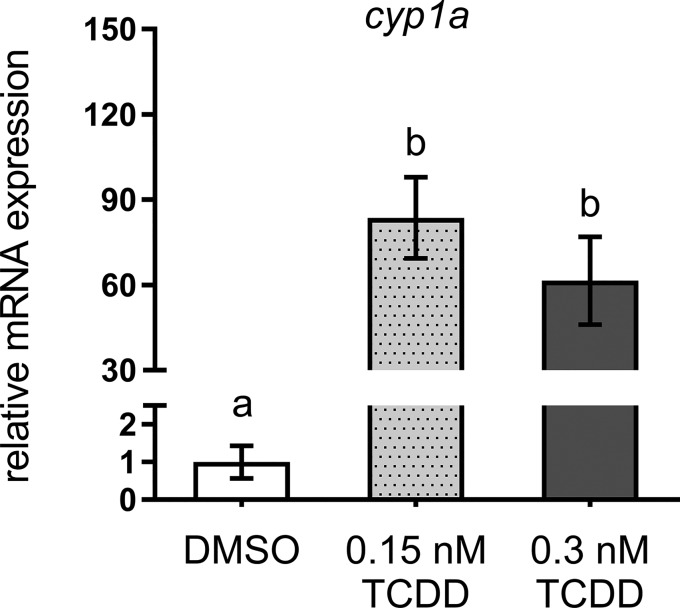
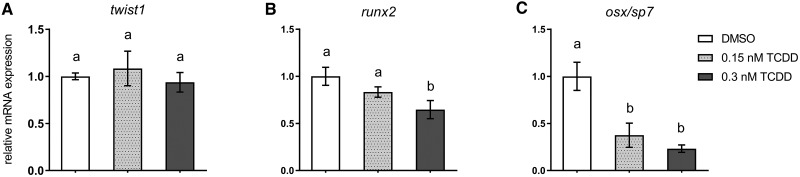
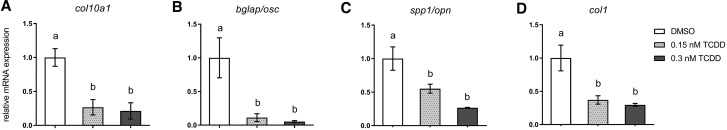
qPCR was used to anchor observed deficits in bone mineralization to expression changes in selected genes within the osteoblast gene regulatory network within isolated axial tissues of DMSO- and TCDD-treated medaka. To confirm TCDD-mediated AhR activation, cyp1a expression, a canonical marker of TCDD exposure was measured. At 20 days postexposure cyp1a expression was significantly induced (>50-fold) in both 0.15 and 0.3 nM-treated medaka (DMSO vs 0.15 nM TCDD, P ≤ .003; DMSO vs 0.3 nM TCDD, P ≤ .02) (Figure 4) compared with DMSO controls. To assess modulation within the osteoblast gene regulatory network, expression of select transcriptional regulators and markers of osteoblast differentiation and maturation was measured. twist1, a marker of mesenchymal/osteoprogenitor cells in the IVL was not significantly impacted by TCDD exposure (Figure 5A). Expression of runx2, an early osteoblastogenic transcription factor, was significantly downregulated with 0.3 nM TCDD (P ≤ .04) compared with DMSO controls (Figure 5B). Downstream of twist1 and runx2, however, osx/sp7 displayed a significant, concentration-dependent response with expression reduced 2.7- and 4.3-fold in 0.15 and 0.3 nM TCDD, respectively, compared with DMSO controls (0.15 nM TCDD, P ≤ .02; 0.3 nM TCDD, P ≤ .008) (Figure 5C). TCDD-mediated reduction in osx/sp7 suggests that maturation of osteoblasts is impacted. Therefore, expression of markers of terminally differentiated osteoblasts was measured. collagen type X alpha 1 (col10a1), a marker of osteoblasts and hypertrophic chondrocytes, demonstrated a significant 3.8- and 4.7-fold reduction in 0.15 nM TCDD- and 0.3 nM TCDD-treated medaka (0.15 nM TCDD, P ≤ .004; 0.3 nM TCDD, P ≤ .001), respectively (Figure 6A). The most dramatic effect occurred in expression of bglap, encoding an ECM protein, whose expression is regulated downstream of osx/sp7 and runx2. When compared with controls, bglap was significantly reduced 9- and 19-fold with 0.15 and 0.3 nM TCDD treatment, respectively (0.15 nM TCDD, P ≤ .05; 0.3 nM TCDD, P ≤ .04) (Figure 6B), although the 2 treatments were not statistically different from each other. Other ECM markers, including spp1 and col1, were attenuated with both TCDD treatments compared with DMSO controls; however, there was no significant difference between 0.15 and 0.3 nM TCDD treatment groups (Figs. 6C and 6D). A full summary of qPCR results is available in Supplementary Table S3.
FIG. 4.
Relative cyp1a expression in 20 dpf DMSO- and TCDD-exposed medaka. Statistical assessment was conducted using 1-way ANOVA with a Tukey post hoc analysis, n = 3–4 per treatment, letters indicate significant differences between groups.
FIG. 5.
qPCR analysis of select osteogenic transcription factors from axial tissue isolated from Orange-red medaka at 20 dpf. Statistical assessment was conducted using 1-way ANOVA with a Tukey post hoc analysis, n = 3–4 per treatment, letters indicate significant differences between groups (P < .05).
FIG. 6.
qPCR analysis of select ECM markers from axial tissue isolated from Orange-red medaka at 20 dpf. Statistical assessment was conducted using 1-way ANOVA with a Tukey post hoc analysis, n = 3–5 per treatment, letters denote significant differences between groups (P < .05).
TCDD Significantly Alters Global Inflammatory and Musculoskeletal Disease Pathways
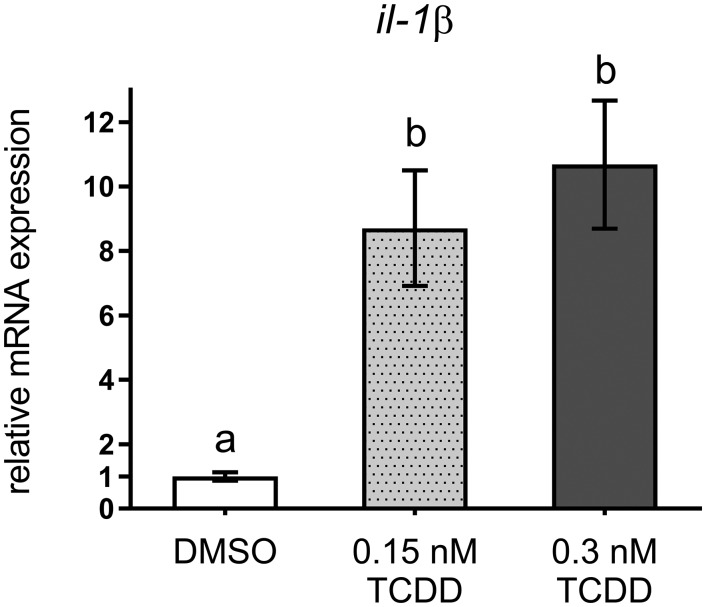
A global RNA-Seq analysis was conducted using RNA isolated from the axial region of DMSO- and 0.3 nM TCDD-exposed medaka to identify additional targets within the osteochondral pathway potentially impacted by TCDD exposure. In total, 597 genes were significantly up- or down-regulated (q < 0.05) (for a complete list, refer to GEO accession no. GSE87168). Within this dataset, AhR-mediated genes were enriched with marked upregulation of ahr, ahrr (AhR repressor), cyp1a, and cyp1b as markers of AhR transactivation. Interestingly, the inflammatory mediators il-1β and cxcl-14 were also significantly upregulated. Expression of il-1β was validated using qPCR and revealed a significant 8- and 10-fold induction with 0.15 nM (P < 0.02) and 0.3 nM TCDD treatment (P < .01) (Figure 7). RNA-Seq also identified numerous osteochondral genes with significantly reduced expression in the TCDD-exposed samples. Specifically, we observed significant reduction in osx/sp7 and dlx3, transcriptional regulators of osteoblastogenesis, and reduced expression of the chondrogenic regulator sox9b. Likewise, downstream ECM genes associated with bone and cartilage development were also significantly downregulated (col10a1, col5a2, col1a1, col11a1, col2a1, sparc/osteonectin). Other observations of interest included TCDD-mediated reduction in the Wnt pathway inhibitors, dkk3 and sfrp2, and induction of the osteoclastogenic inhibitor, opg-a.
FIG. 7.
qPCR analysis of il-1β from axial tissue isolated from Orange-red medaka at 20 dpf. Letters indicate statistical significance (P < .05) between groups analyzed by 1-way ANOVA with a Tukey post hoc analysis, n = 4–5 per treatment.
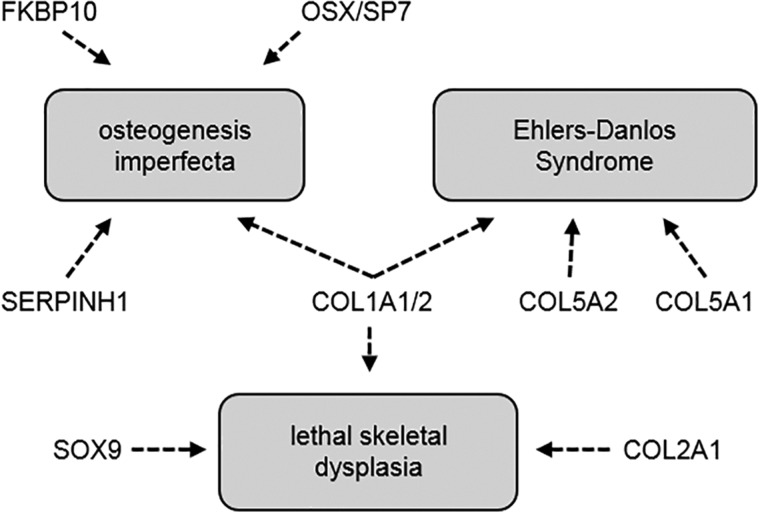
Differentially regulated genes were evaluated using IPA and were enriched among several musculoskeletal disease and developmental pathways. Arthropathy and arthritis were the 2 most heavily enriched musculoskeletal disorders with 51 and 49 genes, respectively, and were predicted to be increased in the TCDD-exposed group (z-score > 2.0). Also enriched were pathways associated with altered bone mineralization and osteoclastogenesis (Table 1). Furthermore, expression of osx/sp7, sox9, numerous collagen genes, and genes encoding ECM synthesis and transport were also downregulated by TCDD in this dataset. Mutations in several of these genes are associated with human skeletal maladies including osteogenesis imperfecta, Ehlers-Danlos syndrome, and other lethal skeletal dysplasias (Figure 8). Taken together, the RNA-Seq data displays concordant findings with the above qPCR data, and provides additional insight into gene dysregulation associated with musculoskeletal disease pathways.
TABLE 1.
IPA Highlighting a Subset of Affected Pathways Including Osteochondral Diseases and/or Altered Functional Osteogenic States
| Categories | Disease/Function | P-value | Predicted Activation State | Activation z-Score | Number of Genes |
|---|---|---|---|---|---|
| Connective tissue disorders, skeletal and muscular disorders | Arthropathy | 4.73E-04 | Increased | 2.769 | 51 |
| Connective tissue disorders, inflammatory disease, skeletal and muscular disorders | Arthritis | 9.78E-04 | Increased | 2.769 | 49 |
| Cell death and survival | Cell Death Of Connective Tissue Cells | 1.77E-06 | Decreased | −2.354 | 41 |
| Skeletal and muscular disorders | Myopathy | 1.48E-07 | N.P. | 1.093 | 40 |
| Skeletal and muscular system development/function | Mineralization Of Bone | 4.93E-04 | N.P. | 1.091 | 12 |
| Skeletal and muscular system development/function | Osteoclastogenesis | 8.95E-05 | N.P. | 1.061 | 10 |
RNA-Seq was performed on RNA isolated from the axial region of 20 dpf larvae exposed to DMSO or 0.3 nM TCDD at 4 hpf. For each Disease/Functional state, activation z-scores < −2.0 were predicted to be “Decreased”; Activation z-scores > 2.0, “Increased”; N.P. = not predicted.
FIG. 8.
Shared targets of human skeletal anomalies associated with heterozygous/LOF mutations and downregulation of orthologous genes in 0.3 nM TCDD-treated medaka.
DISCUSSION
In teleosts, TCDD is a potent teratogen known to elicit pericardial edema and other embryolethal phenotypes at concentrations in the nanomolar range (Antkiewicz et al., 2005; Carney et al., 2006; Dong et al., 2010; Teraoka et al., 2002). Of interest, several studies have investigated the effect of sublethal TCDD concentrations on teleost skeletal development as a means to assess the relationships between AhR activation and skeletal dysplasias (Baker et al., 2013, 2014a,b). In this study, we establish that embryonic exposure to TCDD, a legacy contaminant and potent AhR ligand, results in significant deficits in perichordal ossification of vertebrae during larval development.
Our findings indicate that TCDD exposure attenuates ossification of the centra and neural and hemal arches as evidenced qualitatively by whole-mount alizarin red staining and quantitatively from an ImageJ assessment of ALC- and calcein-stained transgenic medaka. A similar study in zebrafish previously demonstrated that TCDD inhibits perichondral ossification, i.e. ossification around a cartilage template, in the craniofacial skeleton (Burns et al., 2015). Other studies have also established the role of TCDD in disrupting cell-rich hyaline cartilage and extracellular matrix synthesis in craniofacial (Burns et al., 2015; Planchart and Mattingly, 2010; Teraoka et al., 2006; Xiong et al., 2008) and axial structures (Baker et al., 2014a,b; Dong et al., 2012). To contrast, our results demonstrate that TCDD inhibits perichordal ossification in vertebrae lacking a bone fide cartilage scaffold, which suggests that multipotent MSCs or osteoblasts undergoing differentiation are potential targets of TCDD toxicity.
In teleosts, notochordal cells direct the initial mineralization and segmentation of the chordacentra along the vertebral column (Grotmol et al., 2005). Confocal imaging of transgenic medaka used in this experiment illustrates positive ALC and calcein staining of centra in a segmented pattern, indicating that early mineralization of the notochordal sheath does not appear to be impacted following TCDD exposure. Given the fact that chordal ossification is present early, we argue that AhR-mediated events affect perichordal ossification that drives the lateral growth of the vertebral body in the rostral and caudal orientations. In twist:EGFP fish at 20 dpf, sclerotome-derived MSCs appeared highly dispersed within a larger IVL area compared with controls, and, in the highest TCDD treatment, EGFP+ cells were observed in middle of the centra beyond the IVL space. Based on prior evidence, these twist:EGFP+ cells are likely in the process of differentiating into perichordal osteoblasts (Renn et al., 2013). Though distribution of twist:EGFP+ MSCs appear to impacted by TCDD exposure, mRNA expression of twist1 remains unchanged. This observation suggests several possibilities. First, TCDD may not specifically target osteoprogenitor cells localized to the IVL and impacts more specifically the terminal maturation of osteoblasts. Second, TCDD may impact the ability of twist:EGFP+ osteoprogenitor cells to differentiate to osteoblasts without altering their motility across and within the centra from the IVL. Alternatively, it may be possible that both of these processes are impacted by TCDD. Based on evidence from TCDD-exposed tg(col10a1:nlGFP) and tg(osx/sp7:mCherry) individuals, it is more likely that TCDD targets intermediate to terminal differentiation of osteoblasts. Structures undergoing perichordal ossification at the centrum periphery and along the neural and hemal arches revealed a reduction in nlGFP+ signal intensity in tg(col10a1:nlGFP) individuals and a concentration-dependent reduction in mCherry+ cells in tg(osx/sp7:mCherry) individuals exposed to TCDD. These cellular observations are further supported at the molecular level where we demonstrate reduced expression of osx/sp7 and col10a1 in TCDD-treated medaka. Collectively, these data suggest that TCDD inhibits intermediate to terminal stages of osteoblast differentiation with the possibility of also attenuating differentiation of osteoprogenitors to osteoblasts. This may thus provide an explanation for observed TCDD-mediated deficits in vertebral body ossification.
Our findings are consistent with mammalian studies investigating bone development following exposure to TCDD or other AhR ligands. Developmental exposure to TCDD in rats adversely affects the structural and mechanical properties of bone (Miettinen et al., 2005), and numerous in vitro studies have confirmed TCDD-mediated inhibition of osteoblast differentiation in murine cell models (Gierthy et al., 1994; Korkalainen et al., 2009; Ryan et al., 2007; Singh et al., 2000; Yu et al., 2014). In humans, elevated exposure to polychlorinated dibenzo-p-dioxins and furans during breastfeeding has been strongly associated with hypomineralization of teeth (Alaluusua et al., 1996). Moreover, children living in the TCDD-contaminated zones in Seveso, Italy display an increased incidence of tooth enamel defects (Alaluusua et al., 2004). Cementoblasts, the osteoblast-like cells responsible for forming of mineralized tissue surrounding the tooth-root surface, are regulated by OSX/SP7 (Cao et al., 2012), suggesting a possible linkage between TCDD exposure and altered dentition and bone formation observed in humans.
To further investigate the observed phenotypes in TCDD-treated medaka larvae, qPCR was conducted on 20-dpf axial tissue for select targets within the osteogenic gene regulatory network. In addition to diminished expression of osx/sp7, we further illustrate that downstream markers of terminally differentiated osteoblasts including bglap, spp1, col1, and col10a1, are significantly diminished by TCDD in this experiment. The morphological results we observed associated with diminished osx/sp7 expression following TCDD treatment recapitulate the phenotype observed in osx/sp7 morpholino (MO)-knockdown medaka. Specifically, osx/sp7 MOs display reduced formation of mineralized vertebra (Renn and Winkler, 2014). Our observations are also consistent with defects in ossification found in Osx/Sp7−/− mice (Nakashima et al., 2002). Similarly, humans haploinsufficient in the OSX/SP7 allele with osteogenesis imperfecta Type VII experience low bone mass and bone fragility associated with an increased risk of bone fracture (Lapunzina et al., 2010). Moreover, a genome-wide association study of more than 1500 children revealed a strong association between reduced bone mineral density and 4 single-nucleotide polymorphisms (SNPs) at the OSX/SP7 gene locus (Timpson et al., 2009). To the authors’ knowledge, repression of osx/sp7 and runx2 as transcriptional regulators of osteoblast differentiation is the first mechanism to explain how TCDD inhibits bone formation in medaka.
Given the multiple targets of TCDD toxicity, RNA-Seq was conducted on DMSO- and 0.3 nM TCDD-treated medaka to identify additional pathways impacted by dioxin exposure. As expected, numerous AhR-responsive genes involved in xenobiotic metabolism were heavily enriched with TCDD treatment, a finding similar to a gene array analysis of TCDD-treated adult medaka (Volz et al., 2006). In addition to a suite of osteogenic targets osx/sp7, bglap, sparc/osteonectin, and col10a1, TCDD exposure attenuates expression of FKBP10 and SERPINH1, genes associated with posttranslational modification and transport of collagens that comprise bone ECM. In combination with OSX/SP7, mutations in both FKBP10 and SERPINH1 are linked to severe osteogenesis imperfecta (Alanay et al., 2010; Christiansen et al., 2010), which offers further support for the bone phenotype we observed in TCDD-treated medaka.
TCDD may also impact osteogenesis through additional mechanisms. Interestingly, il-1b expression was upregulated >8-fold with TCDD exposure, which may offer additional insight into the putative role of inflammatory regulators in osteoblast maturation and bone formation. Inflammation is associated with adult degenerative bone disease outcomes including rheumatoid arthritis and delayed fracture repair, however, its precise role in bone formation is not yet fully understood. Inhibitory effects of Il1b on differentiation of MC3T3-E1 osteoblast-like cells and MSCs derived from mice have been observed in vitro (Lacey et al., 2009; Taichman and Hauschka, 1992). Moreover, IL1B in human MG-63 osteoblast-like cells is known to induce expression of osteoprotegerin (OPG; TNFRS11B) via the p38 and ERK pathways (Lambert et al., 2007). OPG functions as a decoy receptor that binds RANKL to prevent osteoclast differentiation thereby slowing bone resorption by mature osteoclasts (Simonet et al., 1997). We observed significant upregulation of opg-a, the medaka orthologue of human OPG, in TCDD-exposed larvae. This finding was unexpected as endogenous osteoclast activity is not present in the axial skeleton of medaka until 3–4 weeks postfertilization (To et al., 2012) under normal conditions. It is possible that upregulation of opg-a may serve as a compensatory mechanism to prevent bone resorption in larvae lacking sufficient bone development, such as when exposed to TCDD.
In addition to TCDD, other AhR ligands present in the environment elicit similar inhibitory effects on osteoblast development. Of these AhR agonists, the polycyclic aromatic hydrocarbons 3-methlycholanthrene and benzo[a]pyrene have received the most attention due to their presence in direct and second-hand cigarette smoke. These ligands, as well as cigarette smoke condensate, all demonstrate inhibitory effects on osteoblast differentiation and function in vitro (Gullihorn et al., 2005; Korkalainen et al., 2009; Naruse et al., 2002; Nishimura et al., 2009) and in mice in vivo (Herlin et al., 2013; Naruse et al., 2002). Environmental exposure to xenobiotics such as those found in cigarette smoke, are increasingly associated with various skeletal diseases in humans. For example, smoking is known to trigger or exacerbate the pathogenesis of rheumatoid arthritis and osteoporosis (Lee et al., 2013), and an increasing body of evidence points to the role of the AhR in the manifestation of these disease states (Kobayashi et al., 2008; Lahoti et al., 2014, 2013; Nguyen et al., 2013). However, the interaction between developmental exposure to xenobiotic stressors and congenital skeletal diseases and the onset of adverse skeletal outcomes is less well established. In the present work, TCDD exposure attenuates bone formation in the axial skeleton in medaka during larval stages. The phenotypic analysis we present is anchored to a thorough assessment of differentially expressed markers and regulators of osteogenesis. Based on these data, we further hypothesize that exposure to ubiquitous AhR ligands during critical windows of development may contribute to the onset of developmental and/or adult skeletal dysplasias including rheumatoid arthritis and osteoporosis. Given the fact that skeletal development is highly conserved across vertebrate phyla, we additionally demonstrate that teleosts are suitable models to improve our mechanistic understanding of environmental exposures and their pathological role in human skeletal diseases.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
ACKNOWLEDGMENTS
The authors would like to extend their gratitude to Dr Jeff Tucker, Dr Anita Buettner, and Dr Eva Johannes for their assistance with confocal imaging and analysis. We thank Dr Akira Kudo for providing the twist:EGFP line used in this study. Many thanks also to the members of the Medaka National BioResource Project (NBRP), National Institute of Basic Biology National (NIBB) (Japan) for facilitating collaboration among the members of the Japanese medaka research community. We would also like to thank all current and former members of the Kullman laboratory who assisted with maintenance of the medaka facility: Erin Yost, PhD; Erin Kollitz, PhD; Crystal Lee Pow, PhD; Debabrata Mahaprata, DVM; Hong Li, Gwijun Kwon; Jeanne Burr.
FUNDING
The research reported in this publication was supported by the College of Sciences, North Carolina State University and the National Institute of Environment Health Sciences (NIEHS) under award number P30ES025128 for the North Carolina State University Center for Human Health and the Environment.
REFERENCES
- Alaluusua S., Calderara P., Gerthoux P. M., Lukinmaa P. L., Kovero O., Needham L., Patterson D. G., Tuomisto J., Mocarelli P. (2004). Developmental dental aberrations after the dioxin accident in Seveso. Environ. Health Perspect. 112, 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaluusua S., Lukinmaa P. L., Vartiainen T., Partanen M., Torppa J., Tuomisto J. (1996). Polychlorinated dibenzo-p-dioxins and dibenzofurans via mother’s milk may cause developmental defects in the child’s teeth. Environ. Toxicol. Pharmacol. 1, 193–197. [DOI] [PubMed] [Google Scholar]
- Alanay Y., Avaygan H., Camacho N., Utine G. E., Boduroglu K., Aktas D., Alikasifoglu M., Tuncbilek E., Orhan D., Bakar F. T., et al. (2010). Mutations in the Gene Encoding the RER Protein FKBP65 Cause Autosomal-Recessive Osteogenesis Imperfecta. Am. J. Hum. Genet. 86, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkiewicz D. S., Burns C. G., Carney S. A., Peterson R. E., Heideman W. (2005). Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377. [DOI] [PubMed] [Google Scholar]
- Apschner A., Schulte-Merker S., Witten P. E. 2011. Not all bones are created equal – using zebrafish and other teleost species in osteogenesis research, 3rd ed, Methods in Cell Biology. Elsevier Inc, Amsterdam, Netherlands. [DOI] [PubMed] [Google Scholar]
- Baker T. R., Peterson R. E., Heideman W. (2014a). Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol. Sci. 138, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. R., Peterson R. E., Heideman W. (2014b). Adverse effects in adulthood resulting from low-level dioxin exposure in juvenile zebrafish. Endocr. Disruptors (Austin), 2, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. R., Peterson R. E., Heideman W. (2013). Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol. Sci. 135, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek P., Kern B., Yang X., Schrock M., Sosic D., Hong N., Wu H., Yu K., Ornitz D. M., Olson E. N., et al. (2004). A twist code determines the onset of osteoblast differentiation. Dev. Cell 6, 423–435. [DOI] [PubMed] [Google Scholar]
- Burns F. R., Peterson R. E., Heideman W. (2015). Dioxin disrupts cranial cartilage and dermal bone development in zebrafish larvae. Aquat. Toxicol. 164, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L. (2009). The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 622, 611–622. [DOI] [PubMed] [Google Scholar]
- Cao Z., Zhang H., Zhou X., Han X., Ren Y., Gao T., Xiao Y., de Crombrugghe B., Somerman M. J., Feng J. Q. (2012). Genetic evidence for the vital function of Osterix in cementogenesis. J. Bone Miner. Res. 27, 1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. A., Chen J., Burns C. G., Xiong K. M., Peterson R. E., Heideman W. (2006). Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 70, 549–561. [DOI] [PubMed] [Google Scholar]
- Christiansen H. E., Schwarze U., Pyott S. M., AlSwaid A., Al Balwi M., Alrasheed S., Pepin M. G., Weis M. A., Eyre D. R., Byers P. H. (2010). Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am. J. Hum. Genet. 86, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Hinton D. E., Kullman S. W. (2012). TCDD disrupts hypural skeletogenesis during medaka embryonic development. Toxicol. Sci. 125, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Matsumura F., Kullman S. W. (2010). TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias latipes) embryos. Toxicol. Sci. 118, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A., Kishida M. G., Kimmel C. B., Keynes R. J. (2015). Building the backbone: The development and evolution of vertebral patterning. Development 142, 1733–1744. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Silkworth J. B., Tassinari M., Stein G. S., Lian J. B. (1994). 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits differentiation of normal diploid rat osteoblasts in vitro. J. Cell. Biochem. 54, 231–238. [DOI] [PubMed] [Google Scholar]
- Grotmol S., Nordvik K., Kryvi H., Totland G. K. (2005). A segmental pattern of alkaline phosphatase activity within the notochord coincides with the initial formation of the vertebral bodies. J. Anat. 206, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullihorn L., Karpman K., Lippiello L. (2005). Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J. Orthop. Trauma 19, 17–22. [DOI] [PubMed] [Google Scholar]
- Hammond C. L., Moro E. (2012). Using transgenic reporters to visualize bone and cartilage signaling during development in vivo. Front. Endocrinol. (Lausanne) 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanno K., Oda S., Mitani H. (2010). Effects of dioxin isomers on induction of AhRs and CYP1A1 in early developmental stage embryos of medaka (Oryzias latipes). Chemosphere 78, 830–839. [DOI] [PubMed] [Google Scholar]
- Herlin M., Finnilä M. A. J., Zioupos P., Aula A., Risteli J., Miettinen H. M., Jämsä T., Tuukkanen J., Korkalainen M., Håkansson H., et al. (2013). New insights to the role of aryl hydrocarbon receptor in bone phenotype and in dioxin-induced modulation of bone microarchitecture and material properties. Toxicol. Appl. Pharmacol. 273, 219–226. [DOI] [PubMed] [Google Scholar]
- Hermanns P., Lee B. (2002). Transcriptional dysregulation in skeletal malformation syndromes. Am. J. Med. Genet. 106, 258–271. [PubMed] [Google Scholar]
- Imai Y., Youn M. Y., Inoue K., Takada I., Kouzmenko A., Kato S. (2013). Nuclear receptors in bone physiology and diseases. Physiol. Rev. 93, 481–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamatsu T. (2004). Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 121, 605–618. [DOI] [PubMed] [Google Scholar]
- Karsenty G., Kronenberg H. M., Settembre C. (2009). Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648. [DOI] [PubMed] [Google Scholar]
- King-Heiden T. C., Mehta V., Xiong K. M., Lanham K. A., Antkiewicz D. S., Ganser A., Heideman W., Peterson R. E. (2012). Reproductive and developmental toxicity of dioxin in fish. Mol. Cell. Endocrinol. 354, 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Murata K., Naruse K., Tanaka M. (Eds.), 2009. Medaka: Biology, Management, and Experimental Protocols, 1st ed Wiley, Hoboken, NJ. [Google Scholar]
- Kirkham G. R., Cartmell S. H. 2007. Genes and proteins involved in the regulation of osteogenesis In Topics in Tissue Engineering (Ashammakhi N., Reis R., Chiellini E., Eds.), pp. 1–22. Available at: http://www.oulu.fi/spareparts/ebook_topics_in_t_e_Vol15502/. [Google Scholar]
- Kneissel M., Baron R. (2013). WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 19, 179–192. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Okamoto H., Iwamoto T., Toyama Y., Tomatsu T., Yamanaka H., Momohara S. (2008). A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford) 47, 1317–1322. [DOI] [PubMed] [Google Scholar]
- Komori T. (2010). Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 339, 189–195. [DOI] [PubMed] [Google Scholar]
- Korkalainen M., Kallio E., Olkku A., Nelo K., Ilvesaro J., Tuukkanen J., Mahonen A., Viluksela M. (2009). Dioxins interfere with differentiation of osteoblasts and osteoclasts. Bone 44, 1134–1142. [DOI] [PubMed] [Google Scholar]
- Krakow D., Rimoin D. L. (2010). The skeletal dysplasias. Genet. Med. 12, 327–341. [DOI] [PubMed] [Google Scholar]
- Lacey D. C., Simmons P. J., Graves S. E., Hamilton J. A. (2009). Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 17, 735–742. [DOI] [PubMed] [Google Scholar]
- Lahoti T. S., Hughes J. M., Kusnadi A., John K., Zhu B., Murray I. A., Gowda K., Peters J. M., Amin S. G., Perdew G. H. (2014). Aryl hydrocarbon receptor antagonism attenuates growth factor expression, proliferation, and migration in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Pharmacol. Exp. Ther. 348, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoti T. S., John K., Hughes J. M., Kusnadi A., Murray I., Krishnegowda G., Amin S., Perdew G. (2013). Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann. Rheum. Dis. 72, 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C., Oury C., Dejardin E., Chariot A., Piette J., Malaise M., Merville M. P., Franchimont N. (2007). Further insights in the mechanisms of interleukin-1beta stimulation of osteoprotegerin in osteoblast-like cells. J. Bone Miner. Res. 22, 1350–1361. [DOI] [PubMed] [Google Scholar]
- Lapunzina P., Aglan M., Temtamy S., Caparrós-Martín J. A., Valencia M., Letón R., Martínez-Glez V., Elhossini R., Amr K., Vilaboa N., et al. (2010). Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am. J. Hum. Genet. 87, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Patel R., Biermann J. S., Dougherty P. J. (2013). The musculoskeletal effects of cigarette smoking. J. Bone Joint. Surg. 95, 850–859. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Bhattaram P. 2010. Vertebrate Skeletogenesis, in: Koopman, P. (Ed.), Current Topics in Developmental Biology: Organogenesis in Development. Elsevier Inc., San Diego. pp. 291–317. doi:10.1016/s0070-2153(10)90008-2. [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Marcellini S., Henriquez J. P., Bertin A. (2012). Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: Cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. BioEssays News Rev. Mol. Cell. Dev. Biol. 34, 953–962. [DOI] [PubMed] [Google Scholar]
- McCollum C. W., Ducharme N. A., Bondesson M., Gustafsson J. A. (2011). Developmental toxicity screening in zebrafish. Birth Defects Res. Part C, Embryo Today Rev. 93, 67–114. [DOI] [PubMed] [Google Scholar]
- Miettinen H. M., Pulkkinen P., Jämsä T., Koistinen J., Simanainen U., Tuomisto J., Tuukkanen J., Viluksela M. (2005). Effects of in utero and lactational TCDD exposure on bone development in differentially sensitive rat lines. Toxicol. Sci. 85, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29. [DOI] [PubMed] [Google Scholar]
- Naruse M., Ishihara Y., Miyagawa-Tomita S., Koyama A., Hagiwara H. (2002). 3-Methylcholanthrene, which binds to the arylhydrocarbon receptor, inhibits proliferation and differentiation of osteoblasts in vitro and ossification in vivo. Endocrinology 143, 3575–3581. [DOI] [PubMed] [Google Scholar]
- Ng G. H. B., Gong Z. (2013). GFP transgenic medaka (Oryzias latipes) under the inducible cyp1a promoter provide a sensitive and convenient biological indicator for the presence of TCDD and other persistent organic chemicals. PLoS One 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. T., Nakahama T., Kishimoto T. (2013). Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin. Immunopathol. 35, 637–644. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Nishimura H., Ito T., Miyata C., Izumi K., Fujimaki H., Matsumura F. (2009). Dioxin-induced up-regulation of the active form of vitamin D is the main cause for its inhibitory action on osteoblast activities, leading to developmental bone toxicity. Toxicol. Appl. Pharmacol. 236, 301–309. [DOI] [PubMed] [Google Scholar]
- Nordvik K., Kryvi H., Totland G. K., Grotmol S. (2005). The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. J. Anat. 206, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli I. M., Castilla E. E., Barbosa-Neto J. G. (1986). The birth prevalence rates for the skeletal dysplasias. J. Med. Genet. 23, 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchart A., Mattingly C. J. (2010). 2,3,7,8-Tetrachlorodibenzo- p-dioxin upregulates FoxQ1b in zebrafish jaw primordium. Chem. Res. Toxicol. 23, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn J., Büttner A., To T. T., Chan S. J. H., Winkler C. (2013). A col10a1: NlGFP transgenic line displays putative osteoblast precursors at the medaka notochordal sheath prior to mineralization. Dev. Biol. 381, 134–143. [DOI] [PubMed] [Google Scholar]
- Renn J., Winkler C. (2014). Osterix/Sp7 regulates biomineralization of otoliths and bone in medaka (Oryzias latipes). Matrix Biol. 34, 193–204. [DOI] [PubMed] [Google Scholar]
- Renn J., Winkler C. (2009). Osterix-mCherry transgenic medaka for in vivo imaging of bone formation. Dev. Dyn. 238, 241–248. [DOI] [PubMed] [Google Scholar]
- Ryan E. P., Holz J. D., Mulcahey M., Sheu T. J., Gasiewicz T. A., Puzas J. E. (2007). Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J. Bone Miner. Res. 22, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., et al. (1997). Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89, 309–319. [DOI] [PubMed] [Google Scholar]
- Singh S. U., Casper R. F., Fritz P. C., Sukhu B., Ganss B., Girard B., Savouret J. F., Tenenbaum H. C. (2000). Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. J. Endocrinol. 167, 183–195. [DOI] [PubMed] [Google Scholar]
- Sinha K. M., Zhou X. (2013). Genetic and molecular control of osterix in skeletal formation. J. Cell. Biochem. 114, 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes N. S., Padilla S., Knudsen T. B. (2011). Zebrafish-as an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C, Embryo Today Rev. 93, 256–267. [DOI] [PubMed] [Google Scholar]
- Taichman R. S., Hauschka P. V. (1992). Effects of interleukin-1beta and tumor necrosis factor-alpha on osteoblastic expression of osteocalcin and mineralized extracellular matrix invitro. Inflammation 16, 587–601. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Dong W., Ogawa S., Tsukiyama S., Okuhara Y., Niiyama M., Ueno N., Peterson R. E., Hiraga T. (2002). 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 65, 192–199. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Dong W., Okuhara Y., Lwasa H., Shindo A., Hill A. J., Kawakami A., Hiraga T. (2006). Impairment of lower jaw growth in developing zebrafish exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and reduced hedgehog expression. Aquat. Toxicol. 78, 103–113. [DOI] [PubMed] [Google Scholar]
- Timpson N. J., Tobias J. H., Richards J. B., Soranzo N., Duncan E. L., Sims A. M., Whittaker P., Kumanduri V., Zhai G., Glaser B., et al. (2009). Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum. Mol. Genet. 18, 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T. T., Witten P. E., Renn J., Bhattacharya D., Huysseune A., Winkler C. (2012). Rankl-induced osteoclastogenesis leads to loss of mineralization in a medaka osteoporosis model. Development 141–150. [DOI] [PubMed] [Google Scholar]
- Truong L., Reif D. M., Mary L. S., Geier M. C., Truong H. D., Tanguay R. L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl R. W., Chernoff N., Rogers J. M. (2007). Altered axial skeletal development. Birth Defects Res. B Dev. Reprod. Toxicol. 472, 451–472. [DOI] [PubMed] [Google Scholar]
- Volz D. C., Hinton D. E., Law J. M., Kullman S. W. (2006). Dynamic gene expression changes precede dioxin-induced liver pathogenesis in medaka Fish. Toxicol. Sci. 89, 524–534. [DOI] [PubMed] [Google Scholar]
- Xiong K. M., Peterson R. E., Heideman W. (2008). Aryl Hydrocarbon Receptor-Mediated Down-Regulation of Sox9b Causes Jaw Malformation in Zebrafish Embryos. Mol. Pharmacol. 74, 1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutake J., Inohaya K., Kudo A. (2004). Twist functions in vertebral column formation in medaka, Oryzias latipes. Mech. Dev. 121, 883–894. [DOI] [PubMed] [Google Scholar]
- Yu H., Du Y., Zhang X., Sun Y., Li S., Dou Y., Li Z., Yuan H., Zhao W. (2014). The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway. Toxicol. Appl. Pharmacol. 280, 502–510. [DOI] [PubMed] [Google Scholar]
- Zhang G. (2009). An evo-devo view on the origin of the backbone: Evolutionary development of the vertebrae. Integr. Comp. Biol. 49, 178–186. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Hu J. (2007). Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol. Sci. 95, 356–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.