Abstract
Tuberculosis is responsible for >2 million deaths a year, and the number of new cases is rising worldwide. DNA vaccination combined with Mycobacterium bovis bacillus Calmette Guérin (BCG) represents a potential strategy for prevention of this disease. Here, we used a heterologous prime-boost immunization approach using a combination of DNA plasmids and BCG in order to improve the efficacy of vaccination against Mycobacterium tuberculosis infection in mice. As model antigens, we selected the M. tuberculosis Apa (for alanine-proline-rich antigen) and the immunodominant Hsp65 and Hsp70 mycobacterial antigens combined with BCG. We demonstrated that animals injected with a combination of DNA vectors expressing these antigens, when boosted with BCG, showed increased specific antimycobacterial immune responses compared to animals vaccinated with BCG alone. More importantly, the protection achieved with this regimen was also significantly better than with BCG alone.
Recently, the World Health Organization estimated that there are ∼8 million new cases of tuberculosis (TB) in the world annually, causing >2 million deaths (3). TB is the greatest cause of death due to a single pathogen, and it is a leading killer among people infected with human immunodeficiency virus or AIDS. Mycobacterium bovis bacillus Calmette Guérin (BCG) is the only vaccine available for clinical use and protects children against miliary tuberculosis (13); however, it has shown variable levels of efficacy against pulmonary TB, as demonstrated by several clinical trials (7). For example, a major trial in The United Kingdom showed >75% protection (9); however, trials in south India and Malawi demonstrated that BCG fails to consistently protect against pulmonary TB (7, 19). BCG has been used for >6 decades, and >3 billion doses have been administered; therefore, it is one of the most used vaccines worldwide. Current research interest has been directed to improving the immunogenicity of BCG (2); recently, recombinant BCG exporting ESAT-6 was found to confer enhanced protection against tuberculosis compared with BCG (20). Similarly, it has been demonstrated that recombinant BCG vaccine expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induced greater protective immunity against tuberculosis than the conventional BCG vaccine (12). A different approach is the heterologous expression of cytokines in BCG (1, 17) or bacterial proteins, such as Listeria monocytogenes listeriolysin, which improved the capacity to stimulate antimycobacterial T-cell responses (10). An interesting complementary approach to improving BCG immunogenicity is its combination in prime-boost immunization protocols (8, 14): priming with a DNA vaccine expressing antigen 85B and boosting with BCG improved its protective efficacy in a murine M. tuberculosis challenge model (5). However, it is still an open question whether further-improved levels of protective immunity in mice could be obtained by using plasmid DNA combinations associated with BCG boosting. Here, we show that it is possible to significantly improve BCG efficacy in mice by first priming a robust antimycobacterial immune response using the M. tuberculosis Apa (alanine-proline-rich antigen) and the mycobacterial Hsp65 and Hsp70 antigens delivered by DNA injection, followed by conventional BCG vaccination. Hsp65 and Hsp70 genes were selected based on previous studies showing induction of protection against tuberculosis infection in mice by immunization with DNA encoding individual antigens (16, 26, 27). The mycobacterial Apa secretion antigen, also known as the 45/47-kDa protein complex (23), is composed of mannosylated proteins with up to nine identified glycoforms (11, 24). Apa was selected because of its ability to be recognized by the immune responses in animals inoculated with live BCG and the high level of immunogenicity previously described (23, 24). The results of this vaccination protocol are consistent with previous encouraging data initially reported in cattle (25).
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C57BL/6 and BALB/c mice were obtained from the specific-pathogen-free unit at the National Institute of Medical Research. Experiments were carried out in the United Kingdom according to the Home Office Animal Scientific Act of 1986.
pCMV4.Apa.
Primers for the amplification of the entire coding sequence of Apa, including the N-terminal signal peptide, were designed to include a BamHI and a NotI site for directional cloning in pCMV4 (27). PCRs were performed using 32 ng of genomic DNA of M. tuberculosis H37Rv per 50-μl reaction mixture diluted in PCR buffer [10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris (pH 8.74), 0.1% Triton 100, 100 μg of bovine serum albumin/ml] (Sigma, Dorset, United Kingdom). Amplifications were done using 2.5 U of Pfu DNA polymerase (Stratagene, La Jolla, Calif.) per reaction. The annealing temperature was 55°C with an extension time of 1.5 min. The primers used were CMV4.Apa forward (5′-ATTGGATCCGCCATGCATCAGGTGGAC-3′) and CMV4.Apa reverse (5′-TATGCGGCCGCCTCAGGCCGGTAAG-3′). A 1-kb DNA product was obtained and gel purified for further cloning using the QIAquick gel extraction kit (QIAgen, Dorking, West Sussex, United Kingdom). Vector pCMV4 was digested with BamHI and NotI and gel purified. Ligation reactions were performed using 1 μl of T4 DNA ligase in 10 μl of ligation buffer (Roche Diagnostic, East Sussex, United Kingdom). DNA plasmids were amplified using Escherichia coli TOP10 supercompetent cells (Invitrogen, Paisley, United Kingdom). Restriction enzyme analysis identified the correct constructs.
pQE60.Apa.
The bacterial expression vector pQE60 was obtained from QIAgen. This vector allows fusion of the cloned sequence with a six-His tag in the C-terminal part of the recombinant protein. The vector was gel purified and double digested with BamHI and NcoI. Primers amplifying the entire coding sequence of the apa gene, including the N-terminal signal sequence, and containing BamHI and NcoI restriction sites were designed as described previously (6). Recombinant colonies were selected on ampicillin plates, and restriction enzyme analysis was performed to identify positive clones. Ni-nitrilotriacetic acid affinity chromatography (QIAgen) was used to obtain the recombinant Apa antigen. Immobilized polymixin B in agarose (Detoxi-Gel Endotoxin Removing Gel; Pierce Chemical Co., Rockford, Ill.) was used to reduce the endotoxin levels of the recombinant Apa antigen to 5 to 6 endotoxin units/mg of protein.
pCMV4.65 and pCMV70.
The pCMV4.65 and pCMV70 DNA constructs were described previously (16, 27). The plasmids were used only when endotoxin levels were <0.1 enzyme unit/μg of DNA.
DNA transfection.
Transient transfections of cell lines were performed as described previously (26). Briefly, pCMV4.Apa, diluted in serum-free Optimem medium (Invitrogen), was incubated at room temperature (RT) for 45 min. Lipofectin reagent (Gibco-BRL, Paisley, United Kingdom), was diluted in the same medium and also incubated at RT for 45 min. DNA and liposome solutions were mixed gently and left undisturbed at RT for a further 15 min. At the end of this period, 28 ml of medium was added to the DNA-liposome mixture, and the mixture was transferred to one 175-cm2 flask containing CV1 cells (European Collection of Cell Cultures, Salisbury, United Kingdom) at 40 to 50% confluency in 15 ml of Optimem. After 16 h of incubation at 37°C, the cell monolayer was washed and new medium was added. Supernatants were recovered 48 h later, filtered through 0.2-μm-pore-size membranes, and concentrated by lyophilization. The cells were collected for preparation of the cell extract, which was then assayed for specific gene expression.
Protein separation and Western blotting.
Sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (one-dimensional polyacrylamide gel electrophoresis under denaturing conditions) was performed using a Mighty Small II Mini-Gel System (Hoeffer Scientific Instruments, Little Chalfont, United Kingdom). Protein samples were denatured in sample buffer (for 10 ml, 2.2 ml of 0.5 M Tris, pH 6.8, 2.25 g of SDS, 1.77 ml of glycerol, 0.4 ml of 2-β-mercaptoethanol, and 2 mg of bromophenol blue) and added to the gel, along with molecular weight markers. After electrophoresis, the gels were used for immunoblotting as described previously (6). Briefly, the polyvinylidene difluoride membranes were incubated in blocking buffer (Tris-buffered saline; 10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Tween 20, plus 10% skim milk and 2 mM EDTA) for 2 h at RT. After being washed once in Tris-buffered saline-0.1% Tween 20 for 15 min, the blots were incubated with the first antibody for 1 h. For the detection of Apa protein, a specific monoclonal antibody (MAb), 6A3 (4), was used at 1:250 dilution. After the membrane was washed, a 1:1,500 dilution of an anti-mouse immunoglobulin G-alkaline phosphatase conjugate (DAKO, Ely, United Kingdom) was applied to the membrane for 1 h. Finally, the membrane was washed and developed after the addition of substrate nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma) diluted in water.
Enrichment and culture of CD4+ or CD8+ T cells.
Cell suspensions were made from spleens. Enrichment of CD4+- and CD8+-T-cell subpopulations was obtained by negative selection using a mouse T Cell Subset Column kit (R&D Systems, Oxon, United Kingdom) according to the manufacturer's instructions. Briefly, erythrocyte-depleted splenocytes were resuspended in column wash buffer, and a specific monoclonal antibody mix (R&D Systems) was added to the cells. The mixture was incubated at RT for 15 min. Following two washes, the antibody-treated cells were loaded into the column. Up to 90% pure CD4+ or CD8+ populations were eluted; after being washed, the cells were plated at 4 × 105 to 5 × 105/ml with an equivalent number of irradiated spleen cells in 250 μl of AIM-V medium (Gibco-BRL) supplemented with 80 μM 2-β-mercaptoethanol and 2% fetal calf serum (FCS; Advanced Protein Products, Brierly Hill, United Kingdom). The cells were cultured for up to 72 h at 37°C with 6 μg of recombinant Apa/ml, 10 μg of purified protein derivative (PPD) (VLA Laboratories, New Addlestone, United Kingdom)/ml, or 5 μl of sterile saline; the supernatants were collected after 24, 48, and 72 h; filtered through 0.2-μm-pore-size filters; and kept at −70°C. When cells from M. tuberculosis-infected animals were isolated, procedures were carried out using microbiological class I cabinets in the containment III unit of the National Institute of Medical Research.
Cytokine levels and ELISPOT assays.
Cytokines were assayed using commercial sandwich enzyme-linked immunosorbent assay (ELISA) kits for the presence of gamma interferon (IFN-γ) or interleukin 2 (IL-2) (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom, or R&D Systems), according to the manufacturer's protocols. Enzyme-linked immunospot (ELISPOT) plates (MAIP S45; Millipore, Bedford, Mass.) were coated with 100 μl of 10-μg/ml anti-IFN-γ MAb R4-6A2 (BD Pharmingen, Oxford, United Kingdom)/well diluted in calcium-magnesium-free sterile phosphate-buffered saline (PBS) (Gibco-BRL), pH 7.4, and incubated overnight at 4°C. The plates were washed twice with 200 μl of AIM-V medium (Gibco-BRL)/well supplemented with 2% FCS (Advanced Protein Products) and blocked with AIM-V-10% FCS for 2 h at 37°C. Splenic CD4+- or CD8+-T-cell suspensions plus antigen-presenting cells were incubated with 10 μg of PPD/ml at ∼106 cells/ml in AIM-V-2% FCS for 48 h at 37°C and 5% CO2. The cells were resuspended, counted, and added in 100-μl volumes at various serial dilutions to ELISPOT plates. The plates were incubated for 16 h at 37°C and 5% CO2 before being washed. Biotinylated anti-IFN-γ MAb (clone XMG 1.2; BD-Pharmingen) was added to the plates for 2 h. After the plates were washed, 100 μl of streptavidin-alkaline phosphatase (BD-Pharmingen)/well diluted 1:1,000 in PBS-0.1% Tween 20 plus bovine serum albumin was added for 1 h at RT. Spot counting was performed after the addition of nitroblue tetrazolium-BCIP substrate solution (Sigma). The results were expressed as the mean number of cytokine-producing cells per 105 total cells subtracted from the controls. Each sample was assayed in triplicate. The controls included wells without cells (background) and unstimulated cells.
Vaccination and challenge infection.
Fifty to 75 μg of each plasmid DNA/50 μl of saline was injected intramuscularly three times at 3-week intervals; the DNA constructs pCMV4.Apa, pCMV4.65, and pCMV.70 were injected simultaneously in different anatomical sites. The boost with ∼106 CFU of BCG vaccine (Glaxo, Stevenage, United Kingdom) injected intradermally at the base of the tail was performed 1 month after priming with DNA. Twelve to 15 weeks after the BCG boost, the animals were either used for immune response analysis or challenged with 105 CFU of M. tuberculosis H37Rv (obtained from our original culture stock) injected intravenously. Five weeks later, the bacterial load in the lungs was evaluated. Briefly, the organs were weighed and homogenized in PBS using a Mini-Bead Beater (BioSpec Products, Bartlesville, Okla.). Serial 10-fold dilutions of the homogenates were plated on Middlebrook 7H11 Bacto Agar (Difco Laboratories, Surrey, United Kingdom). The colonies were counted 3 to 4 weeks later, and the results were expressed as CFU per lung.
Statistical analysis.
Statistical analysis was performed for cytokine responses (ELISA titers and ELISPOT counts) and bacterial CFU data using Student's t test. A P value of <0.05 was considered significant.
RESULTS
Expression and immunogenicity of the mycobacterial Apa antigen.
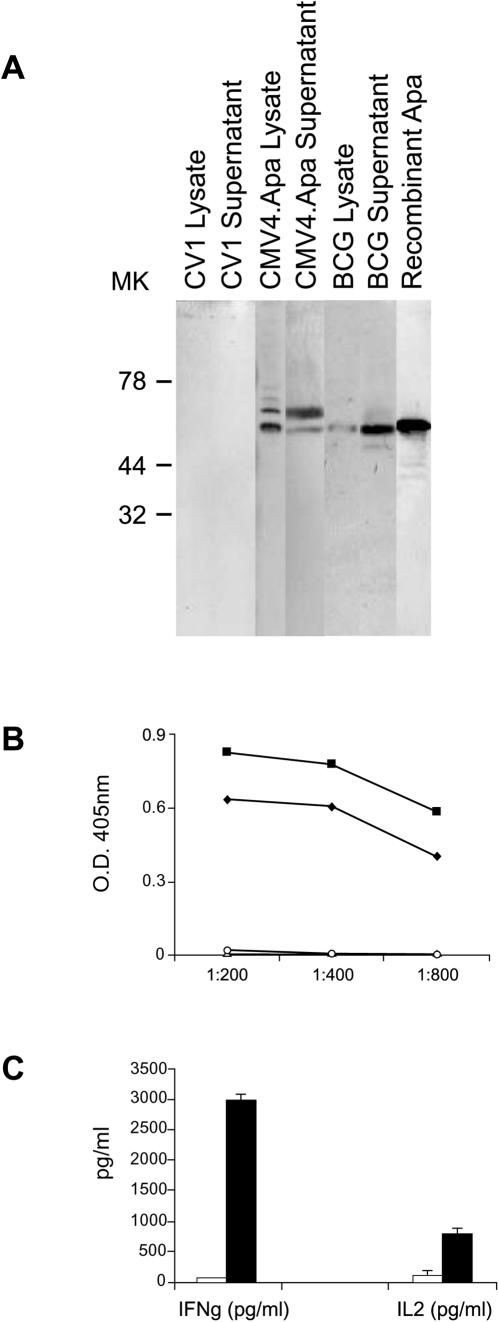
The expression and immunogenicity of mycobacterial Hsp65 and Hsp70 have been previously described (16, 26, 27). We cloned the mycobacterial Apa antigen in the CMV4 mammalian expression vector (27) and initially confirmed its expression following in vitro DNA transfection of CV1 cells (Fig. 1A). The results after Western blot analysis using the Apa-specific monoclonal antibody 6A3 demonstrated the expression of two products in the cell lysates and concentrated supernatants of Apa-transfected CV1 cells but not in the cell extracts or supernatants of vector-transfected cells (Fig. 1A). These two products could possibly be attributed to alternative starting codons and/or posttranslational glycosylation (6). Consistent with the gene expression results, we detected antigen-specific immunoglobulin G antibody responses after a series of intramuscular Apa-DNA injections (Fig. 1B). In addition, spleen CD4+ T cells from Apa-DNA-injected mice specifically secreted IL-2 and IFN-γ in vitro in response to recombinant Apa antigen stimulation (Fig. 1C), demonstrating that this DNA construct is highly immunogenic in mice.
FIG. 1.
M. tuberculosis Apa antigen expression by Apa DNA-transfected cells. (A) Western blot analysis of Apa antigen expression by transfected CV1 cells. Cell extracts or supernatants were prepared as described in Materials and Methods; proteins were separated by SDS-polyacrylamide gel electrophoresis, blotted on nitrocellulose membranes, and incubated with the specific anti-Apa monoclonal antibody 6A3. The positive controls were BCG lysate and recombinant Apa antigen. (B and C) Specific immune responses induced after Apa DNA injection. Humoral and cellular responses were determined 4 weeks after four injections of Apa DNA. A control group of mice received CMV4 vector only. MK, molecular markers (in kilodaltons). (B) Apa-specific antibody levels in pooled sera from Apa DNA (▪), CMV4 vector (○), or saline-vaccinated mice (▵) were determined using the recombinant Apa antigen; (⧫), positive control MAb 6A3. ELISA readings were assayed in triplicate with a standard deviation of <20%. (C) IFN-γ or IL-2 produced by immune T cells from Apa DNA-vaccinated mice in response to recombinant Apa antigen stimulation. The cytokines were not detected in unstimulated cultures. The filled bars (Apa DNA-vaccinated mice) and empty bars (CMV4 vector-vaccinated mice) represent the means of triplicate wells plus standard errors. The results shown are one representative out of two or three separate experiments.
Specific CD4+- and CD8+-T-cell responses induced after DNA-BCG boost vaccination.
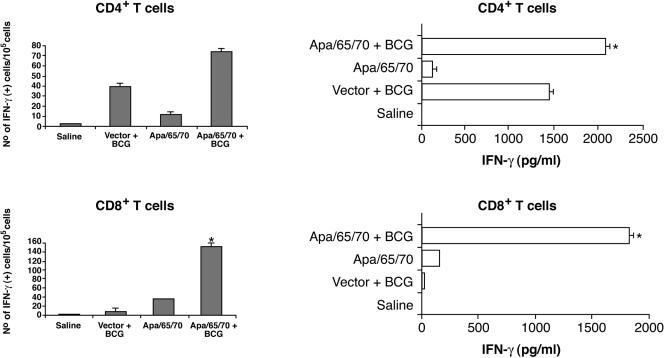
We next sought to examine the immune responses of mice injected with Apa DNA in combination with DNA gene constructs expressing the immunodominant Hsp65 and Hsp70 antigens. Groups of BALB/c mice were injected intramuscularly three times with plasmid DNA constructs expressing these mycobacterial antigens and were boosted with BCG 4 weeks later. Groups of control mice received CMV4 vector or saline or CMV4 vector plus BCG. Twelve to 15 weeks after BCG injection, we tested by ELISPOT (Fig. 2, left panel) and conventional ELISA (Fig. 2, right panel) the antigen-specific immune responses of the vaccinated mice by measuring specific IFN-γ production by immune splenic CD4+ or CD8+ T cells stimulated in vitro with mycobacterial PPD. We demonstrated specific CD4+- and CD8+-T-cell responses using cells obtained from Apa/65/70 DNA-vaccinated mice and showed that the PPD-specific T-cell reactivity was consistently more robust with cells obtained from Apa/65/70-BCG than with cells originating from CMV4 vector-BCG-vaccinated mice (Fig. 2). These results suggest specific enhanced priming of M. tuberculosis-reactive T cells in mice vaccinated with the DNA combination and BCG boost.
FIG. 2.
Specific IFN-γ responses induced after DNA-BCG boost vaccination. Cytokine responses were assessed 12 to 15 weeks after BCG injection by ELISPOT (left panels) or ELISA (right panels). Mycobacterial PPD was used as a specific antigen. IFN-γ was not detected in cultures stimulated with saline. The groups were Apa/65/70 DNA, Apa/65/70 DNA plus BCG, CMV4 vector plus BCG, and saline. The bars represent the means plus standard errors (error bars). The results shown are one representative experiment out of two or three separate experiments. *, P < 0.05; Student's t test versus CMV4 vector plus BCG.
Protection against M. tuberculosis infection by DNA-BCG boost immunization.
We next tested whether vaccinated BALB/c mice were protected after M. tuberculosis H37Rv challenge. Twelve to 15 weeks after BCG injection, the groups of mice primed with the Apa/65/70 plasmids showed increased protection in comparison to the saline control group (Fig. 3A). In addition, when the Apa/65/70 plasmid cocktail was combined with BCG, the animals exhibited significantly better protection in the lungs than animals vaccinated with BCG alone. We then confirmed the effect of this vaccination regimen using C57BL/6 mice, frequently used for preclinical testing of new vaccine candidates against tuberculosis (18). Similar to the results obtained with BALB/c mice, C57BL/6 mice primed with Apa/65/70 DNA plasmids displayed significant protection (Fig. 3B). Additionally, when this vaccine cocktail was combined with BCG, the protection obtained was significantly improved compared to animals vaccinated with BCG plus empty vector (Fig. 3B). Interestingly, preliminary results suggested that removal of the Apa DNA construct from the immunization protocol resulted in significantly diminished protection (J. C. Ferraz and R. E. Tascon, unpublished data).
FIG. 3.
Protection against M. tuberculosis infection by DNA plus BCG boost immunization. Groups of 5 to 15 BALB/c (A) or C57BL/6 (B) mice were vaccinated three times with Apa/65/70 DNA or Apa/65/70 DNA plus BCG. Controls were vaccinated with CMV4 vector or saline or CMV4 vector plus BCG. Twelve to 15 weeks after BCG injection, the mice were infected with 105 CFU of M. tuberculosis H37Rv. Six weeks later, the number of live bacteria in the lungs was determined and expressed as CFU per lung. The bars represent the mean CFU plus standard error (error bars). The results shown are one representative out of two separate experiments. *, P < 0.05; Student's t test for Apa/65/70 plus BCG versus CMV4 vector plus BCG and Apa/65/70 versus saline. **, P < 0.01; Student's t test for Apa/65/70 plus BCG versus CMV4 vector plus BCG and Apa/65/70 versus CMV4 vector.
DISCUSSION
In this work, we showed that vaccination of BALB/c or C57BL/6 mice with three priming doses of a plasmid DNA cocktail encoding the mycobacterial antigens Apa, Hsp65, and Hsp70 followed by one intradermal injection of BCG 1 month later resulted in highly significant reduction in the M. tuberculosis bacterial load after challenge infection. In addition, our results showed that the improved protection over BCG correlated with a significant increase in the frequency of IFN-γ-secreting T cells, particularly CD8+ T cells, in response to PPD before challenge. However, this correlation with protection was not observed in animals examined after M. tuberculosis challenge (6). Recently, it was reported that the M. tuberculosis Apa antigen stimulated IFN-γ-secreting CD4+ and CD8+ T cells from PPD-positive individuals and conferred protection in the guinea pig model of tuberculosis infection (15); these observations and our results suggest that reactivity against Apa antigen may be an important component of an effective protective immune response to M. tuberculosis infection. The protective efficiency of the plasmid-DNA combination Apa/65/70 combined with BCG has recently been tested in cattle (25). In that model, vaccination resulted in significant enhancement of six pathological and microbiological parameters of protection (the number of animals with lung lesions, the mean lung lesion score, the number of animals with lymph node lesions, the mean lymph node lesion score, the mean number of lymph nodes with lesions per animal, and the mean lymph node bacterial count after challenge with M. bovis), while vaccination with BCG alone enhanced only two parameters of protection. Our results described here are consistent with these observations using a different model of M. tuberculosis infection and support the concept that a combination of vaccines may be better than a single vaccine for protection against tuberculosis. Importantly, this vaccination regimen for generating highly effective protective immunity can also be used to identify in vitro correlates of antimycobacterial protection. We are investigating in more detail the role of the Apa antigen in this vaccination protocol, and in particular, whether priming of T-cell responses to this antigen was promoted by an indirect adjuvant effect induced by the mycobacterial heat shock antigens. Although there is concern that microbial heat shock antigens used in DNA vaccination protocols may induce autoimmune pathology, we and others have demonstrated protection of rats from autoimmune arthritis by immunization with naked DNA encoding heat shock protein 65 (21, 22). Nonetheless, this important question should be studied in more detail before heat shock antigen combination vaccines advance to clinical testing. Other antigen combinations, and alternatively, safer polynucleotide delivery platforms, like self-replicative RNA (28), should also be investigated concomitantly. Strategies such as fusion of different immunoprotective epitopes in polynucleotide constructs may also offer increased vaccine efficacy when combined with successfully engineered BCG.
Acknowledgments
We are grateful to the Medical Research Council for support. Jose Candido Ferraz was supported by CNPq-Brazil.
We thank Silvia Ragno for critical reading of the manuscript.
The authors declare that they have no competing financial interest.
Editor: F. C. Fang
REFERENCES
- 1.Biet, F., L. Kremer, I. Wolowczuk, M. Delacre, and C. Locht. 2000. Mycobacterium bovis BCG producing interleukin-18 increases antigen-specific gamma interferon production in mice. Infect. Immun. 70:6549-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietrich, G., J. F. Viret, and J. Hess. 2003. Mycobacterium bovis BCG-based vaccines against tuberculosis: novel developments. Vaccine 21:667-670. [DOI] [PubMed] [Google Scholar]
- 3.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 4.Espitia, C., R. Espinosa, R. Saavedra, R. Mancilla, F. Romain, A. Laqueyrerie, and C. Moreno. 1995. Antigenic and structural similarities between Mycobacterium tuberculosis 50- to 55-kilodalton and Mycobacterium bovis BCG 45- to 47-kilodalton antigens. Infect. Immun. 63:580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferraz, C. Ph.D. thesis. University College London, London, United Kingdom. (Submitted.)
- 7.Fine, P. E. M. 2000. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 8.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 9.Hart, P. D., and I. Sutherland. 1977. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Final report to the Medical Research Council. BMJ 2:293-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess, J., D. Miko, A. Catic, V. Lehmensiek, D. G. Russell, and S. H. Kaufmann. 1998. Mycobacterium bovis Bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 95:5299-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn, C., A. Namane, P. Pescher, M. Rivière, F. Romain, G. Puzo, O. Barzu, and G. Marchal. 1999. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J. Biol. Chem. 274:32023-32030. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jason, J., L. K. Archibald, O. C. Nwanyanwu, P. N. Kazembe, J. A. Chatt, E. Norton, H. Dobbie, and W. R. Jarvis. 2000. Clinical and immune impact of Mycobacterium bovis BCG vaccination scarring. Infect. Immun. 70:6188-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, P., R. R. Amara, V. K. Challu, V. K. Chadda, and V. Satchidanandam. 2003. The Apa protein of Mycobacterium tuberculosis stimulates gamma interferon-secreting CD4+ and CD8+ T cells from purified protein derivative-positive individuals and affords protection in a guinea pig model. Infect. Immun. 71:1929-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. E. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15:834-838. [DOI] [PubMed] [Google Scholar]
- 17.Murray, P. J., A. Aldovini, and R. A. Young. 1996. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc. Natl. Acad. Sci. USA 93:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 3:115-118. [DOI] [PubMed] [Google Scholar]
- 19.Ponnighaus, J. M., P. E. M. Fine, J. A. Sterne, R. J. Wilson, E. Msosa, P. J. Gruer, P. A. Jenkins, S. B. Lucas, N. G. Liomba, and L. Bliss. 1992. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339:636-639. [DOI] [PubMed] [Google Scholar]
- 20.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 21.Quintana, F. J., P. Carmi, F. Mor, and I. R. Cohen. 2002. Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J. Immunol. 169:3422-3428. [DOI] [PubMed] [Google Scholar]
- 22.Ragno, S., M. J. Colston, D. B. Lowrie, V. R. Winrow, D. R. Blake, and R. E. Tascon. 1997. Protection of rats from adjuvant arthritis by immunization with naked DNA encoding for mycobacterial heat shock protein 65. Arthritis Rheum. 40:277-283. [DOI] [PubMed] [Google Scholar]
- 23.Romain, F., A. Laqueyrerie, P. Militzer, P. Pescher, P. Chavarot, M. Lagranderie, G. Auregan, M. Gheorghiu, and G. Marchal. 1993. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect. Immun. 61:742-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romain, F., C. Horn, P. Pescher, A. Namane, M. Riviere, G. Puzo, O. Barzu, and G. Marchal. 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 67:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 27.Tascon, R. E., M. J. Colston, E. Stavropoulos, S. Ragno, D. Gregory, and D. B. Lowrie. 1997. Protection against tuberculosis by plasmid DNA, p. 181-185. In G. Gregoriadis, B. McCormack, and A. C. Allison (ed.), Vaccine design: the role of cytokine networks, vol. 293. Kluwer Academic Publishers, Hingham, Mass.
- 28.Xue, T., E. Stavropoulos, M. Yang, S. Ragno, H. M. Vordermeier, M. Chambers, R. G. Hewinson, D. B. Lowrie, M. J. Colston, and R. E. Tascon. Protective immune responses against M. tuberculosis infection conferred by RNA encoding MPT83 antigen. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]