Abstract
Protection against invasive amebiasis was achieved in the gerbil model for amebic liver abscess by oral immunization with live attenuated Yersinia enterocolitica expressing the Entamoeba histolytica galactose-inhibitable lectin that has been fused to the Yersinia outer protein E (YopE). Protection was dependent on the presence of the YopE translocation domain but was independent from the antibody response to the ameba lectin.
The intestinal protozoan parasite Entamoeba histolytica, the causative agent of human amebiasis, is endemic in large parts of the world and is considered to be responsible for tens of millions of cases of dysentery and liver abscess each year (22). Morbidity and mortality associated with amebic infection have persisted despite the availability of effective therapy, suggesting that interventions designed to reduce or eliminate disease are needed. Since humans are the only relevant host for E. histolytica, a sufficient vaccine could potentially eradicate amebiasis.
A leading candidate for a vaccine to prevent amebiasis is the E. histolytica galactose- and N-acetylgalactosamine-inhibitable surface lectin (12). Various independent vaccination trials of intraperitoneal immunization with either the purified native molecule or recombinantly expressed sections of the 170-kDa heavy subunit revealed substantial protection in the gerbil model for amebic liver abscess (1, 9-11, 18, 24). In contrast to systemic application, oral vaccination of gerbils with the lectin, either fused to the B subunit of cholera toxin (9) or somatically expressed in an attenuated Salmonella strain, revealed only little protection against liver abscess formation (11, 23). Thus, it appears that the delivery system for an oral amebiasis vaccine has to be improved.
Recent progress in the development of bacterial live carrier vaccines has been made by the use of the type III secretion systems (T3SS) for heterologous antigen delivery (4). The Yersinia enterocolitica T3SS has been used successfully to translocate heterologous proteins into host cells (7, 20). This translocation was achieved by fusion of proteins to at least 50 amino acids (aa) of the N terminus of YopE (19). However, shortening of the YopE N terminus to 18 amino acid residues abolished translocation into host cells but led to secretion of the chimeric proteins into the culture medium (16, 17, 20). Recently, it was shown for mice that oral application of recombinant Yersinia cells expressing listeriolysin via T3SS resulted in a protective immune response against listeria infection (14, 15).
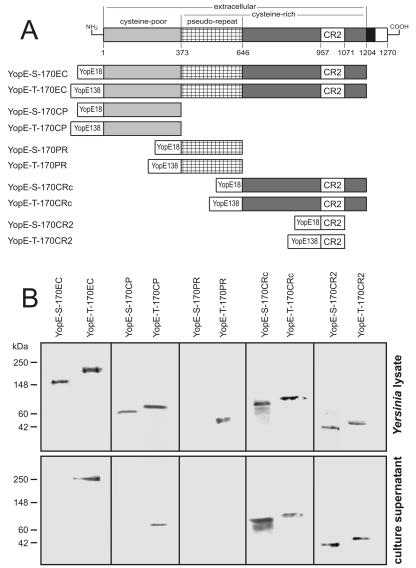
In order to assess the vaccine potential of YopE-directed antigen delivery against amebiasis, we generated attenuated recombinant Yersinia enterocolitica O8 cells, which either secrete or translocate YopE-lectin hybrid proteins via the T3SS. DNA fragments, encoding various segments of the 170-kDa heavy subunit of the E. histolytica surface lectin, were ligated in frame to the sequences for the 18-aa YopE secretion (YopE-S) or the 138-aa YopE secretion and translocation (YopE-T) domain and cloned into the Yersinia expression plasmid pACYC184 (15) conferring chloramphenicol resistance (Fig. 1A). Plasmids were transformed into attenuated Yersinia strain WA irp1 (abolished yersiniabactin production) (13), as this strain was found to colonize the intestine and Peyer's patches of orally infected gerbils but did not disseminate to other organs and kill the animals as wild-type cells do. With the exception of YopE-S-170PR transformants, recombinant Yersinia cells were able to express all of the various fusion proteins, as revealed by Western blots prepared from bacterial lysates and developed with antilectin immune serum (Fig. 1B, upper panel). In addition, a number of the various hybrid proteins were released and could be detected in yersinia culture supernatants (Fig. 1B, lower panel). Two of the four secreted proteins were released only in the presence of the YopE secretion and translocation domain. Consistent with release via the T3SS pathway, coculture experiments of recombinant bacteria with HeLa cells (15) revealed that only fusion proteins containing the YopE secretion and translocation domain were targeted to the HeLa cell cytosol (data not shown).
FIG. 1.
Expression and secretion of YopE-lectin hybrid proteins. (A) Shown are the structural domains of the E. histolytica 170-kDa surface lectin as they have been defined previously (10, 21). Numbering refers to amino acid residues and indicates the boundaries of fragments recombinantly expressed as fusions with the YopE secretion (YopE18) or the YopE secretion and translocation (YopE138) domain. (B) Immunoblots of lysates and culture supernatants from Yersinia cells transformed with the various YopE-lectin expression plasmids, which were developed with an antilectin immune serum.
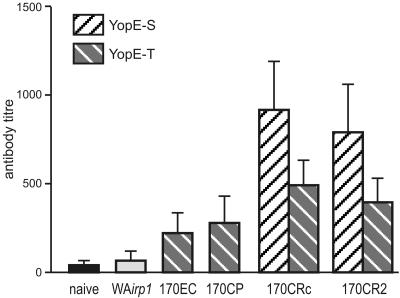
Recombinant Yersinia cells able to secrete or translocate YopE-lectin fusion proteins were used for oral vaccination of gerbils. Intragastric application of 109 CFU resulted in long-lasting intestinal colonization for at least 30 days. However, culturing of reisolated bacteria under chloramphenicol selection indicated differences in the stability of the expression plasmid between the various transformants. Whereas most of the reisolated bacteria (50 to 70%) from the majority of transformants were chloramphenicol resistant for up to at least 14 days of colonization, all Yersinia cells initially expressing YopE-T-170EC or YopE-T-170CR2 did not grow in the presence of chloramphenicol, even when they were reisolated as early as 3 days postinoculation (data not shown). Accordingly, a vaccination scheme was applied in which gerbils were infected with repeated doses of recombinant Yersinia cells given once per week for four consecutive weeks. Subsequently, animals were treated with antibiotics to eliminate remaining bacteria and then challenged by intrahepatic inoculation of 105 axenically cultured E. histolytica trophozoites (3). Seven days later, animals were sacrificed, and the livers were entirely removed, sectioned, and inspected for the presence of abscesses. In cases where abscesses were present, the weight of abscesses relative to total liver weight was determined. Animals vaccinated with the attenuated nontransformed Yersinia strain WA irp1 served as controls. The results indicated clear differences between control animals and those vaccinated with Yersinia cells expressing the various hybrid YopE-lectin proteins. In all groups, some degree of protection was observed. However, differences in the levels of protection were statistically significant only with Yersinia cells transformed either with YopE-T-170CP or YopE-T-170CR2 (Table 1). The protective potential of 170CR2 to inhibit amebic liver abscess in gerbils has been shown previously (9, 10). However, the potential of 170CP to induce protective immunity is surprising, as previous studies have shown that high titers of serum immunoglobulin G antibodies to this fragment exacerbate amebic disease (10). Interestingly, analysis of antibody responses after oral infection with recombinant Yersinia cells indicated that the observed protection was most likely mediated by antibody-independent immune mechanisms, as antibody titers were relatively low compared to those from previous intraperitoneal vaccination trials with recombinant ameba lectin (10) and there was no correlation between the degree of protection and titers of antibodies (Fig. 2). Because of the lack of immunological markers in the gerbil model, we can only speculate about the mechanism responsible for the observed protection. Recently, it has been shown that mice orally immunized with Yersinia cells expressing YopE chimeric proteins under control of the YopE translocation domain but not under control of the YopE secretion domain develop CD8 and CD4 T cells which secrete high levels of gamma interferon and tumor necrosis factor alpha upon stimulation with corresponding antigens (14). On the other hand, it is well documented that activation of macrophages leading to the production of nitric oxide is important to control amebic infection (2, 5, 6, 8, 17). Thus, immunization with Yersinia cells expressing YopE-lectin hybrid proteins under control of the YopE translocation domain might induce the production of gamma interferon and tumor necrosis factor alpha by specifically stimulated CD4 T cells, which will result in activation of macrophages and finally in host defense against invading E. histolytica. Thus, the application of gram-negative bacteria delivering appropriate ameba antigen via the T3SS might constitute a promising new strategy for the development of an oral amebiasis vaccine.
TABLE 1.
Protection of gerbils from amebic liver abscess by oral vacccination with recombinant Y. enterocolitica WA irp1 expressing various YopE-lectin fusion proteins
| Vaccine group | No. of gerbils with liver abscess/no. of gerbils challenged | % of gerbils protecteda | Size of liver abscess in nonprotected animals (% of control)a |
|---|---|---|---|
| WA irp1 (control) | 46/48 | 4.2 | |
| YopE-T-170EC | 6/10 | 40.0 | 68.6 |
| YopE-T-170CP | 3/10 | 70.0 A | 45.3 C |
| YopE-S-170CRc | 13/14 | 7.1 | 52.9 |
| YopE-T-170CRc | 8/11 | 27.3 | 82.4 |
| YopE-S-170CR2 | 10/15 | 33.3 | 58.7 |
| YopE-T-170CR2 | 5/14 | 64.3 B | 34.8 D |
Significant differences from WA irp1 control, as determined by Fisher's exact test, are indicated by A, P < 0.002; B, P < 0.001; C, P < 0.05; and D, P < 0.005.
FIG. 2.
Serum immunoglobulin G antibody response to the ameba lectin in gerbils following oral infection with WA irp1 transformants expressing the various YopE-lectin hybrid proteins. Noninfected (naive) and nontransformed WA irp1 served as controls.
Acknowledgments
We thank Claudia Marggraff and Ina Hennings for skillful technical assistance.
H.R., J.H., and E.T. were supported by the Deutsche Forschungsgemeinschaft (priority program “new vaccination strategies”; RU838/1-2, HE1297/9-2, and TA110/7-2).
Editor: J. B. Bliska
REFERENCES
- 1.Chadee, K., and E. Meerovitch. 1984. The Mongolian gerbil (Meriones unguiculatus) as an experimental host for Entamoeba histolytica. Am. J. Trop. Med. Hyg. 33:47-54. [DOI] [PubMed] [Google Scholar]
- 2.Denis, M., and K. Chadee. 1989. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect. Immun. 57:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 4.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 5.Ghadirian, E., and M. Denis. 1992. In vivo activation of macrophages by IFN-gamma to kill Entamoeba histolytica trophozoites in vitro. Parasite Immunol. 14:397-404. [DOI] [PubMed] [Google Scholar]
- 6.Ghadirian, E., and A. Salimi. 1993. In vitro effect of recombinant interferon gamma in combination with LPS on amoebicidal activity of murine Kupffer cells. Immunobiology 188:203-219. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi, C. A., A. Roggenkamp, A. Rakin, R. Zumbihl, L. Leitritz, and J. Heesemann. 1998. In vitro and in vivo expression studies of yopE from Yersinia enterocolitica using the gfp reporter gene. Mol. Microbiol. 30:865-882. [DOI] [PubMed] [Google Scholar]
- 8.Lin, J. Y., R. Seguin, K. Keller, and K. Chadee. 1994. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect. Immun. 62:1534-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotter, H., F. Khajawa, S. L. Stanley, Jr., and E. Tannich. 2000. Protection of gerbils from amebic liver abscess by vaccination with a 25-mer peptide derived from the cysteine-rich region of Entamoeba histolytica galactose-specific adherence lectin. Infect. Immun. 68:4416-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotter, H., T. Zhang, K. B. Seydel, S. L. Stanley, Jr., and E. Tannich. 1997. Identification of an epitope on the Entamoeba histolytica 170-kD lectin conferring antibody-mediated protection against invasive amebiasis. J. Exp. Med. 185:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann, B. J., B. V. Burkholder, and L. A. Lockhart. 1997. Protection in a gerbil model of amebiasis by oral immunization with Salmonella expressing the galactose/N-acetyl d-galactosamine inhibitable lectin of Entamoeba histolytica. Vaccine 15:659-663. [DOI] [PubMed] [Google Scholar]
- 12.McCoy, J. J., B. J. Mann, and W. A. Petri, Jr. 1994. Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect. Immun. 62:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelludat, C., A. Rakin, C. A. Jacobi, S. Schubert, and J. Heesemann. 1998. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J. Bacteriol. 180:538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rüssmann, H., U. Gerdemann, E. I. Igwe, K. Panthel, J. Heesemann, S. Garbom, H. Wolf-Watz, and G. Geginat. 2003. Attenuated Yersinia pseudotuberculosis carrier vaccine for simultaneous antigen-specific CD4 and CD8 T-cell induction. Infect. Immun. 71:3463-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russmann, H., A. Weissmuller, G. Geginat, E. I. Igwe, A. Roggenkamp, A. Bubert, W. Goebel, H. Hof, and J. Heesemann. 2000. Yersinia enterocolitica-mediated translocation of defined fusion proteins to the cytosol of mammalian cells results in peptide-specific MHC class I-restricted antigen presentation. Eur. J. Immunol. 30:1375-1384. [DOI] [PubMed] [Google Scholar]
- 16.Schesser, K., E. Frithz-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 178:7227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seydel, K. B., S. J. Smith, and S. L. Stanley, Jr. 2000. Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect. Immun. 68:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soong, C. J., K. C. Kain, M. Abd-Alla, T. F. Jackson, and J. I. Ravdin. 1995. A recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 19.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 21.Tannich, E., F. Ebert, and R. D. Horstmann. 1991. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh, J. A. 1986. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev. Infect. Dis. 8:228-238. [DOI] [PubMed] [Google Scholar]
- 23.Zhang, T., and S. L. Stanley, Jr. 1996. Oral immunization with an attenuated vaccine strain of Salmonella typhimurium expressing the serine-rich Entamoeba histolytica protein induces an antiamebic immune response and protects gerbils from amebic liver abscess. Infect. Immun. 64:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, T., and S. L. Stanley, Jr. 1994. Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect. Immun. 62:2605-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]