Abstract
More than a million childhood diarrhoeal episodes occur worldwide each year, and in developed countries a considerable part of them are caused by viral infections. In this study, we aimed to search for genetic variants associated with diarrhoeal disease in young children by meta-analyzing genome-wide association studies, and to elucidate plausible biological mechanisms. The study was conducted in the context of the Early Genetics and Lifecourse Epidemiology (EAGLE) consortium. Data about diarrhoeal disease in two time windows (around 1 year of age and around 2 years of age) was obtained via parental questionnaires, doctor interviews or medical records. Standard quality control and statistical tests were applied to the 1000 Genomes imputed genotypic data. The meta-analysis (N = 5758) followed by replication (N = 3784) identified a genome-wide significant association between rs8111874 and diarrhoea at age 1 year. Conditional analysis suggested that the causal variant could be rs601338 (W154X) in the FUT2 gene. Children with the A allele, which results in a truncated FUT2 protein, had lower risk of diarrhoea. FUT2 participates in the production of histo-blood group antigens and has previously been implicated in the susceptibility to infections, including Rotavirus and Norovirus. Gene-set enrichment analysis suggested pathways related to the histo-blood group antigen production, and the regulation of ion transport and blood pressure. Among others, the gastrointestinal tract, and the immune and neuro-secretory systems were detected as relevant organs. In summary, this genome-wide association meta-analysis suggests the implication of the FUT2 gene in diarrhoeal disease in young children from the general population.
Introduction
Diarrhoea, defined as three or more loose stools within the previous 24 h, is probably one of the most common symptoms in children, with an estimated 1370 million annual episodes in children younger than 5 years in 2010 (1). Two percent of these episodes progress to severe disease, and 700 000 episodes lead to death, mainly in low-income countries (1). In developed countries, diarrhoeal disease is a common reason for attendance at a general practitioner, especially in children under 5 years of age (2).
Several pathogens can account for infections associated with diarrhoeal disease, including viruses, bacteria and parasites. The GEMS study (Global Enteric Multicenter Study), conducted as a case–control study in seven African and Asian sites, identified Rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli, and Shigella as most responsible attributable pathogens for cases of moderate-to-severe diarrhoea (3). In developed countries, improvements in public health infrastructure (water and sewage management), has caused a shift in the main causes of acute paediatric diarrhoea, moving from bacterial and parasite aetiologies to viruses. Rotavirus is the primary cause of diarrhoeal disease globally, and is responsible for almost half of the gastroenteritis cases requiring hospitalization in Western countries, followed by Norovirus, Adenovirus and Salmonella (4). The peak age for infection with Rotavirus is between 3 months and 2 years, coinciding with reduced protection by transplacental transfer of maternal antibodies (5) and the end of the lactation period (6). At the age of 5, almost all children have been infected with Rotavirus, and progressively develop natural immunity against this virus (7). Enteric pathogens damage small bowel enterocytes and cause impaired intestinal absorption, low grade fever and watery diarrhoea as a result of the deregulation of ion transport and stimulation of the enteric nervous system (8,9). In children, factors other than enteric pathogens can cause diarrhoea, including among others extra-intestinal infections, intolerances or food allergies (10), nutrient deficiencies, antimicrobials, or hereditary diseases, such as cystic fibrosis, but these represent a small proportion of all cases of diarrhoea.
The exposure to infectious agents plays a major role in the acquisition of the pathogen and development of diarrhoeal disease. However, not all individuals are equally susceptible to infection, and if infected, they may differ in their immunological response. Host genetic factors can explain part of the differences in susceptibility and response to infection. In a study on families with adopted children, it was shown that premature death due to infection of the biological parent, but not of the adopting parent, increased the relative risk of death due to infection in the adopted child by 5.8-fold, a higher risk than observed for vascular disease or cancer (11). Twin studies on infectious diseases have shown higher correlation between monozygotic than dizygotic twins (12–14). Heritability for early childhood diarrhoea was estimated to be 54% in a pedigree-based design in Brazil (15).
Candidate gene studies for infectious gastroenteritis have identified genetic variants in genes involved in the innate and acquired immune responses and in genes that participate in the production of histo-blood group antigens (HBGAs), which serve as receptors for numerous pathogens. In particular, the non-secretor phenotype, associated with null or inactivating mutations in FUT2 gene resulting in a lack of certain antigens in secretions and epithelial mucosa, confers strain-specific protection against Norovirus (16–18) and Rotavirus (19). A review of the associations between enteric pathogens and genetic variants can be found elsewhere (20,21).
The aims of the present study were: (1) to identify genetic variants that confer susceptibility to diarrhoeal disease in young children from the general population of developed countries through a genome-wide association meta-analysis, and (2) to elucidate potential biological mechanisms involved in diarrhoeal disease using pathway analysis approaches.
Results
Sample
Four different traits, any diarrhoea and doctor’s confirmed diagnosis of diarrhoea around 1 year of age (D1Y and DD1Y) and around 2 years of age (D2Y and DD2Y), were explored. Samples analyzed for each outcome in the discovery and in the replication phase are shown in Table 1. Information on diarrhoea was collected through questionnaires, doctor interviews or medical records (see Supplementary Material, Annex A).
Table 1.
Samples included in the study at age 1 year and at age 2 years by diarrhoeal disease definition and study phase
| Age 1 year |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhoea (D1Y) |
Doctor diagnosis of diarrhoea (DD1Y) |
||||||||||
| Cohort | Assessment (period in months)a | N total | N cases | % cases | Cohort | Assessment (period in months)a | N total | N cases | % cases | ||
| Discovery | ALSPAC (Disc)d | Questionnaire (6–18) | 3363 | 2001 | 59.5 | ALSPAC (Disc)d | Questionnaire (6–18) | 3363 | 893 | 26.6 | |
| Generation R | Questionnaire (6–12)c | 2033 | 469 | 23.1 | Generation R | Questionnaire (6–12)c | 2033 | 179 | 8.8 | ||
| INMA_SAB | Questionnaire (6–14) | 362 | 223 | 61.6 | COPSAC2000 | Doctor interview (6–12) | 345 | 81 | 23.5 | ||
| LISAplus | Questionnaire (6–12) | 662 | 186 | 28.1 | |||||||
| TOTAL | 5758 | 2693 | 46.8 | TOTAL | 6403 | 1339 | 21.8 | ||||
| Replication | ALSPAC (Repl) | Questionnaire (6–18) | 3361 | 2047 | 60.9 | ALSPAC (Repl) | Questionnaire (6–18) | 3361 | 871 | 25.9 | |
| MoBa | Questionnaire (6–18)c | 407 | 255 | 62.6 | COPSAC2010 | Doctor interview (6–12) | 547 | 244 | 44.6 | ||
| INMA_VAL | Questionnaire (0–12) | 334 | 149 | 44.6 | |||||||
| CHOPb | Medical records (6–18) | 3223 | 147 | 4.6 | |||||||
| TOTAL | 3768 | 2302 | 61.1 | TOTAL | 7465 | 1411 | 18.9 | ||||
| Age 2 years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhoea (D2Y) |
Doctor diagnosis of diarrhoea (DD2Y) |
||||||||||
| Cohort | Assessment (period in months)a | N total | N cases | % cases | Cohort | Assessment (period in months)a | N total | N cases | % cases | ||
| Discovery | ALSPAC (Disc)d | Questionnaire (18–30) | 3189 | 1746 | 54.8 | ALSPAC (Disc) | Questionnaire (18–30) | 3189 | 514 | 16.1 | |
| Generation R | Questionnaire (18–24)c | 2058 | 943 | 45.8 | Generation R | Questionnaire (18–24)c | 2058 | 190 | 9.2 | ||
| INMA_SAB | Questionnaire (12–24) | 361 | 166 | 46 | COPSAC2000 | Doctor interview (18–24) | 319 | 86 | 27 | ||
| LISAplus | Questionnaire (18–24) | 667 | 200 | 30 | |||||||
| TOTAL | 5608 | 2855 | 50.9 | TOTAL | 6233 | 990 | 20.6 | ||||
| Replication | ALSPAC (Repl)d | Questionnaire (18–30) | 3187 | 1759 | 55.2 | ALSPAC (Repl) | Questionnaire (18–30) | 3187 | 485 | 15.2 | |
| INMA_VAL | Questionnaire (12–14) | 329 | 213 | 64.7 | COPSAC2010 | Doctor interview (18–24) | 518 | 271 | 52.3 | ||
| GINIplusd | Questionnaire (12–24) | 794 | 328 | 41.3 | |||||||
| CHOPb | Medical records (18–30) | 3223 | 190 | 5.9 | |||||||
| Total | 3516 | 1972 | 56.1 | Total | 7722 | 1274 | 16.5 | ||||
Period referred in the questionnaire, medical record or doctor interview.
Only children not vaccinated against Rotavirus were included.
It refers specifically to gastric flu or gastroenteritis.
Statistically significant differences in the proportions of diarrhoeal disease among males and females (P-value < 0.05) [ALSPAC-D1Y-Discovery: 61.2% males versus 57.7% females; ALSPAC–DD1Y-Discovery: 29.2% versus 23.8%; ALSPAC-D2Y-Discovery: 56.6% versus 52.8%; ASLPAC-D2Y-Replication: 58.5% versus 51.7%; and GINIplus-DD2Y-Replication: 46.7% versus 36.0%].
In the discovery phase, 46.8% of the children had had at least one diarrhoeal episode around the age of 1 year (D1Y), while this proportion decreased to 21.8% for the cases diagnosed by a doctor (DD1Y). The proportions for children around the age of two years were 50.9% for diarrhoeal disease (D2Y) and 20.6% for doctor’s diagnosis (DD2Y). Similar frequencies of diarrhoea were observed in the replication samples. The number of diarrhoea cases in Generation R and CHOP was lower than in other cohorts. For some of the diarrhoeal definitions, ALSPAC and GINIplus showed higher proportion of cases among males than among females.
Discovery phase
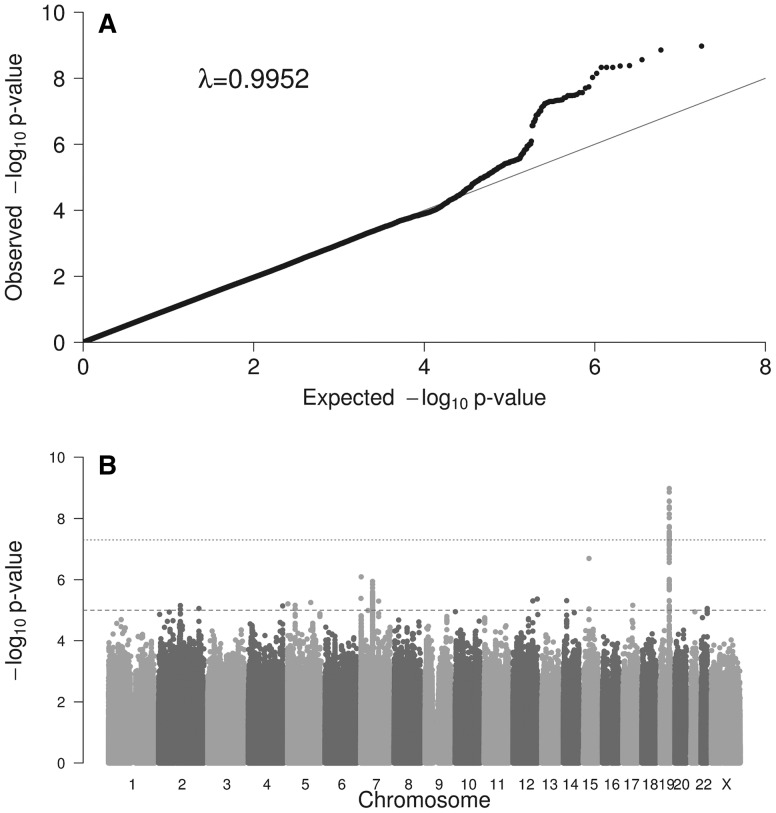
The Q–Q plots and the Manhattan plots for each outcome are shown in Figure 1 (D1Y) and in the Supplementary Material, Figures S1 (DD1Y), S2 (D2Y) and S3 (DD2Y). Genomic inflation factor, lambda (λ), ranged from 0.9952 to 1.0031.
Figure 1.
(A) Quantile-quantile (Q-Q) plots showing the probability values from GWAS meta-analysis of diarrhoeal disease at age 1 year (D1Y). The line indicates the distribution under the null hypothesis. Lambda value is shown. (B) Manhattan plot of the GWAS meta-analysis of diarrhoeal disease at age 1 year (D1Y). The x-axis represents the autosomal chromosomes and the y-axis represents –log10(P). The dotted line indicates genome-wide significance (P = 5.00E−08), and the dashed line indicates suggestive genome-wide significance (P = 1.00E−05).
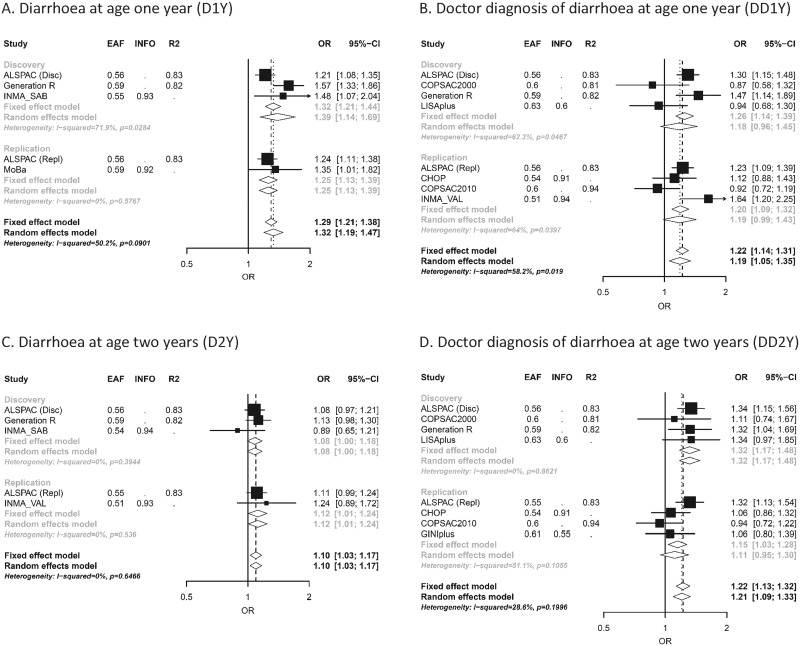
For D1Y, the meta-analysis of 5,758 samples revealed a genome-wide significant variant, rs8111874, at 19q13.33 [odds ratio (OR) (95% confidence interval [CI]) = 1.32 (1.21–1.44); P-value = 1.06E−09] (Table 2 and Fig. 2)]. This lead SNP was also nominally associated with DD1Y [OR (95%CI) = 1.26 (1.14–1.39); P-value = 1.05E−05] and with DD2Y [OR (95%CI) = 1.32 (1.17–1.48); P-value = 4.6E−07] and with D2Y [OR (95%CI) = 1.08 (1.00–1.18); P-value = 6.56E−02]. In D1Y, the G allele increased the risk of diarrhoeal disease in all the studies (P-value for heterogeneity = 2.99E−02, Table 2).
Table 2.
Results from the fixed effect meta-analysis for diarrhoeal disease at age 1 year (D1Y) by discovery and replication phase.
| Discoverya |
Replicationb |
Combined |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | rs_number | Chr | Pos | EA | NEA | EAF | N | OR | lCI | uCI | P-value | P-value het | Effects | N variants/ locus | Gene | N | OR | lCI | UCI | p-value | N | OR | lCI | UCI | p-value | p-value het |
| 19:49168942:SNP | rs8111874 | 19 | 49168942 | G | A | 0.57 | 5758 | 1.32 | 1.21 | 1.44 | 1.06E−09 | 2.99 E − 02 | +++ | 71 | NTN5 and SEC1P (intronic) | 3768 | 1.25 | 1.13 | 1.39 | 1.69 E − 05 | 9526 | 1.29 | 1.21 | 1.38 | 8.05 E − 14 | 9.01 E − 02 |
| 15:50562847:SNP | rs62020330 | 15 | 50562847 | A | G | 0.97 | 5396 | 2.16 | 1.61 | 2.88 | 2.02 E − 07 | 5.05 E − 02 | ++? | 2 | 4.7kb 5' of HDC | 3195 | 0.99 | 0.69 | 1.43 | 9.72 E − 01 | 8591 | 1.60 | 1.27 | 2.00 | 4.97 E − 05 | 1.35 E − 03 |
| 7:2930941:SNP | rs1713926 | 7 | 2930941 | C | T | 0.69 | 5758 | 1.37 | 1.21 | 1.55 | 8.06 E − 07 | 5.68 E − 01 | +++ | 1 | 15kb 3' of CARD11 | 1956 | 1.07 | 0.92 | 1.25 | 3.58 E − 01 | 7714 | 1.24 | 1.13 | 1.37 | 1.04 E − 05 | 1.23 E − 01 |
| 7:63621349:SNP | rs139755348 | 7 | 63621349 | C | T | 0.73 | 5758 | 0.79 | 0.71 | 0.87 | 1.14 E − 06 | 9.29 E − 01 | — | 89 | 46kb 5' of ZNF735 | 3768 | 1.06 | 0.95 | 1.19 | 2.96 E − 01 | 9526 | 0.89 | 0.83 | 0.96 | 2.59 E − 03 | 2.60 E − 03 |
| 7:1599067:SNP | rs112411182 | 7 | 1599067 | T | C | 0.95 | 5396 | 1.7 | 1.36 | 2.13 | 4.10 E − 06 | 8.75 E − 01 | ++? | 1 | 3kb 5' of TMEM184A | 3768 | 0.78 | 0.60 | 1.02 | 7.26 E − 02 | 9164 | 1.23 | 1.04 | 1.46 | 1.76 E − 02 | 1.38 E − 04 |
| 12:130325960:SNP | rs34180477 | 12 | 130325960 | G | A | 0.93 | 5396 | 0.58 | 0.46 | 0.73 | 4.30 E − 06 | 4.93 E − 01 | –? | 1 | TMEM132D (intronic) | 3009 | 1.21 | 0.96 | 1.53 | 1.04 E − 01 | 8405 | 0.84 | 0.71 | 0.98 | 3.24 E − 02 | 1.55 E − 05 |
| 14:38210286:SNP | rs74731421 | 14 | 38210286 | G | A | 0.97 | 5396 | 1.81 | 1.4 | 2.33 | 4.87 E − 06 | 8.07 E − 01 | ++? | 1 | 146kb 5' of FOXA1 | 3768 | 0.91 | 0.69 | 1.22 | 5.42 E − 01 | 9164 | 1.34 | 1.11 | 1.63 | 2.32 E − 03 | 6.59 E − 03 |
| 12:106438211:INDELc | 12 | 106438211 | R | D | 0.52 | 5758 | 1.26 | 1.14 | 1.39 | 4.95 E − 06 | 8.07 E − 01 | +++ | 1 | 19kb of 3' NUAK1 | 407 | 1.20 | 0.85 | 1.68 | 3.01 E − 01 | 6165 | 1.26 | 1.14 | 1.38 | 2.73 E − 06 | 9.15 E − 01 | |
| 7:96366342:SNP | rs12704876 | 7 | 96366342 | T | C | 0.51 | 5758 | 0.83 | 0.77 | 0.9 | 5.04 E − 06 | 8.45 E − 01 | — | 1 | 27kb 5' of SHFM1 | 3768 | 1.10 | 1.00 | 1.20 | 4.94 E − 02 | 9526 | 0.93 | 0.88 | 0.99 | 2.74 E − 02 | 2.32 E − 04 |
| 5:1695532:SNP | rs79411306 | 5 | 1695532 | C | T | 0.95 | 5396 | 0.55 | 0.42 | 0.71 | 6.17 E − 06 | 4.86 E − 01 | –? | 1 | 13kb 3' of MIR4277 | 3136 | 0.89 | 0.71 | 1.13 | 3.55 E − 01 | 8532 | 0.72 | 0.60 | 0.85 | 1.79 E − 04 | 3.61 E − 02 |
| 5:40474267:SNP | rs116560909 | 5 | 40474267 | C | T | 0.97 | 5396 | 1.9 | 1.44 | 2.52 | 6.90 E − 06 | 5.52 E − 01 | ++? | 2 | 206kb 5' of PTGER4 | 3768 | 1.08 | 0.78 | 1.49 | 6.63 E − 01 | 9164 | 1.50 | 1.21 | 1.85 | 2.03 E − 04 | 1.74 E − 02 |
| 17:52598239:INDELc | 17 | 52598239 | R | D | 0.94 | 5396 | 1.57 | 1.29 | 1.92 | 6.90 E − 06 | 6.76 E − 01 | ++? | 1 | 380kb 5' of TOM1L1 | 407 | 1.29 | 0.59 | 2.80 | 5.20 E − 01 | 5803 | 1.56 | 1.28 | 1.88 | 6.00 E − 06 | 8.13 E − 01 | |
| 2:116776614:SNP | rs12615869 | 2 | 116776614 | A | G | 0.94 | 5396 | 1.54 | 1.28 | 1.87 | 7.02 E − 06 | 5.62 E − 01 | ++? | 2 | 174kb 3' of DPP10 | 3768 | 1.11 | 0.90 | 1.37 | 3.38 E − 01 | 9164 | 1.33 | 1.16 | 1.53 | 6.57 E − 05 | 1.25 E − 01 |
| 4:184501675:SNP | rs7662749 | 4 | 184501675 | C | T | 0.72 | 5758 | 0.81 | 0.74 | 0.89 | 7.24 E − 06 | 8.53 E − 01 | — | 1 | 59kb 3' of RWDD4 | 3768 | 1.02 | 0.92 | 1.14 | 6.55 E − 01 | 9526 | 0.90 | 0.84 | 0.96 | 1.69 E − 03 | 2.69 E − 02 |
| 22:49732481:SNP | rs5770255 | 22 | 49732481 | G | A | 0.76 | 5758 | 1.23 | 1.12 | 1.35 | 8.77 E − 06 | 7.32 E − 01 | +++ | 2 | 281kb 3' of C22orf34 | 3768 | 0.94 | 0.84 | 1.05 | 2.83 E − 01 | 9526 | 1.10 | 1.03 | 1.19 | 5.95 E − 03 | 4.69 E − 03 |
Variants with a p-value < 1. E − 05 in the discovery phase are shown.
Cohort designs: ALSPAC, COPSAC2010, Generation R, INMA_VAL, INMA_GIP, LISAplus, GINIplus and MOBA are unselected population-based birth cohorts. COPSAC2000 is a prospective clinical study of a birth cohort of infants born to mothers with a history of asthma. CHOP is a random collection of paediatric patients from a hospital centre.
Only the most significant variant per locus is shown. Variants in the same locus are defined in a 1 Mb window. N variants/locus indicates the number of SNPs in the locus with a P-value < 1E−05.
Only genetic variants with a minimal of 5000 samples are shown.
EA: effect allele; NEA: non effect allele; EAF: effect allele frequency; N: sample size; OR: odds ratio; lCI: 95% lower confidence interval; uCI: 95% upper confidence interval; P-value het: P-value for the heterogeneity test.
Cohorts in the discovery phase are included in alphabetical order: ALSPAC (Disc), Generation R and INMA_SAB.
Cohorts included in the replication phase are: ALSPAC (Repl) and MoBa.
ALSPAC (Repl) has no data for these markers.
Bonferroni correction for 15 variants considered in the replication phase: P-value = 3.33E − 03.
Figure 2.
Forest plots for rs8111874 at 19q13.33 for the four diarrhoeal outcomes: (A) Diarrhoea at age 1 year (D1Y); (B) Doctor diagnosis of diarrhoea at age 1 year (DD1Y); (C) Diarrhoea at age 2 years (D2Y); (D) Doctor diagnosis of diarrhoea at age 2 years (DD2Y). In the vertical panel, the studies participating in the discovery or replication phase are presented. In the horizontal lines, the sizes of the boxes represent precision and the lines the confidence intervals. The diamond shapes represent the pooled effect estimates, for both the fixed- and random-effect models. The horizontal axis shows the scale of the effect estimates. The effect allele is G, and the other allele is A.
Another locus at 4q21.23, was associated with DD1Y at genome-wide significance [N = 6403, OR (95%CI) = 1.31 (1.19–1.44); P-value = 2.92E−08] (Supplementary Material, Table S1). The lead variant, rs1481779, was located in an intron of ARHGAP24 gene. This variant was also nominally associated with D1Y [OR (95%CI) = 1.09 (1.01–1.19); P-value = 3.7E−02], but not with D2Y or DD2Y.
Variants with a P-value < 1E−05 are shown in Table 2 (D1Y) and in Supplementary Material, Tables S1 (DD1Y), S2 (D2Y) and S3 (DD2Y). With the exception of the signal at 19q13.33, no major overlap was observed among the suggestive variants for each outcome (data not shown). A description of the potential function of the genetic variants and genes in each loci detected at a P-value < 1E−05 can be found in Supplementary Material, Tables S4–S7.
Replication phase
Seventy-two loci (P-value < 1E-05 in any of the four diarrhoea definitions) were followed for replication in an independent dataset (Table 1). After multiple-testing adjustment, the SNP rs8111874 at chromosome 19q13.33 was associated with D1Y [replication: OR (95%CI) = 1.25 (1.13–1.39); P-value = 1.69E−05] (Table 2), and it was nominally associated for the other diarrhoea outcomes (Fig. 2). Other variants at the same locus were also associated with DD1Y and DD2Y (Supplementary Material, Tables S1 and S3). None of the variants in other loci replicated, and neither of them reached genome-wide significance in the combined analyses. Results of the replication phase can be found in Table 2 (D1Y) and in Supplementary Material, Tables S1 (DD1Y), S2 (D2Y) and S3 (DD2Y).
Chromosome 19 locus: FUT2 gene
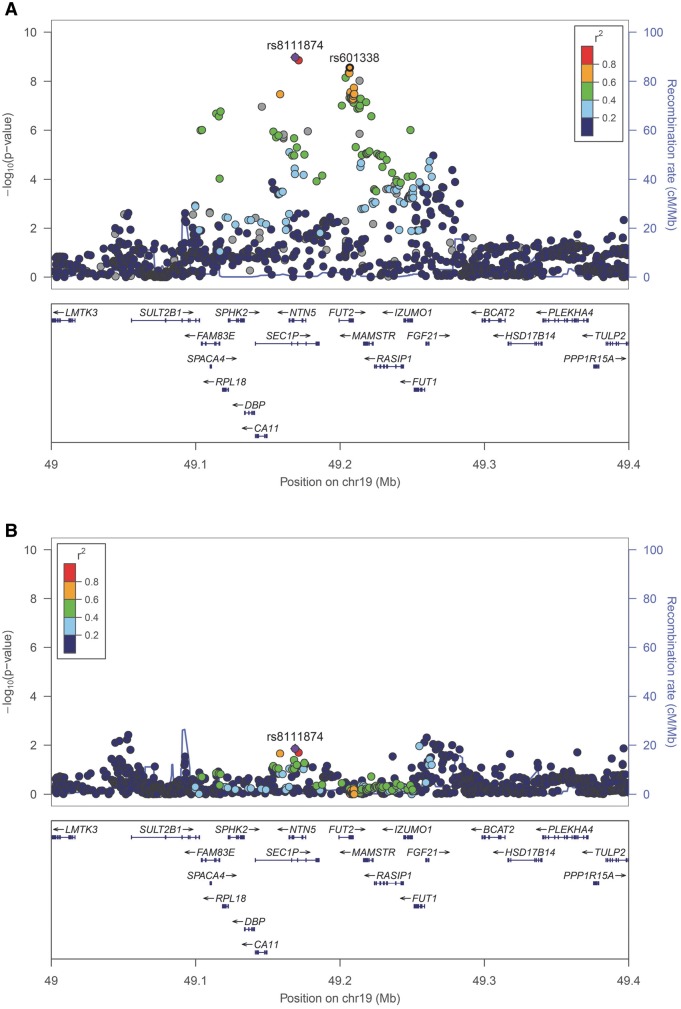
The regional association plot of rs8111874 at 19q13.33 shows a linkage disequilibrium block that overlaps several genes, including FUT2 (Fig. 3A). FUT2 participates in the production of histo-blood group antigens (HBGAs) and contains a stop mutation (rs601338, W154X) known to confer protection against certain infections. We conditioned the top SNP at 19q13.33 (rs8111874) on the stop mutation (rs601338) and vice versa: the odds ratios from these analyses were attenuated (Table 3). After conditioning to rs601338, no secondary signals were observed in the region (window size 1 Mb) (Fig. 3B). The forest plots for rs601338 (W154X) are shown in Supplementary Material, Figure S4.
Figure 3.
(A) Regional association of 19q13.33 locus (top SNP: rs8111874, chr19:49168942) with diarrhoeal disease at age 1 year (D1Y) (N = 5758). The top variant is indicated with a diamond in purple and the flanking variants in circles, coloured according to their linkage disequilibrium (LD). Variant rs601338 is shown in a black circle. The plot was constructed using the 1000 Genomes CEU population (Northern and Western European ancestry). (B) Regional association of 19q13.33 locus (top SNP: rs8111874, chr19:49168942) with diarrhoeal disease at age 1 year (D1Y) conditioned to rs601338 (N = 5758). The associations of the genetic variants in the region were attenuated.
Table 3.
Conditional analysis at 19q13.33 locus (rs8111874 and rs601338)
| Crude results for rs8111874 |
Results for rs8111874 conditioned to rs601338 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | rs_number | EA | NEA | EAF | IMP | Outcome | OR | lCI | uCI | P-value | N | OR | lCI | uCI | P-value |
| 19:49168942:SNP | rs8111874 | G | A | 0.57 | 0.82-0.93 | D1Y | 1.32 | 1.21 | 1.44 | 1.06 E − 09 | 5214.7 | 1.08 | 1.01 | 1.14 | 1.40 E − 02 |
| DD1Y | 1.26 | 1.13 | 1.39 | 1.17 E − 05 | 5576.7 | 1.05 | 0.98 | 1.12 | 2.00 E − 01 | ||||||
| D2Y | 1.08 | 0.99 | 1.18 | 6.56 E − 02 | 5385.9 | 1.00 | 0.95 | 1.06 | 9.12 E − 01 | ||||||
| DD2Y |
1.32 |
1.17 |
1.48 |
2.74 E − 06 |
5672.7 |
1.08 |
1.00 |
1.17 |
4.77 E − 02 |
||||||
|
Crude results for rs601338 |
Results for rs601338 conditioned to rs8111874 |
||||||||||||||
|
Marker |
rs_number |
EA |
NEA |
EAF |
IMP |
Outcome |
OR |
lCI |
uCI |
P-value |
OR |
lCI |
uCI |
P-value |
|
| 19:49206674:SNP | rs601338 | G (W) | A (X) | 0.52 | 1 | D1Y | 1.28 | 1.18 | 1.39 | 2.74 E − 09 | 6224.8 | 1.06 | 1.00 | 1.12 | 3.93 E − 02 |
| DD1Y | 1.25 | 1.14 | 1.37 | 1.99 E − 06 | 6872.8 | 1.07 | 1.01 | 1.14 | 2.48 E − 02 | ||||||
| D2Y | 1.10 | 1.02 | 1.18 | 1.84 E − 02 | 6471.1 | 1.04 | 0.99 | 1.09 | 1.40 E − 01 | ||||||
| DD2Y | 1.27 | 1.14 | 1.4 | 5.90 E − 06 | 6976.7 | 1.06 | 0.99 | 1.13 | 1.16 E − 01 | ||||||
EA: effect allele; NEA: non effect allele; EAF: effect allele frequency; IMP: imputation quality (from–to); D1Y: any diarrhoea at age one year; DD1Y: doctor’s confirmed diagnosis of diarrhoea at age 1 year; D2Y any diarrhoea at age two years; DD2y doctor’s confirmed diagnosis of diarrhoea at age 2 years. Distance between SNPs is 37.7 kb.
Enrichment analysis
Two prediction programs, the Meta-Analysis Gene-set Enrichment of variaNT Associations (MAGENTA) and the Data-driven Expression Prioritized Integration for Complex Traits (DEPICT) were used to investigate gene-sets enriched among the variants with the lowest P-values. Using MAGENTA, the ‘KEGG_GLYCOSPHINGOLIPID_BIOSYNTHESIS_GLOBO_SERIES’ gene-set was identified in DD2Y at a 5% False Discovery Rate (FDR) (Supplementary Material, Table S8). Six out of 14 genes of this pathway had SNPs in the 95th percentile of lowest P-values. This gene-set was also weakly associated with diarrhoea at age 1 year (D1Y). Other gene-sets with nominal evidence are listed in Supplementary Material, Table S8, including the ’KEGG_GLYCOSPHINGOLIPID_BIOSYNTHESIS_GANGLIO_SERIES’ and the blood pressure regulatory gene-set ‘KEGG_RENIN_ANGIOTENSIN_SYSTEM’.
The top 10 gene-sets detected with DEPICT for each outcome are shown in Supplementary Material, Table S9. The gene-sets ‘ENSG00000147955—SIGMAR1 PPI subnetwork’ (involved in ion channels regulation and modulation of neurotransmitter release), ‘MP:0001675—abnormal ectoderm development’, ‘GO:0007492—endoderm development’ and ‘ENSG00000140612—SEC11A PPI subnetwork’ (component of the microsomal signal peptidase complex) remained after multiple-testing for DD1Y. Genetic variants showing suggestive P-values were linked to genes with enriched expression in the gastrointestinal tract (D1Y), in the immune system (DD1Y) and in the neuro-secretory system (D2Y), among others (Supplementary Material, Table S10). Exclusion of genetic variants in 1 Mb around the FUT2 locus gave similar gene-sets and tissue/organs, but P-values were attenuated (data not shown).
Overlap with known variants and genes for related diseases
We compared the results from this study with variants reported in the literature as associated with inflammatory bowel disease (IBD) risk (22) and with viral infection and response to vaccination (23–36). Nine out of the 162 variants identified in IBD were nominally associated with D1Y (Supplementary Material, Table S11). Two of these variants passed the multiple-testing correction: one of them located in the FUT2 gene (P-value = 4.67E−09, opposite effect direction), and the other one in CARD11 locus (P-value = 1.52E−05, same effect direction). In addition, one out of 139 variants associated with infections was also associated with D2Y after correction for multiple-testing (Supplementary Material, Table S12). Specifically, the A allele of rs17793829, located in TTC7B gene, was associated with higher anti Cytomegalovirus IgG titer (31) and higher risk for D2Y (P-value = 2.12E-04). Finally, we evaluated 86 genes retrieved from OMIM (Online Mendelian Inheritance in Man) with the entry ‘diarrhoea’. None of them was associated with childhood diarrhoeal disease after multiple testing correction (Supplementary Material, Table S13).
Discussions
This study suggested the implication of the FUT2 locus in the diarrhoeal risk and provided evidences supporting the role of the histo-blood group antigen (HBGA) production and the regulation of ion transport pathways. The gastrointestinal tract and the immune and neuro-secretory systems were detected as relevant organs.
The genome-wide association meta-analysis followed by replication identified an association between rs8111874 mapping to the 19q13.33 locus and diarrhoea around 1 year of age (D1Y). Although with different strength, the association was also observed in all the different diarrhoeal outcomes investigated. The rs8111874 variant is located in an intronic region of the NTN5 (Netrin 5) and SEC1P (Secretory Blood Group 1, Pseudogene) genes and close to FUT2, previously associated with susceptibility to infection. FUT2 encodes the Fucosyltransferase 2 enzyme that participates in the production of histo-blood group antigens (HBGAs) by catalyzing the addition of a fucose residue in α1,2 linkage to the galactose of O- or N-glycoproteins and globo-, ganglio- or lacto-series of glycolipids (37). The FUT2 enzymatic activity is polymorphic, exhibiting the non-secretor phenotype (lack of certain antigens in the gut and epithelial mucosa) when inactivating mutations are present in the FUT2 gene. The most common FUT2 inactivating variant in Caucasians (Europeans and Iranians) and in Africans, is the stop mutation W154X (rs601338), while in Asians it is the missense variant A385T (rs1047781) (38). In order to investigate whether the signal observed at 19q13.33 locus could be caused by the stop mutation W154X (rs601338), the lead SNP in the region (rs8111874) was conditioned to the inactivating mutation and vice versa. The magnitudes of the effects of the conditional analyses were substantially attenuated, suggesting the presence of one single association signal. Children with the A allele at rs601338 (W154X), which results in a truncated FUT2 protein and the non-secretor phenotype, had lower risk of diarrhoeal disease during the first years of life. In agreement with our results from a population based design, the non-secretor phenotype has been associated with protection against Rotavirus (19), Norovirus (16–18) and Helicobacter pylori (39,40) in small settings of very well characterized hospitalized subjects. It is known that Norovirus (41) and Rotavirus (42–44) bind to the antigen associated with the FUT2 secretor phenotype to enter the cells. In contrast, the FUT2 non-secretor phenotype has been associated with higher risk of urinary tract infections (45), acute pyelonephritis (46), oral or vaginal Candida infections (12,47,48), Haemophilus influenza (49), Neisseria meningitides and Streptococcus pneumoniae infections (50). In addition, non-secretor individuals are at a higher risk of developing autoimmune diseases such as inflammatory bowel disease (22,51), psoriasis (52,53), and Behcet’s disease (54) and they also have higher vitamin B12 plasma levels (55,56). Recently, the non-secretor phenotype has been associated with gut microbiota at both the compositional and functional level (57). Non-secretors have lower species richness than the secretors (58), and the secretion status is a modifying factor for gut microbiota composition in Chron’s disease (59). Fucose from fucosylated proteins synthesized by FUT2 in response to the activation of the innate immunity can be used by microbes as an energy source, and this has been shown to reduce bacterial infection and downregulate the expression of virulence genes (60,61). Fucosylation appears to be a protective mechanism to maintain host-microbial interactions during pathogen-induced stress, but on the other hand, it facilitates viral entrance into the cells.
Although the non-secretor phenotype related mutation in FUT2 (W154X, rs601338) is the most likely causal variant of childhood diarrhoeal disease at 19q13.33 locus, the functional role of other variants in the region cannot be completely ruled out based on statistical analysis. Another variant, located in an intron of the ARHGAP24 gene (rs1481779), reached genome-wide significance in DD1Y, but it could not be replicated. ARHGAP24 codes a Rho GTPase-activating protein involved in cell polarity, cell morphology and cytoskeletal organization; and SNPs in it have been reported to be associated with blood pressure regulation (62).
In order to gain insight into potential molecular mechanisms underlying diarrhoeal disease in young children, we performed enrichment studies. The analysis using two different programs identified several gene-sets at 5% FDR. The ‘KEGG_GLYCOSPHINGOLIPID_BIOSYNTHESIS-GLOBO_SERIES’ gene-set contains genes related to the production of histo-blood group antigens (HBGAs) from globo-series glycolipids and was at least nominally associated with several definitions of diarrhoea (D1Y and DD2Y). Apart from FUT2, already discussed above, A4GALT (Alpha 1,4-Galactosyltransferase) in the gene-set also showed some evidence of association with D2Y. A similar gene-set, ‘KEGG_GLYCOSPHINGOLIPID_BIOSYNTHESIS-GANGLIO_SERIES’, was also identified.
A second gene-set consisting of a SIGMAR1 protein interaction network (ENSG00000147955—SIGMAR1 PPI subnetwork) was detected for DD1Y. SIGMAR1 is an endoplasmic reticulum-resident two-transmembrane chaperone that regulates voltage-gated ion channels including calcium, sodium and potassium channels (63). We also identified other pathways related to hydro-electrolytic balance such as the ‘KEGG_RENIN_ ANGIOTENSIN_SYSTEM’, the ‘GO:0005227—calcium activated cation channel activity’, the ‘GO:0015299—solute:hydrogen antiporter activity’ and the ‘ENSG00000187446—ENSG00000187446 PPI subnetwork’, related to cell pH regulation by controlling plasma membrane-type Na(+)/H(+) exchange activity (64). Deregulation of transport of ions is a central mechanism in the pathophysiology of enteric pathogens (8,9). Host genetic variants might compensate or accelerate watery stools after enteric infection.
Other gene-sets related to ectoderm and endoderm development were observed for DD1Y: ‘MP:0001675—abnormal ectoderm development’ and ‘GO:0007492—endoderm development’. The endoderm is the inner most germ layer that develops into the gastrointestinal tract, the lungs and associated tissues. Finally, the ‘ENSG00000140612—SEC11A PPI subnetwork’ was also identified. SEC11A is a component of the microsomal signal peptidase complex which removes signal peptides from nascent proteins as they are translocated into the lumen of the endoplasmic reticulum.
As expected, the gastrointestinal tract and the immune system were detected as relevant organs for diarrhoeal disease. The gastrointestinal tract was identified with the general definition of diarrhoea around 1 year of age (D1Y); while doctor diagnosis of diarrhoeal disease (DD1Y), probably more related to severe cases, highlighted the immune system. In addition, the neuro-secretory system was found to be associated with diarrhoea around 2 years of age (D2Y). The central nervous system communicates with the intestine through the hypothalamic–pituitary–adrenal axis. The autonomic enteric nervous system regulates gastrointestinal motility, secretion, barrier function and inflammatory response at the mucosa (65). Indeed, gene-sets related to neurotransmission were also identified: ‘Panther—Nicotinic_ acetylcholine_receptor_signaling_pathway’, ‘GO:0090278—negative regulation of peptide hormone secretion’ or a protein network regulating neurotransmitter release ‘ENSG00000147955—SIGMAR1 PPI subnetwork’. Several organs, such as the urogenital and musculoskeletal systems, not a priori of relevance for diarrhoeal disease, were identified for DD2Y.
Finally, given the known opposite effect of the FUT2 locus in infectious diseases compared to autoimmune diseases, we decided to compare our GWAS results with the results reported in the largest GWAS of IBD, for which an infectious component has been suspected (22). Besides the FUT2 locus, where we confirmed the opposite direction of the association, the same allele in a SNP at CARD11 locus, was associated with higher risk of IBD and higher risk of D1Y. CARD11 (Caspase Recruitment Domain Family, Member 11) is involved in the co-stimulatory signal essential for T-cell receptor (TCR)-mediated T-cell activation. We also compared our results with several GWAS of viral infections and response to vaccination (23–36). The TTC7B (Tetratricopeptide Repeat Domain 7B) locus, associated with higher anti Cytomegalovirus IgG titer (31), a marker of either new infection or frequent viral reactivation, was associated with higher risk for D2Y. Other studies have reported the participation of TTC7B in Chikungunya virus infection (66), and a paralog of TTC7B, TTC7A, has been implicated in a rare hereditary disease characterized by intestinal obstructions and profound immune defects (67).
The main limitations of the study are the low specificity of the phenotype definition, and the lack of underlying etiological information for the defined episodes. Information on diarrhoeal disease was retrieved using standard epidemiological tools for population-based birth cohort studies: questionnaires (ALSPAC, Generation R, GINIplus and LISAplus, INMA_VAL and INMA_SAB and MoBa), medical interviews (COSPAC) and medical records (CHOP). Apart from medical record definitions, the most specific assessment of infectious gastroenteritis was in Generation R and in MoBa studies, where the questionnaires included the following statement: ‘infections of the stomach/intestine or gastric flu’. In fact, Generation R showed a low number of diarrhoeal disease cases and a high effect of the variant at FUT2 locus, suggesting less misclassification problems. We acknowledge that misclassification and heterogeneity (i.e. infection by different underlying aetiologies, bacterial species or viral strains or seasonality) may have decreased the statistical power in the discovery phase and might explain the lack of replication of suggestive hits besides the FUT2 locus. Furthermore, cohorts with different study designs participated in the analyses: mainly population-based birth cohorts, but also a random collection of paediatric children from a hospital centre (CHOP, replication), and a population-based birth cohort of infants born to mothers with a history of asthma (COPSAC2000, discovery). The potential relationship between asthma and infection diseases might have affected the associations. To the best of our knowledge, there are no population-based studies of this sample size with molecular diagnosis of infectious gastroenteritis. Although we analyzed all available samples from the EArly Genetics and Life course Epidemiology (EAGLE) consortium following a flexible inclusion criteria the sample size is still intermediate for genome-wide scale studies. Therefore, increasing the specificity of the outcome and the sample size in population-based designs might reveal novel loci for childhood diarrhoeal disease and confirm the role of host genetics in infectious diseases during the first years of life. Finally, although diarrhoeal disease in young children is mainly caused by Rotavirus, which is a wide-spread virus producing seasonal break-outs in all the countries included in this study, we cannot completely exclude the possibility that the identified variants reflect different levels of exposure to the virus, rather than a higher susceptibility to infection.
In summary, the genome-wide association meta-analysis of diarrhoeal disease in children suggested the implication of the FUT2 locus at the population level, and has pointed to W154X (rs601338) as the most likely causal variant. The histo-blood group antigen (HBGA) production and the regulation of ion transport were plausible underlying biological mechanisms accounting for part of the host genetic variability of diarrhoeal disease.
Materials and Methods
Sample and diarrhoeal disease definition
This study was performed within the framework of the Early Genetics and Life course Epidemiology (EAGLE) Consortium (http://www.wikigenes.org/e/art/e/348.html) and it was divided in two phases: discovery and replication. The following population-based birth cohorts or studies settled up in developed countries participated in the discovery and/or replication phases (Table 1): the Avon Longitudinal Study of Parents And Children (ALSPAC), the Children's Hospital of Philadelphia (CHOP) study, the Copenhagen prospective studies on asthma in childhood (COPSAC2000 and COPSAC2010), the Generation R study, the Influence of Life-style related factors on the development of the Immune System and Allergies in East and West Germany plus the influence of traffic emissions and genetics (LISAplus) study, the Study on the influence of Nutrition Intervention plus Air pollution and Genetic on Allergy development (GINIplus), the Infancia y Medio Ambiente (INMA) project and the national Norwegian Mother and Child Cohort Study (MoBa). Cohorts were allocated in the discovery or in the replication set with the aim of making both sets comparable. Diarrhoeal disease was defined in two different time windows: around age 1 year (from 0 months to 18 months) and around age 2 years (from 12 to 30 months). At each time point diarrhoea (D1Y and D2Y) and doctor diagnosis of diarrhoea (DD1Y and DD2Y) were studied. Data were collected from parental questionnaires, doctor interviews or medical records. A detailed description of diarrhoeal disease definitions in each cohort at each time point is described in the Supplementary Material, Annex A. A comparison of the year of initiation of the cohort vs. the year of introduction of Rotavirus vaccination in each country can be seen in Supplementary Material, Annex B. The vaccine was introduced in the USA at the time when children from CHOP study were enrolled, and thus we excluded vaccinated children, identified through medical records, from the analysis.
Each cohort obtained the ethical approval from the respective Ethical Committees and a written consent including permission to perform GWAS analyses was obtained from participating parents.
Genotyping, quality control and imputation
Genotypes within each cohort were collected using high-density SNP arrays on Illumina (ALSPAC, CHOP, COPSAC2000, COPSAC2010, Generation R, INMA_SAB, INMA_VAL, MoBa) and Affymetrix (GINIplus and LISAplus) platforms. Each cohort imputed up to ∼30 M variants using MACH (68) or IMPUTE2 (69) considering the 1000 Genomes Project CEU release March 2012 (http://mathgen.stats.ox.ac.uk/impute/ALL_1000G_phase1integrated_v3_impute.tgz) as the reference population panel. More details on the process followed by each cohort are described in Supplementary Material, Annex A.
Analysis, meta-analysis and replication
The study included only at term Caucasian singletons and children with congenital anomalies were excluded. The association between diarrhoeal disease and the variant dose was assessed in each study using logistic regression analyses assuming an additive genetic model. Sex and principal components accounting for genetic sub-stratification were added as covariates. Chromosome X was analysed under the same statistical model but without sex adjustment. More details on the programs used by each study to perform the analysis can be found in Supplementary Material, Annex A.
Only variants with a Minor Allele Frequency (MAF) ≥0.01 and with a quality of imputation ≥ 0.4 (INFO) or ≥ 0.3 (R2) were considered. Due to the limited sample size of some cohorts, an additional filtering based on expected minor allele counts (EMAC) was performed. This parameter is related to both the sample size and the quality of imputation (2*N*MAF*quality of imputation). Variants that did not reach an EMAC ≥ 50 were excluded. After quality control, from 5.4 to 8.7 million variants were kept for the analysis in each cohort. The genomic inflation factor lambda (λ) was calculated for each study. A summary of the quality control procedure is shown in Supplementary Material, Table S14. Marker names and alleles were harmonized among studies.
A fixed effect meta-analysis weighted by inverse variance was conducted using GWAMA (70). The genomic control approach was applied to the meta-analysis results. Only variants with data for at least 5000 samples were considered. Genome-wide level of significance was defined at P-value ≤ 5E−08 and suggestive associations at P-value ≤1E−05. Quantile–quantile (Q–Q) plots, calculation of lambda (λ) and Manhattan plots were performed in R software environment version 3.2.3 (71). Regional association plots were performed with Locus Zoom (72).
In total 72 variants were followed for replication in an independent dataset. They were selected among the four outcomes based on the statistical significance (P-value < 1E−05). Association P-values from the replication phase were corrected for multiple testing using Bonferroni correction (for each trait independently). Exclusion of CHOP cohort, as it comprises potentially vaccinated children, from the replication phase did not revealed any new replicated genetic variant, and the association of replicated variants was maintained (data not shown).
Conditional analysis
We conditioned the analysis of the leading SNP identified at 19q13.33 (rs8111874) to a stop mutation situated 37.7 kb apart (rs601338, W154X) using the GCTA program (73). As reference, we used the INMA 1000 Genomes imputation [restricted to variants with MAF > 0.01 and imputation quality (INFO) >0.8].
A similar analysis was performed to search for secondary signals in ±500 kb surrounding the stop mutation (rs601338) or the top SNP at 19q13.33 (rs8111874). In this case, the significance threshold was calculated by Bonferroni correction, where the number of independent tests was the effective number of variants in this region estimated using Nyholt’s procedure and the 1000G reference data for Europeans (74).
Annotation and enrichment analysis
Genetic variants annotation (nearest gene, eQTLs, protein binding, and regulatory features) was done with the HaploReg v4.1 program (75). In addition, a second search for eQTLs and for expression levels in tissues was performed with Genotype-Tissue Expression (GTEx) data (http://www.gtexportal.org/; date last accessed July 2016). GeneCard (http://www.genecards.org; date last accessed July 2016) and the USC genome browser (http://genome.ucsc.edu/; date last accessed July 2016) were used to search for gene functions and GWAS signals, respectively.
Two different tools were used to explore gene-set enrichment analysis. We performed an analysis with MAGENTA software that uses genome-wide summary statistics (76). Briefly, first, the program links variants to genes considering flanking regions and then computes the gene-set enrichment comparing the variants with the lowest P-values (95th percentile) versus the rest. Gene-set databases evaluated in MAGENTA were Panther, KEGG (Kyoto Encyclopaedia of Genes and Genomes) and Ingenuity. In addition, we used DEPICT to identify enriched genes-sets as well as tissues/cell types where genes from associated loci are highly expressed (77). In this case, only variants associated with diarrhoea with a P-value ≤ 1E−05 and a sample size >5000 were considered. Both programs estimate adjusted P-values using the FDR method. In the case of MAGENTA, FDR was applied within each database.
Overlap with known variants and genes for related diseases
We investigated the association between diarrhoea and known variants for inflammatory bowel disease (IBD) (22). We also looked at variants associated with viral infection susceptibility, disease progression and response to vaccination against different viruses (23–36). Variants were selected from GWAS retrieved from the GWAS catalog (http://www.ebi.ac.uk/gwas/, April 2015). All variants reported in European populations, regardless of their statistical significance, and their replication status, were evaluated. Finally, we evaluated 86 genes retrieved from OMIM (Online Mendelian Inheritance in Man; July 2016) with the entry ‘diarrhoea’. To test the association of these ‘candidate’ genes, we performed a gene-based analysis using VEGAS2 (Versatile Gene based Association Study, http://vegas2.qimrberghofer.edu.au/) (78), considering linkage disequilibrium patterns described in European populations and a flanking region of ±50 kb around the gene. The corrected statistical significance level was calculated using Bonferroni correction accounting for the number of variants/genes within each analysis (inflammatory bowel disease, viral infection, or hereditary diarrhoeal diseases) as independent tests.
Genome-wide summarized results of the discovery phase can be found at INMA’s web page (Infancia and Medio Ambiente project, http://www.proyectoinma.org/) and at the EAGLE consortium web page (http://www.wikigenes.org/e/art/e/348.html).
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
ALSPAC
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. GWAS data was generated by Sample Logistics and Genotyping Facilities at the Wellcome Trust Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. This publication is the work of the authors and Carolina Bonilla will serve as guarantor for the contents of this paper.
CHOP
We thank the network of primary care clinicians, their patients and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium (PeRC) at The Children’s Hospital of Philadelphia. Rosetta Chiavacci, Elvira Dabaghyan, Hope Thomas, Kisha Harden, Andrew Hill, Kenya Fain, Crystal Johnson-Honesty, Cynthia Drummond, Shanell Harrison and Sarah Wildrick, Cecilia Kim, Edward Frackelton, George Otieno, Kelly Thomas, Cuiping Hou, Kelly Thomas and Maria L. Garris provided expert assistance with genotyping or data collection and management. We would also like to thank Smari Kristinsson, Larus Arni Hermannsson and Asbjörn Krisbjörnssonof Raförninnehf for their extensive software design and contribution.
COPSAC
We express our deepest gratitude to the children and families of the COPSAC2000 and COPSAC2010 study cohorts for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team.
GENERATION R
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the Faculty of Social Sciences of the Erasmus University, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR). We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam. The generation and management of GWAS genotype data for the Generation R Study were done at the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, The Netherlands. We thank Pascal Arp, Mila Jhamai, Marijn Verkerk, Lizbeth Herrera and Marjolein Peters for their help in creating, managing and QC of the GWAS database. Also Karol Estrada and Carolina Medina-Gomez for their support in creation and analysis of imputed data. We would like to thank Karol Estrada, Fernando Rivadeneira and Anis Abuseiris (Erasmus MC Rotterdam, The Netherlands), for their help in creating GRIMP, and we thank Big GRID for access to their grid computing resources.
GINIplus
The authors thank all the families for their participation in the GINIplus study. Furthermore, we thank all members of the GINIplus Study Group for their excellent work. The GINIplus Study group consists of the following: Institute of Epidemiology I, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg (Heinrich, J., Brüske, I., Schulz, H., Flexeder, C., Zeller, C., Standl, M., Schnappinger, M., Sußmann, M., Thiering, E., Tiesler, C.); Department of Paediatrics, Marien-Hospital, Wesel (Berdel D, von Berg A); Ludwig-Maximilians-University of Munich, Dr von Hauner Children’s Hospital (Koletzko S); Child and Adolescent Medicine, University Hospital rechts der Isar of the Technical University Munich (Bauer CP, Hoffmann U); IUF-Environmental Health Research Institute, Düsseldorf (Schikowski T, Link E, Klümper C).
LISAplus
The authors thank all the families for their participation in the LISAplus study. Furthermore, we thank all members of the LISAplus Study Group for their excellent work. The LISAplus Study group consists of the following: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology I, Munich (Heinrich, J., Schnappinger, M., Brüske, I., Sußmann, M., Lohr, W., Schulz, H., Zeller, C., Standl, M.); Department of Paediatrics, Municipal Hospital ‘St. Georg’, Leipzig (Borte, M., Gnodtke, E.); Marien Hospital Wesel, Department of Pediatrics, Wesel (von Berg, A., Berdel, D., Stiers, G., Maas, B.); Paediatric Practice, Bad Honnef (Schaaf B); Helmholtz Centre of Environmental Research—UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig (Lehmann, I., Bauer, M., Röder, S., Schilde, M., Nowak, M., Herberth, G., Müller, J., Hain, A.); Technical University Munich, Department of Paediatrics, Munich (Hoffmann, U., Paschke, M., Marra, S.); Clinical Research Group Molecular Dermatology, Department of Dermatology and Allergy, Technische Universität München (TUM), Munich (Ollert M).
INMA
The authors would like to thank all the participants for their generous collaboration. The authors are grateful to Silvia Fochs, Anna Sànchez, Maribel López, NuriaPey, Muriel Ferrer, Amparo Quiles, Sandra Pérez, Gemma León, Elena Romero, Maria Andreu, Nati Galiana, Maria Dolores Climent, Amparo Cases and Cristina Capo for their assistance in contacting the families and administering the questionnaires. A full roster of the INMA project investigators can be found at http://www.proyectoinma.org/presentacion-inma/listado-investigadores/en_listado-investigadores.html. Some of the DNA extractions and genotyping were performed at the Spanish National Genotyping Centre (CEGEN-Barcelona).
MOBA (Mother and Child Cohort of NIPH)
We are grateful to all the participating families in Norway who take part in this ongoing cohort study. Researchers interested in using data or biological material from MoBa must obtain approval from the Scientific Management Committee and from the Regional Committee for Medical and Health Research Ethics. Researchers will be required to follow the terms of an Assistance Agreement containing a number of clauses designed to ensure protection of privacy and compliance with relevant laws. For further information, contact the principal investigator of MoBa, Per Magnus (per.magnus@fhi.no).
Conflict of Interest statement. None declared.
Funding
ALSPAC
The UK Medical Research Council and the Wellcome Trust [102215/2/13/2] and the University of Bristol provide core support for ALSPAC. G.DS.’s and N.J.T.’s works are supported by the UK Medical Research Council Integrative Epidemiology Unit at the University of Bristol [MC_UU_12013_1 and MC_UU_12013/3, respectively].
CHOP
This research was financially supported by the Daniel B. Burke Chair for Diabetes Research, an Institute Development Award from the Children’s Hospital of Philadelphia, a Research Development Award from the Cotswold Foundation and the National Institutes of Health [R01 HD056465].
COPSAC
COPSAC is funded by private and public research funds all listed on www.copsac.com. The Lundbeck Foundation [R16-A1694]; The Danish Ministry of Health [903516]; Danish Council for Strategic Research [0603-00280B]; and The Capital Region Research Foundation have provided core support for COPSAC. Genotyping was supported by The Danish Council for Independent Research [10-082884 and 271-08-0815]. No pharmaceutical company was involved in the study. The funding agencies did not have any role in design and conduct of the study; collection, management and interpretation of the data; or preparation, review or approval of the manuscript.
GENERATION R
The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport. V.W.V.J. received grants from the Netherlands Organization for Health Research and Development [ZonMw 907.00303, ZonMw 916.10159, VIDI 016.136.361], and from the European Research Council [ERC-2014-CoG-648916].
GINIplus
The GINIplus study was mainly supported for the first 3 years of the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm). The 4-year, 6-year, 10-year and 15-year follow-up examinations of the GINIplus study were covered from the respective budgets of the five study centres (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich and from 6 years onwards also from IUF—Leibniz Research-Institute for Environmental Medicine at the University of Düsseldorf) and a grant from the Federal Ministry for Environment [IUF Düsseldorf, FKZ 20462296]. Further, the 15-year follow-up examination was supported by the European Commission [FP7-HEALTH-2010-261357], as well by the companies Mead Johnson and Nestlé.
LISAplus
The LISAplus study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and in addition from Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research - UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Paediatric Practice, Bad Honnef for the first 2 years. The 4-year, 6-year, 10-year and 15-year follow-up examinations of the LISAplus study were covered from the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Paediatric Practice, Bad Honnef, IUF—Leibniz-Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment [IUF Düsseldorf, FKZ 20462296]. Further, the 15-year follow-up examination was supported by the European Commission [FP7-HEALTH-2010-261357].
INMA
This project was funded by grants from Instituto de Salud Carlos III: FIS-FEDER [CB06/02/0041, G03/176, PI041436, PI081151, PI041705, PI061756, PI091958 and PS09/00432, 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, 09/02647, 11/01007, 11/02591, 11/02038, 13/1944, 13/2032] and Miguel Servet-FEDER [CP11/0178, MS15/0025, and CP11/00269]; Spanish Ministry of Science and Innovation [SAF2008-00357]; European Commission [FP7-HEALTH-2010-261357, FP7-ENV-2011-282957]; Fundació La Marató de TV3; Generalitat de Catalunya [CIRIT 1999SGR 00241] and Conselleria de Sanitat Generalitat Valenciana. The work at X.E.'s laboratory was funded by ‘Retos de la Sociedad 2013: Europa Redes y Gestores’ Programme from the Spanish Ministry of Economy and Competitiveness [SAF2013-49108-R], the Generalitat de Catalunya [AGAUR 2014 SGR-1138], the European Commission [FP7/2007-2013-262055]. X.E.'s lab acknowledges support of the MINECO, ‘Centro de Excelencia Severo Ochoa 2013-2017’ [SEV-2012-0208].
MOBA (Mother and Child Cohort of NIPH)
This work was supported by grants from the Norwegian Research Council [FUGE 183220/S10, FRIMEDKLI-05 ES236011], Swedish Medical Society [SLS 2008-21198], Jane and Dan Olsson Foundations and Swedish government grants to researchers in the public health service [ALFGBG-2863, ALFGBG-11522, ALFGBG-426411], Swedish Medical Research Council [2015-02559] and the European Commission [HEALTH-F4-2007-201413]. The Norwegian Mother and Child Cohort Study was also supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS [N01-ES-75558], NIH/NINDS [UO1 NS 047537-01 and UO1 NS 047537-06A1] and the Norwegian Research Council/FUGE [151918/S10; FRI-MEDBIO 249779].
Authors contributions
Study design: M.B., M.S., N.V-T., J.P.B., F.D.M., F.B., S.L., H.S., V.W.V.J., H.H., S.F.A.G., K.B., H.B., H.A.M., G.DS., H.B, A.J., B.J., J.H., X.E., J.S. Phenotyping: A.J., J.P.B., N.H.V., N.R.F., B.J., F.D.M., F.B., J.C.K-dJ, H.M.W., S.L., E.T., S.B., J.A., H.H., S.F.A.G., J.B., K.B., H.B., J.H., J.S. Genotyping: M.B., C.M-G., C.M.T.T., F.R., S.R., B.J., J.K., N.J.T., D.M.E., J.W., H.H., S.F.A.G., J.B., K.B., H.B., X.E. Analysis: M.B., M.S., N.V-T., C.M-G., C.B., T.S.A., J.P.B., F.R., F.D.M., J.C.K., J.B. Writing manuscript: M.B., M.S., Q.B., X.E., J.S. Revising and reviewing paper: M.B., M.S., Q.B., N.V-T., C.M-G., C.B., T.S.A., A.J., J.P.B., C.M.T.T., F.R., S.R., N.H.V., N.R.F., B.J., F.D.M., F.B., J.K., J.C.K-dJ, H.M.W., S.L., E.T., S.B., N.J.T., J.A., H.S., V.W.V.J., D.M.E., J.W., H.H., S.F.A.G., J.B., K.B., H.B., G.DS., H.A.M., J.H., X.E., J.S.
References
- 1.Walker C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A., O'Brien K.L., Campbell H., Black R.E. (2013) Global burden of childhood pneumonia and diarrhoea. Lancet, 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott E.J. (2007) Acute gastroenteritis in children. BMJ, 334, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet, 382, 209–222. [DOI] [PubMed] [Google Scholar]
- 4.Wiegering V., Kaiser J., Tappe D., Weissbrich B., Morbach H., Girschick H.J. (2011) Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int. J. Infect. Dis., 15, e401–e407. [DOI] [PubMed] [Google Scholar]
- 5.Ray P.G., Kelkar S.D., Walimbe A.M., Biniwale V., Mehendale S. (2007) Rotavirus immunoglobulin levels among Indian mothers of two socio-economic groups and occurrence of rotavirus infections among their infants up to six months. J. Med. Virol., 79, 341–349. [DOI] [PubMed] [Google Scholar]
- 6.Prameela K.K., Vijaya L.R. (2012) The importance of breastfeeding in rotaviral diarrhoeas. Malays. J. Nutr., 18, 103–111. [PubMed] [Google Scholar]
- 7.Velazquez F.R., Matson D.O., Guerrero M.L., Shults J., Calva J.J., Morrow A.L., Glass R.I., Pickering L.K., Ruiz-Palacios G.M. (2000) Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis., 182, 1602–1609. [DOI] [PubMed] [Google Scholar]
- 8.Ramig R.F. (2004) Pathogenesis of intestinal and systemic rotavirus infection. J. Virol., 78, 10213–10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg H.B., Estes M.K. (2009) Rotaviruses: from pathogenesis to vaccination. Gastroenterology, 136, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapar N., Sanderson I.R. (2004) Diarrhoea in children: an interface between developing and developed countries. Lancet, 363, 641–653. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen T.I., Nielsen G.G., Andersen P.K., Teasdale T.W. (1988) Genetic and environmental influences on premature death in adult adoptees. N. Engl. J. Med., 318, 727–732. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Aryeh H., Blumfield E., Szargel R., Laufer D., Berdicevsky I. (1995) Oral Candida carriage and blood group antigen secretor status. Mycoses, 38, 355–358. [DOI] [PubMed] [Google Scholar]
- 13.Burgner D., Jamieson S.E., Blackwell J.M. (2006) Genetic susceptibility to infectious diseases: big is beautiful, but will bigger be even better? Lancet Infect. Dis., 6, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill A.V. (2006) Aspects of genetic susceptibility to human infectious diseases. Annu. Rev. Genet., 40, 469–486. [DOI] [PubMed] [Google Scholar]
- 15.Pinkerton R.C., Oria R.B., Kent J.W., Jr., Kohli A., Abreu C., Bushen O., Lima A.A., Blangero J., Williams-Blangero S., Guerrant R.L. (2011) Evidence for genetic susceptibility to developing early childhood diarrhea among shantytown children living in northeastern Brazil. Am. J. Trop. Med. Hyg., 85, 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorven M., Grahn A., Hedlund K.O., Johansson H., Wahlfrid C., Larson G., Svensson L. (2005) A homozygous nonsense mutation (428G–>A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J. Virol., 79, 15351–15355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsson B., Kindberg E., Buesa J., Rydell G.E., Lidon M.F., Montava R., Abu Mallouh R., Grahn A., Rodriguez-Diaz J., Bellido J., et al. (2009) The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 Norovirus infection. PLoS One, 4, e5593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindberg E., Akerlind B., Johnsen C., Knudsen J.D., Heltberg O., Larson G., Bottiger B., Svensson L. (2007) Host genetic resistance to symptomatic norovirus (GGII.4) infections in Denmark. J. Clin. Microbiol., 45, 2720–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imbert-Marcille B.M., Barbe L., Dupe M., Le Moullac-Vaidye B., Besse B., Peltier C., Ruvoen-Clouet N., Le Pendu J. (2014) A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J. Infect. Dis., 209, 1227–1230. [DOI] [PubMed] [Google Scholar]
- 20.Petri W.A., Jr., Miller M., Binder H.J., Levine M.M., Dillingham R., Guerrant R.L. (2008) Enteric infections, diarrhea, and their impact on function and development. J. Clin. Invest., 118, 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores J., Okhuysen P.C. (2009) Genetics of susceptibility to infection with enteric pathogens. Curr. Opin. Infect. Dis., 22, 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature, 491, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limou S., Le Clerc S., Coulonges C., Carpentier W., Dina C., Delaneau O., Labib T., Taing L., Sladek R., Deveau C., et al. (2009) Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis., 199, 419–426. [DOI] [PubMed] [Google Scholar]
- 24.Le Clerc S., Limou S., Coulonges C., Carpentier W., Dina C., Taing L., Delaneau O., Labib T., Sladek R., Deveau C., et al. (2009) Genomewide association study of a rapid progression cohort identifies new susceptibility alleles for AIDS (ANRS Genomewide Association Study 03). J. Infect. Dis., 200, 1194–1201. [DOI] [PubMed] [Google Scholar]
- 25.Fellay J., Ge D., Shianna K.V., Colombo S., Ledergerber B., Cirulli E.T., Urban T.J., Zhang K., Gumbs C.E., Smith J.P., et al. (2009) Common genetic variation and the control of HIV-1 in humans. PLoS Genet., 5, e1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbeck J.T., Gottlieb G.S., Winkler C.A., Nelson G.W., An P., Maust B.S., Wong K.G., Troyer J.L., Goedert J.J., Kessing B.D., et al. (2010) Multistage genomewide association study identifies a locus at 1q41 associated with rate of HIV-1 disease progression to clinical AIDS. J. Infect. Dis., 201, 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereyra F., Jia X., McLaren P.J., Telenti A., de Bakker P.I., Walker B.D., Ripke S., Brumme C.J., Pulit S.L., Carrington M., et al. (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science, 330, 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bol S.M., Moerland P.D., Limou S., van Remmerden Y., Coulonges C., van Manen D., Herbeck J.T., Fellay J., Sieberer M., Sietzema J.G., et al. (2011) Genome-wide association study identifies single nucleotide polymorphism in DYRK1A associated with replication of HIV-1 in monocyte-derived macrophages. PLoS One, 6, e17190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troyer J.L., Nelson G.W., Lautenberger J.A., Chinn L., McIntosh C., Johnson R.C., Sezgin E., Kessing B., Malasky M., Hendrickson S.L., et al. (2011) Genome-wide association study implicates PARD3B-based AIDS restriction. J. Infect. Dis., 203, 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D., McKay J.D., Clifford G., Gaborieau V., Chabrier A., Waterboer T., Zaridze D., Lissowska J., Rudnai P., Fabianova E., et al. (2011) Genome-wide association study of HPV seropositivity. Hum. Mol. Genet., 20, 4714–4723. [DOI] [PubMed] [Google Scholar]
- 31.Kuparinen T., Seppala I., Jylhava J., Marttila S., Aittoniemi J., Kettunen J., Viikari J., Kahonen M., Raitakari O., Lehtimaki T., et al. (2012) Genome-wide association study does not reveal major genetic determinants for anti-cytomegalovirus antibody response. Genes Immun., 13, 184–190. [DOI] [PubMed] [Google Scholar]
- 32.Liu L., Li J., Yao J., Yu J., Zhang J., Ning Q., Wen Z., Yang D., He Y., Kong X., et al. (2011) A genome-wide association study with DNA pooling identifies the variant rs11866328 in the GRIN2A gene that affects disease progression of chronic HBV infection. Viral Immunol., 24, 397–402. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy R.B., Ovsyannikova I.G., Pankratz V.S., Haralambieva I.H., Vierkant R.A., Poland G.A. (2012) Genome-wide analysis of polymorphisms associated with cytokine responses in smallpox vaccine recipients. Hum. Genet., 131, 1403–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duggal P., Thio C.L., Wojcik G.L., Goedert J.J., Mangia A., Latanich R., Kim A.Y., Lauer G.M., Chung R.T., Peters M.G., et al. (2013) Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Ann. Intern. Med., 158, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaren P.J., Coulonges C., Ripke S., van den Berg L., Buchbinder S., Carrington M., Cossarizza A., Dalmau J., Deeks S.G., Delaneau O., et al. (2013) Association study of common genetic variants and HIV-1 acquisition in 6,300 infected cases and 7,200 controls. PLoS Pathog., 9, e1003515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy R.B., Ovsyannikova I.G., Haralambieva I.H., Lambert N.D., Pankratz V.S., Poland G.A. (2014) Genome-wide SNP associations with rubella-specific cytokine responses in measles-mumps-rubella vaccine recipients. Immunogenetics, 66, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Ruvoen N., Clement M., Le Pendu J. (2001) ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie, 83, 565–573. [DOI] [PubMed] [Google Scholar]
- 38.Ferrer-Admetlla A., Sikora M., Laayouni H., Esteve A., Roubinet F., Blancher A., Calafell F., Bertranpetit J., Casals F. (2009) A natural history of FUT2 polymorphism in humans. Mol. Biol. Evol., 26, 1993–2003. [DOI] [PubMed] [Google Scholar]
- 39.Ikehara Y., Nishihara S., Yasutomi H., Kitamura T., Matsuo K., Shimizu N., Inada K., Kodera Y., Yamamura Y., Narimatsu H., et al. (2001) Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol. Biomarkers Prev., 10, 971–977. [PubMed] [Google Scholar]
- 40.Azevedo M., Eriksson S., Mendes N., Serpa J., Figueiredo C., Resende L.P., Ruvoen-Clouet N., Haas R., Boren T., Le Pendu J., et al. (2008) Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol., 215, 308–316. [DOI] [PubMed] [Google Scholar]
- 41.Ruvoen-Clouet N., Belliot G., Le Pendu J. (2013) Noroviruses and histo-blood groups: the impact of common host genetic polymorphisms on virus transmission and evolution. Rev. Med. Virol., 23, 355–366. [DOI] [PubMed] [Google Scholar]
- 42.Hu L., Crawford S.E., Czako R., Cortes-Penfield N.W., Smith D.F., Le Pendu J., Estes M.K., Prasad B.V. (2012) Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature, 485, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang P., Xia M., Tan M., Zhong W., Wei C., Wang L., Morrow A., Jiang X. (2012) Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J. Virol., 86, 4833–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramani S., Cortes-Penfield N.W., Hu L., Crawford S.E., Czako R., Smith D.F., Kang G., Ramig R.F., Le Pendu J., Prasad B.V., et al. (2013) The VP8* domain of neonatal rotavirus strain G10P[11] binds to type II precursor glycans. J. Virol., 87, 7255–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinane D.F., Blackwell C.C., Brettle R.P., Weir D.M., Winstanley F.P., Elton R.A. (1982) ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br. Med. J. (Clin. Res. Ed.), 285, 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishitoya S., Yamamoto S., Mitsumori K., Ogawa O., Terai A. (2002) Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int., 89, 851–854. [DOI] [PubMed] [Google Scholar]
- 47.Thom S.M., Blackwell C.C., MacCallum C.J., Weir D.M., Brettle R.P., Kinane D.F., Wray D. (1989) Non-secretion of blood group antigens and susceptibility to infection by Candida species. FEMS Microbiol. Immunol., 1, 401–405. [DOI] [PubMed] [Google Scholar]
- 48.Chaim W., Foxman B., Sobel J.D. (1997) Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J. Infect. Dis., 176, 828–830. [DOI] [PubMed] [Google Scholar]
- 49.Blackwell C.C., Jonsdottir K., Hanson M.F., Weir D.M. (1986) Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet, 2, 687.. [DOI] [PubMed] [Google Scholar]
- 50.Blackwell C.C., Jonsdottir K., Hanson M., Todd W.T., Chaudhuri A.K., Mathew B., Brettle R.P., Weir D.M. (1986) Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet, 2, 284–285. [DOI] [PubMed] [Google Scholar]
- 51.McGovern D.P., Jones M.R., Taylor K.D., Marciante K., Yan X., Dubinsky M., Ippoliti A., Vasiliauskas E., Berel D., Derkowski C., et al. (2010) Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum. Mol. Genet., 19, 3468–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellinghaus D., Ellinghaus E., Nair R.P., Stuart P.E., Esko T., Metspalu A., Debrus S., Raelson J.V., Tejasvi T., Belouchi M., et al. (2012) Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet., 90, 636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang H., Jin X., Li Y., Jiang H., Tang X., Yang X., Cheng H., Qiu Y., Chen G., Mei J., et al. (2014) A large-scale screen for coding variants predisposing to psoriasis. Nat. Genet., 46, 45–50. [DOI] [PubMed] [Google Scholar]
- 54.Xavier J.M., Shahram F., Sousa I., Davatchi F., Matos M., Abdollahi B.S., Sobral J., Nadji A., Oliveira M., Ghaderibarim F., et al. (2013) FUT2: filling the gap between genes and environment in Behcet's disease? Ann. Rheum. Dis., 74, 618–624. [DOI] [PubMed] [Google Scholar]
- 55.Grarup N., Sulem P., Sandholt C.H., Thorleifsson G., Ahluwalia T.S., Steinthorsdottir V., Bjarnason H., Gudbjartsson D.F., Magnusson O.T., Sparso T., et al. (2013) Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PLoS Genet., 9, e1003530.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazra A., Kraft P., Lazarus R., Chen C., Chanock S.J., Jacques P., Selhub J., Hunter D.J. (2009) Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum. Mol. Genet., 18, 4677–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong M., McHardy I., Ruegger P., Goudarzi M., Kashyap P.C., Haritunians T., Li X., Graeber T.G., Schwager E., Huttenhower C., et al. (2014) Reprograming of gut microbiome energy metabolism by the FUT2 Crohn's disease risk polymorphism. Isme J., 8, 2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wacklin P., Tuimala J., Nikkila J., Sebastian T., Makivuokko H., Alakulppi N., Laine P., Rajilic-Stojanovic M., Paulin L., de Vos W.M., et al. (2014) Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS One, 9, e94863.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rausch P., Rehman A., Kunzel S., Hasler R., Ott S.J., Schreiber S., Rosenstiel P., Franke A., Baines J.F. (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl. Acad. Sci. USA, 108, 19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto Y., Obata T., Kunisawa J., Sato S., Ivanov I.I., Lamichhane A., Takeyama N., Kamioka M., Sakamoto M., Matsuki T., et al. (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science, 345, 1254009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickard J.M., Maurice C.F., Kinnebrew M.A., Abt M.C., Schenten D., Golovkina T.V., Bogatyrev S.R., Ismagilov R.F., Pamer E.G., Turnbaugh P.J., et al. (2014) Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature, 514, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato N., Loh M., Takeuchi F., Verweij N., Wang X., Zhang W., Kelly T.N., Saleheen D., Lehne B., Mateo Leach I., et al. (2015) Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet., 47, 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinoshita M., Matsuoka Y., Suzuki T., Mirrielees J., Yang J. (2012) Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res., 1452, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang T., Hisamitsu T., Mori H., Shigekawa M., Wakabayashi S. (2004) Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: tightly bound Ca2+ ions as important structural elements. Biochemistry, 43, 3628–3636. [DOI] [PubMed] [Google Scholar]
- 65.de Jonge W.J. (2013) The Gut's Little Brain in Control of Intestinal Immunity. ISRN Gastroenterol., 2013, 630159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourai M., Lucas-Hourani M., Gad H.H., Drosten C., Jacob Y., Tafforeau L., Cassonnet P., Jones L.M., Judith D., Couderc T., et al. (2012) Mapping of Chikungunya virus interactions with host proteins identified nsP2 as a highly connected viral component. J. Virol., 86, 3121–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen R., Giliani S., Lanzi G., Mias G.I., Lonardi S., Dobbs K., Manis J., Im H., Gallagher J.E., Phanstiel D.H., et al. (2013) Whole-exome sequencing identifies tetratricopeptide repeat domain 7A (TTC7A) mutations for combined immunodeficiency with intestinal atresias. J. Allergy Clin. Immunol., 132, 656–664. e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Willer C.J., Ding J., Scheet P., Abecasis G.R. (2010) MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol., 34, 816–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howie B.N., Donnelly P., Marchini J. (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet., 5, e1000529.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magi R., Morris A.P. (2010) GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics, 11, 288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R-Core-Team. (2015) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- 72.Pruim R.J., Welch R.P., Sanna S., Teslovich T.M., Chines P.S., Gliedt T.P., Boehnke M., Abecasis G.R., Willer C.J. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics, 26, 2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J., Lee S.H., Goddard M.E., Visscher P.M. (2011) GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet., 88, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nyholt D.R. (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet., 74, 765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward L.D., Kellis M. (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res., 40, D930–D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segre A.V., Groop L., Mootha V.K., Daly M.J., Altshuler D. (2010) Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet, 6, e1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pers T.H., Karjalainen J.M., Chan Y., Westra H.J., Wood A.R., Yang J., Lui J.C., Vedantam S., Gustafsson S., Esko T., et al. (2015) Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun., 6, 5890.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra A., Macgregor S. (2015) VEGAS2: software for more flexible gene-based testing. Twin Res. Hum. Genet., 18, 86–91. [DOI] [PubMed] [Google Scholar]