Abstract
Whether folic acid fortification and supplementation at the population level have led to a higher prevalence of vitamin B-12 deficiency in the absence of anemia remains to be examined among a nationally representative sample of older U.S. adults. We assessed the prevalence of low vitamin B-12 status in the absence of anemia or macrocytosis before and after fortification among adults aged >50 y using cross-sectional data from the NHANES 1991–1994 (prefortification) and 2001–2006 (postfortification). We compared the prefortification and postfortification prevalence of multiple outcomes, including serum vitamin B-12 deficiency (<148 pmol/L) and marginal deficiency (148–258 pmol/L) with and without anemia (hemoglobin <130 g/L for men, <120 g/L for women) and with and without macrocytosis (mean cell volume >100 fL) using multinomial logistic regression, adjusting for age, sex, ethnicity, body mass index, C-reactive protein, and vitamin B-12 supplement use. Prefortification and postfortification serum vitamin B-12 deficiency without anemia [4.0 vs. 3.9%; adjusted prevalence ratio (aPR) (95% CI): 0.98 (0.67, 1.44)] or without macrocytosis [4.2 vs. 4.1%; aPR (95% CI): 0.96 (0.65, 1.43)] remained unchanged. Marginal deficiency without anemia [25.1 vs. 20.7%; aPR (95% CI): 0.82 (0.72, 0.95)] or without macrocytosis [25.9 vs. 21.3%; aPR (95%CI): 0.82 (0.72, 0.94)] were both significantly lower after fortification. After fortification, higher folic acid intake was associated with a lower prevalence of low serum B-12 status in the absence of anemia or macrocytosis. Results suggest that the prevalence of low serum B-12 status in the absence of anemia or macrocytosis among older U.S. adults did not increase after fortification. Thus, at the population level, we found no evidence to support concerns that folic acid adversely affected the clinical presentation of vitamin B-12 deficiency among older adults.
Introduction
Before the discovery and isolation of vitamin B-12, the practices of consuming raw liver and liver extract injections were well-known, effective treatments for pernicious anemia (PA)6, a type of megaloblastic anemia—found mainly in older adults—characterized by macrocytosis and, if untreated, irreversible spinal cord damage (1). At the time, it was unknown that megaloblastic anemias could result from either folate or vitamin B-12 deficiency. Early reports of folic acid effectively correcting the megaloblastic anemia of pregnancy rapidly led to its use as a treatment for PA (2). In hindsight, this was a mistake because the underlying problem in PA results from vitamin B-12 deficiency. Anemia improved initially but relapsed eventually inmost patients with PA who were taken off liver therapy and placed on high-dosage folic acid (3). Folic acid was also unable to effectively prevent or treat PA-associated neurologic complications (4,5).
The presence of neurologic complications in the absence of anemia among patients raised concerns that folic acid might have an adverse effect on vitamin B-12 deficiency. As reviewed previously, early case reports suggested that folic acid might directly cause neurologic damage or indirectly allow neurologic damage to progress without effective therapy by treating the anemia and making the underlying deficiency harder to detect (3,6). However, evidence of neuropathy in the absence of anemia among patients with PA who have not been treated with folic acid indicates that the appearance of neurologic complications without anemia could be unrelated to folic acid (7). Furthermore, in the early case reports, patients whose anemia improved after being administered folic acid could have had concurrent folate deficiency and thus responded to treatment (8).
Nevertheless, among countries that are considering or have introduced folic acid fortification for neural tube defects prevention, questions remain about whether exposure to higher levels of folic acid in fortified foods and supplements would affect the clinical presentation of vitamin B-12 deficiency, delay its diagnosis, and result in an increase in the proportion of individuals with low serum vitamin B-12 status in the absence of anemia or macrocytosis, in particular, among older adults who are more likely to have low vitamin B-12 status and higher folic acid intake than the rest of the population (9,10). Reviews of published scientific evidence indicate that amounts of folate (11–13) and folic acid (3) (i.e., <1 mg/d) typically consumed through diet or vitamin supplements are unlikely to be associated with neurologic complications in PA. However, several studies investigating the effects of folic acid fortification on the clinical presentation of vitamin B-12 deficiency have reported mixed findings (14–20). In the United States, the hematologic presentation of vitamin B-12 deficiency after folic acid fortification remains to be assessed in a representative population of older adults. Defining low vitamin B-12 status often poses a challenge because of the lack of agreement regarding the most appropriate cutoffs and biomarker combinations, as well as the low specificity of clinical signs. Here, we examined the prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis among older adults before and after mandatory folic acid fortification. Because an individual’s folate status could vary widely, depending on the various different sources of folic acid consumed (10,21), we also stratified the analyses by the source and amount of folic acid from fortified foods and supplements to assess whether folic acid consumption patterns after fortification affected the hematologic presentation of vitamin B-12 deficiency.
Methods
Study design and population
NHANES are cross-sectional surveys conducted periodically from the 1960s to 1994 and continuously in 2-y cycles since 1999. Using nationally representative samples of non-institutionalized civilians, NHANES monitor the health and nutritional status of the U.S. population. At the mobile examination center, survey participants gave informed consent, reported their health status and dietary intake, underwent physician-administered physical examinations, and provided blood samples for biomarker analyses. Survey protocols were approved by the National Center for Health Statistics Research Ethics Review Board. Additional details of the survey have been discussed previously (22).
To compare the prevalence of low vitamin B-12 status in the absence of anemia or macrocytosis before and after fortification, we used data from NHANES III Phase 2, 1991–1994 (prefortification), the years for which serum vitamin B-12 measurements were available, and data combined from NHANES 2001–2002, 2003–2004, and 2005–2006 (postfortification). To estimate the prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis by source of folic acid intake and amount consumed from supplements, we used data from only postfortification years NHANES 2001–2006. Data from NHANES 1999–2000 were excluded from the analyses because folic acid intake was reported as part of the combined variable total folate intake (folic acid + natural food folate), and thus, its potential effects on the prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis could not be assessed alone.
After limiting our analyses to adults aged >50 y, the initial sample size was 3614 before fortification and 6516 after fortification. To rule out other causes and factors potentially affecting anemia, we excluded participants who had liver disease (before: 115; after: 375), renal dysfunction (before: 195; after: 419), heavy alcohol use (before: 163; after: 316), or treatment for anemia <3 mo before survey participation (before: 115; after: 231) (23,24). Liver disease was defined as alanine aminotransferase >40 U/L (25). Renal disease was defined as serum creatinine >131 µmol/L for men and >115 µmol/L for women (26). Using the CDC definition, heavy alcohol use was defined as >2 drinks/d for men and >1 drink/d for women (27). Only participants with complete serum vitamin B-12, hemoglobin, and mean cell volume (MCV) measurements were included in the study. The final analytic sample size (and proportion of initial sample) was 2911 (80.5%) before fortification and 4946 (75.9%) after fortification.
Laboratory measurements
Hematologic indicators, B vitamins, and other biomarker analyses were performed on venous-blood and whole-blood hemolysate samples. Hemoglobin and MCV were measured using the Coulter S-plus Jr in NHANES III Phase 2 and Coulter MAXM hematology flow cytometer in NHANES 2001–2006. Based on the WHO definition, anemia was defined as hemoglobin <130 g/L for men and <120 g/L for women (28). Macrocytosis was defined using the common clinical characterization of MCV >100 fL (24). Participants were categorized as being without anemia if their hemoglobin concentrations were above the cutoff (≥130 g/L for men and ≥120 g/L for women) and without macrocytosis if their MCV was ≤100 fL. Serum and RBC folate and serum vitamin B-12 concentrations were measured using the QuantaPhase II radioassay (Bio-Rad Laboratories). Normal serum vitamin B-12 status was defined as serum B-12 >258 pmol/L. Low serum vitamin B-12 status was defined as 2 groups: 1) serum vitamin B-12 deficiency (<148 pmol/L); and 2) marginal deficiency (148–258 pmol/L) (29–31). Serum alanine aminotransferase and creatinine were measured as 1 of 22 analytes in a routine biochemistry profile analysis using the Hitachi multichannel analyzers in NHANES III Phase 2 (model 737) and NHANES 2001–2002 (model 704) and the Beckman Synchron LX20 in NHANES 2003–2006. Serum creatinine was determined using the Jaffe reaction across all years. As recommended by NHANES analytic guidelines, the Deming regression equation was applied to NHANES III, 2001–2002, and 2005–2006 to account for serum creatinine measurement error across survey years (32). C-reactive protein (CRP), an indicator of inflammation that might cause anemia by decreasing erythropoiesis, was quantified by latex-enhanced nephelometry on a Behring Nephelometer (33). Additional details on specimen processing and laboratory methods have been described previously (22).
Nutrient Intake
In the United States, folic acid can be found in fortified foods and dietary supplements. Among foods, enriched cereal grain products (ECGPs) are required to be fortified with 140 µg of folic acid/100 g of flour, whereas ready-to-eat cereals (RTEs) can be voluntarily fortified up to 400 µg/serving (12,34). Supplements most commonly provide 400 µg of folic acid per pill. Vitamin B-12 can be found naturally in animal-source foods or synthetically added at variable amounts to supplements and fortified foods (e.g., RTEs) (35).
We estimated the total daily intake of folic acid and vitamin B-12 from foods and supplements for each participant using nutritional information from 24-h dietary recalls and data on supplement use. Detailed methods have been reported previously and are briefly described below (10). The daily amount of each nutrient consumed from foods was estimated using a single 24-h dietary recall in NHANES 2001–2002 and 2 separate 24-h dietary recalls in each of the 2-y cycles of NHANES 2003–2006. We estimated the average daily intake of folic acid or vitamin B-12 from supplements based on each participant’s use of dietary supplements containing either folic acid or vitamin B-12, respectively, within the previous 30 d. Total daily folic acid or vitamin B-12 intake was reported as micrograms per day and defined as the sum of the nutrient consumed (folic acid from fortified foods and vitamin B-12 from natural and synthetic sources) plus the average daily intake of the nutrient from supplements.
To examine whether the prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis differed by folic acid intake from different sources, we divided the postfortification participants into 4 mutually exclusive folic acid intake groups. Individuals who consumed ECGPs and no other foods fortified with folic acid were classified as ECGP only. Those who consumed ECGPs and RTEs but no supplements containing folic acid were classified as ECGP + RTE, and those who consumed ECGPs and supplements containing folic acid but no RTEs were classified as ECGP + SUP. Finally, those who consumed all 3 sources were classified as ECGP + RTE + SUP. To examine whether the prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis differed by the amount of folic acid consumed from supplements, we separated postfortification participants into 3 categories by the amount of folic acid reportedly consumed daily from supplements: 0 µg/d (no supplements containing folic acid), 1–200 µg/d, and >200 µg/d.
Covariates
Covariates considered in the analyses included sex, age, race/ethnicity, BMI, smoking status, and CRP. Race/ethnicity was divided into 4 categories: 1) non-Hispanic whites; 2) non-Hispanic blacks; 3) Mexican Americans; 4) and other. Smoking status, reported by the participants at the time of their interview, was defined as yes/no. CRP was dichotomized; abnormal was defined as >10 mg/L (36,37). Participant BMIs were calculated by dividing their measured weight (kilograms) by their height (square meters). Age and BMI were treated as continuous variables.
Statistical analyses
All statistical analyses, with the exception of the usual intakes of folic acid and vitamin B-12 (discussed below), were performed using SAS-Callable SUDAAN (version 10; 2008, Research Triangle Institute) to account for the complex survey design of NHANES. Using multiple linear regression, we estimated geometric means (least squares means) and 95% CIs for serum and RBC folate and vitamin B-12 adjusting for age, sex, race/ethnicity, smoking status, and BMI. These biomarker concentrations were log-transformed to normalize their distributions. Statistically significant differences were evaluated using Satterthwaite-adjusted F tests for continuous variables and χ2 tests for categorical variables. To determine the prevalence of low serum vitamin B-12 status in the presence or absence of anemia or macrocytosis, we examined multiple outcomes each separated into mutually exclusive and exhaustive categories. In one model, the outcome was divided into five categories: 1) vitamin B-12 deficiency without anemia; 2) vitamin B-12 deficiency with anemia; 3) marginal deficiency without anemia; 4) marginal deficiency with anemia; and 5) normal serum vitamin B-12 status (including those with and without anemia). In a separate model, an analogous outcome was assessed for macrocytosis in place of anemia. Multinomial logistic regression (38) was used to estimate the prevalence (e.g., predictive margins), adjusted prevalence ratios (aPRs), and 95% CIs by fortification period, source of folic acid intake, and amount of folic acid consumption from supplements. Estimates were adjusted for age, sex, race/ethnicity, BMI, and CRP (as well as oral vitamin B-12 supplement use in comparisons between prefortification and postfortification time periods). Statistical significance for all tests was established at α = 0.05. To account for differential nonresponse and noncoverage, as well as the oversampling of specific groups in survey design, appropriate samples weights were used for all analyses (39,40). Mobile examination center sample weights were used for comparisons between NHANES III Phase 2 and NHANES 2001–2006. Dietary subsample weights were used for prevalence estimates of low vitamin B-12 status in the absence of anemia or macrocytosis in NHANES 2001–2006 by source of folic acid intake and amount of folic acid consumed from supplements. Estimates with relative standard errors (RSEs) ≥30% were considered statistically unreliable and were reported with a footnote for RSE = 30–39% and not reported at all for RSE ≥40%.
From the total daily folic acid and vitamin B-12 intake (described above, Nutrient intake), we estimated the usual daily intake of each nutrient using the Software for Intake Distribution Estimation (PC-SIDE version 1.02; Iowa State University, Ames, IA). As has been described previously (41,42), usual daily nutrient intake estimates require at least a subsample of participants to have 2 d of dietary recall to estimate both the within- and between-person variations. In our analytic sample, participants from NHANES 2001–2002 provided nutrient intake information for only 1 d; however, most of the participants from NHANES 2003–2006 had 2 independent days of intake data, allowing us to estimate usual nutrient intake for the entire sample. Usual daily folic acid and vitamin B-12 intakes were estimated stratified by folic acid intake group (ECGP, ECGP + RTE, ECGP + SUP, ECGP + RTE + SUP) and folic acid supplement use (0, 1–200, and >200 µg/d) after adjusting for intake day and day of the week. SEEs were obtained using jackknife replicate weights calculated based on a combination of day 2 dietary weights for individuals with 2 d of dietary recall data and day 1 dietary weights for individuals with only 1 d of dietary recall data. Details of PC-SIDE methodology are have been described previously (41,42). Comparisons of usual daily intakes of folic acid and vitamin B-12 within folic acid intake and folic acid supplement use groups were made using z tests. Six comparisons were made across the 4 folic acid intake groups, and 3 comparisons were made across the 3 folic acid supplement use groups.
Results
Among participants aged >50 y, the prevalence of marginal serum vitamin B-12 deficiency was significantly lower after compared with before fortification (Table 1). We observed no significant differences in the prevalence of serum vitamin B-12 deficiency (P = 0.23). Both serum vitamin B-12 concentration and the use of supplements containing vitamin B-12 were significantly higher after fortification. Furthermore, as expected, serum and RBC folate concentrations were higher after fortification. No significant differences were observed in the prevalence of anemia or macrocytosis across fortification periods. Additional characteristics of the analytic sample are presented in Table 1.
TABLE 1.
Characteristics of U.S. adults aged >50 y before and after folic acid fortification in NHANES1
| Before fortification (1991–1994) |
After fortification (2001–2006) |
|
|---|---|---|
| n, unweighted | 2911 | 4946 |
| Age, y | 65.4 ± 0.5 | 64.2 ± 0.3* |
| Female, % | 55.8 (52.8, 58.7) | 55.9 (54.4, 57.4) |
| Race/ethnicity, % | ||
| White, non-Hispanic | 81.4 (76.7, 85.3) | 80.4 (76.5, 83.7) |
| Black, non-Hispanic | 8.1 (6.4, 10.2) | 8.6 (6.8, 10.9) |
| Mexican American | 2.8 (2.1, 3.6) | 3.7 (2.6, 5.3) |
| Other | 7.7 (4.9, 11.9) | 7.3 (5.5, 9.5) |
| Low serum vitamin B-12 status,2 % | ||
| Marginal deficiency | 27.7 (24.6, 31.0) | 20.9 (19.3, 22.5)** |
| Deficiency | 4.8 (3.6, 6.5) | 3.8 (3.1, 4.8) |
| Serum vitamin B-12, pmol/L | 310 (302, 318) | 352 (344, 360)*** |
| Vitamin B-12 supplement use, % | 29.0 (26.1, 32.0) | 41.0 (38.7, 43.5)*** |
| Serum folate, nmol/L | 16.0 (15.1, 16.9) | 33.0 (32.2, 33.8)*** |
| RBC folate, nmol/L | 464 (440, 490) | 696 (682, 710)*** |
| Anemia,3% | 5.2 (4.3, 6.4) | 4.9 (4.1, 5.7) |
| Macrocytosis,4% | 1.9 (1.3, 3.0) | 2.5 (2.0, 3.1) |
Values are percentages (95% CIs) or geometric means (95% CIs). Age is reported as mean ± SE. Analyses were weighted to account for the complex sampling design of NHANES and excluded participants with liver disease, renal dysfunction, heavy alcohol use, and treatment of anemia <3 mo before survey participation. Surveys were compared using χ2 test for categorical variables and Satterthwaite-adjusted F test for continuous variables adjusting for age, sex, race/ethnicity, smoking status, and BMI (variables being analyzed were not treated as a potential confounder for their own analyses). Different from before fortification, *P < 0.05, **P < 0.001, ***P < 0.0001.
Serum vitamin B-12 concentrations for marginal deficiency were defined as 148–258 pmol/L; deficiency was defined as <148 pmol/L.
Anemia was defined as hemoglobin <130 g/L for men and <120 g/L for women.
Macrocytosis was defined as mean corpuscular volume >100 fL.
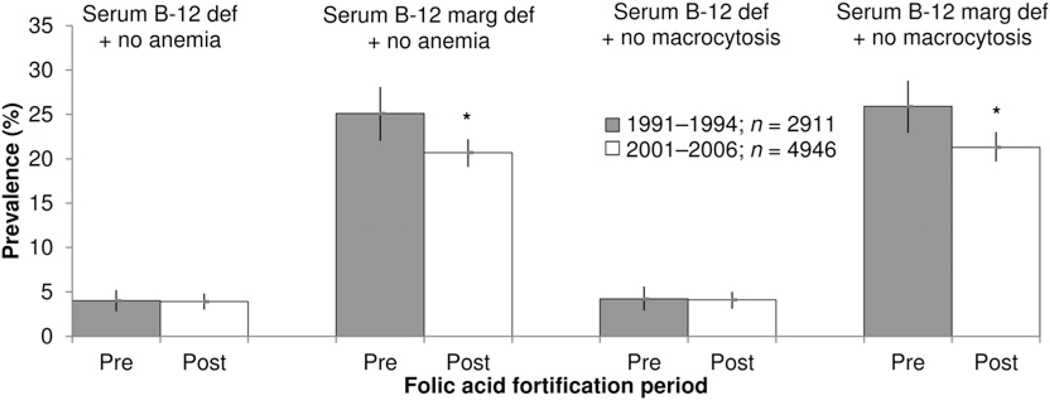
Overall, we did not observe a higher prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis among those aged >50 y after fortification when compared with before fortification (Fig. 1; Supplemental Table 1). The prevalence of serum vitamin B-12 deficiency without anemia remained the same (before: 4.0%; after: 3.9%; aPR: 0.98; 95% CI: 0.67, 1.44), whereas the prevalence of marginal deficiency without anemia was significantly lower after fortification (before: 25.1%; after: 20.7%; aPR: 0.82; 95% CI: 0.72, 0.95). Similarly, no difference in serum vitamin B-12 deficiency without macrocytosis was observed (before: 4.2%; after: 4.1%; aPR: 0.96; 95% CI: 0.65, 1.43), and the prevalence of marginal deficiency without macrocytosis was lower after fortification (before: 25.9%; after: 21.3%; aPR: 0.82; 95%CI: 0.72, 0.94). In addition, the prevalence of serum vitamin B-12 deficiency and marginal deficiency with anemia did not differ significantly before and after fortification (Supplemental Table 1).
FIGURE 1.
Prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis among U.S. adults aged >50 y in NHANES before and after mandatory folic acid fortification. Bars denote 95% CIs. *P < 0.001 based on prevalence estimate differences in serum vitamin B-12 deficiency (<148 pmol/L) and marginal deficiency (148–258 pmol/L) without anemia (hemoglobin ≥ 130 g/L for men, ≥120 g/L for women) or without macrocytosis (MCV ≤ 100 fL) using multinomial logistic regression adjusted for age, sex, race/ethnicity, BMI, C-reactive protein, smoking, and oral vitamin B-12 supplement use. Participants with liver disease, renal dysfunction, heavy alcohol use, and treatment of anemia <3 mo before survey participation were excluded from the analyses. Analyses were weighted to account for the complex sampling design of NHANES. MCV, mean cell volume; Serum B-12 def + no anemia, serum vitamin B-12 <148 pmol/L without anemia; serum B-12 def + no macrocytosis, serum vitamin B-12 <148 pmol/L without macrocytosis; serum B-12 marg def + no anemia, serum vitamin B-12 of 148–258 pmol/L without anemia; serum B-12 def + no macrocytosis, serum vitamin B-12 of 148–258 pmol/L without macrocytosis.
After fortification, as expected, usual daily folic acid intake was significantly higher as groups consumed more sources of folic acid (ECGP only > ECGP + RTE > ECGP + SUP > ECGP + RTE + SUP) and higher amounts of supplemental folic acid (0 > 1–200 > >200 µg/d) (Table 2). Similarly, usual daily vitamin B-12 intake also increased among groups consuming more sources of folic acid and increasing amounts of supplemental folic acid. Almost all users of supplements containing folic acid also consumed supplements containing vitamin B-12 (≥97.0%; Table 2). Lower prevalence of serum vitamin B-12 deficiency and marginal deficiency were associated with the consumption of RTEs and/or SUP and with higher daily intake of folic acid from supplements (Table 2).
TABLE 2.
Usual folic acid and vitamin B-12 intake and status among U.S. adults aged >50 y, NHANES 2001–20061
| Folic acid intake group2 | Folic acid supplement intake3 | ||||||
|---|---|---|---|---|---|---|---|
| ECGP only | ECGP + RTE | ECGP + SUP | ECGP + RTE + SUP | 0 µg/d | 1–200 µg/d | >200 µg/d | |
| Folic acid content | 140 µg / 100 g | ≤400 µg / serving | Most common 400 µg | — | — | — | — |
| n, unweighted | 1910 (36.7) | 767 (16.4) | 1269 (29.2) | 714 (17.7) | 2677 (53.1) | 366 (8.7) | 1617 (38.2) |
| Usual folic acid intake,2,3 µg/d | 113 (88, 143)a | 273 (255, 292)b | 488 (401, 595)c | 667 (545, 839)d | 151 (110, 207)a | 294 (219, 391)b | 596 (504, 754)c |
| Usual vitamin B-12 intake,2,3 µg/d | 3.7 (2.5, 11.5)e | 13.9 (5.2, 27.6)f | 21.2 (10.0, 35.1)g | 26.4 (12.9, 49.5)g | 4.6 (3.1, 15.5)a | 12.6 (6.9, 30.6)a,b | 25.8 (12.3, 42.0)b |
| Vitamin B-12 supplement use, % | 2.9 (2.1, 4.0) | 4.6 (3.0, 7.1) | 97.2 (95.4, 98.3) | 97.1 (94.4, 98.5) | 3.4 (2.6, 4.5) | 97.7 (94.4, 99.1) | 97.0 (95.2, 98.2) |
| Serum vitamin B-12 <148 pmol/L,2,3% | 5.8 (4.1, 7.5)h | 4.5 (2.6, 6.4)h | 2.2 (1.1, 3.4)i | 1.7 (0.9, 2.4)i | 5.3 (4.1, 6.9)h | 4.2 (2.0, 8.8)h,i,4 | 1.5 (1.0, 2.4)i |
| Serum vitamin B-12 148–258 pmol/L,2,3% | 32.1 (28.6, 35.6)h | 24.3 (20.3, 28.2)i | 11.6 (8.6, 14.6)j | 10.0 (7.6, 12.4)j | 29.6 (27.1, 32.2)h | 15.7 (11.8, 20.4)i | 10.0 (8.1, 12.3)j |
Values are percentages (95% CIs) and median usual folic acid and vitamin B-12 intakes (25th and 75th percentiles). Usual folic acid and vitamin B-12 intake were estimated using PC-SIDE (Department of Statistics, Iowa State University) with jackknife replicate weights after adjusting for intake day and day of the week. Analyses were weighted to account for the complex sampling design of NHANES and excluded participants with incomplete dietary and supplement use data, as well as those with liver disease, renal dysfunction, heavy alcohol use, and treatment of anemia <3 mo before survey participation. ECGP, enriched cereal grain product; RSE, relative standard error; RTE, ready-to-eat cereal; SUP, supplement containing folic acid.
Participants were categorized into 4 folic acid intake groups: 1) ECGP-only group consumed enriched cereal grain products only, excluding ready-to-eat cereals and supplements containing folic acid; 2) ECGP + RTE group consumed enriched cereal grain products plus ready-to-eat cereals; 3) ECGP + SUP group consumed enriched cereal grain products plus supplements containing folic acid; 4) ECGP + RTE + SUP group consumed enriched cereal grain products, ready-to-eat cereals, and supplements containing folic acid. Values across folic acid intake groups within a row are significantly different and marked by different superscript letters (a < b < c < d) at P < 0.010 (z test) and (e < f < g) at P < 0.015 (z test). Values across folic acid intake groups within a row are significantly different and marked by different superscript letters (h > i > j) at P < 0.009 using multinomial logistic regression adjusting for age, race/ethnicity, BMI, and C-reactive protein.
Participants were also categorized into 3 categories by the amount of daily folic acid reportedly consumed from supplements: 0 µg/d (non-users of supplements or users of supplements containing no folic acid), 1–200 µg/d, and >200 µg/d. Values across folic acid supplement intake groups within a row are significantly different and marked by different superscript letters (a < b < c) at P < 0.010 (z test). Values across folic acid supplement intake groups within a row are significantly different and marked by different superscript letters (h > i > j) at P < 0.03 using multinomial logistic regression adjusting for age, race/ethnicity, BMI, and C-reactive protein.
Estimates with relative standard error (RSE) ≥30% were considered statistically unreliable and reported for RSE = 30–39%.
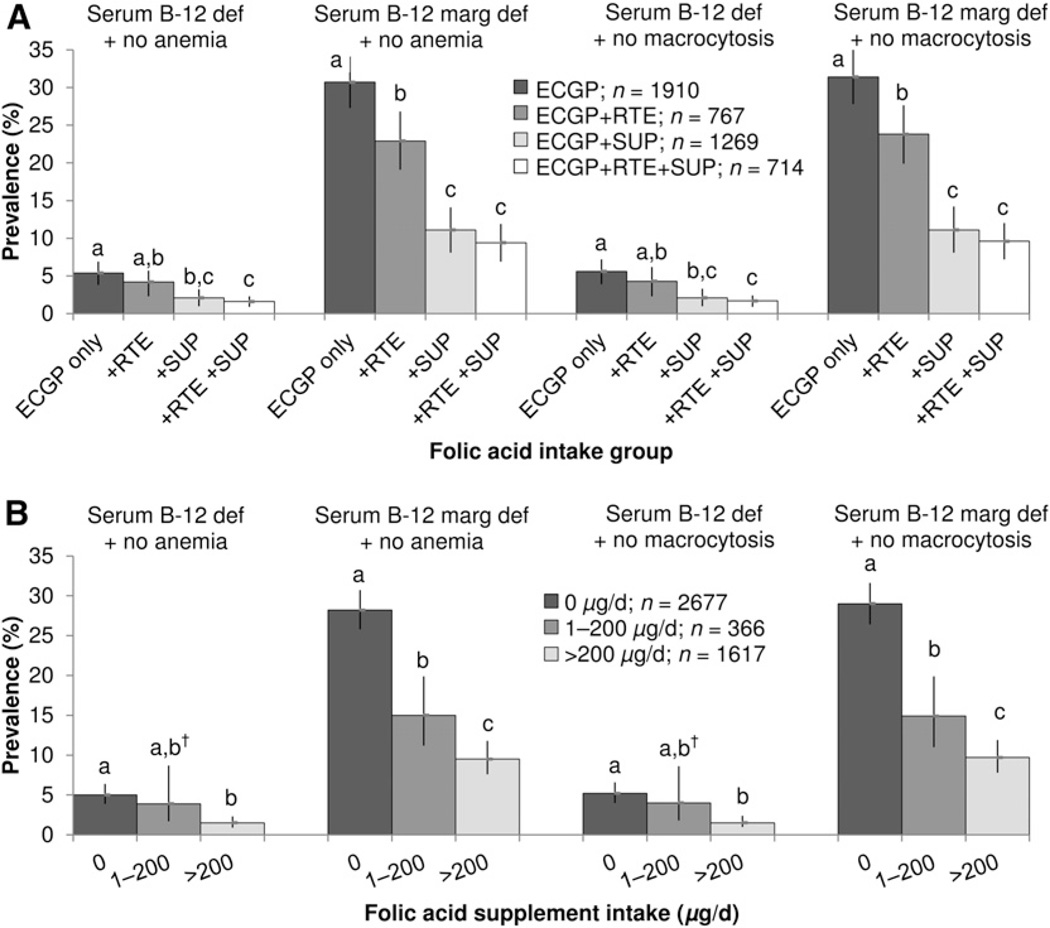
Analyses by source of folic acid intake in the postfortification period showed that consuming additional sources of folic acid was associated with a lower prevalence of serum vitamin B-12 deficiency in the absence of anemia (Fig. 2A; ECGP only: 5.4%; ECGP + RTE: 4.2%; ECGP + SUP: 2.1%; ECGP + RTE + SUP: 1.6%) or macrocytosis (Fig. 2A; ECGP only: 5.6%; ECGP + RTE: 4.3%; ECGP + SUP: 2.1%; ECGP + RTE + SUP: 1.7%). Compared with the ECGP-only group, those consuming ECGP + RTE + SUP were ~70% less likely to have serum vitamin B-12 deficiency without anemia or without macrocytosis (P = 0.0001 for both). Analogous results were observed for serum vitamin B-12 marginal deficiency without anemia (Fig. 2A; ECGP only: 30.7%; ECGP + RTE: 22.9%; ECGP + SUP: 11.1%; ECGP + RTE + SUP: 9.4%) or without macrocytosis (Fig. 2A; ECGP only: 31.4%; ECGP + RTE: 23.8%; ECGP + SUP: 11.1%; ECGP + RTE + SUP: 9.6%).
FIGURE 2.
Postfortification prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis among U.S. adults aged >50 y in NHANES by folic acid intake group (A) and folic acid supplement intake group (B). Bars denote 95% CIs. Estimates with RSEs ≥30% were considered statistically unreliable and were reported with a dagger for RSE = 30–39%. Values across folic acid intake groups and folic acid supplement intake groups are significantly different and marked by different superscript letters (a > b > c) at P < 0.04 using multinomial logistic regression adjusting for age, race/ethnicity, BMI, and C-reactive protein. Participants with liver disease, renal dysfunction, heavy alcohol use, and treatment of anemia <3 mo before survey participation were excluded from the analyses. ECGP, enriched cereal grain product; RSE, relative standard error; RTE, ready-to-eat cereal; serum B-12 def + no anemia, serum vitamin B-12 <148 pmol/L without anemia; serum B-12 def + no macrocytosis, serum vitamin B-12 <148 pmol/L without macrocytosis; serum B-12 marg def + no anemia, serum vitamin B-12 of 148–258 pmol/L without anemia; serum B-12 def + no macrocytosis, serum vitamin B-12 of 148–258 pmol/L without macrocytosis; SUP, supplement containing folic acid; 0 µg/d, non-users and users of supplements containing no folic acid.
Analyses by folic acid supplement use showed that increasing consumption of supplemental folic acid after fortification was associated with a lower prevalence of serum vitamin B-12 deficiency without anemia (Fig. 2B; 0 µg/d: 5.0%; 1–200 µg/d: 3.9%; >200 µg/d: 1.5%) or without macrocytosis (Fig. 2B; 0 µg/d: 5.2%; 1–200 µg/d: 4.0%; >200 µg/d: 1.5%). Compared with those who consumed no folic acid from supplements, those who consumed >200 µg/d folic acid from supplements had significantly lower prevalence of serum vitamin B-12 deficiency and marginal deficiency without anemia or without macrocytosis (P < 0.0001). Likewise, similar results were observed for marginal deficiency without anemia (Fig. 2B; 0 µg/d: 28.2%; 1–200 µg/d: 15.0%; >200 µg/d: 9.5%) or without macrocytosis (Fig. 2B; 0 µg/d: 29.0%; 1–200 µg/d: 14.9%; >200 µg/d: 9.7%).
Discussion
Examining the hematologic presentation of serum vitamin B-12 deficiency before and after folic acid fortification is one way to evaluate the influence of fortification on those with low vitamin B-12 status. Our study is the first to assess this issue among nationally representative older U.S. adults. In our analyses of cross-sectional NHANES data, we found no evidence that the prevalence of serum vitamin B-12 deficiency or marginal deficiency in the absence of anemia or macrocytosis was higher after fortification compared with before fortification among older U.S. adults.
Our results are in agreement with previous reports from both voluntary and mandatory fortification settings which found no changes in anemia indicators and no evidence of deteriorating vitamin B-12 status among those with improved folate status (16,17). Our analyses are also consistent with a U.S. Veterans Affairs hospital-based report which found no significant differences in the proportion of adults with low serum vitamin B-12 (<258 pmol/L) without anemia from before to after fortification (14). Results from that report remained unchanged even after restricting the analyses to older adults (>60 y) or using a more conservative cutoff for low serum vitamin B-12 status (<150 pmol/L).
Our findings were not consistent with those from a different U.S. hospital-based study, in which the proportion of adults with low serum vitamin B-12 without macrocytosis were higher after fortification (18). However, differences in sampling and population characteristics might have accounted for much of the divergent findings between the 2 studies. For example, whereas our study examined a nationally representative sample of adults aged >50 y, the hospital-based study examined a convenience sample of individuals (fortification period: before, n = 86; peri, n = 138; after, n = 409) with racial/ethnic distribution, age, and health profiles that potentially differ from the general population (18). Furthermore, due to the lack of blood folate data, an overall improvement in population folate status (i.e., lower folate deficiency anemia) could not be ruled out in that study to explain the MCV improvements from before to after fortification.
In our study, we also observed that postfortification participants with higher usual intakes of folic acid (i.e., ECGP + RTE, ECGP + SUP, and ECGP + RTE + SUP groups or 1–200 and >200 µg/d groups) were less likely to have low serum vitamin B-12 status without anemia or without macrocytosis when compared with those with lower folic acid intake (i.e., ECGP alone or no folic acid from supplements). Because most RTEs and supplements containing folic acid also have added vitamin B-12 (10,43), older adults who are able to absorb the vitamin normally would have a higher vitamin B-12 intake and better vitamin B-12 status from consuming these products. Consistent with previously published NHANES data (10), our findings confirm that, as folic acid intake increased among older adults, usual vitamin B-12 intake and vitamin B-12 supplement use also increased, and the prevalence of serum vitamin B-12 deficiency and marginal deficiency were lower. It should be noted that individuals with PA would be unable to obtain sufficient vitamin B-12 from the low dosages typically found in RTEs and supplements and thus would require high pharmacologic doses of the vitamin to achieve adequate vitamin B-12 status.
Major strengths of our study include the large sample size available for analysis and that it was nationally representative of the U.S. population aged >50 y. The vast amount of data collected by NHANES allowed us to adjust for potential confounders (i.e., vitamin B-12 supplement use, markers for inflammation) and exclude from the analyses individuals with conditions (i.e., heavy alcohol use, liver disease) that could affect vitamin B-12, hemoglobin, and MCV values but were not ruled out in previous published analyses.
Several limitations should be considered when interpreting the results of our study. First, our analyses are based on comparisons of group-level cross-sectional data before and after fortification; thus, causal inferences with respect to changes in vitamin B-12 deficiency status cannot be made at the level of the individual. However, because controlled trials with folic acid among vitamin B-12–deficient populations would be unethical, comparisons of population prevalence across time periods are a valuable resource for assessing the influence of fortification on vitamin B-12 status. A similar approach has been used to examine potential changes in vitamin B-12 status associated with mandatory folic acid fortification in smaller non-representative samples (14,17,18). Second, because anemia and macrocytosis could only be defined as concurrently occurring with, rather than resulting from, low serum vitamin B-12 status, some misclassification might have occurred, but the exclusion of individuals with heavy alcohol use, liver disease, renal disease, and recent treatment for anemia from our analyses would limit the misclassification based on these other causes of anemia or macrocytosis. It should be noted that, by excluding individuals with heavy alcohol use, we might have also excluded some at higher risk of PA attributable to gastric atrophy from chronic alcohol use. Third, we did not include cognitive/neurologic function data from NHANES as outcomes in our analyses because of their lack of sensitivity and specificity to vitamin B-12 deficiency. Fourth, using solely serum vitamin B-12 <148 pmol/L to define deficiency might not be adequate (44). A recent expert roundtable discussion on vitamin B-12 biomarkers in NHANES recommended that biochemical vitamin B-12 deficiency be defined using one biomarker that directly measures vitamin B-12 in blood (i.e., serum vitamin B-12 or holotranscobalamin) and one functional indicator (i.e., methylmalonic acid or total homocysteine) to minimize sensitivity/specificity issues with using any individual marker alone (45). However, the use of different cutoffs and their combinations resulted in widely different prevalence estimates of low vitamin B-12 status and suggested that “[t]he most appropriate cutoffs for assessing vitamin B-12 status when using NHANES data remain elusive” (46). The lack of a formal method to compare the different methylmalonic acid assays in NHANES III Phase 2 and NHANES 2001–2004 (not measured in 2005–2006) also precluded the use of this functional indicator in our analyses (47). Fifth, self-reported folic acid and vitamin B-12 intakes in the dietary recalls and supplement use data might differ from actual intakes and fail to show patterns of irregular supplement consumption. Sixth, the amount of folic acid and vitamin B-12 reported in the nutritional database might be different from the actual amount found in foods. Last, despite the large study sample, the sample sizes were still small among some subgroups, resulting in unstable estimates.
In conclusion, we examined the hematologic presentation of serum vitamin B-12 deficiency before and after folic acid fortification and found no evidence of a higher prevalence of serum vitamin B-12 deficiency or marginal deficiency in the absence of anemia or macrocytosis among nationally representative U.S. adults aged >50 y after mandatory folic acid fortification. In the time period after fortification, we did not observe a higher prevalence of serum vitamin B-12 deficiency or marginal deficiency in the absence of anemia or macrocytosis with higher folic acid intake from foods and supplements. Better serum vitamin B-12 status was seen among adults with higher folic acid intake, which might reasonably be explained by higher intakes of vitamin B-12 also found in fortified foods and supplements (10). Our findings provide reassurance and help assuage concerns that exposure to higher levels of folic acid in fortified foods and supplements would adversely affect the clinical presentation of vitamin B-12 deficiency at the population level and delay its diagnosis. More importantly, advancements in medical and laboratory technology have allowed for the identification of vitamin B-12 deficiency specifically without having to rely solely on hematologic indices. The routine use of these diagnostic tools along with efforts to educate clinicians about the risk factors and varied clinical presentations of vitamin B-12 deficiency will allow for the timely diagnosis and treatment of this condition and the prevention of complications related to untreated vitamin B-12 deficiency.
Supplementary Material
Acknowledgments
We thank Godfrey P. Oakley Jr. (Department of Epidemiology, Emory University, Atlanta, GA) for his helpful comments and review of the manuscript and Mary E. Cogswell (National Center for Chronic Disease and Health Promotion, CDC, Atlanta, GA) for her advice on NHANES data analysis. Y.P.Q., A.N.D., C.M.P., and R.J.B. designed research; Y.P.Q. analyzed data and wrote the paper. Y.P.Q., A.N.D., C.M.P., H.C.H., and R.J.B. were involved in data interpretation and the critical revision of the manuscript for important intellectual content. Y.P.Q. had primary responsibility for final content. All authors have read and approved the final manuscript.
Footnotes
Supported by the CDC and in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. Findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC.
Author disclosures: Y. P. Qi, A. N. Do, H. C. Hamner, C. M. Pfeiffer, and R. J. Berry, no conflicts of interest.
Supplemental Table 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org
Abbreviations used: aPR, adjusted prevalence ratio; CRP, C-reactive protein; ECGP, enriched cereal grain product; MCV, mean cell volume; PA, pernicious anemia; RSE, relative standard error; RTE, ready-to-eat cereal; SUP, supplement containing folic acid.
Literature Cited
- 1.Chanarin I. Historical review: a history of pernicious anaemia. Br J Haematol. 2000;111:407–415. doi: 10.1046/j.1365-2141.2000.02238.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffbrand AV, Weir DG. The history of folic acid. Br J Haematol. 2001;113:579–589. doi: 10.1046/j.1365-2141.2001.02822.x. [DOI] [PubMed] [Google Scholar]
- 3.Savage DG, Linedenbaum J. Folate-coblamin interactions. In: Bailey LB, editor. Folate in health and disease. New York: Marcel Dekker; 1995. pp. 237–285. [Google Scholar]
- 4.Vilter CF, Vilter RW, Spies TD. The treatment of pernicious and related anemias with synthetic folic acid; observations on the maintenance of a normal hematologic status and on the occurrence of combined system disease at the end of one year. J Lab Clin Med. 1947;32:262–273. [PubMed] [Google Scholar]
- 5.Ross JF, Belding H, Paegel BL. The development and progression of subacute combined degeneration of the spinal cord in patients with pernicious anemia treated with synthetic pteroylglutamic (folic) acid. Blood. 1948;3:68–90. [PubMed] [Google Scholar]
- 6.Dickinson CJ. Does folic acid harm people with vitamin B-12 deficiency? QJM. 1995;88:357–364. [PubMed] [Google Scholar]
- 7.Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine. 1991;70:229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Dudley GM, Coltman CA., Jr Resolution of ineffective erythropoiesis of pernicious anemia and “strongly suggestive” folate lack in response to folic acid. Am J Clin Nutr. 1970;23:147–155. doi: 10.1093/ajcn/23.2.147. [DOI] [PubMed] [Google Scholar]
- 9.Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J, Kaye K, Lindenbaum J, Stabler SP. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–1204. [PubMed] [Google Scholar]
- 10.Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, Pfeiffer CM, Berry RJ. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Clin Nutr. 2010;92:1001. doi: 10.3945/ajcn.2009.28401. Corrected and republished from: Am J Clin Nutr. 2010;91:64–72. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Food labeling: health claims and label statements; folate and neural tube defects. Fed Regist. 1996;61:8752–8781. [Google Scholar]
- 12.Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; folic acid (folacin) Fed Regist. 1996;61:8797–8807. [PubMed] [Google Scholar]
- 13.Scientific Committee on Food. Opinion of the scientific committee on food on the tolerable upper intake level of folate. Brussels (Belgium): European Commission; 2000. [Google Scholar]
- 14.Mills JL, Von Kohorn I, Conley MR, Zeller JA, Cox C, Williamson RE, Dufour DR. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am J Clin Nutr. 2003;77:1474–1477. doi: 10.1093/ajcn/77.6.1474. [DOI] [PubMed] [Google Scholar]
- 15.Ray JG, Vermeulen MJ, Langman LJ, Boss SC, Cole DE. Persistence of vitamin B-12 insufficiency among elderly women after folic acid food fortification. Clin Biochem. 2003;36:387–391. doi: 10.1016/s0009-9120(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 16.Metz J, McNeil AR, Levin M. The relationship between serum cobalamin concentration and mean red cell volume at varying concentrations of serum folate. Clin Lab Haematol. 2004;26:323–325. doi: 10.1111/j.1365-2257.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, West R, Randell E, Longerich L, O’Connor KS, Scott H, Crowley M, Lam A, Prabhakaran V, McCourt C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth. 2004;4:20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyckoff KF, Ganji V. Proportion of individuals with low serum vitamin B-12 concentrations without macrocytosis is higher in the post folic acid fortification period than in the pre folic acid fortification period. Am J Clin Nutr. 2007;86:1187–1192. doi: 10.1093/ajcn/86.4.1187. [DOI] [PubMed] [Google Scholar]
- 19.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010;91:1733–1744. doi: 10.3945/ajcn.2009.28671. [DOI] [PubMed] [Google Scholar]
- 21.Yeung L, Yang Q, Berry RJ. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA. 2008;300:2486–2487. doi: 10.1001/jama.2008.742. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. National Health and Nutrition Examination Survey questionnaires, datasets, and related documentation. [cited 2012 April 4]; Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 23.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 24.Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225–234. doi: 10.1053/j.seminhematol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 26.Landry D, Bazari H. Goldman’s Cecil Medicine. In: Goldman L, Schafer A, editors. Approach to the patient with renal disease. 24th ed. Philadelphia: Saunders Elsevier; 2011. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Alcohol and public health. [cited 2012 April 4]; Available from: http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- 28.World Health Organization Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 29.Chanarin I. The megaloblastic anemias. 2nd ed. Oxford, UK: Blackwell Scientific; 1979. [Google Scholar]
- 30.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology. 2003:62–81. doi: 10.1182/asheducation-2003.1.62. [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–1647. [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Food standards: amendment of standards of indentity for enriched grain products to require addition of folic acid, final rule. 21 CFR Parts 136, 137, and 139. Fed Regist. 1996;61:8781–8797. [Google Scholar]
- 35.Institute of Medicine. DRI dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate vitamin B-12, pantothenic acid, biotin and choline. Washington: National Academy Press; 1998. [PubMed] [Google Scholar]
- 36.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019–1025. doi: 10.1016/s0736-4679(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 37.Cusick SE, Mei Z, Freedman DS, Looker AC, Ogden CL, Gunter E, Cogswell ME. Unexplained decline in the prevalence of anemia among US children and women between 1988–1994 and 1999–2002. Am J Clin Nutr. 2008;88:1611–1617. doi: 10.3945/ajcn.2008.25926. [DOI] [PubMed] [Google Scholar]
- 38.Research Triangle Institute. SUDAAN Language Manual, Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 39.National Center for Health Statistics. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey, NHANES III (1988–1994) [cited 2012 April 4]; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 40.National Center for Health Statistics. Analytic and reporting guidelines. The National Health and Nutrition Examination Survey (NHANES) [cited 2012 April 4]; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.
- 41.Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr. 1997;127:1106–1112. doi: 10.1093/jn/127.6.1106. [DOI] [PubMed] [Google Scholar]
- 42.Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr. 2003;133:601S–608S. doi: 10.1093/jn/133.2.601S. [DOI] [PubMed] [Google Scholar]
- 43.Berry RJ, Carter HK, Yang Q. Cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;86:265–267. doi: 10.1093/ajcn/86.1.265. author reply 7–9. [DOI] [PubMed] [Google Scholar]
- 44.Stabler SP. Screening the older population for cobalamin (vitamin B-12) deficiency. J Am Geriatr Soc. 1995;43:1290–1297. doi: 10.1111/j.1532-5415.1995.tb07408.x. [DOI] [PubMed] [Google Scholar]
- 45.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94:313S–321S. doi: 10.3945/ajcn.111.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94:552–561. doi: 10.3945/ajcn.111.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–450. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.