Abstract
Johne's disease of cattle is widespread and causes significant economic loss to producers. Control has been hindered by limited understanding of the immune response to the causative agent, Mycobacterium avium subsp. paratuberculosis, and lack of an effective vaccine and sensitive specific diagnostic assays. The present study was conducted to gain insight into factors affecting the immune response to M. avium subsp. paratuberculosis. A persistent proliferative response to M. avium subsp. paratuberculosis purified protein derivative and soluble M. avium subsp. paratuberculosis antigens was detected in orally infected neonatal calves 6 months postinfection (p.i.) by flow cytometry (FC). CD4+ T cells with a memory phenotype (CD45R0+) expressing CD25 and CD26 were the predominant cell type responding to antigens. Few CD8+ T cells proliferated in response to antigens until 18 months p.i. γδ T cells did not appear to respond to antigen until 18 months p.i. The majority of WC1+ CD2− and a few WC1− CD2+ γδ T cells expressed CD25 at time zero. By 18 months, however, subsets of γδ T cells from both control and infected animals showed an increase in expression of CD25, ACT2, and CD26 in the presence of the antigens. Two populations of CD3− non-T non-B null cells, CD2+ and CD2−, proliferated in cell cultures from some control and infected animals during the study, with and without antigen. The studies clearly show multicolor FC offers a consistent reliable way to monitor the evolution and changes in the immune response to M. avium subsp. paratuberculosis that occur during disease progression.
Johne's disease, paratuberculosis, is a chronic wasting disease of the intestine caused by Mycobacterium avium subsp. paratuberculosis. It causes significant economic loss to producers, especially the dairy industry, due to increase in forage consumption, decreased milk production, and early culling due to poor health of affected animals (reviewed in reference 51). The disease has been difficult to control because of the lack of sensitivity and specificity of available diagnostic assays (5) and the lack of an efficacious vaccine (4, 33, 37). Available assays, such as M. avium subsp. paratuberculosis antigen enzyme-linked immunosorbent assays (ELISAs) and the gamma interferon (IFN-γ) assays, vary in their capacity to detect infected animals in the early stages of the disease (reviewed in reference 51). Available vaccines have been shown to reduce the severity of pathology but not stop shedding of bacteria (reviewed in reference 33). Consequently, there is both a need to develop better diagnostic assays and a vaccine that, at a minimum, stops shedding during the productive life of dairy cattle. At this juncture, information remains limited on the factors affecting the immune response to M. avium subsp. paratuberculosis, and it is unlikely that it will be possible to make progress on the development of a vaccine until the immune response to M. avium subsp. paratuberculosis is better understood. Studies to date have shown the time course of disease is characterized by a long latency period (2 to 5 years) with no clinical signs of disease. No defined time point has been determined during this latency period when immune control of infection begins to deteriorate and animals begin shedding bacteria. It is thought that loss of immune control is associated with a depression of cell-mediated immunity (CMI) and an increase in the antibody response to M. avium subsp. paratuberculosis antigens. Comparison of the tissue distribution and functional status of lymphocyte subsets in animals at different stages of disease supports this supposition (38). Peripheral and intestinal CMI responses were decreased in symptomatic compared to asymptomatic animals (38). Disease progression appeared to be associated with local loss of CD4+ T cells and an increase in γδ T cells (11, 38). The underlying defect that could be associated with a depression in CMI is dysregulation of macrophage function mediated by M. avium subsp. paratuberculosis (56, 61, 65). Disruption of gene activation and signaling through genes encoding tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-12, and IFN-γ and their receptors could lead to a breakdown in the immune response controlling infection (27, 36, 46). Studies with genetically susceptible and knockout mice with defects in αβ and γδ T-cell receptors and defects in CD4 and CD8 coreceptors have shown that CD4+ T cells with a type 1 cytokine profile (8) play a critical role in maintenance of protection (38). The effector mechanisms used by CD4+ T cells include secretion of IFN-γ, which activates bactericidal activity in macrophages, and secretion of lymphotoxin, perforin, and granulysin (10, 52). Disruption could also affect effector activity of CD8+ T cells (10).
To gain further insight into the factors affecting the immune response to M. avium subsp. paratuberculosis, it will be essential to use experimentally infected cattle where the evolution of an immune response can be monitored from the time of first exposure to the time of shedding and progression to clinical disease. One question that must be answered to make this approach successful, however, is whether animals can be uniformly infected by the oral route or whether it will be necessary to directly inoculate animals to ensure they become infected. An initial study has clearly shown direct inoculation of a large dose of M. avium subsp. paratuberculosis into the tonsils leads to infection and the appearance of humoral and lymphoproliferative responses to M. avium subsp. paratuberculosis antigens (60). An antibody response was detected to two proteins (∼50 and ∼60 kDa) within 7 to 14 days of exposure and by 134 days of exposure to lipoarabinomannan (LAM). A persistent lymphoproliferative response to LAM and soluble M. avium subsp. paratuberculosis antigens was evident by 5 to 6 months. CD4+ T cells with a memory phenotype (CD45R0+) that expressed an activation molecule (CD26) were the predominant cell type responding to both antigens. IFN-γ response above control values was evident by 194 days, while a nitric oxide response in macrophages was evident by 160 days.
The present study was conducted to determine if animals can also be uniformly infected by the oral route and whether flow cytometry (FC) can be used, in conjunction with commercial M. avium subsp. paratuberculosis antibody ELISAs, IFN-γ assays, and fecal sampling, to monitor the evolution of the immune response to M. avium subsp. paratuberculosis antigens during the early and late stages of infection. As reported here, high-dose exposure by the oral route also leads to uniform infection and the appearance of a lymphoproliferative response to M. avium subsp. paratuberculosis antigens with similar kinetics to those noted in animals directly inoculated with M. avium subsp. paratuberculosis. The commercial ELISAs remained negative during the course of the study. The IFN-γ assay yielded inconsistent results. Fecal shedding was not detected at the time of writing of this report.
MATERIALS AND METHODS
Animals.
Three newborn Holstein calves were obtained from the Washington State University M. avium subsp. paratuberculosis-free dairy herd for the present study. The five newborn calves experimentally infected with M. avium subsp. paratuberculosis were obtained from a dairy in the middle of the state. The calves were given daily doses of M. avium subsp. paratuberculosis (107 bacteria) by the oral route for 7 days, starting at 24 to 48 h after birth. The calves were housed and maintained according to the Institutional Animal Care and Use committee guidelines and the Association for Assessment and Accreditation of Laboratory Animal Care. The three M. avium subsp. paratuberculosis-free animals were housed separately and served as controls.
MHC typing.
Major histocompatibility complex (MHC) typing was performed by hybridization of biotinylated, genomic PCR products from MHC class I, DRB3, and DQA genes to short, 15- to 22-bp, oligonucleotide microarrays as previously described (47). The only significant modification from our previously published procedure was that instead of using heminested PCR for the class I and DRB3 genes, all of the biotinylated PCR products were generated by single-round amplification. Data were interpreted using Cytofile matrix analysis software (15). Although the class I typing was performed by microarray hybridization, the serological names have been used to denote the haplotypes (Table 1). The class II haplotypes are indicated using the D-region haplotype nomenclature (16, 42, 47, 53). The official allele names assigned by the Bovine Leukocyte Antigen Nomenclature Committee of the International Society for Animal Genetics were used for the class II alleles present in each haplotype (14, 54).
TABLE 1.
BoLA typing results
| Steer | Class I haplotypea | Class IIa haplotypeb | DRB3 allelec | DQA1 allelec | DQA2 allelec | DQB allelesc |
|---|---|---|---|---|---|---|
| C1 | A11 | DH24A | 0101d | 0101d | 0101d | |
| A13 | DH23A | 2703d | 0101d | 22031d | 0103d + 1803d | |
| C2 | A15 (A8) | DH22H | 1101d | 10011d | wsu2-2e | 1002d + 1402d |
| A20 | DH08A | 1201d | 12011d | 2201d | 1005 + 1201d | |
| C3 | A14 (A8) | DH11A | 0902d | 0204d | 0301 | |
| A20 | DH08A | 1201d | 12011d | 2201d | 1005 + 1201d | |
| 58 | A12 (A30) | DH16A | 1501d | 10011d | 22021d | 0102 + 1101 |
| A15 (A8) | DH22H | 1101d | 10011d | wsu2-2e | 1002d + 1402d | |
| 59 | A14 (A8) | DH27A | 14011d | 1401d | 1401d | |
| Blank | New | 3701f | NDg | ND | ND | |
| 61 | A13 | DH23A | 2703d | 0101d | 22031d | 0103d + 1803d |
| A14 (A8) | DH11A | 0902d | 0204d | 0301 | ||
| 62 | A12 (A30) | DH16A | 1501d | 10011d | 22021d | 0102 + 1101 |
| A14 (A8) | DH11A | 0902d | 0204d | 0301 | ||
| 63 | w44 | DH27A | 14011d | 1401d | 1401d |
Although the class I typing was done by microarray hybridization, the serological names have been used for the class I haplotypes (14).
Exon 2 sequence for this haplotype was confirmed at Washington State University (J. Y. Park, J. Norimine, and C. J. Davies, unpublished data).
New allele that does not yet have an official name from the BoLA Nomenclature Committee (J. Norimine and C. J. Davies, unpublished data).
The class II alleles from this haplotype have not been sequenced. This allele was assigned on the basis of microarray typing and needs confirmation.
ND, not determined.
Blood processing for tissue culture and FC.
Peripheral blood mononuclear cells (PBMC) used in cultures were obtained from buffy coat fractions of blood collected in acid citrate dextrose (ACD) separated by density gradient centrifugation with Accu-Paque (density, 1.086; Accurate Chemical & Scientific Corp., Westbury, N.Y.). Residual erythrocytes were lysed with H2O. Dead cells and debris were removed from cultured cells by density gradient centrifugation before use in FC.
For analysis of the composition of peripheral blood at the initiation of culture, 10 ml of blood was collected in ACD and lysed in Tris-buffered NH4Cl to remove erythrocytes. The resultant cells were washed several times in phosphate-buffered saline (PBS) containing 20% ACD (PBS-ACD) to remove excess platelets and then used in FC as described elsewhere (18).
Antigens.
M. avium subsp. paratuberculosis purified protein derivative (PPD) Johnin was obtained from the National Veterinary Service Laboratory. Live bacteria used in the study and soluble antigen (SAg) were prepared from a field isolate (8544) used in previous studies (29). M. avium subsp. paratuberculosis was grown as previously described (60) and harvested by centrifugation at 1,500 × g for 10 min at 4°C, washed three times in PBS, suspended in PBS (pH 7.4) containing 0.01% Tween 80, and irradiated with exposure to a 60Co source for 26 h, using the fixed tube at a distance of 5.5 in. from base, in a Plexiglas rack (2.5 Mrad). The mycobacterial concentration was determined spectrophotometrically as that producing an optical density at 600 nm of 0.2 of M. avium subsp. paratuberculosis cells, approximately 108/ml. Irradiated cells were sonicated at the settings of output control 6, duty cycle 60 continuous, using a cup sonicator three times for 3 min, and with a 3-min rest on ice between rounds of sonication. Soluble M. avium subsp. paratuberculosis antigen was prepared by vortexing M. avium subsp. paratuberculosis cells mixed with the same amount (vol/vol) of zirconia-silica beads (Biospec Products, Inc., Bartlesville, Okla.) with three to five cycles of 2 min, with 1 min rest on ice in between cycles, and harvesting supernatant following centrifugation at 4°C at maximum speed. Phenylmethylsulfonyl fluoride was added to soluble M. avium subsp. paratuberculosis antigen at a final concentration of 1 mM to prevent protein degradation. Protein concentration of SAg was calculated by measuring the optical density at 280 nm.
Cell culture and IFN-γ ELISA.
PBMC were resuspended in RPMI 1640 medium supplemented with 13% bovine calf serum, 2-mercaptoethanol, and antibiotics and then distributed in T75 tissue culture flasks (3 × 106 cells/ml). Two flasks each were stimulated with M. avium subsp. paratuberculosis PPD (20 μg/ml; RPMI 1640 complete medium) or SAg (4 μg/ml). Two additional flasks were cultured without stimulation. At 6 or 7 days poststimulation, the cultures were collected and subjected to density gradient centrifugation to remove dead cells and then labeled for FC as described elsewhere (18). Medium from the cultures was collected after 1, 3, and 6 days of culture to assay for the presence of IFN-γ.
Whole blood cultures were set up with fresh heparinized blood according to the protocol recommended in the BOVIGAM IFN-γ test kit (CSL Veterinary, Parkville, Victoria, Australia) to test for the presence of IFN-γ after 24 h of culture. Two milliliters of blood was dispensed into each well of a 24-well tissue culture plate. Antigen (40 μg of commercial Johnin PPD) was added to triplicate wells for stimulation. Wells without antigen were used as controls. At 24 h, 100 μl of supernatant plasma or cultured medium was collected by centrifugation and tested for the presence of IFN-γ by using the BOVIGAM bovine IFN-γ test kit according to the protocols recommended by the manufacturer.
ELISA for antibody to M. avium subsp. paratuberculosis.
Serological responses were evaluated by using commercially available PARACHEK Johne's absorbed ELISA (CSL Veterinary) with absorbed serum and HerdChek M. paratuberculosis according to the protocols recommended by the manufacturer.
Serum samples were also submitted to the diagnostic laboratory for evaluation with an antibody ELISA (IDEXX Labs., Westbrook, Maine).
Detection of M. avium subsp. paratuberculosis with RT-PCR.
Cecal lymph nodes were obtained at 1 year postinfection to test for the presence of M. avium subsp. paratuberculosis by reverse transcription-PCR (RT-PCR). Total RNA from bovine cecal lymph nodes was prepared using TRIzol reagent (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. The first-strand cDNA was prepared using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen) with oligo(dT)12-18 as a primer. The PCR mixture contained 1× PCR buffer, a 0.2 mM concentration of each deoxynucleoside triphosphate, a 0.1 μM concentration each of the IS900-specific forward (5′-CCGCTAATTGAGAGATGCGATTGG-3′) and reverse (5′-AATCAACTCCAGCAGCGCGGCCTCG-3′) primers, 1.25 U of Taq DNA polymerase (Invitrogen), and 1 μl of first-strand cDNA template. Thermocycler conditions included 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min. Amplification of the 229-bp PCR product was confirmed by electrophoresis in 2% agarose gel.
FC.
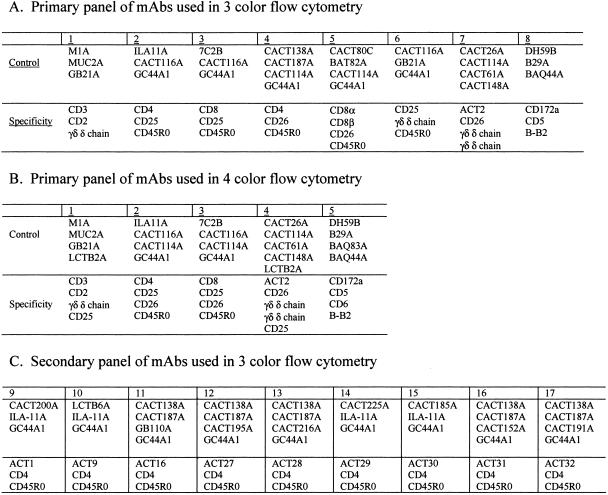
The monoclonal antibodies (MAbs) used in the present study are shown in Table 2. The combinations of MAbs used in the three- and four-color analyses are shown in Fig. 1. In some combinations, two MAbs of the same specificity and isotype were used to increase the intensity of the fluorescent signal. MAbs specific for the δ chain of the γδ T-cell receptor were used in combination with MAbs to CD3 and CD2 to distinguish αβ T cells from the CD2+ WC1− and CD2− WC1+ γδ T-cell subsets. WC1 (workshop cluster 1) is a unique molecule expressed on a subset of γδ T cells only found in Artiodactyla (20). Previous studies have shown the WC1− subset is the same as the δ chain+ CD2+ WC1− subset and that the WC1+ subset is the same as the δ chain+ CD2− subset (17). WC1 is used in the text with CD2 when describing results obtained on the CD2+ WC1− and CD2− WC1+ populations of γδ T cells. All MAbs were used at 15 μg/ml. The same combinations of MAbs were used throughout the study. In brief, 106 cells were incubated for 15 min in 96-well conical bottom assay plates in the respective cocktails of MAbs (50 μl of each MAb) in a final concentration of 250 μl of first wash buffer (PBS-20% ACD containing 0.5% horse serum, 0.09% azide, and 0.1% phenol red), washed three times, and then incubated for an additional 15 min in cocktails of isotype-specific second-step reagents conjugated with fluorescein, phycoerythrin, phycoerythrin-Cy5 (Tricolor), or Cy5 (Caltag, Burlingame, Calif., and Southern Biotechnology Associates, Birmingham, Ala.). Following incubation, the cells were washed two times in PBS-ACD without phenol red, fixed in 2% PBS-buffered formaldehyde, and kept in the refrigerator until examined.
TABLE 2.
MAbs used to determine the composition of PBMC at time zero and after 6 to 7 days of culture with antigens
| MAb | Ig isotype | Specificity (reference) |
|---|---|---|
| MM1A | G1 | CD3 (21, 43) |
| MUC2A | G2a | CD2 (35, 39) |
| BAT76A | G2a | CD2 (24, 39) |
| ILA-11A | G2a | CD4 (3) |
| CACT138A | G1 | CD4 (7) |
| CACT187A | G1 | CD4 (this report) |
| B29A | G2a | CD5 (19) |
| BAQ83A | G2b | CD6 (this report) |
| 7C2B | G2a | CD8α? (this report) |
| CACT80C | G1 | CD8α (44) |
| BAT82A | G1 | CD8β (44) |
| GB21A | G2b | γδ T cells δ chain specific (17) |
| CACT61A | M | γδ T cells δ chain specific (17, 48) |
| CACT148A | M | γδ T cells δ chain specific (17) |
| GC44A1 | G3 | CD45R0 (unpublished; reactivity identical to IL-116 [6]) |
| BAQ44A | M | B cells (19, 34) |
| DH59B | G1 | CD172a (2, 22, 57) |
| LCTB2A | G3 | CD25 (23) |
| CACT116A | G1 | CD25 (45) |
| CACT114A | G2b | CD26 (41) |
| CACT200A | G1 | ACT1 (identical to B7A1 [IgM] [23]) |
| CACT26A | G1 | ACT2 (23) |
| LCTB6A | G1 | ACT9 (23) |
| GB110A | M | ACT16 (23) |
| CACT195A | M | ACT27 (this report) |
| CACT216A | M | ACT28 (this report) |
| CACT225A | G1 | ACT29 (this report) |
| CACT185A | G1 | ACT30 (this report) |
| CACT152A | M | ACT31 (this report) |
| CACT191A | M | ACT32 (this report) |
FIG. 1.
Summary of MAb combinations used to analyze the composition of PBMC following culture with PPD and M. avium subsp. paratuberculosis SAg.
A FACSort flow cytometer equipped with argon and red lasers, a Macintosh Quadra computer, and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) was used to collect data. FCS Express software (De Novo Software, Thornton, Ontario, Canada) was used to analyze the data.
Analysis of the proliferative response to M. avium subsp. paratuberculosis antigens.
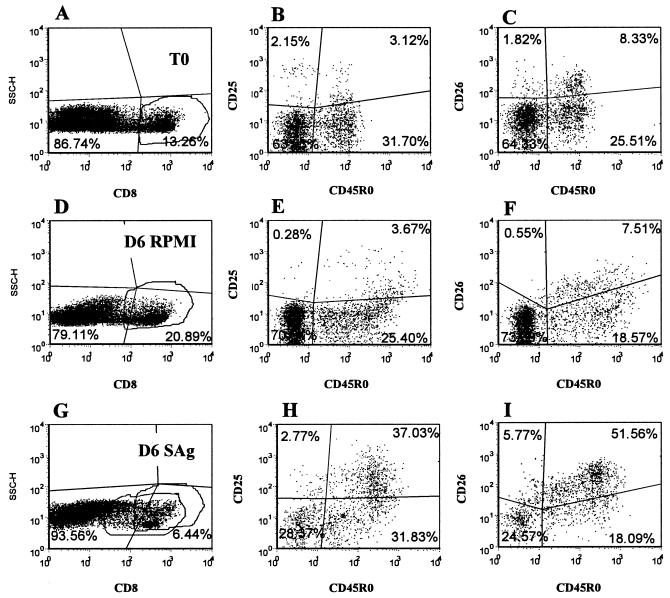
Following oral inoculation, blood was collected every 2 weeks for 2 months, once a month to 14 months, and every 2 months thereafter and processed for cell culture, the IFN-γ assay, and FC. Sera were collected at each time point for later analysis. In the first year, three-color FC was used to collect data using the primary panel of MAbs shown in Fig. 1A. In subsequent studies, four-color FC was used to collect data, using the primary panel of MAbs shown in Fig. 1B. A secondary panel of MAbs was used at 50 weeks postexposure to determine if additional molecules were upregulated on CD4+ T cells stimulated with M. avium subsp. paratuberculosis antigens (Fig. 1C). Selective gating was used to obtain results on specific subpopulations of lymphocytes. For time zero (T0), data were collected on 20,000 cells (see Fig. 2 and 3, below). Granulocytes and nonspecific signal were excluded at the time of data acquisition. Gate 1 was used to define small lymphocytes. Gate 2 was used to define monocytes and large lymphocytes at the start of culture and large lymphocytes undergoing blastogenesis and proliferation on days 6 and 7 of culture (see Fig. 2). For analysis of cultured cells, data were collected with and without additional gates. An additional gate was placed on CD3+ cells at the time of analysis to analyze the composition of αβ and γδ T cells. In the first months of the study, gates were placed on CD4+ and CD8+ T cells at the time of data acquisition because of the low frequency of these subpopulations in unstimulated cultures. A gate was placed on γδ T cells at the time of analysis. Exclusion gates were place on CD3 and CD5 T cells to analyze null cells present in gate 2 (see Fig. 4, below). MAbs specific for CD25 and CD26 were used to monitor the activation status of αβ and γδ T cells and other cells in the cultures (23, 40). An additional MAb was used to monitor the upregulation of a molecule, ACT2, uniquely expressed on activated γδ T cells (Table 2; Fig. 1) (23). Figures 2, 3, and 5 to 7 (below) are representative FC dot plot profiles of cells obtained from an animal that had been infected for 18 months. Figure 4 is a representative set of profiles from a control animal showing the characteristics of CD3− CD2+ and CD3− CD2− populations of null cells that were present in some cultures of cells from control and infected animals. Summary data on the response of control and infected animals to PPD and SAg are shown below in Fig. 8 to 10.
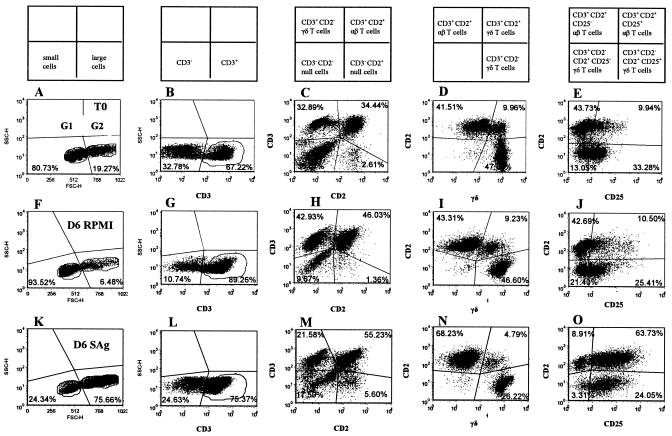
FIG. 2.
Representative dot plot profiles of PBMC labeled with four MAbs (Fig. 1B, MAb set 1) before (T0) and after 6 days of culture in RPMI alone or with M. avium subsp. paratuberculosis SAg. The phenotypes of cell subsets present in each quadrant in the dot plot profiles are shown in boxes above columns A to E. In profiles A, F, and K, the quadrants show the division between small lymphocytes in gate 1 and large cells in gate 2 and the relative frequencies of cells in each gate before and after culture. The profiles B, G, and L, with gates placed only on 1 and 2, show the frequency of CD3+ cells at each time point. The profiles in C, H, and M, with gates placed only on 1 and 2, show the frequency of CD3− CD2− null cells, CD3+ CD2− γδ+ T cells, CD2+ αβ and CD2+ γδ T cells, and CD3− CD2+ null cells. At T0, the CD3− CD2− null population contains CD3− CD2− null cells, B cells, monocytes, and dendritic cells. After 6 to 7 days of culture, this double negative population includes residual B cells and CD3− CD2− null cells. The profiles D, I, and N, with gates on 1, 2, and CD3+ cells, show the frequency of CD3+ αβ T cells, CD2+ WC1− γδ T cells, and CD2− WC1+ γδ T cells. The profiles E, J, and O, with gates on 1, 2, and CD3+ cells, show the frequency of CD2− CD25− γδ T cells, CD2+ CD25− αβ T cells plus CD2+ WC1− γδ T cells, CD2+ CD25+ αβ T cells plus CD2+ WC1− γδ T cells, and CD2− WC1+ CD25+ γδ T cells.
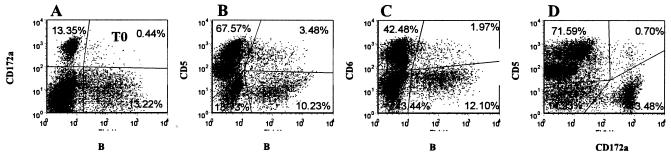
FIG. 3.
Representative dot plot profiles of PBMC labeled with four MAbs at the start of culture (T0) (Fig. 1B, MAb set 5). Data were collected in gates 1 and 2. Profile A, anti-CD172a versus anti-B-B2, shows the frequency of CD172a+ monocytes in the upper left quadrant, CD172a+ B-B2+ cells in the upper right quadrant, and CD172a− B-B2+ cells in the lower right quadrant. Profile B, anti-CD5 versus anti-B-B2, shows the frequency of CD5+ T cells in the upper left quadrant, CD5+ B-B2+ B cells in the upper right quadrant, and CD5− B-B2+ B cells in the lower right quadrant. Profile C, anti-CD6 versus anti-B-B2, shows the frequency of CD6+ T cells in the upper left quadrant, CD6+ B-B2+ cells in the upper right quadrant, and CD6− B-B2+ cells in the lower right quadrant. Profile D, anti-CD5 versus anti-CD172a, shows CD5+ T cells in the upper left quadrant, CD5+ CD172a+ cells in the upper right quadrant, and CD172a+ monocytes in the lower right quadrant.
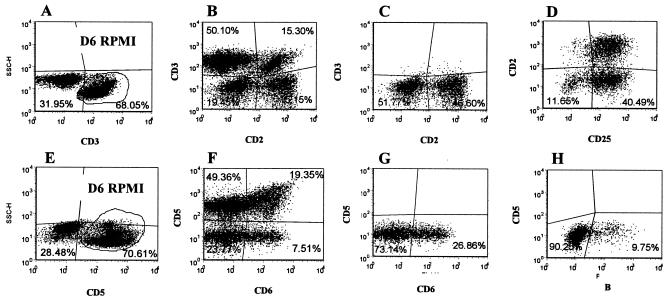
FIG. 4.
Representative dot plot profiles of PBMC from a control animal labeled with four MAbs after 6 days of culture in RPMI (Fig. 1B, MAb sets 1 [A to D] and 5 [E to H]). Dot plot profiles A to D, with selective and exclusion gates on 2 and CD3, show that null cells comprise two unique phenotypically distinct CD3− CD2− and CD3− CD2+ populations of cells. Profile A shows the frequency of CD3− and CD3+ cells present in gate 2. Profile B shows that the cells in gate 2 comprise four populations: CD3− CD2− null cells in the lower left quadrant, CD3+ CD2− γδ T cells in the upper left quadrant, CD3+ CD2+ αβ and γδ T cells in the upper right quadrant, and CD3− CD2+ null cells in the lower right quadrant. Profile C, with an exclusion gate on CD3, shows the frequency of CD3−, CD2− CD3−, and CD2+ populations of null cells in gate 2. Profile D, with an exclusion gate on CD3, shows that both null cell populations, CD3− CD2+ cells in the upper right quadrant and CD3− CD2− cells in the lower right quadrant, express CD25. Dot plot profiles E to H, with selective and exclusion gates on 2 and CD5, show that the two null cell populations do not express CD5 and that a subset of the null cells expresses CD6. Profile E shows the frequency of CD5− and CD5+ cells in gate 2. Profile F shows the cells in gate 2 comprise four populations: CD5− CD6− null cells in the lower left quadrant, CD5+ γδ T cells in the upper left quadrant, CD2+ CD6+ T cells in the upper right quadrant, and CD5− CD6+ null cells in the lower right quadrant. Profile G, with an exclusion gate on CD5, shows the frequency of CD6− and CD6+ null cells in gate 2. Profile H, with an exclusion gate on CD5, shows the frequency of residual B-B2+ B cells present in the culture.
FIG. 5.
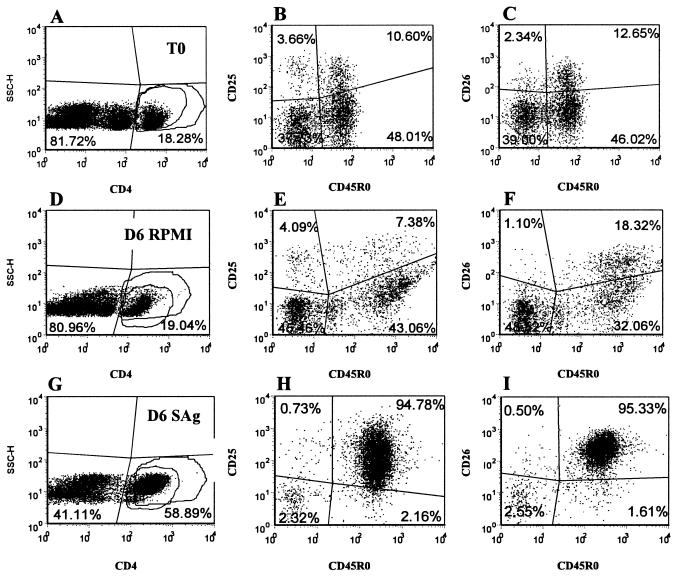
Representative dot plot profiles of CD4+ T cells from an infected animal labeled with four MAbs at T0 and after 6 days of culture in RPMI alone or with SAg (Fig. 1B, MAb set 2). A gate was placed on CD4+ T cells to analyze the expression of CD25 and CD26 on naïve and memory T cells present in gates 1 and 2. Profiles A, D, and G, SSC versus anti-CD4, show the frequency of CD4+ cells in the cell preparations in gates 1 and 2. Profiles B, E, and H, anti-CD25 versus anti-CD45R0, with a gate on CD4, show the frequency of CD25− naïve (lower left quadrant), CD25+ naïve (upper left quadrant), CD25+ memory (upper right quadrant), and CD25− memory (lower right quadrant) T cells. Profiles C, F, and I, anti-CD26 versus anti-CD45R0, with a gate on CD4, show the frequency of CD26− naïve (lower left quadrant), CD26+ naïve (upper left quadrant), CD26+ memory (upper right quadrant), and CD26− memory (lower right quadrant) T cells.
FIG. 7.
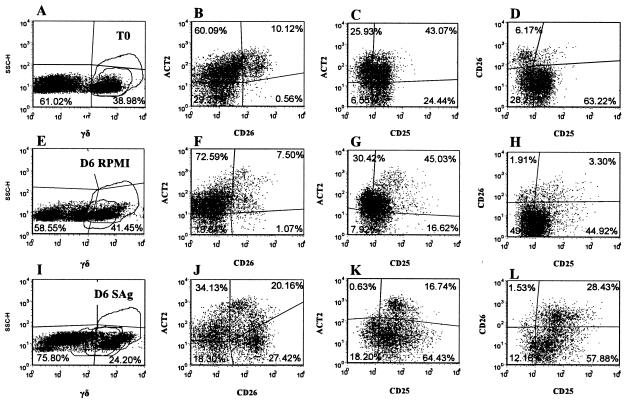
Representative dot plot profiles of γδ+ T cells from an infected animal labeled with four MAbs at T0 and after 6 days of culture in RPMI alone or with SAg (Fig. 1B, MAb set 4). A gate was placed on γδ+ T cells to analyze the expression of ACT2, CD25, and CD26 on γδ T cells present in gates 1 and 2. Previous studies have shown these cells are CD45R0 positive (data not shown). Profiles A, E, and I, SSC versus anti-γδ T cells, show the frequency of γδ T cells present in gates 1 and 2. Profiles B, F, and J, anti-ACT2 versus anti-CD26, with a gate on γδ T cells, show the frequency ACT2− CD26− (lower left quadrant), ACT2+ CD26− (upper left quadrant), ACT2+ CD26+ (upper right quadrant), and ACT2− CD26+ (lower right quadrant) γδ T cells. Profiles C, G, and K, anti-ACT2 versus anti-CD25, with a gate on γδ T cells, show the frequency of ACT2− CD25− (lower left quadrant), ACT2+ CD25− (upper left quadrant), ACT2+ CD25+ (upper right quadrant), and ACT2− CD25+ (lower right quadrant) γδ T cells. Profiles D, H, and I, anti-CD26 versus anti-CD25, with a gate on γδ T cells, show the frequency CD26− CD25− (lower left quadrant), CD26+ CD25− (upper left quadrant), CD26+ CD25+ (upper right quadrant), and CD26+ CD25− (lower right quadrant) γδ T cells.
FIG. 8.
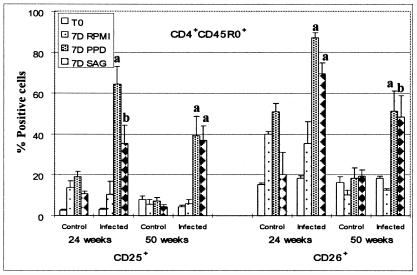
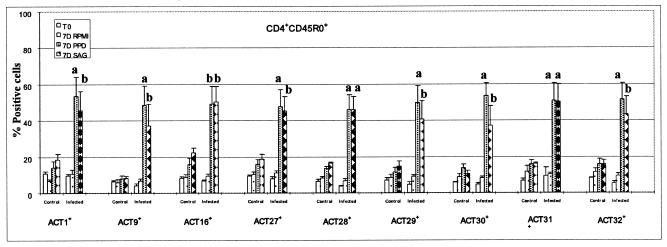
Summary of FC analysis of CD25 and CD26 expressed on CD4+ CD45R0+ T cells stimulated with PPD and M. avium subsp. paratuberculosis SAg. Significant differences were noted between control animals and animals infected with M. avium subsp. paratuberculosis as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05).
FIG. 10.
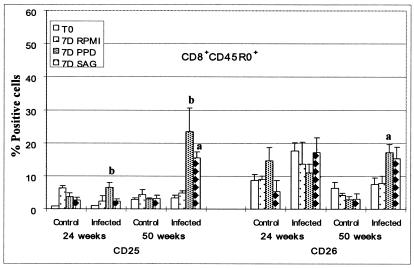
Summary of FC analysis of CD25 and CD26 expressed on CD8+ CD45R0+ T cells stimulated with PPD and M. avium subsp. paratuberculosis SAg. The significant differences between control animals and animals infected with M. avium subsp. paratuberculosis are as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05).
Statistics.
Data were analyzed by either repeated-measures, one-way analysis of variance followed by a Tukey-Kramer multiple comparison test or with Student's t test.
RESULTS
FC analysis of the proliferative response to M. avium subsp. paratuberculosis antigens.
At the initiation of culture, CD4+ and CD8+ T cells comprised, on average, ∼16 and 10%, of the ungated cells, respectively. CD2+ αβ+ T cells comprised ∼42% of the CD3 gated cells. CD2+ γδ+ and CD2− γδ+ T cells comprised ∼4 and 47% of the CD3+ gated cells, respectively (Fig. 2). CD3− CD2+ cells comprised ∼1 to 3% of the ungated population of cells. The CD3− CD2− cells—B cells, monocytes, dendritic, and null cells included in the CD3− CD2− population—comprised ∼38% of the ungated cells (Fig. 2). B cells, both CD5+ and CD5− populations, comprised a variable proportion of cells at the start of culture (Fig. 3).
The composition of cultured cells changed with time following infection with M. avium subsp. paratuberculosis. Based on side and forward light scatter, lymphocytes were small and fell into gate 1 at the start of culture. Up to 5 to 6 months, both αβ and γδ T cells remained small in cultures of unstimulated and stimulated cells from control and infected animals cultured for 7 days. Only 6 to 10% of lymphocytes fell into the large lymphocyte gate (i.e., gate 2 in Fig. 2). In cultures from some control and infected animals, however, populations of CD3− CD2− and CD3− CD2+ null cells increased in size and proliferated. These cells proliferated in the presence and absence of antigen. As shown in representative profiles in Fig. 4, examination of cells present in the large lymphocyte gate (i.e., gate 2) with an exclusion gate on CD3 revealed that both CD3− CD2− and CD3− CD2+ null cell populations expressed CD25. Analysis of the CD172A, CD5, CD6, and B-cell combination showed that in cultures where few CD3− CD2− and CD3− CD2+ cells proliferated, persisting B cells accounted for most of the null cells present in the culture. An exclusion gate on CD5, used with cultures where these populations proliferated, showed that the CD3− CD2− CD5− cells did not express B-B2, a marker of B cells. A subset, however, expressed CD6 (Fig. 4). Together, these findings demonstrated that a population of non-T, non-B cells from mononuclear cell cultures of young calves proliferate and express CD25 without specific stimulation. Thus, selective gates were placed on CD3, CD4, CD8, and γδ T cells to exclude null cells when analyzing the response of CD4+, CD8+, and γδ T cells to M. avium subsp. paratuberculosis antigens.
Up to 5 months, there was little difference in the composition of cultures of unstimulated and stimulated PBMC taken from control and infected animals. The majority of CD4+, CD8+, and γδ T cells were present in the small lymphocyte gate (i.e., gate 1 in Fig. 2). By 5 months a response to M. avium subsp. paratuberculosis antigens was evident in some infected animals. At 6 months, cells from all infected animals proliferated in the presence of M. avium subsp. paratuberculosis PPD and SAg. After 6 to 7 days of culture in the presence of antigens, 70 to 80% of the T cells were present in the large lymphocyte gate (i.e., gate 2 in Fig. 2). Analysis of gated CD4+ T cells showed that the majority of CD4+ T cells fell into the large lymphocyte gate and that they were CD45R0+, CD25+, and CD26+ (Fig. 5). CD45R0− cells were present in the small lymphocyte gate (i.e., gate 1 in Fig. 2) and were negative for CD25 and CD26. As shown below in Fig. 8, the proportion of CD45R0+ CD25+ and CD26+ T cells from infected animals was significantly higher at 24 and 50 weeks than in respective samples from control animals. Further examination at 50 weeks showed additional activation molecules were also upregulated in response to stimulation with M. avium subsp. paratuberculosis PPD and SAg (see Fig. 9).
FIG. 9.
Summary of FC data on nine activation molecules upregulated on CD4+ CD45R0+ T cells 50 weeks following stimulation with PPD and M. avium subsp. paratuberculosis SAg. The significant differences between control animals and animals infected with M. avium subsp. paratuberculosis are as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05).
The proliferative response of CD8+ T cells was minimal to 50 weeks postexposure to M. avium subsp. paratuberculosis. They comprised less than 10% of cells in stimulated and unstimulated cultures from control and infected animals. At 50 weeks, some CD8+ T cells from infected animals were beginning to respond to antigen (see Fig. 10). Although their frequency in cultures was still low at 18 months, examination of CD8+ cells from infected animals showed some cells were responding to antigens (Fig. 6). Selective gating showed the responding cells were increased in size, CD45R0+, CD25+, and CD26+. Small cells in gate 1 were CD45R0−, CD25−, and CD26−.
FIG. 6.
Representative dot plot profiles of CD8+ T cells from an infected animal labeled with four MAbs at T0 and after 6 days of culture in RPMI alone or with SAg (Fig. 1B, MAb set 3). A gate was placed on CD8+ T cells to analyze the expression of CD25 and CD26 on naïve and memory T cells present in gates 1 and 2. Profiles A, D, and G, SSC versus anti-CD8, show the frequency of CD8+ cells in the cell preparations in gates 1 and 2. Profiles B, E, and H, anti-CD25 versus anti-CD45R0, with a gate on CD8, show the frequency of CD25− naïve (lower left quadrant), CD25+ naïve (upper left quadrant), CD25+ memory (upper right quadrant), and CD25− memory (lower right quadrant) T cells. Profiles C, F, and I, anti-CD26 versus anti-CD45R0, with a gate on CD8, show the frequency of CD26− naïve (lower left quadrant), CD26+ naïve (upper left quadrant), CD26+ memory (upper right quadrant), and CD26− memory (lower right quadrant) T cells.
As noted in Fig. 2, γδ T cells comprised a large proportion of cells present at the initiation and after 6 to 7 days of culture. The majority of the γδ T cells were CD2− WC1+. The frequency of this subset varied from 40 to 50% of the total T-cell population in both control and infected animals. The CD2+ WC1− population varied from 5 to 10%. The relative proportions of these subsets did not change in unstimulated cultures. The frequencies of both populations were lower in antigen-stimulated cultures from infected animals containing proliferating CD4+ T cells (Fig. 2). γδ T cells expressed CD25 and ACT2 at low levels (Fig. 7). They did not express CD26. Eighteen months postinfection, γδ T cells in cultures from control and infected animals exhibited an activated phenotype in the presence of PPD and SAg (Fig. 2 and 7). Selective gating on small and large cells with a gate on the CD2+ WC1− populations showed 10% of the cells in the small lymphocyte gate expressed CD25 at T0, 6% expressed CD25 after 6 days of culture without antigen, and 13% expressed CD25 6 days after culture with antigen, respectively. At the same time points, 50, 64, and 84% of the γδ T cells in the large lymphocyte gate expressed CD25 (Fig. 2). Selective gating on small and large cells with a gate on CD2− WC1+ cells showed 70% of the cells in the small lymphocyte gate expressed CD25 at T0, 52% expressed CD25 after 6 days of culture without antigen, and 81% expressed CD25 at 6 days after culture with antigen. At the same time points, 86, 96, and 98% of the cells in the large lymphocyte gate expressed CD25 (Fig. 2). In the presence of antigen, subsets coexpressing ACT2 and CD26 or CD26 alone were readily distinguished. Selective gating showed that the cells expressing higher levels of ACT2 and CD26 were present in the large lymphocyte gate, indicating that they were activated and proliferating.
IFN-γ and ELISA assays.
As noted in a previous study (5), IFN-γ responses did not yield consistent results over time (data not shown). Some infected animals tested positive at some time points and negative at other time points during the course of the study. No positive results were obtained with the absorbed ELISA for M. avium subsp. paratuberculosis-specific antibody with infected animals (data not shown). Serum samples submitted to the Washington State Diagnostic Laboratory and examined by HerdChek ELISA (IDEXX Labs) were also negative. M. avium subsp. paratuberculosis was not detected in any of the fecal samples submitted to the diagnostic laboratory throughout the period of investigation.
Detection of M. avium subsp. paratuberculosis in tissue biopsies.
To determine if M. avium subsp. paratuberculosis could be detected in infected animals responding to M. avium subsp. paratuberculosis antigens, PCR was performed on cecal lymph node tissue taken at 12 months postexposure from the control and infected animals. Similar to findings in previous studies, M. avium subsp. paratuberculosis was not detected in tissues from every animal (60). Lymph nodes from two of the infected animals were positive by RT-PCR (data not shown).
Effect of MHC haplotype on the immune response to M. avium subsp. paratuberculosis.
At the time of writing of this report, no clear differences were detected in the immune response of cattle with different MHC genotypes (Table 1) to M. avium subsp. paratuberculosis as analyzed by FC. Therefore, data were combined for statistical analysis of the response of individual subpopulations of cells to M. avium subsp. paratuberculosis antigens.
DISCUSSION
The results presented here extend previous observations and reveal the importance of using multicolor FC for in vitro analysis of the immune response to M. avium subsp. paratuberculosis. Using the intratonsillar inoculation model, we were able to show animals can be uniformly infected and used to examine the evolution of an immune response to M. avium subsp. paratuberculosis (60). Fecal culture and IS900 RT-PCR confirmed the animals were infected. Light shedding was detected between 146 to 271 days postinoculation (one to four colonies/agar slant). At the termination of the study at 320 days, M. avium subsp. paratuberculosis was isolated from tonsil, duodenum, ileum, and jejunum and the ileal, jejunal, and spiral colon lymph nodes. M. avium subsp. paratuberculosis was not obtained from some tissues in each of the animals examined, similar to the findings of Koets et al. (38). Sequential sampling and culture of PBMC with LAM and SAg revealed a recall proliferative response to M. avium subsp. paratuberculosis was detectable by 6 months after infection by tritiated thymidine incorporation and FC. Phenotypic analysis by FC showed CD4+ T cells expressing CD45R0 and an activation molecule, CD26, were the predominant cell type present in the cultures responding to antigen. The IFN-γ ELISA demonstrated the presence of IFN-γ in culture supernatants. Intracellular staining showed CD4+ T cells were the main cell type positive for IFN-γ.
Infection by the oral route yielded similar results with the kinetics of appearance of a proliferative response to M. avium subsp. paratuberculosis antigens occurring in the same time frame. In contrast to the variable results observed with the IFN-γ and serological ELISAs, a persistent strong cellular recall response to M. avium subsp. paratuberculosis antigens was observed with PBMC from all five infected animals at all time points tested 6 months after infection. This is the first consistent indication of infection in animals still fecal culture negative and inconsistently positive in the IFN-γ and antibody-based diagnostic ELISAs. This finding would be expected in animals where the immune response to M. avium subsp. paratuberculosis is still controlling but not eliminating M. avium subsp. paratuberculosis, as suggested in the recent National Academy of Science report on Johne's disease (51).
FC analysis confirmed CD4+ T cells are the predominant population of cells responding to antigens in the first year of infection and, further, revealed the complexity of cultures maintained over a 6- to 7-day period in the absence and presence of antigen. Analysis of CD4+ and CD8+ T cells showed each population can be divided into three subpopulations based on expression of CD45R0, a marker for memory T cells (6, 28), and CD25 and CD26, markers used to determine the state of cell activation (Fig. 5 and 6) (9, 32). CD4+ CD45R0+ T cells comprised ∼50% of CD4+ T cells at the start of culture; ∼4 to 10% expressed CD25 at low levels; 10 to 20% expressed CD26 at levels similar to the values obtained in animals 13 to 302 days of age. Following stimulation, the CD4+ memory T cells increased in size and began to proliferate. By 6 to 7 days, the majority of CD4+ T cells expressed CD25 and CD26 at high levels (Fig. 5 and 8). They also expressed nine additional activation molecules (Fig. 9). After 6 to 7 days of culture, few naïve CD4+ T cells were present in the culture. As noted at the beginning of the culture, the naïve cells were small and negative for CD25 or CD26, indicating they had not been activated. Analysis of CD8+ T cells showed CD8+ CD45R0+ T cells comprised ∼30% of the CD8+ T cells at the start of culture, ∼1% expressed CD25 at low levels, and ∼10% expressed CD26. Up to a year, there appeared to be little change in size or composition of the CD8+ T-cell population in stimulated cultures (Fig. 10). However, by 18 months, antigen-stimulated CD8+ T cells showed an increase in size and expression of CD25 and CD26, suggesting that a progressive change in the immune response to M. avium subsp. paratuberculosis was beginning to occur (Fig. 6). Selective gating on large cells in gate 2 showed it was the CD45R0+ memory T cells that increased in size and expressed CD25 and CD26.
The response of γδ T cells to M. avium subsp. paratuberculosis antigens was not clearly defined in the present study. Analysis of the frequency and activation status of γδ T cells in the first months of the study showed the two major subpopulations of γδ T cells comprised a large proportion of the cells in culture in the presence and absence of M. avium subsp. paratuberculosis antigens at all time points during the study. The CD2+ γδ+ population corresponds to the CD2+ WC1− population, and the CD2− γδ+ population corresponds to the CD2− WC1+ population. These populations are distinct (17). They have different patterns of trafficking in blood and tissues (26, 62-64). The frequency of CD2− WC1+ cells is high in blood and low in intestinal epithelial and lymphoid tissues and skin. CD2− WC1+ cells express CD62L (L selectin) at high levels, whereas the CD2+ WC1− population expresses a low level of CD62L. The latter population is present at a low frequency in blood and most tissues but high in the spleen. The relative proportions of these two populations of γδ T cells remained similar in unstimulated and antigen-stimulated cultures of cells. At the initiation of culture, CD2− WC1+ γδ T cells were the predominant population expressing CD25. The majority of these cells were present in the small lymphocyte gate, indicating they were not activated. CD2+ WC1− γδ T cells expressing CD25 were found mainly in the large lymphocyte gate, suggesting they were activated. There appeared to be minimal expression of ACT2 or CD26 on either population of cells in cultures prepared during the first year of the study. However, at 18 months, subsets of γδ T cells in cultures of cells from both control and infected animals either coexpressed ACT2 and CD26 or expressed CD26 alone. Cells expressing these molecules were present in the large lymphocyte gate, indicating they were proliferating.
Of particular interest, the study revealed the presence of two populations of null cells: one, CD3− CD2+, considered to be NK cells and one that was CD3− CD2−. The latter population has not been previously reported. The appearance of these two populations of cells in unstimulated and antigen-stimulated cultures from control and infected animals was not anticipated. The predominant population in most cultures where proliferation occurred was CD3− CD2−. It was also CD5− B-B2−, and a subset expressed CD6. Culture conditions promoting proliferation of these populations of cells are not known, but it is clear that the random appearance of this population in cultures of PBMC could account for the high background noted in studies where tritiated thymidine has been used to detect cell proliferation. The data obtained in this study would have been difficult to interpret based on tritiated thymidine incorporation. The use of exclusion gating permitted us to analyze the antigen-specific responses of lymphocyte subsets in all cultures.
Limited information is available on whether there is any association of disease resistance or susceptibility to M. avium subsp. paratuberculosis linked to a given MHC haplotype. The data obtained here did not reveal any difference in the time of appearance or magnitude of the proliferative response to PPD or SAg. Further studies are needed with additional infected animals with different MHC haplotypes to determine if there are MHC-linked associations affecting the rate of disease progression.
Elucidation of how M. avium subsp. paratuberculosis modulates the immune response and evades immune elimination is crucial to understanding what strategies need to be employed to develop an effective vaccine. The general view is that protection is mediated through CMI against M. avium subsp. paratuberculosis and that disease progression correlates with an apparent shift in the dominant immune response to M. avium subsp. paratuberculosis from a strong CMI response to a humoral response. Cumulative data from studies of Mycobacterium tuberculosis and M. avium subsp. paratuberculosis indicate that CD4+ T cells with a type 1 cytokine profile and CD8+ T cells are the primary cells mediating protection against intracellular pathogens. The role of γδ T cells remains unclear, especially in Artiodactyla, where two phenotypically distinct complex subpopulations exist. As mentioned, the subsets differ in tissue distribution and patterns of trafficking. The effector mechanisms of CD4+ cells are associated with secretion of IFN-γ, which activates bactericidal activity in macrophages, lymphotoxin, perforin, and granulysin (10). The effector mechanisms of CD8+ T cells and γδ T cells are less clear, but there is evidence that CD8+ and γδ T cells also secrete granulysin (10, 55). Their presence in M. tuberculosis lesions has suggested they may synergize with CD4+ T cells in mediating protection. Dysregulation could involve disruption of cell signaling essential for maintenance of CMI at multiple steps, beginning with the first encounter and uptake of the pathogen by dendritic cells and macrophages (25, 58). Uptake and sequestration of M. avium subsp. paratuberculosis could involve the same receptors as described for M. tuberculosis and associated disruption in phagosome lysosome fusion. Retention of TACO (tryptophan-aspartate-containing coat protein) in the phagosome appears to retard fusion with lysosomes and protects replicating bacteria from bactericidal oxygen and nitrous oxide intermediates produced by activated macrophages (49, 50). Alterations in the cytokine and chemokine profiles could affect innate immunity and the way antigens are processed and presented to T cells (27, 36, 46, 58). Progressive changes attributable to dissemination and spread of M. avium subsp. paratuberculosis and increased antigenic load could induce alterations in signaling mediated through cytokines and chemokines that modulate the response to M. avium subsp. paratuberculosis antigens. Recent gene profiling has revealed changes in gene expression that could account for alterations in cell signaling mediated through cell receptors and altered patterns of cytokine and chemokine secretion (12, 13). Significant changes were noted in expression of multiple genes in cultures of PBMC from infected animals incubated with live M. avium subsp. paratuberculosis. Some genes exhibited increased expression in cells from infected animals, while others exhibited a decrease in expression. Of particular interest, changes were noted in the expression of IFN-γ, IL-10, and CD154 (CD40L). M. avium subsp. paratuberculosis-mediated arrest of phagosome maturation also appears to involve members of the rab family of small GTPases, which confer fusion competence in the endocytic pathway (30, 56, 59). More specific changes that might be associated with M. avium subsp. paratuberculosis-mediated changes in gene expression and potential mechanisms of pathogenesis have been found in tissue samples taken from the ileum of M. avium subsp. paratuberculosis-infected animals. A large increase in expression of IL-1α and TNF receptor-associated factor 1 were found in tissues from infected animals (1). Increases in secretion of IL-1α could lead to local toxicity and reduced proinflammatory signaling mediated by TNF-α. Whether specific changes in gene expression can be associated with functional changes in CMI and humoral responses to M. avium subsp. paratuberculosis are not yet clear. Comparative analysis using single-fluorescence FC did not reveal any statistically significant differences in blood lymphocyte and monocyte populations between infected and uninfected animals (12). However, a more comprehensive study by Koets et al. (38) has shown that progressive changes in the frequency of CD4+ T cells and γδ T cells might correlate with changes in gene activation. Using immunohistochemistry and single-fluorescence FC, their studies with (i) normal animals, (ii) animals at the asymptomatic but shedding stage of disease, (iii) symptomatic animals at the end stage of disease, and (iv) animals vaccinated with a live vaccine showed differences exist in the frequency of CD4+ T cells and γδ T cells in the lesional areas of the ileal lamina propria in symptomatic compared to asymptomatic animals. Using tritiated thymidine incorporation, they also showed an overall lower proliferative response to M. avium subsp. paratuberculosis heat shock protein 70, PPD, whole bacteria, and concanavalin A in symptomatic compared to normal, asymptomatic shedders and to vaccinated animals.
These recent observations are beginning to provide insight into the immune response to M. avium subsp. paratuberculosis and potential mechanisms by which M. avium subsp. paratuberculosis modulates the response during the course of disease. With the higher resolution obtained with multicolor FC combined with gene profiling, it should be possible to elucidate how M. avium subsp. paratuberculosis affects immune recognition at the level of antigen-presenting cells and the events during immune recognition and development of an immune response to M. avium subsp. paratuberculosis. The finding that a proliferative response to M. avium subsp. paratuberculosis is not readily detected until 5 months after infection by direct inoculation or the oral route suggests M. avium subsp. paratuberculosis interferes with pathways of activation associated with antigen uptake by antigen-presenting cells. It will be important to determine if M. avium subsp. paratuberculosis uses DC-SIGN (CD209) similar to M. tuberculosis to dysregulate antigen processing and/or disrupt toll-like receptor-mediated signaling (31, 58).
Acknowledgments
This study was funded in part by grants USDA-NRICGP 2002-35204-11688 and 2003-05165, USDA-APHIS-VS 03-9100-0788-GR, and 03-9100-07-GR, intramural support USDA Animal Health WNV-00150. Hye Cheong Koo was supported in part by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University, the Brain-Korea 21 Project in Agricultural Biotechnology, and the Washington State University Monoclonal Antibody Center.
Editor: J. D. Clements
REFERENCES
- 1.Aho, A. D., A. M. McNulty, and P. M. Coussens. 2003. Enhanced expression of interleukin-1α and tumor necrosis factor receptor-associated protein 1 in ileal tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 71:6479-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, B., C. Sánchez, R. Bullido, A. Marina, J. Lunney, F. Alonso, A. Ezquerra, and J. Domínguez. 2000. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens 55:342-351. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, C. L., A. J. Teale, J. Naessens, B. M. Goddeeris, N. D. MacHugh, and W. I. Morrison. 1986. Characterisation of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J. Immunol. 136:4385-4391. [PubMed] [Google Scholar]
- 4.Barletta, R. G., R. O. Donis, O. Chacon, H. Shams, and J. D. Cirillo. 2000. Vaccines against intracellular pathogens. Subcell. Biochem. 33:559-599. [DOI] [PubMed] [Google Scholar]
- 5.Barrington, G. M., J. M. Gay, I. S. Eriks, W. C. Davis, J. E. Evermann, C. Emerson, J. L. O'Rourke, M. J. Hamilton, and D. S. Bradway. 2003. Temporal patterns of diagnostic results in serial samples from cattle with advanced paratuberclosis infections. J. Vet. Diagn. Investig. 15:195-200. [DOI] [PubMed] [Google Scholar]
- 6.Bembridge, G. P., N. D. MacHugh, D. Mckeever, E. Awino, P. Sopp, R. A. Collins, K. I. Gelder, and C. J. Howard. 1995. CD45RO expression on bovine T cells: relation to biological function. Immunology 86:537-544. [PMC free article] [PubMed] [Google Scholar]
- 7.Bensaid, A., and M. Hadam. 1991. Bovine CD4. Vet. Immunol. Immunopathol. 27:51-54. [DOI] [PubMed] [Google Scholar]
- 8.Bloom, B. R., P. Salgame, and B. Diamond. 1992. Revisiting and revising suppressor T cells. Immunol. Today 13:131-135. [DOI] [PubMed] [Google Scholar]
- 9.Boonacker, E., and N. Van. 2003. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 82:53-73. [DOI] [PubMed] [Google Scholar]
- 10.Canaday, D. H., R. J. Wilkinson, Q. Li, C. V. Harding, R. F. Silver, and W. H. Boom. 2001. CD4+ and CD8+ T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J. Immunol. 167:2734-2742. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini, R. J. 1996. Immunology: resistance to paratuberculosis. Vet. Clin. North Am. Food Anim. Pract. 12:313-343. [DOI] [PubMed] [Google Scholar]
- 12.Coussens, P. M., C. J. Colvin, G. J. Rosa, L. Perez, and M. D. Elftman. 2003. Evidence for a novel gene expression program in peripheral blood mononuclear cells from Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 71:6487-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coussens, P. M., C. J. Colvin, K. Wiersma, A. Abouzied, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies, C. J., L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Crockett, J. J. van der Poel, and V. P. Rutten. 1997. Nomenclature for factors of the BoLA system, 1996. Anim. Genet. 28:159-168. [Google Scholar]
- 15.Davies, C. J., I. Joosten, L. Andersson, M. A. Arriens, D. Bernoco, B. Bissumbhar, G. Byrns, M. J. T. van Eijk, B. Kristensen, H. A. Lewin, S. Mikko, A. L. G. Morgan, N. E. Muggli-Crockett, P. R. Nilsson, R. A. Oliver, C. A. Park, J. J. van der Poel, M. Polli, R. L. Spooner, and J. A. Stewart. 1994. Polymorphism of bovine MHC class II genes. Joint report of the Fifth International Bovine Lymphocyte Antigen (BoLA) Workshop, Interlaken, Switzerland, August 1992. Eur. J. Immunogenet. 21:259-289. [DOI] [PubMed] [Google Scholar]
- 16.Davies, C. J., I. Joosten, D. Bernoco, M. A. Arriens, J. Bester, G. Ceriotti, S. Ellis, E. J. Hensen, H. C. Hines, P. Horin, B. Kristensen, H. A. Lewin, D. Meggiolaro, A. L. G. Morgan, M. Morita, P. R. Nilsson, R. A. Oliver, A. Orlova, H. Ostergard, C. Park, H. Schuberth, M. Simon, R. L. Spooner, and J. A. Stewart. 1994. Polymorphism of bovine MHC class I genes. Joint report of the Fifth International Bovine Lymphocyte (BOLA) Workshop, Interlaken, Switzerland, August 1992. Eur. J. Immunogenet. 21:239-258. [DOI] [PubMed] [Google Scholar]
- 17.Davis, W. C., W. C. Brown, M. J. Hamilton, C. R. Wyatt, J. A. Orden, A. M. Khalid, and J. Naessens. 1996. Analysis of monoclonal antibodies specific for the γδ TcR. Vet. Immunol. Immunopathol. 52:275-283. [DOI] [PubMed] [Google Scholar]
- 18.Davis, W. C., J. E. Davis, and M. J. Hamilton. 1995. Use of monoclonal antibodies and flow cytometry to cluster and analyze leukocyte differentiation molecules. Methods Mol. Biol. 45:149-167. [DOI] [PubMed] [Google Scholar]
- 19.Davis, W. C., M. J. Hamilton, Y. H. Park, R. A. Larsen, C. R. Wyatt, and K. Okada. 1990. Ruminant leukocyte differentiation molecules, p. 47-70. In O. Barta (ed.), MHC, differentiation antigens and cytokines in animals and birds. Monographs in animal immunology. Bar-Lab, Inc., Blacksburg, Va.
- 20.Davis, W. C., L. R. Heirman, M. J. Hamilton, S. M. Parish, G. M. Barrington, A. Loftis, and M. Rogers. 2000. Flow cytometric analysis of an immunodeficiency disorder affecting juvenile llamas. Vet. Immunol. Immunopathol. 74:103-120. [DOI] [PubMed] [Google Scholar]
- 21.Davis, W. C., N. D. MacHugh, Y. H. Park, M. J. Hamilton, and C. R. Wyatt. 1993. Identification of a monoclonal antibody reactive with the bovine orthologue of CD3 (BoCD3). Vet. Immunol. Immunopathol. 39:85-91. [DOI] [PubMed] [Google Scholar]
- 22.Davis, W. C., S. Marusic, H. A. Lewin, G. A. Splitter, L. E. Perryman, T. C. McGuire, and J. R. Gorham. 1987. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet. Immunol. Immunopathol. 15:337-376. [DOI] [PubMed] [Google Scholar]
- 23.Davis, W. C., J. Naessens, W. C. Brown, J. A. Ellis, M. J. Hamilton, G. H. Cantor, J. I. R. Barbosa, W. Ferens, and G. A. Bohach. 1996. Analysis of monoclonal antibodies reactive with molecules upregulated or expressed only on activated lymphocytes. Vet. Immunol. Immunopathol. 52:301-311. [DOI] [PubMed] [Google Scholar]
- 24.Davis, W. C., and G. S Splitter. 1991. Bovine CD2(BoCD2). Vet. Immunol. Immunopathol. 27:43-50. [DOI] [PubMed] [Google Scholar]
- 25.Demangel, C., and W. J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell. Biol. 78:318-324. [DOI] [PubMed] [Google Scholar]
- 26.Doherty, M., H. F. Bassett, P. J. Quinn, W. C. Davis, A. P. Kelley, and M. L. Monaghan. 1996. A sequential study of the bovine tuberculin reaction. Immunology 87:9-14. [PMC free article] [PubMed] [Google Scholar]
- 27.Dorman, S. E., and S. M. Holland. 2000. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 11:321-333. [DOI] [PubMed] [Google Scholar]
- 28.Dutton, R. W., L. M. Bradley, and S. L. Swain. 1998. T cell memory. Annu. Rev. Immunol. 16:201-223. [DOI] [PubMed] [Google Scholar]
- 29.Eriks, I. S., K. T. Munck, T. E. Besser, G. H. Cantor, and V. Kapur. 1996. Rapid differentiation of Mycobacterium avium and M. paratuberculosis by PCR and restriction enzyme analysis. J. Clin. Microbiol. 34:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geijtenbeek, T. B. H., D. J. E. B. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. F. Van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 32.Gorrell, M. D., V. Gysbers, and G. W. McCaughan. 2001. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 54:249-264. [DOI] [PubMed] [Google Scholar]
- 33.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hein, W. R., L. Dudler, and C. R. Mackay. 1989. Surface expression of differentiation antigens on lymphocytes in the ileal and jejunal Peyer's patches of lambs. Immunology 68:365-370. [PMC free article] [PubMed] [Google Scholar]
- 35.Howard, C. J., and J. Naessens. 1993. Summary of workshop findings for cattle (tables 1 and 2). Vet. Immunol. Immunopathol. 39:25-48. [DOI] [PubMed] [Google Scholar]
- 36.Jouanguy, E., R. Döffinger, S. Dupuis, A. Pallier, F. Altare, and J.-L. Casanova. 1999. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and man. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 37.Kalis, C. H. J., J. W. Hesselink, H. W. Barkema, and M. T. Collins. 2001. Use of long-term vaccination with a killing vaccine to prevent fecal shedding of Mycobacterium avium subsp. paratuberculosis in dairy herds. Am. J. Vet. Res. 62:270-274. [DOI] [PubMed] [Google Scholar]
- 38.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Müller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen, R. A., M. L. Monaghan, Y. H. Park, M. J. Hamilton, J. A. Ellis, and W. C. Davis. 1990. Identification and characterization of monoclonal antibodies reactive with bovine, caprine, and ovine T-lymphocyte determinants by flow microfluorimetry. Vet. Immunol. Immunopathol. 25:195-200. [DOI] [PubMed] [Google Scholar]
- 40.Lee, S. U., W. A. Ferens, W. C. Davis, M. J. Hamilton, Y. H. Park, K. Dzuewanowska, L. K. Fox, J. Naessens, and G. A. Bohach. 2001. Identity of activation molecule 3 on superantigen-stimulated cells is CD26. Infect. Immun. 69:7190-7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee, S. U., Y. H. Park, W. C. Davis, M. J. Hamilton, J. Naessens, and G. A. Bohach. 2002. Molecular characterization of bovine CD26 upregulated by a staphylococcal superantigen. Immunogenetics 54:216-220. [DOI] [PubMed] [Google Scholar]
- 42.Lewin, H. A., G. C. Russell, and E. J. Glass. 1999. Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol. Rev. 167:145-158. [DOI] [PubMed] [Google Scholar]
- 43.MacHugh, N. D., J. K. Mburu, M. J. Hamilton, and W. C. Davis. 1998. Characterisation of a monoclonal antibody recognising the CD3ɛ chain of the bovine T cell receptor complex. Vet. Immunol. Immunopathol. 61:25-35. [DOI] [PubMed] [Google Scholar]
- 44.MacHugh, N. D., and P. Sopp. 1991. Bovine CD8 (BoCD8). Vet. Immunol. Immunopathol. 27:65-69. [DOI] [PubMed] [Google Scholar]
- 45.Naessens, J., M. Sileghem, N. MacHugh, Y. H. Park, W. C. Davis, and P. Toye. 1992. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology 76:305-309. [PMC free article] [PubMed] [Google Scholar]
- 46.Ottenhoff, T. H. M., T. de Boer, C. E. Verhagen, F. A. W. Verreck, and J. T. van Dissel. 2000. Human deficiencies in type 1 cytokine receptors reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Microbes Infect. 2:1559-1566. [DOI] [PubMed] [Google Scholar]
- 47.Park, Y. H., Y. S. Joo, J. Y. Park, J. S. Moon, S.-H. Kim, N. H. Kwon, J. S. Ahn, W. C. Davis, and C. J. Davies. 2004. Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J. Vet. Sci. 5:29-39. [PubMed] [Google Scholar]
- 48.Parsons, K. R., G. Crocker, P. Sopp, C. J. Howard, and W. C. Davis. 1993. Identification of mAb specific for the γ/δ TCR. Vet. Immunol. Immunopathol. 39:161-167. [DOI] [PubMed] [Google Scholar]
- 49.Pieters, J. 2001. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr. Opin. Immunol. 13:37-44. [DOI] [PubMed] [Google Scholar]
- 50.Raupach, B., and S. H. E. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 51.Rideout, B. A., S. T. Brown, W. C. Davis, J. M. Gay, R. A. Giannella, M. E. Hines II, W. D. Hueston, and L. J. Hutchinson. 2003. Diagnosis and control of Johne's disease. The National Academies Press, Washington, D.C. [PubMed]
- 52.Roach, D. R., H. Briscoe, B. Saunders, M. P. France, S. Riminton, and W. J. Britton. 2001. Secreted lymphotoxin-α is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell, G. C. 2000. Sequence duplication at the 3′ end of BoLA-DQB genes suggest multiple allelic lineages. Immunogenetics 52:101-106. [DOI] [PubMed] [Google Scholar]
- 54.Russell, G. C., C. J. Davies, L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Crockett, and J. J. van der Poel. 1997. BoLA class II nucleotide sequences, 1996: report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 28:169-180. [Google Scholar]
- 55.Spada, F. M., E. P. Grant, P. J. Peters, M. Sugita, A. Melian, D. S. Leslie, H. K. Lee, E. van Donselaar, D. A. Hanson, A. M. Krensky, O. Majdic, S. A. Porcelli, C. T. Morita, and M. B. Brenner. 2000. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J. Exp. Med. 191:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tooker, B. C., J. L. Burton, and P. M. Coussens. 2002. Survival tactics of M. paratuberculosis in bovine macrophage cells. Vet. Immunol. Immunopathol. 87:429-437. [DOI] [PubMed] [Google Scholar]
- 57.Tumas, D. B., A. L. Brassfield, A. S. Tavernor, M. T. Hines, W. C. Davis, and T. C. McGuire. 1994. Monoclonal antibodies to the equine CD2 T lymphocyte marker, a pan-granulocyte/monocyte marker and a unique pan-B lymphocyte marker. Immunobiology 192:48-64. [DOI] [PubMed] [Google Scholar]
- 58.van Kooyk, Y., and T. B. H. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 59.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 60.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson, E., M. K. Aydintug, and M. A. Jutila. 1999. A circulating bovine γδ T cell subset, which is found in large numbers in the spleen, accumulates inefficiently in an artificial site of inflammation: correlation with lack of expression of E-selectin ligands and L-selectin. J. Immunol. 162:4914-4919. [PubMed] [Google Scholar]
- 63.Wilson, E., J. F. Hedges, E. C. Butcher, M. Briskin, and M. A. Jutila. 2002. Bovine γδ T cell subsets express distinct patterns of chemokine responsiveness and adhesion molecules: a mechanism for tissue-specific γδ T cell subset accumulation. J. Immunol. 169:4970-4975. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, E., B. Walcheck, W. C. Davis, and M. A. Jutila. 1998. Preferential tissue localization of bovine γδ T cell subsets defined by anti-T cell receptor for antigen antibodies. Immunol. Lett. 64:39-44. [DOI] [PubMed] [Google Scholar]
- 65.Zur Lage, S., R. Goethe, A. Darji, P. Valentin-Weigand, and S. Weiss. 2003. Activation of macrophages and interference with CD4+ T-cell stimulation of Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium. Immunology 108:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]