Key Points
Microenvironmental interactions upregulate CD20 expression in CLL cells through the CXCR4/SDF-1 axis.
Ibrutinib treatment causes downregulation of CD20 in CLL cells.
Abstract
Agents targeting B-cell receptor (BCR) signaling-associated kinases such as Bruton tyrosine kinase (BTK) or phosphatidylinositol 3-kinase can induce mobilization of neoplastic B cells from the lymphoid tissues into the blood, which makes them potentially ideal to combine with anti-CD20 monoclonal antibodies (such as rituximab, obinutuzumab, or ofatumumab) for treatment of B-cell lymphomas and chronic lymphocytic leukemia (CLL). Here we show that interactions between leukemia cells and stromal cells (HS-5) upregulate CD20 on CLL cells and that administering ibrutinib downmodulates CD20 (MS4A1) expression in vivo. We observed that CLL cells that have recently exited the lymph node microenvironment and moved into the peripheral blood (CXCR4dimCD5bright subpopulation) have higher cell surface levels of CD20 than the cells circulating in the bloodstream for a longer time (CXCR4brightCD5dim cells). We found that CD20 is directly upregulated by CXCR4 ligand stromal cell-derived factor 1 (SDF-1α, CXCL12) produced by stromal cells, and BTK-inhibitor ibrutinib and CXCR4-inhibitor plerixafor block SDF-1α–mediated CD20 upregulation. Ibrutinib also downmodulated Mcl1 levels in CLL cells in vivo and in coculture with stromal cells. Overall, our study provides a first detailed mechanistic explanation of CD20 expression regulation in the context of chemokine signaling and microenvironmental interactions, which may have important implications for microenvironment-targeting therapies.
Introduction
The introduction of inhibitors of kinases involved in B-cell receptor (BCR) signaling, such as Bruton tyrosine kinase (BTK) or phosphatidylinositol 3-kinase, has been a major therapeutic advance in chronic lymphocytic leukemia (CLL).1,2 The small-molecule inhibitor of BTK kinase, ibrutinib, can disrupt CLL cells’ capacity to interact with cells in the microenvironment by interfering with chemokine-receptor signaling, which is important for the chemotaxis of leukemia B cells to lymphoid tissues, and thus induce their massive and lasting mobilization in the peripheral blood.1-6
The combined use of ibrutinib with anti-CD20 antibodies7-9 or other monoclonal antibodies (mAbs) has been suggested for the treatment of patients with CLL because they use different mechanisms for antileukemia activity. Additionally, we and others have previously shown that microenvironmental interactions protect CLL and lymphoma cells from rituximab-induced cytotoxicity10-12 and chemotherapy-induced apoptosis.11,13 The ibrutinib-induced lymphocytosis suggests that a combinatorial therapy with mAbs might overcome adhesion-mediated antibody resistance and synergize with anti-CD20 mAbs.10-13
Here we examined whether ibrutinib has an effect on the CD20 expression, and this revealed that the CLL cells of patients treated with ibrutinib have lower expression levels of CD20 than the CLL cells of the same patients prior to the therapy. Because ibrutinib interferes with leukemia-cell trafficking to the lymphoid microenvironment, we hypothesized that this downregulation might be because of the loss of stimulation by microenvironmental factors. Indeed, here we described that interactions of CLL cells with stromal cells induce the upregulation of CD20 expression through the CXCR4/SDF-1 axis. We also observed that ibrutinib inhibits SDF-1–induced CD20 expression and, in CLL patients, leads to CD20 downmodulation in vivo.
Study design
Peripheral blood samples were obtained from untreated CLL patients or patients treated with ibrutinib as a single agent (420 mg once daily). CLL cells were separated from the blood samples using negative selection by RosetteSep Human B Cell Enrichment Cocktail (StemCell Technologies) or Ficoll-Paque, followed by magnetic anti-CD3 MicroBeads separation (Miltenyi Biotec). The study was approved by the institutional review board, and samples were obtained with written informed consent. The coculture experiments with immortalized HS-5 stromal cells were performed as previously described.11 Briefly, CLL cells were seeded on plastic or a monolayer of HS-5 cells at a concentration of 2.5 × 106 cells per mL per well. The cells were incubated in RPMI with 10% fetal bovine serum (37°C, 5% CO2) for the indicated time periods and harvested for flow cytometry, gene expression, or immunoblotting analyses (see supplemental Methods, available on the Blood Web site). Statistical analyses were performed using GraphPad Prism Software v 5.0.
Results and discussion
It has been suggested to therapeutically combine BCR-signaling inhibitors with anti-CD20 mAbs. Therefore, we investigated whether ibrutinib affects CD20 expression on CLL cells. We analyzed samples obtained from CLL patients treated with ibrutinib as a single agent (preibrutinib vs postibrutinib; patients’ characteristics are summarized in supplemental Table 1) and observed significant CD20 downmodulation on the CLL cell surface and its messenger RNA (mRNA) levels (Figure 1A and supplemental Figures 1 and 2E-F). Ibrutinib-induced CD20 downmodulation has been also reported by others14 and suggests that CD20 expression might be regulated by a yet unknown mechanism in the context of microenvironmental interactions that are disrupted by ibrutinib. We therefore focused on investigating the effect of microenvironmental interactions on CD20 expression on malignant B cells.
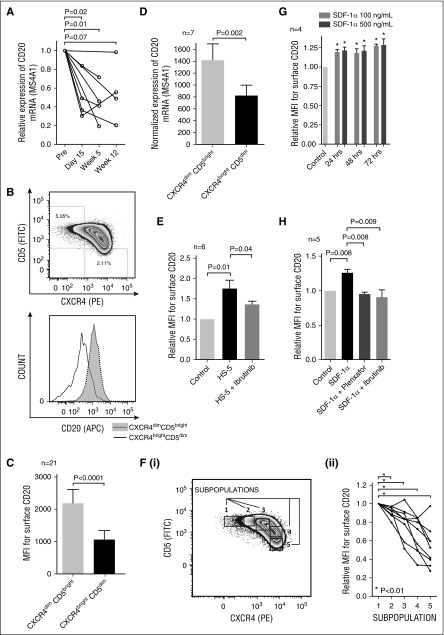
Figure 1.
The effect of microenvironmental interactions on CD20 expression in CLL cells. (A) Relative CD20 mRNA (MS4A1) expression in samples obtained before and during ibrutinib treatment of CLL patients (N = 8 patients; for characterization of CLL samples, see supplemental Table 1, sample no. CLL102-109). Samples were acquired the day before the first administration of ibrutinib (Pre), and on day 15, and/or week 5 and/or week 12 after the first ibrutinib administration. (B) A representative example of the gating of CXCR4dimCD5bright and CXCR4brightCD5dim CLL cell populations analyzed using flow cytometry (top). The histogram of the surface CD20 expression on CLL cells gated in the top panel (bottom). (C) The mean fluorescence intensity (MFI) for the surface CD20 on CXCR4dimCD5bright and CXCR4brightCD5dim CLL cells (N = 21 pairs; for characterization of CLL samples, see supplemental Table 2). The statistical difference was tested by paired Student t test. (D) Normalized CD20 mRNA (MS4A1) expression in sorted CXCR4dimCD5bright and CXCR4brightCD5dim CLL cells (N = 7 pairs; purity >99% CD5+CD19+ cells; for characterization of CLL samples, see supplemental Table 2). The statistical difference was tested by paired Student t test. (E) Freshly obtained CLL cells (N = 6, purity >99% CD5+CD19+ cells; for characterization of CLL samples, see supplemental Table 2) were seeded (2.5 × 106 cells per mL) on plastic (control) or an HS-5 monolayer. CLL cells were pretreated with vehicle (labeled HS-5) or ibrutinib (HS-5 + ibrutinib) for 2 hours prior to being seeded on the HS-5 stromal cells. The CLL cells were treated with ibrutinib (1 μM) prior to seeding on stromal cells to ensure full BTK inhibition before the contact of B cells with HS-5 cells. The control stands for CLL cells treated with vehicle and cultured on plastic with no contact with stromal cells. After 48 hours in cultivation, all cells in the wells were harvested and labeled with anti-CD20 antibody and Annexin-V, and CD20 MFI was analyzed on viable CD105-negative cells (ie, CLL cells) by flow cytometry. CD105 was used as a stromal cell marker. (F) A representative plot showing gating strategy of 5 subpopulations based on CXCR4 and CD5 expression (i). The relative surface CD20 expression in CLL subpopulations gated according to CXCR4/CD5 levels (ii; N = 9 CLL samples). The statistical difference was tested by paired Student t test. (G) Freshly obtained CLL cells (N = 4, purity >99% CD5+CD19+ cells; for characterization of CLL samples, see supplemental Table 2) were seeded on a plastic surface at a concentration of 2.5 × 106 cells per mL and treated with recombinant human SDF-1α (100 or 500 ng/mL), or vehicle (control) and cultured for 24/48/72 hours. After the indicated cultivation period, the CLL cells were harvested and labeled with anti-CD20 antibody and Annexin-V, and CD20 MFI was analyzed on viable cells using flow cytometry. MFI on the control cells was set as 1. *P ≤ .05. (H) Freshly obtained primary CLL cells (N = 5, purity >99% CD5+CD19+ cells; for characterization of CLL samples, see supplemental Table 2) were seeded on plastic (2.5 × 106 cells per mL) and treated with SDF-1α (labeled SDF-1α) or plerixafor and SDF-1α (SDF-1α + plerixafor) or ibrutinib and SDF-1α (SDF-1α + ibrutinib). Ibrutinib (1 μM) or plerixafor (5 μg/mL) was added to the cell culture 2 hours prior to SDF-1α treatment (500 ng/mL) to ensure full BTK/CXCR4 inhibition before the contact of B cells with the SDF-1α chemokine. The control stands for cells that were treated with vehicle and cultured on plastic with no inhibitor or SDF-1α treatment. After 48 hours in cultivation, all cells in the wells were harvested and labeled with anti-CD20 antibody and Annexin-V, and CD20 MFI was analyzed on viable cells using flow cytometry.
We assessed CD20 expression on CLL cell subpopulations defined according to CXCR4 and CD5 levels. We and others have described that CLL cells that have recently exited the lymph node microenvironment and moved into the peripheral blood express lower levels of the chemokine receptor CXCR4 and higher levels of the activation marker CD5.15,16 The CXCR4dimCD5bright CLL cells have approximately twofold higher cell-surface and mRNA expression of CD20 than CXCR4brightCD5dim cells (P < .01; Figure 1B-D). This suggests that changes in surface CD20 levels within immune niches reflect changes in gene transcription. Ibrutinib treatment in vivo also induced a reduction of CXCR4dimCD5bright CLL cell subpopulation (supplemental Figure 2).
We next tested the effect of CLL cell coculture with the stromal cell line HS-511 on CD20 expression with and without ibrutinib treatment. This revealed that surface CD20 levels were significantly upregulated on CLL cells cocultured with the HS-5 cell line compared with control CLL cells cultured on plastic (P < .05; Figure 1E and supplemental Figure 3), and ibrutinib inhibited the upregulation of CD20 (P < .05; Figure 1E).
We further analyzed the CXCR4/CD5 CLL subpopulations and observed that the CD20 expression gradually decreased with CLL cells’ transition from CXCR4dimCD5bright to CXCR4brightCD5dim (P < .01; Figure 1F). This led us to hypothesize that the CXCR4/SDF-1 axis is directly involved in CD20 regulation. Indeed, CLL cells treated with SDF-1α (CXCL12), a ligand for CXCR4 produced by stromal cells, significantly upregulated surface CD20 (Figure 1G). The CD20 upregulation induced by SDF-1α or HS-5 stromal cells was inhibited by plerixafor (a CXCR4 inhibitor) (P < .01; Figure 1H and supplemental Figure 4). Similarly, ibrutinib treatment also inhibited the CD20 upregulation induced by SDF-1α (P < .01; Figure 1H) or induced by CLL cell coculture with stromal cells (P < .05; Figure 1E). This can likely be explained by the previous finding that ibrutinib can directly prevent CXCR4 phosphorylation and alter the function of BTK downstream kinases.3,4,6 Altogether, these data suggest that the CD20 downmodulation induced by ibrutinib is at least partially because of the inhibition of CXCR4/SDF-1α signaling that regulates its levels.
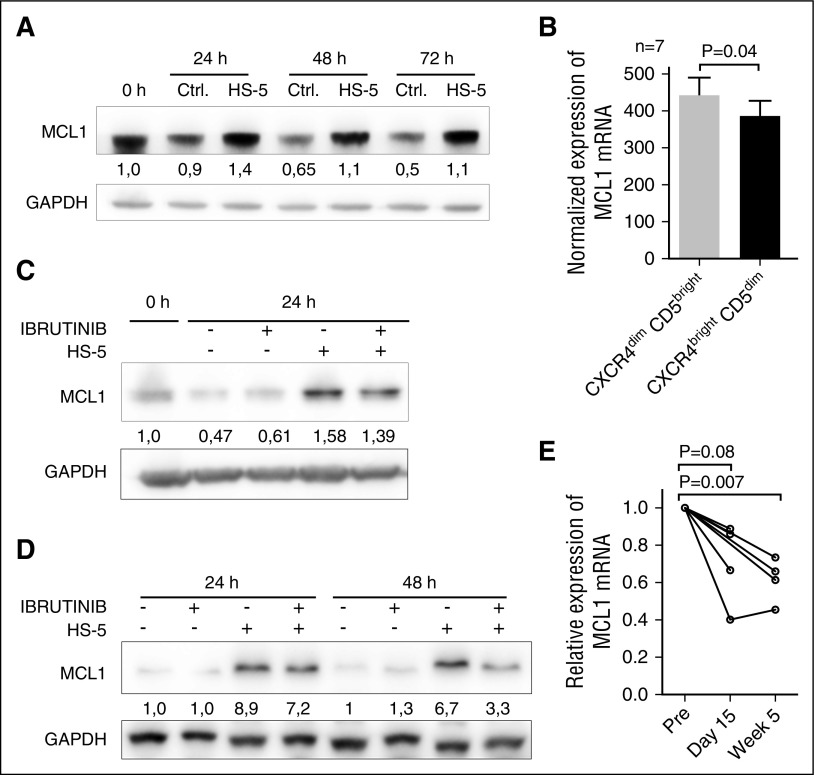
The addition of rituximab to ibrutinib largely mitigates ibrutinib-induced lymphocytosis.7 This implicates that rituximab is able to eliminate circulating CLL cells, at least to some extent, despite lower CD20 levels on ibrutinib-treated patients’ CLL cells. This suggests that ibrutinib potentially has other mechanism(s) of action that facilitate rituximab efficacy when the 2 drugs are given in a combination. We therefore focused on the regulation of antiapoptotic molecules, namely Mcl1, because it has been shown that Mcl1 directly protects CLL cells from rituximab-induced apoptosis and complement dependent cytotoxicity,17 and Mcl1 is rapidly downmodulated by rituximab infusion in vivo.18 In Figure 2A, coculture of CLL cells with HS-5 cells leads to a strong Mcl1 protein upregulation in CLL cells. We also observed higher Mcl1 mRNA levels in the CXCR4dimCD5bright CLL subpopulation that recently exited the lymph node niche (P < .05; Figure 2B). Ibrutinib treatment resulted in significant (P < .05) downmodulation of Mcl1 protein levels in CLL cells (Figure 2C) and the CLL-derived cell line OSU-CLL cocultured with HS-5 (Figure 2D). Ibrutinib had no significant effect on cell viability in these experiments (supplemental Figure 5). Importantly, Mcl1 mRNA levels were also significantly downmodulated after ibrutinib treatment in vivo (Figure 2E), but other antiapoptotic molecules (such as Bcl2, Bcl-XL, Bcl-W, Bcl2A1, or XIAP) were not affected (supplemental Figure 6).
Figure 2.
The effect of microenvironmental interactions and ibrutinib treatment on Mcl1 expression. (A) Representative immunoblot analysis of Mcl1 expression in CLL cells (purity >95% CD5+CD19+ cells) after culture on plastic (Ctrl.) or coculture with HS-5 cells (HS-5) for 24/48/72 hours. The purification of CLL cells after coculture with HS-5 cells was performed using magnetic anti-CD105 MicroBeads (purity >95% CD5+CD19+ cells). The blot images were quantified with UVItec Alliance 4.7 (UVItec Cambridge), and the Mcl1/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the first sample was arbitrarily set at 1. (B) Normalized Mcl1 mRNA expression in sorted CXCR4dimCD5bright and CXCR4brightCD5dim CLL cells (N = 7 pairs, purity >99% CD5+CD19+ cells). (C) Representative immunoblot analysis of Mcl1 expression in CLL cells after culture on plastic or coculture with HS-5 (with or without ibrutinib treatment). The presence of ibrutinib target BTK in CLL was confirmed by immunoblotting (supplemental Figure 8). The CLL cells were pretreated with ibrutinib (10 μM) or vehicle for 2 hours and then washed and seeded on plastic or confluent monolayer of HS-5 cells. The pretreatment of 2 hours with ibrutinib followed by washing was performed to minimize the off-target effects caused by the continuous ibrutinib presence. After the indicated cultivation period, all cells in the wells were harvested, and B-cell purification after coculture with HS-5 cells was performed using magnetic anti-CD105 MicroBeads (purity >95% CD5+CD19+ cells). The blot images were quantified with UVItec Alliance 4.7, and the Mcl1/GAPDH in the first sample was arbitrarily set at 1. Above each panel is a “+” or a “-” in the row labeled “ibrutinib” and/or “HS-5“ to indicate the samples that were exposed to ibrutinib or cocultured with stromal cells. (D) Representative immunoblot analysis of Mcl1 in the OSU-CLL cell line after culture on plastic or coculture with HS-5 (with or without treatment with ibrutinib). The presence of ibrutinib target BTK in OSU-CLL cell line was confirmed by immunoblotting (supplemental Figure 8). The experiment was performed identically as described in panel C for CLL cells. The Mcl1/GAPDH in the control sample was arbitrarily set at 1. (E) Relative Mcl1 mRNA expression in samples obtained before (Pre) and during ibrutinib treatment of CLL patients (time of sampling indicated). The gene expression was analyzed in samples with enough RNA material available (N = 7 patients; for characterization of CLL samples, see supplemental Table 1, sample no. CLL101-103 and 106-109 [other samples did not have enough RNA available]).
In summary, microenvironmental interactions upregulate CD20 expression in CLL B cells through the CXCR4/SDF-1 axis (summarized in supplemental Figure 7). It is likely that inducing CD20 in the context of immune microenvironment is of physiological importance because CD20-knockout B cells have a reduced BCR signaling propensity, and CD20 physically couples with BCR in lipid rafts.19-22 The stroma-induced CD20 upregulation seems to be influenced by transcriptional changes rather than posttranscriptional regulation because microRNAs (miRs) involved in microenvironmental interactions (such as miR-29b, miR-181b, and miR-150)23,24 do not have evolutionary conserved binding sites in CD20 mRNA (data not shown). However, we cannot exclude the possibility that other miRs or microenvironmental factors can also affect CD20 levels. Additionally, our data demonstrate that the CD20 reduction is unlikely to contribute to stromal cell adhesion-mediated rituximab resistance described previously10-12 because the interactions with stroma on the contrary upregulate the rituximab target, CD20. Our findings explain at least partially the mechanism of CD20 downmodulation with ibrutinib treatment and have potentially important implications for combinatorial studies of CD20-targeting antibodies and BCR inhibitors in patients with CLL.
Acknowledgments
This work was supported by the Ministry of Education, Youth, and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601), within special support paid from the National Programme for Sustainability II funds; the Czech Science Foundation (project 16-13334Y); the Ministry of Health of the Czech Republic (grant 16-29622A), all rights reserved; the SoMoPro II Programme (project 4SGA8684), cofinanced by European Union and the South-Moravian Region; the Ministry of Health of the Czech Republic, conceptual development of research organization (FNBr, 65269705, Sup 3/16); the Ministry of Education, Youth, and Sports of the Czech Republic, within program COST CZ (project LD15144); and a research fellowship award granted by the European Hematology Association.
The content of this publication reflects only the authors’ views, and the European Union is not liable for any use that may be made of the information contained therein.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.P. performed experiments, analyzed data, and wrote the manuscript; V.S., K.C., and R.C. performed experiments; M.B. and J.O. performed flow cytometry; J.M., M.S.D., M.D., S.P., T.J.K., M.T., and J.R.B. provided samples and interpreted data; M.M. designed the study, interpreted data, and wrote the manuscript; and all authors edited and approved the manuscript for submission.
Conflict-of-interest disclosure: M.S.D. has received consulting fees from Janssen, Pharmacyclics, and Genentech and institutional research support from Pharmacyclics and Genentech. J.R.B. has served as a consultant for Roche/Genentech, Janssen, and Pharmacyclics. T.J.K. has received consulting fees and honoraria from Janssen and Roche and research funding from Roche. The remaining authors declare no competing financial interests.
Correspondence: Marek Mraz, Central European Institute of Technology, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic; e-mail: marek.mraz@email.cz.
References
- 1.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij MFM, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 4.Ponader S, Chen S-S, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 6.Chen S-S, Chang BY, Chang S, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30(4):833–843. doi: 10.1038/leu.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman SEM, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28(11):2188–2196. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchner M, Brantner P, Stickel N, et al. The microenvironment differentially impairs passive and active immunotherapy in chronic lymphocytic leukaemia - CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151(2):167–178. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
- 11.Mraz M, Zent CS, Church AK, et al. Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin α-4-β-1 (VLA-4) with natalizumab can overcome this resistance. Br J Haematol. 2011;155(1):53–64. doi: 10.1111/j.1365-2141.2011.08794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquez M-E, Hernández-Uzcátegui O, Cornejo A, Vargas P, Da Costa O. Bone marrow stromal mesenchymal cells induce down regulation of CD20 expression on B-CLL: implications for rituximab resistance in CLL. Br J Haematol. 2015;169(2):211–218. doi: 10.1111/bjh.13286. [DOI] [PubMed] [Google Scholar]
- 13.Lwin T, Hazlehurst LA, Li Z, et al. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-kappaB (RelB/p52) in non-Hodgkin’s lymphoma cells. Leukemia. 2007;21(7):1521–1531. doi: 10.1038/sj.leu.2404723. [DOI] [PubMed] [Google Scholar]
- 14.Skarzynski M, Niemann CU, Lee YS, et al. Interactions between ibrutinib and anti-CD20 antibodies: competing effects on the outcome of combination therapy. Clin Cancer Res. 2016;22(1):86–95. doi: 10.1158/1078-0432.CCR-15-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calissano C, Damle RN, Marsilio S, et al. Intraclonal complexity in chronic lymphocytic leukemia: fractions enriched in recently born/divided and older/quiescent cells. Mol Med. 2011;17(11-12):1374–1382. doi: 10.2119/molmed.2011.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui B, Chen L, Zhang S, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124(4):546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain S-RA, Cheney CM, Johnson AJ, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13(7):2144–2150. doi: 10.1158/1078-0432.CCR-06-2294. [DOI] [PubMed] [Google Scholar]
- 18.Byrd JC, Kitada S, Flinn IW, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99(3):1038–1043. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 19.Petrie RJ, Deans JP. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J Immunol. 2002;169(6):2886–2891. doi: 10.4049/jimmunol.169.6.2886. [DOI] [PubMed] [Google Scholar]
- 20.Uchida J, Lee Y, Hasegawa M, et al. Mouse CD20 expression and function. Int Immunol. 2004;16(1):119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 21.Polyak MJ, Li H, Shariat N, Deans JP. CD20 homo-oligomers physically associate with the B cell antigen receptor. Dissociation upon receptor engagement and recruitment of phosphoproteins and calmodulin-binding proteins. J Biol Chem. 2008;283(27):18545–18552. doi: 10.1074/jbc.M800784200. [DOI] [PubMed] [Google Scholar]
- 22.Franke A, Niederfellner GJ, Klein C, Burtscher H. Antibodies against CD20 or B-cell receptor induce similar transcription patterns in human lymphoma cell lines. PLoS One. 2011;6(2):e16596. doi: 10.1371/journal.pone.0016596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mraz M, Chen L, Rassenti LZ, et al. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood. 2014;124(1):84–95. doi: 10.1182/blood-2013-09-527234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musilova K, Mraz M. MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia. 2015;29(5):1004–1017. doi: 10.1038/leu.2014.351. [DOI] [PubMed] [Google Scholar]