Abstract
Proteus mirabilis, an etiologic agent of complicated urinary tract infections, expresses mannose-resistant Proteus-like (MR/P) fimbriae whose expression is phase variable. Here we examine the role of these fimbriae in biofilm formation and colonization of the urinary tract. The majority of wild-type P. mirabilis cells in transurethrally infected mice produced MR/P fimbriae. Mutants that were phase-locked for either constitutive expression (MR/P ON) or the inability to express MR/P fimbriae (MR/P OFF) were phenotypically distinct and swarmed at different rates. The number of P. mirabilis cells adhering to bladder tissue did not appear to be affected by MR/P fimbriation. However, the pattern of adherence to the bladder surface was strikingly different. MR/P OFF colonized the lamina propria underlying exfoliated uroepithelium, while MR/P ON colonized the luminal surfaces of bladder umbrella cells and not the exfoliated regions. Wild-type P. mirabilis was usually found colonizing intact uroepithelium, but it occasionally adhered to exfoliated areas. MR/P ON formed significantly more biofilm than either P. mirabilis HI4320 (P = 0.03) or MR/P OFF (P = 0.05). MR/P OFF was able to form a biofilm similar to that of the wild type. MR/P ON formed a three-dimensional biofilm structure as early as 18 h after the initiation of the biofilm, while MR/P OFF and the wild type did not. After 7 days, however, P. mirabilis HI4320 formed a 65-μm-thick biofilm, while the thickest MR/P ON and MR/P OFF biofilms were only 12 μm thick. We concluded that MR/P fimbriae are expressed by most P. mirabilis cells infecting the urinary tract, dictate the localization of bacteria in the bladder, and contribute to biofilm formation.
Proteus mirabilis is a motile gram-negative bacterium that is commonly associated with complicated urinary tract infections (UTI) in patients with long-term catheterization or with structural or functional abnormalities in the urinary tract (33). This uropathogen expresses several types of fimbriae that promote colonization of the urinary tract. The mannose-resistant Proteus-like (MR/P) fimbria, a surface organelle responsible for mannose-resistant hemagglutination, contributes significantly to colonization and pathogenicity in the CBA mouse model of ascending UTI (9, 14-16, 18). MR/P fimbriae elicit a strong immune response in experimentally infected mice. Indeed, this type of fimbriae was recently shown to be the best immunogen in terms of conferring protection after immunization compared to uroepithelial cell adhesin and P. mirabilis fimbria (28). In addition, studies in our lab indicated that the N-terminal domain of the MrpH tip adhesin of MR/P fimbriae can act as an effective vaccine that prevents P. mirabilis UTI in mice that are intranasally vaccinated (13).
Like that for many other virulence factors (1), the expression of MR/P fimbriae undergoes phase variation (4). The mrp operon carries all of the genes that are necessary for the expression of MR/P fimbriae on the bacterial surface (mrpA, B, C, D, E, F, G, and H), as well as mrpJ, a repressor of flagellin synthesis. A 252-bp invertible element containing a canonical σ70 promoter lies in the intergenic region between mrpA and mrpI; the latter gene encodes a recombinase and is divergently transcribed from the mrp operon (34). This invertible element is flanked by identical 21-bp inverted repeats upon which MrpI acts. MrpI flips the promoter region between the “on” direction, in which the promoter is able to drive expression of the mrp operon, and the “off” direction, in which the promoter is in the opposite orientation and is unable to drive expression. This recombinase-controlled form of phase variation is also observed for type 1 fimbriae of Escherichia coli, which are another important urinary tract virulence factor (1).
For this study, we investigated the role of MR/P fimbriae in biofilm production and colonization during ascending UTI. We discovered that MR/P fimbriae promote initial biofilm formation, with the fimbriae leading to aggregation of the bacteria. We determined that the majority of wild-type P. mirabilis cells produce MR/P fimbriae during ascending UTI. We also observed differential bladder colonization patterns for wild-type P. mirabilis and phase-locked mutants, with the lack of MR/P fimbriae leading to colonization of the lamina propria rather than the uroepithelium. Thus, the expression of MR/P fimbriae leads to bacterial aggregation and umbrella cell colonization while the prevention of MR/P fimbrial expression likely allows secondary adhesins to be expressed, which permit P. mirabilis to adhere to alternative regions of the bladder.
MATERIALS AND METHODS
Chemicals and enzymes.
All enzymes were purchased from Life Technologies (Rockville, Md.), New England Biolabs (Beverly, Mass.), or Roche Molecular Biochemicals (Indianapolis, Ind.). All chemicals were purchased from Sigma-Aldrich (St. Louis, Mo.).
Bacterial strains and media.
The bacterial strains used for this study are listed in Table 1. Luria broth (LB) (10 g of peptone, 5 g of yeast extract, and 10 g of NaCl per liter) and LB agar (LB containing 1.5% [wt/vol] agar) were used as culture media. Nonswarming agar (10 g of peptone, 5 g of yeast extract, 0.5 g of NaCl, 5 ml of glycerol, and 20 g of agar per liter) was used on occasion (5). When necessary, media were supplemented with ampicillin (100 μg/ml). The MR/P phase-locked mutants were created by insertional inactivation with a kanamycin resistance gene of the gene encoding MrpI, the sole mrp operon recombinase, as previously described (15). Inactivation of the recombinase prevents the inversion of the invertible element preceding mrpA; the result is a mutant that either constitutively synthesizes MR/P fimbriae (MR/P ON) or produces no MR/P fimbriae (MR/P OFF).
TABLE 1.
P. mirabilis strains used for this study
| Strain | Relevant genotype | Comments | Reference |
|---|---|---|---|
| P. mirabilis HI4320 | Wild type | Isolated from a patient with CABa | 22 |
| MR/P ON | mrpI::aphA | Phase-locked on; constitutively expresses MR/P fimbriae | 15 |
| MR/P OFF | mrpI::aphA | Phase-locked off; does not express MR/P fimbriae | 15 |
| P. mirabilis HI4320 (pBAC001) | Constitutively expresses GFP from a plasmid | 9 | |
| MR/P ON(pBAC001) | mrpI::aphA (pBAC001) | Constitutively expresses MR/P fimbriae and GFP | This study |
| MR/P OFF(pBAC001) | mrpI::aphA (pBAC001) | Does not express MR/P fimbriae; constitutively expresses GFP | This study |
| E. coli DH5α | pBluescript | DH5α harboring an empty plasmid | 15 |
| pmrpa-j | DH5α harboring the mrp operon | 15 |
CAB, catheter-associated bacteriuria.
Cell culture and standardization of bacterial strains for experiments.
Wild-type P. mirabilis HI4320, MR/P ON, MR/P OFF, wild-type P. mirabilis HI4320(pBAC001), MR/P ON(pBAC001), MR/P OFF(pBAC001), E. coli DH5α, E. coli DH5α(pmrpA-J), and E. coli DH5α(pBluescript) were picked from isolated colonies, inoculated into LB supplemented with antibiotics when necessary for plasmid retention, and cultured with aeration at 37°C overnight. Cultures either were standardized to an optical density at 600 nm (OD600) of 0.5 (∼108 CFU/ml) and used or were spread onto LB agar plates for overnight incubation at 37°C for future inoculation into mice.
Mutant strains constitutively expressing GFP.
Electroporation-competent P. mirabilis MR/P ON and MR/P OFF were transformed with BAC001 encoding green fluorescent protein (GFP) according to standard electroporation protocols (31).
Murine model of ascending UTI.
Female (20 to 22 g, 6 to 8 weeks old) CBA mice (Jackson Laboratory, Bar Harbor, Maine) were inoculated transurethrally as previously described (8) with bacteria grown overnight on LB agar. These colonies were washed from the plate with phosphate-buffered saline (PBS) (0.138 M NaCl, 0.0027 M KCl, pH 7.4) and standardized to 109 CFU (for those producing GFP) or 108 CFU suspended in 0.05 ml of PBS. At the postinoculation times noted for each experiment, the bladders, ureters, and kidneys of the mice were aseptically removed. Bladders and kidneys were weighed and then sliced in half longitudinally. For determinations of the numbers of viable bacteria per gram of tissue, half of each bladder and kidney was homogenized by use of a mortar and pestle and then plated onto LB agar containing ampicillin (100 μg/ml). The plates were incubated overnight at 37°C. Organs containing >105 CFU/g of tissue were used for microscopy and immunohistochemistry.
Tissue preparation for confocal scanning laser microscopy.
Bladders, ureters, and kidneys were fixed in 4% (vol/vol) paraformaldehyde containing 20% (wt/vol) sucrose at 4°C for 24 h. The tissues were embedded in Tissue-Tek OCT compound (Miles, Elkhart, Ind.), frozen in dry ice-ethanol, and held at −80°C until use. Ten-, 15- and 30-μm-thick sections were cut with a cryostat and stored at −20°C until they were used for staining. The tissues were permeabilized by a 30-min room-temperature incubation in 0.1% Triton X. After three 6-min washes in PBS, the tissue sections were incubated with appropriate antibodies.
Staining for MR/P fimbriae.
A rabbit anti-MR/P serum was generated previously in our laboratory, and aliquots of the serum were stored at −20°C (15). Bladder, ureter, and kidney tissues were permeabilized as described above, incubated for 60 min at 37°C with rabbit anti-MrpA (1:200), and washed in PBS three times for 6 min each. The sections were then incubated with a secondary anti-rabbit antibody conjugated to Alexa Fluor 350 (Molecular Probes, Eugene, Oreg.) or Alexa Fluor 568 (1:1,000); this incubation was followed by three washes in PBS as described above. The sections were mounted with Vectashield fluorescent mounting medium (Vector Laboratories, Burlingame, Calif.), Vectashield with DAPI (4′,6′-diamidino-2-phenylindole), or Vectashield with propidium iodide. Negative controls for MR/P binding specificity used the Alexa Fluor 350-conjugated anti-rabbit antibody without the initial step of applying the MR/P antibody.
Staining for ATF.
An antiserum against ambient-temperature fimbriae (ATF) was generated previously in our laboratory (20), and aliquots of the serum were stored at −20°C. Vials of serum were thawed on ice and centrifuged for 1 min, and the supernatants were removed and placed in other tubes for further use. Dilutions of serum (1:100, 1:500, and 1:1,000) were added to permeabilized bladder sections of mice infected with either the ON GFP or OFF GFP strain. Sections were incubated overnight at 4°C, washed three times with PBS, and incubated for 1 h at 37°C with a 1:1,000 dilution of a secondary anti-rabbit antibody conjugated to Alexa Fluor 350 (Molecular Probes). After three more 6-min washes in PBS, the sections were dried and mounted with Vectashield mounting medium.
Confocal scanning laser microscopy.
Slides were examined with a Zeiss LSM410 confocal laser scanning microscope with a 63×, NA 1.4 objective. The GFP and Alexa Fluor 568 signals, Cy3, and propidium iodide were excited with the 488- and 568-nm lines of a 50-mW KrAr laser and detected through 515- to 540-nm band-pass and 590-nm long-pass filters, respectively. A Coherent Enterprise 100-mW argon laser with laser lines of 351 and 364 nm excited the DAPI-stained nuclei and Alexa Fluor 350-conjugated MR/P fimbriae. Signals were detected through a 460-nm long-pass filter.
Assay for MR/P fimbria production during ascending UTI.
The data used to determine the percentage of bacteria that expressed MR/P fimbriae were obtained by examinations of three fields of 63× sections each of bladders, ureters, and kidneys from three mice on days 2 and 4.
Tissue preparation for scanning electron microscopy.
At 4 and 7 days postinoculation, the ureters of mice infected with wild-type P. mirabilis were ligated with surgical thread, the bladders were infused through the urethras with fixative (2% glutaraldehyde, 2% formaldehyde), and then the urethras were ligated. Full bladders were placed in vials of fixative and stored at 4°C for 2 to 4 days. The bladders were removed from the fixative, cut into quarters or eighths, rinsed with PBS three times for 5 min each, and then incubated with 0.1 M sodium cacodylate-3 mM calcium chloride two times for 5 min each. After the bladders were postfixed in 2% osmium tetroxide in 0.1 M sodium cacodylate for 1 h, they were rinsed in distilled water two times for 5 min each and then stained with 2% uranyl acetate for 30 min. The specimens were dehydrated by 5-min incubations in 50, 70, 90, and 100% ethanol. After two additional 5-min incubations in 100% ethanol, the specimens were critical point dried and sputter coated for 2 min with platinum-palladium.
Scanning electron microscopy.
Murine bladders were examined in a Leo field emission scanning electron microscope (Carl Zeiss, Inc.) housed at the Johns Hopkins Microscopy Facility. The bladders were viewed by using a 1- to 2.5-kV operating voltage.
Bladder washout studies for mice experimentally infected with wild-type P. mirabilis HI4320 and phase-locked mutants.
Strains were tested in cochallenge experiments by transurethrally inoculating mice with ∼108 CFU of an equal mixture of wild-type P. mirabilis HI4320 and MR/P ON or wild-type P. mirabilis HI4320 and MR/P OFF. Independent infections with each strain were also examined. Eight female CBA mice were used for each cochallenge experiment. The bladders were washed with 0.05 ml of PBS at 24 h postinoculation and then homogenized. Both the wash fluid and the homogenized bladders were immediately plated (on LB agar [to obtain total CFU] and on LB agar supplemented with kanamycin [20 μg/ml] [to calculate the numbers of mutant bacteria]) to determine the numbers of nonadherent bacteria present in the wash fluid and the numbers of adherent bacteria present in the homogenized bladders. For independent challenges, mice were inoculated as described earlier with either P. mirabilis HI4320, MR/P ON, or MR/P OFF. After 24 h, the bladders were treated as described above, and both the wash fluid and the homogenized bladders were plated on LB agar (P. mirabilis HI4320) or LB agar supplemented with kanamycin (MR/P ON and MR/P OFF). Ten female CBA mice were used for each independent challenge experiment.
Measurement of swarm zone areas for wild-type P. mirabilis HI4320, MR/P ON, and MR/P OFF.
Samples (5 μl) of overnight cultures of wild-type P. mirabilis HI4320, MR/P ON, and MR/P OFF were spotted onto the middle of nine swarming plates. The abilities of the three P. mirabilis strains (wild type, MR/P ON, and MR/P OFF) to swarm were compared by determining the areas of the swarm zones after 12.5 h at 37°C. The areas were determined by using the formula for the area of an ellipse [area = (height × width × π)/4].
Crystal violet biofilm assay.
The assay for biofilm formation used for this study was adapted from the method of O'Toole and Kolter (26) and is based on the ability of bacteria to form biofilms on solid surfaces such as polyvinyl chloride. Standardized cell cultures (20 μl) were added to 180 μl of fresh, filter-sterilized urine in non-tissue-culture-treated polyvinyl chloride U-bottomed 96-well plates (Falcon 3911; Becton Dickinson Labware, Franklin Lakes, N.J.). Wells filled with sterile urine only were included as negative controls. The plates were incubated for 24 h at 30°C. Planktonic bacteria and urine were decanted, and fresh, filter-sterilized urine was added. The plates were incubated for another 24 h at 30°C and then examined for biofilm formation via a crystal violet assay. The urine, as well as any nonadherent bacteria, was decanted from the wells, and any remaining planktonic cells were removed by three rinses with sterile distilled water (dH2O). The wells were air dried, and adherent bacteria were stained for 15 min with 0.1% crystal violet. After three rinses with sterile dH2O, bacterium-bound dye was released by the addition of 200 μl of dimethyl sulfoxide (Sigma-Aldrich Company). One hundred microliters of the dimethyl sulfoxide-crystal violet solution was transferred to Costar 96-well flat-bottomed polystyrene plates (Corning Incorporated, Corning, N.Y.), and the OD595 was determined on an ELX800 universal microplate reader (Bio-Tek Instruments, Inc., Winooski, Vt.).
SEM evaluation of biofilm formation on cover glass.
A standardized culture (20 μl) was added to 180 μl of sterile, fresh, pooled human urine on a no. 1 18-mm-square cover glass (Corning Incorporated Life Sciences, Acton, Mass.). After 18 h, the urine and planktonic cells were decanted, and the cover glass was rinsed three times with sterile dH2O and processed for scanning electron microscopy (SEM) as described above.
Confocal laser scanning microscopic evaluation of biofilm formation on cover glass.
A standardized culture (20 μl) was added to 180 μl of sterile, fresh, pooled human urine on an 18-mm-square cover glass (Corning Incorporated Life Sciences). Every 48 h, the urine and planktonic bacteria were decanted off the cover glass, and fresh sterile urine was added. After 7 days, the urine and planktonic cells were decanted, and the cover glass was rinsed three times with sterile dH2O and examined with a Zeiss LSM410 confocal laser scanning microscope with a 63×, NA 1.4 objective. GFP-expressing bacteria were excited with the 488-nm line of a 50-mW KrAr laser and detected through a 515- to 540-nm band-pass filter.
RESULTS
Production of MR/P fimbriae by P. mirabilis HI4320 infecting the urinary tract.
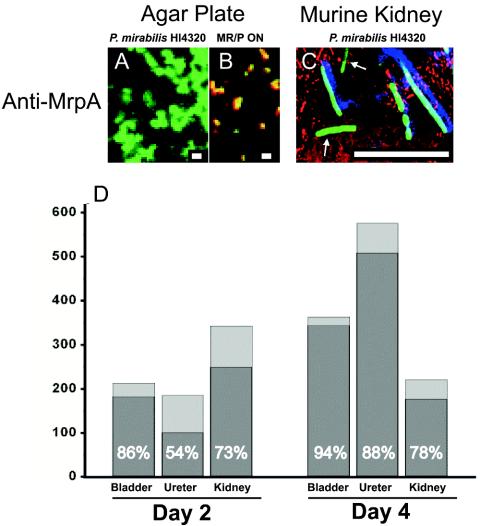
An overnight culture of P. mirabilis HI4320(pBAC001) (200 μl) was spread onto nonswarming LB agar plates and incubated overnight at 37°C in 5% CO2. Colonies were washed from the plates with PBS, the suspension was standardized, and female CBA mice were inoculated with 50 μl (∼109 CFU) of the bacterial suspension as described in Materials and Methods. The bacteria in the inoculum did not produce MR/P fimbriae, as determined by anti-MrpA staining (Fig. 1A) and confocal microscopy. MRP ON(pBAC001) cells were plated and examined for MR/P fimbriation by the same methods as those used for the positive control. MRP ON (green) cells bound by anti-MrpA (red) stained yellow, indicating the colocalization of bacteria and fimbriae (Fig. 1B). At 2 and 4 days postinoculation, the bladders, ureters, and kidneys were removed from the mice, and the infecting bacteria were analyzed for MR/P fimbriation by use of the anti-MrpA antibody and laser scanning confocal microscopy. The antibody bound specifically to bacteria producing MR/P fimbriae (Fig. 1C). In animal experiments, bound anti-MrpA was incubated with Alexa Fluor 350, yielding green-staining GFP-producing bacteria that colocalized with the blue-staining MrpA protein. Fig. 1C shows two bacteria that did not produce MR/P fimbriae (green) and four others that did (green and blue). The majority of P. mirabilis cells (54 to 94%) infecting the urinary tract produced MR/P fimbriae in all three urinary tract organs (Fig. 1D).
FIG. 1.
Expression of MR/P fimbriae in experimentally infected murine urinary tract. (A) An overnight culture of P. mirabilis HI4320(pBAC001) was spread onto nonswarming LB agar plates and incubated overnight at 37°C in 5% CO2. Colonies were washed from the plates with PBS, and bacteria were diluted to a concentration of 109 bacteria/ml. P. mirabilis HI4320(pBAC001) was spread onto a glass slide and incubated with anti-MrpA for 1 h at 37°C. After three 6-min washes, a goat anti-rabbit antibody conjugated to Alexa Fluor 568 was used to visualize MrpA (red). (B) MR/P ON(pBAC001) bacteria were treated in the same manner as a positive control for MR/P production. (C) Female CBA mice were inoculated with 50 μl of the P. mirabilis HI4320(pBAC001) bacterial suspension as described in Materials and Methods. Bacteria in tissue sections (10 μm) were stained with anti-MrpA (blue) and anti-actin (red) antibodies. Six bacteria produced MR/P fimbriae (blue and green), while two bacteria did not produce MR/P fimbriae (green only, arrows). Bars = 1 μm (A and B) and 10 μm (C). (D) Tissues from the experimentally infected mice were used to quantify the percentages of P. mirabilis HI4320(pBAC001) bacteria expressing MR/P fimbriae (percentages are shown in the dark gray portion of each bar). The y axis depicts the total number of bacteria counted.
Effect of MR/P fimbriae on swarming.
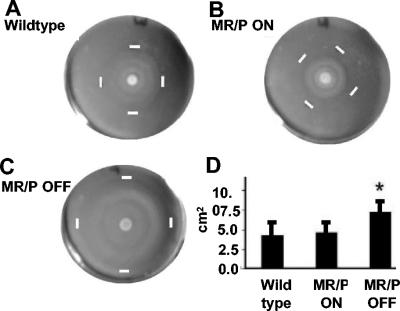
To ascertain the role of MR/P fimbriae, we used mutants that were previously generated in our laboratory (15) that either constitutively produce MR/P fimbriae (MR/P ON) or never express MR/P fimbriae (MR/P OFF). The abilities of the three P. mirabilis strains (wild-type HI4320, MR/P ON, and MR/P OFF) to swarm were compared by determining the areas of the swarm zones (Fig. 2A to C) after 12.5 h at 37°C. The average areas, determined by using the formula for the area of an ellipse (area = height × width × π/4), were 17.26 ± 6.47 cm2, 18.18 ± 5.5 cm2, and 28.5 ± 6.7 cm2 for the wild type, MR/P ON, and MR/P OFF, respectively (Fig. 2D), after 12.5 h. The difference in swarm zone areas was statistically significant when MR/P OFF was compared to either P. mirabilis HI4320 (P = 0.0025) or MR/P ON (P = 0.0028) by Student's two-tailed t test with Welch's correction. Therefore, the prevention of mrp operon transcription allowed faster swarming. However, constitutive expression of the mrp operon, including mrpJ, which encodes a repressor of flagellin synthesis, did not significantly slow the swarming behavior of the MR/P ON strain compared to the wild-type strain (P > 0.05).
FIG. 2.
MR/P OFF swarms faster than MR/P ON or wild-type P. mirabilis. Swarming plates for wild-type P. mirabilis HI4320 (A), MR/P ON (B), and MR/P OFF (C) are shown. Bacteria were spotted onto the center of the agar surface, and the plates were incubated at 37°C. Measurements were taken after 12.5 h. White bars indicate the outer limits of the swarm. (D) The average area of each swarm was determined by using the formula for the area of an ellipse [area = (height × width × π)/4]. Areas were determined for nine swarm zones for each strain. *, P < 0.0028.
MR/P ON enhances biofilm development.
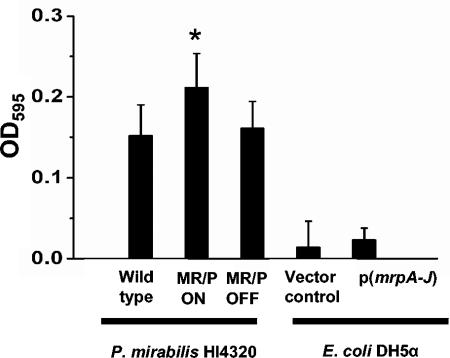
P. mirabilis MR/P ON has shown aggregation properties during studies in our laboratory (unpublished observation), and E. coli DH5α transformed with genes encoding MR/P fimbriae formed pellicles at the air-broth interface when grown statically overnight at 37°C. When cultures were incubated with shaking, aggregates formed on the surface. Since many fimbriae are recognized as being important for biofilm formation (6, 7, 26, 29, 32), we performed a crystal violet biofilm assay (26) with P. mirabilis HI4320, MR/P ON, and MR/P OFF to determine the contribution of MR/P fimbriae to biofilm formation. After 2 days of incubation in sterile urine at 30°C, with fresh urine added at 24 h, MR/P ON formed a significantly larger biofilm than either MR/P OFF or the wild-type strain (P = 0.03 and 0.05, respectively) (Fig. 3). However, the expression of MR/P fimbriae from a plasmid in E. coli DH5α, a laboratory strain of E. coli, was not sufficient to initiate biofilm formation, as the average biofilm formed by E. coli DH5α expressing MR/P fimbriae was not statistically different from that formed by a vector control (P = 0.4).
FIG. 3.
MR/P fimbriae increase biofilm production in P. mirabilis. Aliquots of overnight cultures of wild-type P. mirabilis HI4320, MR/P ON, MR/P OFF, E. coli DH5α (vector control), and DH5α(mrpA-J) were standardized to an OD600 of 0.5 and diluted 1:10 in fresh sterile urine in 96-well plates. After 48 h at 30°C, with decanting of the medium and planktonic bacteria at 24 h, the biofilms were washed with sterile distilled water and a crystal violet biofilm assay was performed as described in Materials and Methods. Data are the averages of quadruplicate experiments that were performed three times. *, P < 0.01 for MR/P ON compared to the wild type and MR/P OFF.
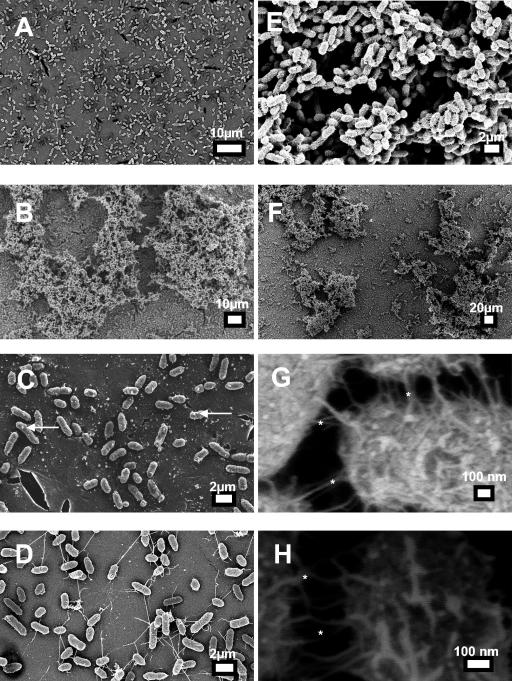
We then used scanning electron microscopy to directly visualize the biofilm development of the three strains, as described in Materials and Methods. Low-power magnification (×3,000) showed MR/P OFF cells adhering to the cover glass, which is the first step in biofilm formation (Fig. 4A). MR/P ON, on the other hand, had formed a biofilm with a typical three-dimensional structure of pillars and channels (Fig. 4B). Wild-type P. mirabilis appeared not to have formed microcolonies but adhered in single colonies to the cover glass (Fig. 4C). No hyperflagellated or elongated swarmer cells were observed in these initial stages of biofilm development. MR/P ON cells appeared to form microcolonies of adherent bacteria after 18 h of incubation which had a fuzzy appearance (Fig. 4E) and appeared to be adhering to each other with channels and spaces throughout the biofilm. A low magnification (×661) showed that MR/P ON formed a layer of bacteria on the cover glass and grew upward into a biofilm community from various areas (Fig. 4F). A higher-power examination of the MR/P ON biofilm showed fimbrial interactions as well as some amorphous substance in the spaces between the cells (Fig. 4G). We examined the interactions of MR/P ON bacteria at a magnification of ×300,000 and detected events consistent with fimbria-fimbria interactions (Fig. 4H).
FIG. 4.
SEM of 18-h biofilm grown on glass. Aliquots of overnight cultures of P. mirabilis HI4320, MR/P ON, and MR/P OFF were standardized to an OD600 of 0.05 and diluted 1:10 in fresh sterile urine on cover glass. The biofilms were incubated for 18 h at 30°C. The biofilms were rinsed with copious amounts of sterile distilled water and then processed for SEM as described in Materials and Methods. (A and D) MR/P OFF; (B and E to H) MR/P ON (asterisks denote surface structures that are consistent in size and shape with fimbriae); (C) wild-type P. mirabilis HI4320.
P. mirabilis HI4320 formed thicker biofilms than MR/P ON and MR/P OFF.
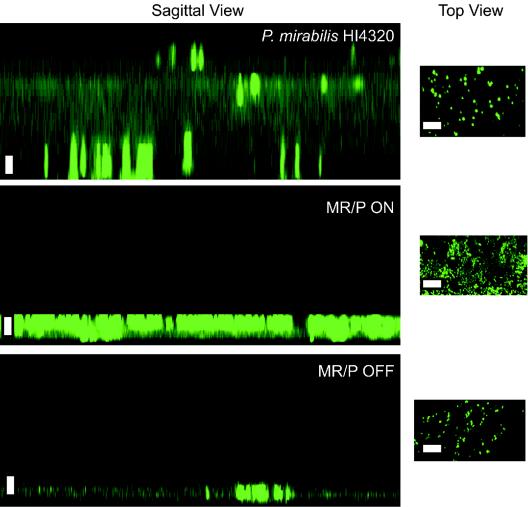
Wild-type P. mirabilis HI4320(pBAC001), MR/P ON(pBAC001), and MR/P OFF(pBAC001) were grown on cover glass for 7 days, and their biofilm depths were examined by laser scanning confocal microscopy (Fig. 5). z sections performed on these biofilms showed that MR/P ON and MR/P OFF formed biofilms averaging ∼10 μm thick while wild-type P. mirabilis formed a biofilm with a maximum thickness of 63 μm (Fig. 5A). Therefore, the constitutive production of MR/P fimbriae or the lack of MR/P fimbria expression prevents maximal biofilm development.
FIG. 5.
Overhead (xy) and sagittal (xz) images of wild-type and MR/P phase-locked mutants. All strains were transformed with pBAC001 (a plasmid encoding GFP) and grown at 30°C in urine placed on a cover glass. The cultures were grown in urine with ampicillin (100 μg/ml) for 7 days without shaking. Saturated urine was replaced with fresh urine and ampicillin every 48 h. After 7 days, each biofilm was rinsed to remove nonadherent bacteria, and the remaining attached cells were examined via confocal microscopy with a 63× oil immersion objective (numerical aperture, 1.4) on a Zeiss LSM410 instrument. Sagittal images were created from a collection of 30 consecutive z-series scans of the wild-type and mutant biofilms. The step size between each z section was 2 μm. Sagittal images were generated with the LSM410 PC software package. Bar = 10 μm.
MR/P fimbriation did not influence the number of P. mirabilis cells adhering to the bladder during experimental infection.
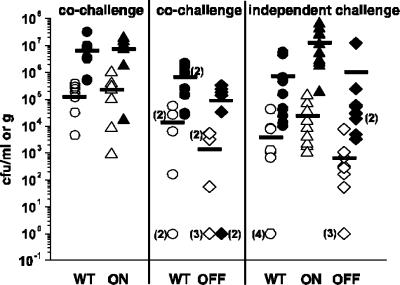
Mice were transurethrally inoculated with bacteria, either in independent challenges or in competition assays, with P. mirabilis HI4320 versus MR/P ON or P. mirabilis HI4320 versus MR/P OFF. After 24 h, the mice were anesthetized and cannulated, and their bladders were washed with 50 μl of PBS. When P. mirabilis HI4320 and MR/P ON were inoculated in a cochallenge experiment, their rates of washout and adherence were similar to those of the independent challenges (i.e., the ratios of adherent cells to washed-out cells were similar) (Fig. 6) and were consistent with data presented earlier in this study demonstrating that the majority of P. mirabilis cells have MR/P fimbriae during UTI. P. mirabilis HI4320 in competition with MR/P OFF colonized tissues in larger numbers, but this difference was not statistically significant. The inability to produce MR/P fimbriae did not impair MR/P OFF's ability to adhere to bladder tissue during vigorous washing. We postulate, however, that the localization of adherence was affected.
FIG. 6.
Numbers of adherent and nonadherent P. mirabilis cells remaining in murine bladders after washing. Strains were tested in cochallenge experiments (eight mice per group) with a transurethrally inoculated, ∼108-CFU equal mixture of wild-type P. mirabilis HI4320 and MR/P ON or wild-type P. mirabilis HI4320 and MR/P OFF. Independent challenges (10 mice/group) were also conducted with each strain. Bladders were washed at 24 h postinoculation and then homogenized. Both the wash fluid and the homogenized bladders were immediately plated to determine the numbers of nonadherent bacteria (open symbols) present in the wash fluid and the numbers of adherent bacteria (closed symbols) present in the homogenized bladders. Bars represent the means for all mice in each group. All values examined by the nonparametric Mann-Whitney test showed no significant differences among the strains' abilities to adhere in the bladder.
Distinct colonization patterns for MR/P ON and MR/P OFF in the murine bladder.
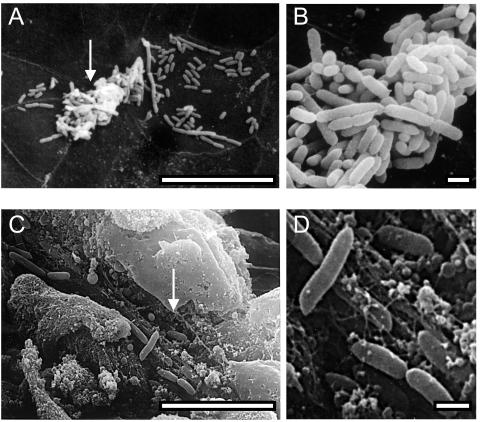
To determine the colonization patterns of the three strains, we examined infected bladders by SEM. Bladders from mice that were infected transurethrally were removed at 2 and 4 days postinoculation and then processed for SEM as described in Materials and Methods. Wild-type P. mirabilis colonized the surfaces of umbrella cells of the bladder (Fig. 7A and B). The bacteria did not invade the epithelium but instead colonized the luminal surfaces both in clusters and as single bacteria. The bacteria were approximately 1 to 2 μm in length and approximately 500 nm wide. The bacteria did not appear to express many flagella. The majority of these bacteria are known to express at least MR/P fimbriae (Fig. 1). Occasionally, wild-type P. mirabilis HI4320 was found colonizing areas of exfoliated tissue (Fig. 7C and D); these wild-type bacteria were found lying in rows rather than in clusters.
FIG. 7.
SEM micrographs of wild-type P. mirabilis HI4320 colonizing the murine bladder. Mice were transurethrally inoculated with 108 CFU of wild-type P. mirabilis HI4320. At 7 days postinoculation, the ureters of mice infected with wild-type P. mirabilis were ligated with surgical thread, the bladders were infused through the urethra with fixative (2% glutaraldehyde, 2% formaldehyde), and then the urethras were tied off. Bladders were aseptically removed and processed for SEM as described in Materials and Methods. Wild-type P. mirabilis HI4320 colonized the bladder uroepithelium (A and B) and, occasionally, areas of exfoliated bladder cells (C and D). Arrows point to areas that are enlarged to the right. Bars = 10 μm (A and C) and 1 μm (B and D).
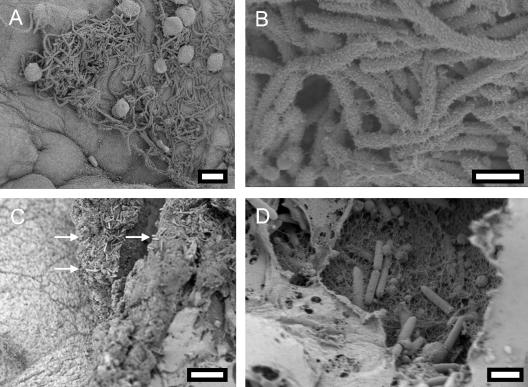
MR/P ON cells were found colonizing the intact bladder epithelium. These bacteria appeared to be heavily fimbriated and elongated (6 to 10 μm) (Fig. 8A). MR/P ON colonized the epithelium in clusters, and high-power magnification revealed that the bacteria were covered with hairy projections. In stark contrast to most wild-type P. mirabilis and MR/P ON cells, MR/P OFF cells were not found colonizing the surfaces of bladder umbrella cells but rather were localized to areas of exfoliation (Fig. 8C). Figure 8C shows an area of a bladder at 4 days postinfection in which the uroepithelium (polyhedral cells on the left side of the micrograph) was disrupted and the lamina propria was exposed (arrows point to bacteria colonizing the lamina propria). Indeed, in a thorough search of the entire luminal surfaces of five bladders, no MR/P OFF bacteria were observed colonizing the intact uroepithelium. However, since the total number of MR/P OFF CFU per gram of bladder tissue was not significantly different from that of the wild type or MR/P ON, we speculated that the bacteria must be localized to a distinct area of the bladder. Indeed, we discovered that the MR/P OFF bacteria colonized the underlying basement membrane of exfoliated areas of the bladder and were not found in clusters as was typical for MR/P ON and some wild-type P. mirabilis colonies. In addition, MR/P OFF did not appear to possess many flagella and had a much smoother surface than MR/P ON (Fig. 8D).
FIG. 8.
SEM micrographs of MR/P ON and MR/P OFF cells colonizing the murine bladder. Mice were transurethrally inoculated with 108 CFU of the mutants in independent experiments. At 4 days postinoculation, the ureters of infected mice were ligated with surgical thread, and the bladders were infused through the urethra with fixative (2% glutaraldehyde, 2% formaldehyde). After the urethras were tied off, the bladders were aseptically removed and processed for SEM as described in Materials and Methods. MR/P ON colonized the bladder uroepithelium (A and B), while MR/P OFF colonized the lamina propria where bladder cells had sloughed off (C [arrows point to MR/P OFF] and D). Bars = 10 μm (A), 2 μm (B and D), and 15 μm (C).
MR/P mutants retain their phenotypes during ascending infection.
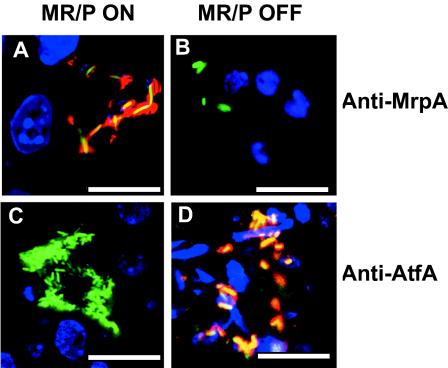
The bladders of mice that were infected with either MR/P ON(pBAC001) or MR/P OFF(pBAC001) were processed for confocal microscopy. Sections (10 μm thick) were stained with a rabbit anti-MrpA antibody, followed by a goat antibody generated against rabbit immunoglobulin G (IgG) and conjugated to Alexa Fluor 568. MR/P ON continued to produce MR/P fimbriae during infection (Fig. 9A), while MR/P OFF did not produce MR/P fimbriae (Fig. 9B). Therefore, it is consistent that the fimbriae seen by field emission scanning electron microscopy (FESEM) on MR/P ON cells were MR/P fimbriae. Interestingly, when examined by confocal microscopy using a specific antiserum, some MR/P OFF cells possessed a possible alternate adhesin, ATF (Fig. 9D), a fimbria type that is not thought to be typically expressed by P. mirabilis, except at 23°C. MR/P ON did not express this type of fimbriae (Fig. 9C).
FIG. 9.
MR/P and ATF expression during ascending UTI. Representative confocal laser scanning micrographs show that the phase-locked mutants continued to either express MR/P fimbriae (MR/P ON) (A) or to not express MR/P fimbriae (MR/P OFF) (B) during experimental UTI. Mice were infected transurethrally with either MR/P ON(pBAC001) or MR/P OFF(pBAC001). After 2 days, the mice were euthanized and their bladders were removed and processed for confocal microscopy as described in Materials and Methods. Sections (10 μm) were stained with a 1:200 dilution of rabbit anti-MrpA, followed by staining with goat anti-rabbit IgG conjugated to Alexa Fluor 568. Tissue sections were mounted with Vectashield containing DAPI. (A) MR/P ON-infected tissue. Green, GFP-expressing P. mirabilis; blue, DAPI-stained bladder cell nuclei; red, rabbit anti-MrpA reacted with goat anti-rabbit IgG conjugated to Alexa Fluor 568; yellow, colocalization of bacteria and fimbriae. (B) MR/P OFF-infected tissue. Blue, DAPI-stained bladder nuclei; green, GFP-expressing P. mirabilis. (C) MR/P ON does not express ATF (green bacteria). (D) MR/P OFF expresses ATF (green bacteria colocalizing with red-stained ATF appear yellow). Sections (10 μm) were stained with a 1:500 dilution of a rabbit anti-ATF serum, followed by staining with goat anti-rabbit IgG conjugated to Alexa Fluor 568. Tissue sections were mounted with Vectashield containing DAPI. For MR/P ON-infected tissue: green, GFP-expressing P. mirabilis; blue, DAPI-stained bladder cell nuclei. For MR/P OFF-infected tissue: green, GFP-expressing P. mirabilis; red, rabbit anti-ATF serum reacted with goat anti-rabbit IgG conjugated to Alexa Fluor 568; blue, DAPI-stained bladder nuclei (D). Bars = 10 μm.
DISCUSSION
We have demonstrated that MR/P fimbriae are produced in all urinary tract organs during P. mirabilis UTI. The proportion of the bacterial population that produced MR/P fimbriae was largest in the bladders (>85%), but the majority of bacteria in the ureters and kidneys also produced these fimbriae. The large proportion of bacteria producing MR/P fimbriae observed in this study is consistent with the results of a published report in which the orientation of the invertible element (15) that controls the transcription of mrp fimbrial genes was quantified. In the previous study, conducted on day 7 postinoculation, the invertible element was found to be in the on orientation in >90% of the bacterial population colonizing the bladders. The present study verifies the prediction that the mrp operon is transcribed in vivo at a high level and reports the first direct demonstration of MR/P fimbrial production in the urinary tract. Since bacteria in inocula lacked fimbriae, the presence of fimbriae in infected tissues suggests that MR/P fimbria expression is important during colonization of the urinary tract.
Previous reports from our laboratory and others indicated that MR/P fimbriae are an important P. mirabilis virulence factor in ascending UTI. One week after transurethral inoculation in the mouse, an mrpA mutant (mrpA encodes the major fimbrial subunit) was outcompeted by the wild-type strain in cochallenge experiments (15). For independent challenges, two outcomes were noted. First, the mrpA mutant was recovered in significantly lower numbers than the parent strain from the urine, bladders, and kidneys in a CBA mouse model of ascending UTI. Overall, colonization was reduced 6-, 28-, and 18-fold, respectively (3). In a second study, a strain that was incapable of making MR/P fimbriae was able to colonize the bladder in significant numbers (15). Our present study supports the latter, more recent experiment. Thus, MR/P fimbriae contribute to but are not absolutely required for colonization of the murine urinary tract. Therefore, colonization likely involves several factors, one of which is MR/P fimbriae.
Use of MR/P phase-locked mutants.
To investigate the possible roles of MR/P fimbriae, we used mutants (15) that either constitutively expressed or lacked MR/P fimbriae. In these mutants, mrpI was insertionally inactivated with a kanamycin cassette via homologous recombination. MrpI is the sole recombinase that is capable of switching the invertible element governing MR/P expression, and thus this insertion created two phase-locked mutants. The first mutant always expressed MR/P fimbriae (MR/P ON), while the other mutant never expressed MR/P fimbriae (MR/P OFF). We tested the ability of the mutants to produce MR/P fimbriae by using several methods. First, a PCR-based assay to determine the orientation of the invertible element showed that the promoter always remained in the expected orientation, the orientation to drive expression of the mrp operon in the MR/P ON strain or the opposite orientation in the MR/P OFF strain (15). The orientation of the invertible element never varied, whether bacteria were cultured on agar or in LB or were inoculated into mice and then plated from bladder and kidney homogenates. Secondly, the ability to agglutinate chicken erythrocytes in the presence of mannose, a phenotype consistent with MR/P fimbria production, was always present in the MR/P ON strain and never occurred in the MR/P OFF strain (15). Thirdly, in this study, antibodies generated against MrpA (the major pilin subunit) bound MR/P ON and did not bind MR/P OFF, regardless of whether the organisms were cultured in LB or on agar or were assayed from sections of infected mouse bladders, ureters, or kidneys.
Swarming motility.
To characterize these mutants, we measured their ability to swarm, a phenotype that is associated with P. mirabilis flagella and inversely associated with MR/P fimbria production (9). On agar, MR/P ON bacteria, which constitutively expressed MR/P fimbriae, were able to swarm as quickly as cells of the parental wild-type strain, which did not produce MR/P fimbriae when cultured on agar. This was an unexpected result because the MR/P ON strain must use energy and resources to synthesize fimbriae, a phenotype that is thought to be unnecessary for growth on agar, yet fimbrial synthesis did not inhibit the swarming motility. MR/P ON bacteria produced both fimbriae and flagella, a phenotype that is not seen with wild-type P. mirabilis. This concurrent production of organelles with functions that are thought to be contradictory provides an interesting insight regarding the regulation of synthesis of these organelles. First, this confirms the published report by Li and colleagues (15) that MrpI is the sole recombinase that is capable of flipping the mrp operon promoter. Since the bacteria continued to express fimbriae in an environment where they are not usually expressed, such as when P. mirabilis was cultured on agar or during swarming, there appears to be no other recombinase that is capable of switching the invertible element.
Secondly, the constitutive expression of mrpJ, which is the last gene in the mrp operon and encodes a repressor of flagellin synthesis (17), did not hinder flagellin production during swarming in this strain. Li and colleagues showed that the constitutive expression of mrpJ from a high-copy-number plasmid reduced swarming (17). However, MR/P ON expresses mrpJ from a single chromosomal copy of the gene. Therefore, this gene is not expected to be highly expressed, and certainly not as highly as from a high-copy-number plasmid. Although the environmental cues for swarming led to an approximately eightfold increase in the expression of flaA (the flagellin gene) (5), this level was not high enough to account for the thousands of flagella on the surfaces of swarmer cells. Perhaps there is some posttranscriptional regulation that aids in the increased numbers of flagella produced on the surfaces of swarmer cells. Since MrpJ is a putative DNA binding protein and hence works at the transcriptional level, its presence would not affect posttranscriptional modifications made during swarming.
The observation that MR/P OFF swarmed significantly faster than the wild type was also unexpected. The numbers of flagella on swarming wild-type bacteria and swarming MR/P OFF bacteria appeared to be similar when viewed by SEM, and thus the reason for the increased velocity of MR/P OFF is not yet understood. On agar, wild-type bacteria do not express MR/P fimbriae, and the MR/P OFF mutant does not express fimbriae either. Therefore, one would hypothesize that the two strains would swarm at similar velocities. This was, however, not the case. The loss of MrpI also does not explain the increased swarming speed of MR/P OFF, because MrpI is not functional in MR/P ON, which swarmed at the same velocity as the wild type. Although it has been documented that wild-type P. mirabilis HI4320 does not produce MR/P fimbriae when cultured on agar, there may be low levels of transcription of the mrp operon, including mrpJ, and thus there may be low levels of MrpJ in the bacteria in the absence of fimbrial production. If this occurs in the wild-type strain, then it almost certainly happens in the MR/P ON strain, wherein the genes of the mrp operon are transcribed and translated. The mechanism by which MrpJ affects motility is unknown. High-level expression of mrpJ from a plasmid represses swarming, and thus there appears to be a role for MrpJ during swarming (17). Currently, however, the ability of the MR/P OFF mutant to swarm at an increased velocity remains unexplained.
Biofilm formation.
Although earlier studies investigated the initial stages of UTI in a mouse model, biofilm formation has been shown to be important for persistent infections, particularly for catheter-associated bacteriuria. Thus, we investigated the contribution of MR/P fimbriae to biofilm formation. Biofilm formation of P. mirabilis on catheter material is a well-documented phenomenon (19, 21, 23, 30). However, it is unknown which gene products are important for P. mirabilis biofilm development. Pratt and Kolter suggested that gene products which are important for biofilm development are also important for pathogenesis (29). Autoaggregation likely aids bacteria in persisting in the host despite actions of the immune system. Bacteria that are adherent to the epithelium and encased by other bacteria may be protected from neutrophils and the activity of complement. Perhaps the autoaggregation of bacteria allows an increased density of bacteria that permits signaling molecules to be passed among the population. This could enable bacteria to determine when they have reached a critical mass, triggering virulence gene expression, such as occurs as the result of quorum sensing in Pseudomonas aeruginosa (12, 27). The ability to autoaggregate is also associated with the ability of Lactobacillus spp. to adhere to the host epithelium (10). Finally, aggregation might permit quantitatively more adherence, with bacteria piling on top of each other. Therefore, we tested the contribution of MR/P fimbriae to biofilm formation and, perhaps, pathogenesis.
The constitutive expression of MR/P fimbriae enhanced biofilm development in a 48-h crystal violet biofilm assay. However, MR/P OFF, which does not express MR/P fimbriae, was able to form a biofilm similar to that formed by wild-type P. mirabilis HI4320. E. coli DH5α expressing MR/P fimbriae constitutively from a plasmid was unable to form biofilms. Therefore, MR/P fimbriae are neither necessary nor sufficient to initiate biofilm formation, yet if they are constitutively expressed, these surface structures enhance biofilm formation.
To examine the structures of the biofilms, we grew wild-type P. mirabilis and the two mutants on cover glass in sterile urine for 18 h at 30°C. The biofilms were rinsed with sterile distilled water and processed for SEM. We then examined the biofilms by SEM. MR/P ON formed a characteristic three-dimensional biofilm structure with channels and mounds, while MR/P OFF and wild-type P. mirabilis were both in the initial stage of adherence and did not form microcolonies. Therefore, the loss of the ability to express MR/P fimbriae did not abolish the ability of the bacteria to form biofilms, as the wild-type and MR/P OFF biofilms were similar. The constitutive expression of MR/P fimbriae, however, leads to quicker biofilm formation. MR/P ON did not merely form microcolonies of aggregated bacteria. The biofilms formed by MR/P ON were consistent with the definition of a biofilm, with mushroom-shaped mounds with channels flowing between them. Perhaps the autoaggregative character of MR/P ON allowed the initial adherence to the coverslip and to each other to occur rapidly, thus allowing the bacteria to quickly move to the next stage of biofilm development, while the wild-type strain and MR/P OFF took more time to undergo these initial steps. Therefore, the constitutive production of MR/P fimbriae enhances biofilm development during the first 48 h.
However, after 7 days of biofilm formation by GFP-expressing bacteria, the wild-type strain created a thick (maximum depth, ∼63 μm) biofilm while both mutants were able to form a biofilm with only one-fifth the thickness of the wild-type biofilm (maximum depth, ∼12 μm). We were able to measure the biofilm thicknesses by taking z sections of the biofilms by confocal scanning laser microscopy. Studies conducted in our laboratory showed that the production of GFP did not affect P. mirabilis growth or biofilm development (data not shown). Therefore, while the constitutive production of MR/P fimbriae on bacterial surfaces enhances biofilm development in the early stages, the ability to switch off the expression of these surface organelles is important for the biofilm to develop fully. Pratt and Kolter (29) studied the development of biofilms and found that many insertional mutations in genes encoding diverse products, such as flagella and type 1 fimbriae, inhibited maximum E. coli biofilm production.
Bladder washout studies.
The ability of MR/P OFF to persist in the bladder despite vigorous washing was a surprising result. Typically, fimbriae produced via the chaperone or usher pathway are adhesive organelles. Therefore, we would have predicted that the lack of this adhesin in MR/P OFF would allow the bacteria to be washed from the bladder more easily than bacteria producing MR/P fimbriae. This was not the case, however, as the proportions of bacteria washed from bladders during our assay were similar for wild-type P. mirabilis, MR/P ON, and MR/P OFF. Two possible explanations for this observation can be offered. First, the washing may have been too vigorous. PBS was delivered into the bladder, withdrawn, and reinjected 10 times. We washed the bladders vigorously because we wanted more turbulence than that provided by the flushing mechanism of micturation. Perhaps if we had adopted a less strenuous protocol, more subtle differences among the strains would have been seen. A second possible explanation is that MR/P OFF cells express surface adhesins that are not normally thought to be important for P. mirabilis during bladder colonization and that these adhesins compensate for the lack of MR/P fimbriae. One of these possible atypically expressed adhesins is ATF. ATF is a fimbrial type that is typically expressed in P. mirabilis at 23°C (20). Zunino and colleagues tested the ability of an ATF null mutant to colonize the urinary tract and found that it colonized as well as the wild type (35). We have shown, by using a rabbit anti-ATF antiserum, that MR/P OFF expresses ATF in bladder tissue. This does not prove, however, that this fimbrial type is the cause of the bladder washout results or results discussed later regarding the colonization patterns of the strains. It is merely an observation that when one fimbrial system is disabled, other adhesins that may not have been expressed previously or that were not necessarily important in healthy wild-type bacteria in this environment are produced.
Colonization of the bladder.
We observed distinct patterns of colonization of P. mirabilis in the presence and absence of MR/P fimbrial expression. We used SEM to qualitatively examine the colonization patterns of P. mirabilis HI4320, MR/P ON, and MR/P OFF in the bladder. MR/P OFF localized exclusively to areas of bladder cell exfoliation where the connective tissue and lamina propria were exposed. Indeed, without MR/P fimbriae, P. mirabilis cells were apparently not able to colonize the luminal surfaces of undamaged epithelium, but instead were found as single bacteria attached to the areas of exfoliation. Bacteria adhered to areas of trauma in which the umbrella cells had exfoliated, leaving the underlying basement membrane exposed. This is consistent with examples in the literature of bacteria binding to areas that are not typically exposed in the lumen of an organ. For example, Kukkonen and colleagues showed type 1 fimbria-mediated adherence to the laminin network in basement membranes in Salmonella enterica and E. coli (11).
In contrast to MR/P OFF, MR/P ON heavily colonized the umbrella (uroepithelial) cells of the bladder. At a high magnification (×250,000), MR/P ON bacteria appeared to interact via fimbria-like appendages. Since these bacteria overproduce MR/P fimbriae and since a rabbit anti-MrpA serum bound MR/P ON cells isolated from Luria-Bertani broth, LB agar, and bladder, ureter, and kidney tissues, these surface appendages were most likely MR/P fimbriae. The evidence gathered in this study of the autoaggregative properties of MR/P fimbriae is not as surprising as the previous results. The autoaggregative properties of MR/P fimbriae were described previously, when the mrp operon was constitutively expressed from a plasmid in E. coli DH5α. The bacteria formed a pellicle at the liquid-air interface of the test tube when grown in LB (15). When MR/P ON was pelleted, it formed tight clumps and was difficult to resuspend (X. Li, personal observation). Here we documented fimbria-fimbria interactions of bacteria constitutively expressing MR/P fimbriae in the urinary tract.
P. mirabilis HI4320 formed colonies on umbrella cells, which was reminiscent of both mutants' colonization patterns. Some wild-type bacteria were found in autoaggregated patterns. An examination of these bacteria at a higher magnification revealed a fuzzy appearance similar to that of MR/P ON cells. Occasionally, P. mirabilis HI4320 colonized areas of exfoliation. These colonies contained bacteria that lay as single cells upon the epithelium. These bacteria had smoother surfaces that were more reminiscent of MR/P OFF cells. This behavior was consistent with the observation that the majority of P. mirabilis bacteria produced MR/P fimbriae in the bladder, so at a population level, the bacteria will either aggregate or bind host tissues singly. There has been much more information published on the colonization patterns of uropathogenic E. coli than on those of P. mirabilis. For example, Hultgren and colleagues have published observations of E. coli invading the bladder uroepithelium and forming foci of intracellular bacteria. They also observed these bacteria reemerging in a filamentous form 6 h later (24). In a recent Science article, they published observations of intracellular E. coli forming biofilms (2). These bacteria produced surface proteins, such as type 1 fimbriae and Ag43, that have been shown to be expressed during biofilm formation. We saw no evidence of P. mirabilis behaving in this manner. In MR/P OFF, perhaps the inability to express MR/P fimbriae allows the expression of alternate adhesins with affinities for receptors on the basal membrane underlying the umbrella cells. Cross-regulation by fimbriae as well as by flagella has been well documented (3, 15, 25, 34). As described earlier, MR/P OFF cells express ATF in vivo, a phenotype which is thought not to occur during wild-type colonization of the urinary tract. The inability to express MR/P fimbriae abolishes binding to the normal bladder epithelium and the ability to autoaggregate, but it appears to allow bacteria to express alternate adhesive organelles, such as laminin, fibronectin, or collagens, so that the bacteria can bind alternative sites in the bladder during ascending UTI. Type 1 fimbriated S. enterica and E. coli have been shown to bind laminin (11); therefore, not only are bacteria able to bind to the luminal side of epithelial cells, but they are also capable of taking advantage of a disrupted epithelium.
In this study, we have shown that MR/P fimbriae, a well-established virulence factor, enhance the autoaggregation of P. mirabilis during ascending UTI. In addition, the majority of infecting bacteria in the bladder, ureters, and kidneys produce these autoaggregative MR/P fimbriae.
Acknowledgments
This work was supported by Public Health Service grant DK49720 from the National Institutes of Health.
Editor: D. L. Burns
REFERENCES
- 1.Abraham, J. M., C. S. Freitag, J. R. Clements, and B. I. Eisenstein. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 82:5724-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani, F. K., G. Massad, C. V. Lockatell, D. E. Johnson, R. G. Russell, J. W. Warren, and H. L. Mobley. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani, F. K., and H. L. Mobley. 1993. Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J. Bacteriol. 175:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belas, R. 1994. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J. Bacteriol. 176:7169-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 7.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen, A. M., C. V. Lockatell, D. E. Johnson, and H. L. T. Mobley. 2003. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect. Immun. 71:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kos, B., J. Suskovic, S. Vukovic, M. Simpraga, J. Frece, and S. Matosic. 2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 94:981-987. [DOI] [PubMed] [Google Scholar]
- 11.Kukkonen, M., T. Raunio, R. Virkola, K. Lahteenmaki, P. H. Makela, P. Klemm, S. Clegg, and T. K. Korhonen. 1993. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol. Microbiol. 7:229-237. [DOI] [PubMed] [Google Scholar]
- 12.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 13.Li, X., C. V. Lockatell, D. E. Johnson, M. C. Lane, J. W. Warren, and H. L. T. Mobley. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect. Immun. 72:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, X., D. E. Johnson, and H. L. Mobley. 1999. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, X., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 45:865-874. [DOI] [PubMed] [Google Scholar]
- 16.Li, X., and H. L. Mobley. 2002. Vaccines for Proteus mirabilis in urinary tract infection. Int. J. Antimicrob. Agents 19:461-465. [DOI] [PubMed] [Google Scholar]
- 17.Li, X., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X., H. Zhao, L. Geymonat, F. Bahrani, D. E. Johnson, and H. L. Mobley. 1997. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect. Immun. 65:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaw, S. J., H. C. Lai, S. W. Ho, K. T. Luh, and W. B. Wang. 2003. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J. Med. Microbiol. 52:19-28. [DOI] [PubMed] [Google Scholar]
- 20.Massad, G., F. K. Bahrani, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 62:1989-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean, R. J., J. R. Lawrence, D. R. Korber, and D. E. Caldwell. 1991. Proteus mirabilis biofilm protection against struvite crystal dissolution and its implications in struvite urolithiasis. J. Urol. 146:1138-1142. [DOI] [PubMed] [Google Scholar]
- 22.Mobley, H. L., and J. Warren. 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25:2216-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, N. S., D. J. Stickler, and C. Winters. 1997. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 80:58-63. [DOI] [PubMed] [Google Scholar]
- 24.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 26.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 27.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrino, R., U. Galvalisi, P. Scavone, V. Sosa, and P. Zunino. 2003. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol. Med. Microbiol. 36:103-110. [DOI] [PubMed] [Google Scholar]
- 29.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 30.Sabbuba, N., G. Hughes, and D. J. Stickler. 2002. The migration of Proteus mirabilis and other urinary tract pathogens over Foley catheters. BJU Int. 89:55-60. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schembri, M. A., K. Kjaergaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-267. [DOI] [PubMed] [Google Scholar]
- 33.Warren, J. W., J. H. Tenney, J. M. Hoopes, H. L. Muncie, and W. C. Anthony. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146:719-723. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23:1009-1019. [DOI] [PubMed] [Google Scholar]
- 35.Zunino, P., L. Geymonat, A. G. Allen, C. Legnani-Fajardo, and D. J. Maskell. 2000. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol. Med. Microbiol. 29:137-143. [DOI] [PubMed] [Google Scholar]