Abstract
The major invasive factor of Yersinia enterocolitica, the invasin (Inv) protein, induces proinflammatory host cell responses, including interleukin-8 (IL-8) secretion from human epithelial cells, by engagement of β1 integrins. The Inv-triggered β1 integrin signaling involves the small GTPase Rac; the activation of MAP kinases, such as p38, MEK1, and JNK; and the activation of the transcription factor NF-κB. In the present study, we demonstrate that Y. enterocolitica YadA, which is a major adhesin of Y. enterocolitica with pleiotropic virulence effects, induces IL-8 secretion in epithelial cells. The abilites of YadA and Inv to promote adhesion to and invasion of HeLa cells and to induce IL-8 production by the cells were investigated by expression of YadA and Inv in Escherichia coli. While YadA mediates efficacious adhesion to HeLa cells, it mediates marginal invasion compared with Inv. Both YadA and Inv trigger comparable levels of IL-8 production. Conformational changes of the YadA head domain by mutation of NSVAIG-S motifs, which abolish collagen binding, also abolish adhesion of Yersinia to HeLa cells and YadA-mediated IL-8 secretion. Furthermore, experiments in which blocking antibodies against β1 integrins were used demonstrate that β1 integrins are crucial for YadA-mediated IL-8 secretion. Inhibitor studies demonstrate the involvement of small GTPases and MAP kinases, such as p38, MEK1, and JNK, indicating that β1 integrin-dependent signaling mediated by Inv or YadA involves similar signaling pathways. These data present YadA, in addition to Inv, YopB, and Yersinia lipopolysaccharide, as a further inducer of proinflammatory molecules by which Y. enterocolitica might promote inflammatory tissue reactions.
Yersinia pseudotuberculosis and Yersinia enterocolitica are enteropathogenic to humans and cause gastrointestinal disorders, such as enterocolitis and mesenteric lymphadenitis, as well as reactive arthritis, erythema nodosum, and septicemia (5, 12). The pathogenicity of Yersinia is determined by a number of virulence factors that are encoded either by genes of the bacterial chromosome or by genes present in the pYV (Yersinia virulence) plasmid (8, 11). The factors encoded by the virulence genes comprise adhesins and invasins, such as invasin (Inv) and Yersinia adhesin A (YadA), as well as the secreted Yersinia outer proteins (Yops) (9, 10).
Previous studies demonstrated that Inv is important in the early phase of intestinal infection (39, 40), most likely for efficient translocation of the bacteria through the M cells and colonization of the Peyer's patches (47). The 192 and 195 carboxy-terminal amino acids of Y. pseudotuberculosis and Y. enterocolitica Inv proteins, respectively, are required for binding to β1 integrins and induction of invasion (28, 31, 46, 58). Beads coated with the C-terminal 195 amino acids of Y. enterocolitica Inv may induce interleukin-8 (IL-8) synthesis by triggering the degradation of IκBα and subsequent translocation of NF-κB p50/p65 and p65/p65 dimers to the nucleus (46). Adhesion of the bacteria to the host cell without internalization is adequate for the induced IL-8 expression (49), suggesting that Inv acts as a ligand for a receptor-mediated signaling pathway. Moreover, it was demonstrated that Rac1 and mitogen-activated protein (MAP) kinases, such as p38 and JNK, are involved in Inv-triggered IL-8 production (21).
Upon infection with Yersinia or other bacteria, such as Salmonella, Shigella, or Escherichia coli (enteropathogenic E. coli), intestinal epithelial cells produce a variety of proinflammatory cytokines, including IL-8 (29, 43). IL-8 is an important chemoattractant and activator of polymorphonuclear leukocytes, and epithelial cells (43) were considered “watchdogs” for the innate and adaptive immune system (15). The production of chemokines may play an ambiguous role in infections, as, e.g., Yersinia is resistant to phagocytosis by polymorphonuclear leukocytes. Therefore, recruitment of these cells may cause tissue disruption, dissemination of the bacteria, and disease rather than contribute to infection control (1, 20, 33).
The inv gene is expressed predominantly at 27°C (38). However, recent work has demonstrated that Inv is also expressed at 37°C at low pH (38). In vivo studies with Inv-deficient mutants revealed that the inv gene is not essential for establishing an infection. Thus, although the initial infection process in the Peyer's patches is delayed upon infection with Y. enterocolitica inv mutant strains, the 50% lethal dose of an inv mutant strain is unaltered (39).
In contrast, YadA appears to be essential for establishing an infection, as yadA-deficient Y. enterocolitica mutants are avirulent in mice (14, 37). YadA mediates adhesion to host cells and confers resistance against complement and defensin bactericidal activity (56). Structural analysis of YadA revealed lollipop-shaped surface projections composed of trimeric YadA proteins (25, 26, 36). Although there is evidence that YadA may bind to host cell β1 integrins, its role in host-pathogen interaction is still unclear. Recent data has suggested that under certain growth conditions (low Ca2+ and high Mg2+ concentrations) or by overexpression of YadA in E. coli, YadA of Y. pseudotuberculosis mediates uptake into host cells, possibly via extracellular-matrix-dependent bridging between YadA and host cell β1 integrins (16). From these data, it was concluded that YadA mediates internalization into host cells under environmental conditions in which Inv is repressed. Although inhibitor studies revealed that Ser/Thr kinases, as well as phosphatidylinositol-3-kinase, are involved in the uptake process, the host cell signaling processes activated by YadA are not clear.
The aim of the study presented here was to investigate whether YadA, like Inv, might induce cytokine production in host cells. Moreover, we wanted to know whether YadA also exploits β1 integrin signaling pathways to accomplish this. Finally, we addressed whether the signaling checkpoints, small GTPases and MAP kinase, are involved in YadA-triggered cytokine production. Our results indicate that YadA in fact induces activation of a signaling pathway leading to NF-κB activation and IL-8 production, suggesting that the induction of inflammatory host cell responses should be added to the list of pleiotropic virulence functions of YadA.
MATERIALS AND METHODS
DNA constructs.
The plasmids pYMS4505 (52), expressing Y. enterocolitica YadA from serotype O:3; pYMS4505-M6 (52); pYL8 (53), expressing Y. enterocolitica YadAO:3 mutants; and pEEV1 (with an additional restriction site compared to pTM100), used as a control, have been described previously and are derived from the expression vector pTM100 (34) (Table 1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Description | Reference or source |
|---|---|---|
| Y. enterocolitica pYV− | Serotype O:8; virulence plasmid-cured derivative of Y. enterocolitica WA-314 | 24 |
| Y. enterocolitica pYV−Δinv | Serotype O:8; virulence plasmid-cured derivative of Y. enterocolitica WA-314; inv gene is knocked out by a kanamycin resistance cassette | 41 |
| E. coli HB101 | F−D(gpt-proA)62 leuB6 glnV44 ara-14 galK2 lacY1 D(mcrC-mrr) rpsL20(Strr) xyl-5 mtl-1 recA13 | 42 |
| pEEV1 | pTM100 derivative with a SacI restriction site engineered in the cat gene | 34 |
| pYMS4505 | yadAYeO3 cloned as a 1,753-bp PCR fragment into the BamHI site of pTM100; Cmr; yadA under control of the interrupted tetracycline resistance gene of the vector | 52 |
| pYMS4505-M6 | pYMS4505 expressing YadA-M6 (V156D, A157D, I158E); Cmr; yadA under control of the interrupted tetracycline resistance gene of the vector | 52 |
| pYL8 | yadAΔ83-104 and the lcrF genes of Y. enterocolitica O:3 cloned into pTM100; Cmr; under control of the natural yadA promoter and lcrF | 53 |
| pNIV 136 | Derivative of pIN-III-ompA2; artificial DNA cloning vector (gi392964, AJ223122) | 19, 49 |
| pInv1914 | Derivative of pNIV136, expressing Inv of Y. enterocolitica O:9 under control of an IPTG-inducible lpp promoter containing a lacUV5 promoter-operator region | 45 |
| pYadAO:8 | Derivative of pNIV136 expressing YadAO:8 under control of an IPTG-inducible lpp promoter containing a lacUV5 promoter-operator region | This study |
The vector pYadAO:8 was constructed by amplifying the yadA gene from pYV plasmid Y. enterocolitica serotype O:8 by PCR using the forward primer 5′-CCATGGGAACTAAAGATTTTAAGATCAGTG-3′ (the NcoI restriction site is underlined) and the reverse primer 5′-AGATCTATTAGGATTAATACAGGCGCAg-3′ (the BglII restriction site is underlined) according to the published sequence (51). The amplified DNA fragments (digested with NcoI and BglII) were cloned into the pNIV136 expression vector, resulting in pYadAO:8.
Bacterial strains and growth conditions.
All of the bacterial strains used are listed in Table 1. Plasmid-cured Y. enterocolitica WA-314 pYV− (24), a Y. enterocolitica WA-314 pYV− Δinv mutant (41), noninvasive E. coli HB101 pNIV136 (49), and E. coli HB101 pInv1914 expressing Y. enterocolitica Inv under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter (49) and E. coli HB101 pYadAO:8 expressing Y. enterocolitica YadA from serotype O:8 under the control of an IPTG-inducible promoter (this work) were grown in Luria-Bertani broth (LB). The same medium was used for the cultivation of E. coli and Y. enterocolitica WA-314 pYV− Δinv pYMS4505 expressing Y. enterocolitica YadA from serotype O:3 and E. coli and Y. enterocolitica WA314 pYV− Δinv pYMS4505-M6 and pYL8 (52), both expressing collagen-binding mutants of YadA, and E. coli and Y. enterocolitica WA314 pYV− Δinv pEEV1 (used as a control).
Cell culture and infection.
Human HeLa cervical epithelial cells (ATCC CCL-2.1; American Type Culture Collection, Manassas, Va.) were maintained in RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 2 mM glutamine (Invitrogen, Karlsruhe, Germany) and 10% fetal calf serum (Sigma, Taufkirchen, Germany). The fibroblast-like GD25 and GD25-β1A cell lines were a kind gift from R. Faessler (MPI, Munich, Germany). The murine GD25 cells were derived from the embryonic stem cell clone G201, which is deficient in the β1 integrin subunit (18). The stably transformed cell line GD25β-1A was obtained by electroporating wild-type integrin β-1A cDNA into GD25 cells (57). GD25 cells which did not express β1 integrins were cultivated in DMEM (Gibco, Karlsruhe, Germany) supplemented with 2 mM glutamine (Invitrogen) and 10% FCS (Sigma), and the GD25-β1A cell line expressing β1 integrins was maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10 μg of puromycin (Sigma)/ml. For infection, cells were grown overnight in 24-well plates and infected with either Y. enterocolitica or E. coli strains expressing Inv, YadA, or YadA mutants. Before infection, the cells were washed three times with prewarmed phosphate-buffered saline (PBS), and fresh RPMI 1640 medium was added. The bacteria were grown overnight in LB at either 27°C for Y. enterocolitica WA-314 pYV− and Y. enterocolitica WA-314 pYV− Δinv or 37°C for Y. enterocolitica WA-314 pYV+ and E. coli strains. The bacteria were diluted 1:10 in LB and grown for another 2 to 3 h at 27 or 37°C. If necessary, IPTG was added to a final concentration of 0.1 mM 30 min before the bacteria were harvested. The bacteria were collected by centrifugation and washed twice with PBS. After determination of the optical density at 600 nm, appropriate dilutions of the bacteria in PBS were performed to infect the cells with a multiplicity of infection (MOI) of 100 bacteria/cell. An MOI of 100 was used in all infection experiments. Monolayers of cells and bacteria were incubated for 1 h at 37°C. After removal of the medium, the cells were washed three times with PBS to remove extracellularly located bacteria, and fresh medium with 100 μg of gentamicin/ml to kill remaining extracellular bacteria was added. After a further 3 h, the supernatants of the cells were collected and the levels of cytokines were determined by enzyme-linked immunosorbent assay (ELISA). TNF-α was a kind gift from G. Adolf (Bender, Vienna, Austria).
Cell adhesion and invasion assays.
For preparation of the cell adhesion and uptake assay, 105 HeLa or GD25 cells per well were seeded and grown overnight in 24-well cell culture plates (Nunc Life Technologies, Wiesbaden, Germany) to a confluent cell layer to avoid adherence of the bacteria to the plastic. Cell monolayers were washed once with phosphate-buffered saline and incubated for 2 h with fresh RPMI 1640 or DMEM without antibiotics before the addition of bacteria (MOI, 100). For blocking studies, increasing concentrations (from 0 to 10 μg/ml) of the anti-human monoclonal β1 integrin (CD29) antibody Lia1/2 (Immunotech, Marseille, France) were added to the medium 0.5 h before infection. For adhesion assays, the cells were washed three times with phosphate-buffered saline 15 min postinfection. The total number of adherent bacteria was determined by cell lysis with 1% Triton X-100 and plating on selective LB agar plates. Bacterial invasion was assessed 15, 30, and 60 min after infection. After this time, the cells were washed three times with phosphate-buffered saline, new medium with 100 μg of gentamicin/ml was added, and the cells were incubated for another 2 h at 37°C and then lysed with 1% Triton X-100 and plated on selective LB agar plates in appropriate dilutions. All adhesion and invasion experiments for each condition were performed in duplicate, and at least three independent experiments were done.
Inhibitors.
Clostridium difficile toxin TcdB10463 was purified as previously described (6, 35). The MAP kinase inhibitors SB202190, PD98059, and SP600125 were purchased from Calbiochem (Schwalbach, Germany).
IL-8 ELISA.
The amount of IL-8 secreted by HeLa cells into the supernatant was determined as previously described (49) using an ELISA with optimal concentrations of a mouse anti-human IL-8 monoclonal antibody (G265-5; BD Biosciences Pharmingen, San Diego, Calif.) and a biotinylated mouse anti-human IL-8 monoclonal antibody (G265-8; BD Biosciences Pharmingen) as the detecting antibody. IL-8 concentrations were calculated from the straight-line portion of a standard curve derived using recombinant human IL-8 (BD Biosciences Pharmingen).
Electrophoretic mobility shift assay (EMSA).
HeLa cells (5 × 106) were infected as described above. At various intervals postinfection, nuclear extracts were prepared as previously described (44). Aliquots of the supernatant containing nuclear proteins were stored at −80°C. Protein concentrations were determined by the Bradford assay. The oligonucleotide probes described below were labeled with [γ-32P]ATP (Amersham Biosciences, Uppsala, Sweden) using T4-polynucleotide kinase (New England Biolabs, Beverly, Mass.) and then purified on a NucTrap probe purification column (Stratagene, Amsterdam, The Netherlands). The following oligonucleotides were used: IL-8-κB wild type (5′-ATCGTGGAATTTCCTCTGA-3′; Metabion, Munich, Germany) and IL-8-κB mutant (5′-ATCCTGCAATGTCGTCTGA-3′; Metabion). Nuclear extracts (3 to 6 μg) were incubated with 30,000 cpm of the 32P-labeled oligonucleotide probe for 45 min on ice in a buffer containing 5% glycerol, 80 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA (pH 8.0), 10 mM Tris-HCl (pH 7.2), and 1 μg of poly(dI-dC) for NF-κB shifts. Samples were resolved on a 5% nondenaturating polyacrylamide gel using 0.5× TBE (25 mM Tris-HCl, 25 mM boric acid, 0.5 mM EDTA) as a running buffer. The gels were transferred to Whatman 3M paper and dried under vacuum. Protein binding was detected by autoradiography.
Immunofluorescence microscopy.
HeLa cells were seeded on coverslips and grown overnight at 37°C. Cells were infected with E. coli pYadAO:8 or E. coli pInv1914 for 2 h. After 3% paraformaldehyde (Sigma) fixation of the monolayers, extracellular E. coli pYadAO:8 was stained with a polyclonal rabbit anti-P1.8 antibody (diluted 1:100) and E. coli pInv1914 was stained with a polyclonal rabbit anti-Inv 3.1 antibody (diluted 1:100), followed by Cy-5-conjugated goat anti-rabbit antibodies (Dianova, Hamburg, Germany). After three washings with phosphate-buffered saline, the cells were permeabilized by 2% Triton X-100 in phosphate-buffered saline and washed, and intracellular bacteria were stained by polyclonal rabbit anti-P1.8 antibody or polyclonal rabbit anti-Inv 3.1 antibody, followed by fluorescein isothiocyanate-conjugated goat anti-rabbit antibodies (Dianova). F-actin was stained by tetramethyl rhodamine isothiocyanate-conjugated phalloidin (Sigma). The fluorescence images were obtained with a DM IRE2 confocal laser scan microscope (Leica, Wetzlar, Germany)
SDS-PAGE and Western blot analysis.
Bacterial strains were lysed in sodium dodecyl sulfate (SDS) sample buffer (∼5 × 107 E. coli bacteria were loaded per lane). Separation of the proteins was performed using SDS-polyacrylamide gel electrophoresis (PAGE). For immunoblotting, the proteins were transferred electrophoretically onto polyvinylidene difluoride membranes (1.2 mA/cm2 for 60 min). The membranes were blocked overnight with PBS-5% milk at 4°C. For detection of Inv, a polyclonal rabbit anti-Inv 3.1 antibody (diluted 1:1,000) and a peroxidase-conjugated secondary anti-rabbit antibody (diluted 1:5,000; Amersham Biosciences) were added for 1 h each. For detection of YadA, a polyclonal rabbit anti-P1.8 antibody (diluted 1:1,000) and a peroxidase-conjugated secondary anti-rabbit antibody (diluted 1:5,000; Amersham Biosciences) were added for 1 h each. The detection was carried out using the ECL detection kit from Amersham Biosciences.
Statistics.
The data shown in the figures are from representative experiments. Comparable results were obtained in at least two additional experiments. Differences between mean values were analyzed using Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
YadA mediates adhesion to and upregulation of IL-8 in HeLa cells.
As previously shown (45, 46, 48, 49), Inv is able to induce proinflammatory cytokines, such as IL-8, and a high number of other gene products (4) in a β1 integrin-dependent manner. Based on reports demonstrating that YadA adheres to and promotes uptake into host cells via β1 integrins (16, 50, 54), we speculated that YadA may also trigger IL-8 secretion.
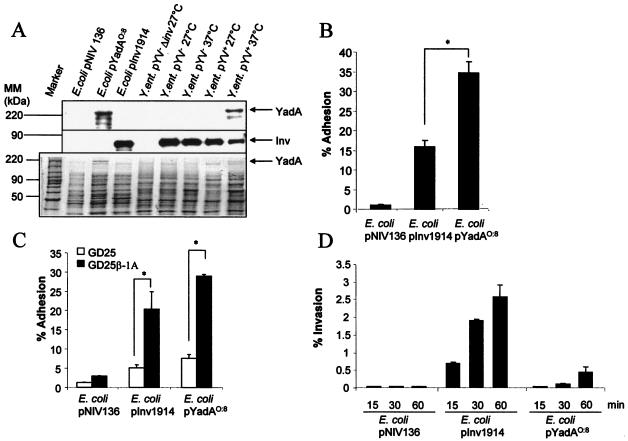
To address this question, YadA and Inv were cloned into the IPTG-inducible vector pNIV136 and transformed into E. coli HB101. Overexpression of YadA and Inv to comparable degrees by E. coli HB101 was confirmed by Western blot analysis and SDS-PAGE, as indicated in Fig. 1A. Since adhesion is a prerequisite for Inv-induced IL-8 secretion, we next compared the adhesion of Inv- and YadA-expressing E. coli to HeLa cells. For this purpose, HeLa cells were infected with E. coli HB101 harboring the vector pNIV136 (vector control), pInv1914 (inv+), or pYadAO:8 (yadA+), and the number of bacteria adhering to epithelial cells was determined 15 min postinfection (Fig. 1B). We found that upon infection of HeLa cells with either E. coli pInv1914 or E. coli pYadAO:8, adherence to HeLa cells was significantly (P < 0.05) increased compared to that with the control strain E. coli HB101 pNIV136 (Fig. 1B). Moreover, YadA overexpressed in E. coli mediated a twofold-higher adhesion to HeLa cells than Inv overexpressed in E. coli.
FIG. 1.
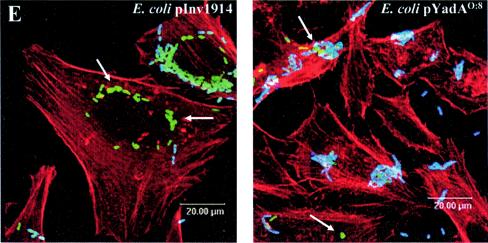
YadA- and Inv-mediated adhesion and invasion. (A) Expression of YadA and Inv of E. coli pYadAO:8, E. coli pInv1914, Y. enterocolitica pYV+, and Y. enterocolitica pYV−. Yersinia and E. coli strains were grown overnight in LB at 27 and 37°C and subcultured for a further 3 h at the indicated temperatures. Subsequently, Western blot analysis using polyclonal antibodies against Inv (middle) and YadA (top) was performed. In parallel, SDS-PAGE (bottom) was performed as a loading control. Inv and YadA expression is indicated by arrows. (B) HeLa cells were infected with Inv- or YadA-expressing bacteria (MOI, 100). Adhesion of bacteria to HeLa cells was determined 15 min postinfection, as described in Materials and Methods. The asterisk indicates a significant difference between adhesion of E. coli pYadAO:8 and that of E. coli pInv1914 to HeLa cells (P < 0.05). The error bars indicate standard deviations. (C) GD25 cells were infected with Inv- or YadA-expressing bacteria (MOI, 100). Adhesion of bacteria to GD25-β1A and GD25 cells was determined 15 min postinfection. The asterisks indicate a significant difference between the adhesion of E. coli pINV1914 and that of E. coli YadAO:8 to GD25 cells versus GD25-β1A cells (P < 0.05). (D) Uptake of bacteria into HeLa cells was analyzed by determining the number of intracellularly viable bacteria using gentamicin killing at the indicated time points. (E) Internalization of E. coli pInv1914 or E. coli pYadAO:8 into HeLa cells was visualized by immunofluorescence using polyclonal antibodies against Inv or YadA, respectively, as indicated in Material and Methods. Overlays of multicolor staining are shown. The cytoskeleton was stained with phalloidin-tetramethyl rhodamine isothiocyanate (red). Extracellularly localized bacteria, blue and green staining; internalized bacteria, exclusively green staining. The arrows indicate examples of intracellularly located bacteria.
To investigate the role of β1 integrins in adhesion, infection experiments with the fibroblast cell line GD25, which lacks β1 integrin expression, and the cell line GD25-β1A, in which β1 integrins are overexpressed, were performed. In general, adhesion to GD25-β1A fibroblasts mediated by YadA was slightly but not significantly higher than adhesion mediated by Inv. Adhesion of E. coli pInv1914 or E. coli pYadAO:8 to GD25 fibroblasts was significantly reduced (75 and 76%) compared to adhesion to GD25-β1A cells (Fig. 1C). These data show that β1 integrins are required for YadA- and Inv-mediated adhesion.
To determine internalization of E. coli pInv1914 or E. coli pYadAO:8, gentamicin killing assays (Fig. 1D) and immunostaining (Fig. 1E) were performed. Inv- or YadA-expressing E. coli strains showed increased internalization compared to E. coli pNIV136 in a time-dependent manner. However, YadA O:8 was much less efficient in mediating internalization than Inv. Immunostaining of E. coli pInv1914- or E. coli pYadAO:8-infected HeLa cells confirmed these results (Fig. 1E). Thus, upon infection with E. coli pYadAO:8, most bacteria were extracellularly located and only a few were internalized.
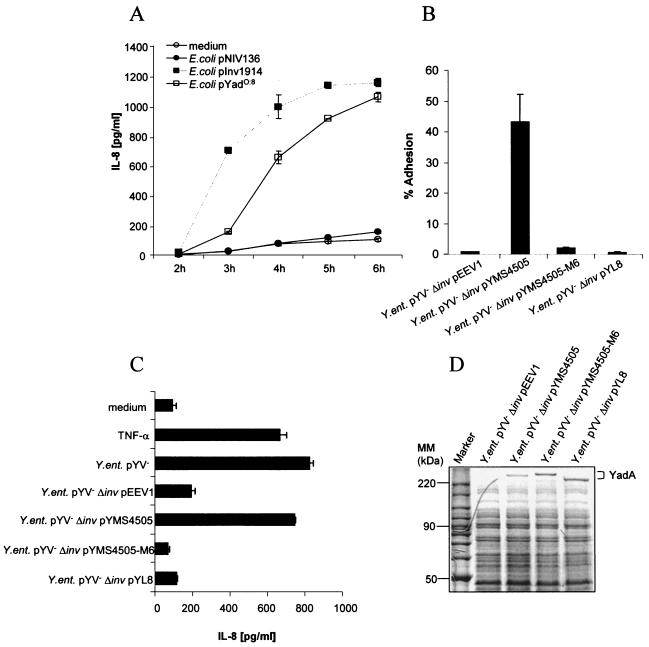
To determine whether YadA triggers IL-8 secretion, HeLa cells were infected with E. coli pNIV136, E. coli pInv1914, or E. coli pYadAO:8, and IL-8 secretion was detected at different time points postinfection. YadA- and Inv-expressing E. coli strains induced comparable amounts of IL-8 6 h postinfection (Fig. 2A). However, the onset of IL-8 secretion induced by YadA was time delayed compared to Inv-induced IL-8 secretion.
FIG. 2.
IL-8 secretion by and adhesion to HeLa cells upon infection with E. coli or Y. enterocolitica expressing YadA and/or Inv (MOI, 100). HeLa cells were infected (A) with E. coli strains or stimulated with TNF-α for the indicated times or (B) with Y. enterocoltica strains for 4 h. IL-8 levels in culture supernatants were determined by ELISA. The error bars indicate standard deviations. (C) HeLa cells were infected with the indicated Yersinia strains for 15 min, and subsequently, adhesion assays were performed as described in Materials and Methods. The experiments were representative of three further independent experiments. (D) SDS-PAGE was performed to show YadA protein expression of Yersinia strains used in panels B and C. E. coli strains were grown in LB at 37°C, and Yersinia strains were grown overnight at 27°C and subcultured for 3 h the next day at 27 or 37°C as indicated. MM, molecular mass.
As described previously, mutation of NSVAIG-S motifs in the head domain of YadA abrogates collagen binding due to conformational change of the structure of the YadA head domain (36, 52). To address whether these structural changes of YadA affect Y. enterocolitica YadA-mediated adhesion and IL-8 induction, HeLa cells were infected with Y. enterocolitica pYV− Δinv transformed with control vector pEEV1, vector pYMS4505 expressing YadA O:3, or vector pYMS4505-M6 or pYL8, which carry YadA O:3 mutants deficient in collagen binding. HeLa cells were infected with different Y. enterocolitica strains, and adhesion, as well as IL-8 secretion, was detected (Fig. 2B and C). Furthermore, SDS-PAGE was performed to show YadA expression of the Yersinia strains used in these experiments (Fig. 2D). Adhesion assays revealed that in contrast to Y. enterocolitica pYV− Δinv pYMS4505 expressing YadA O:3, the strains Y. enterocolitica pYV− Δinv pYMS4505-M6 and Y. enterocolitica pYV− Δinv pYL8, which carry mutations of either the YadA head-forming domain M2/3 or M6, and Y. enterocolitica pYV− Δinv pEEV1, which carries a control vector, were not able to adhere to HeLa cells (Fig. 2B). In a similar manner, IL-8 secretion was significantly increased compared to basal IL-8 levels only upon infection with Y. enterocolitica pYV− Δinv pYMS4505 but not upon infection with Y. enterocolitica pYV− Δinv pYMS4505-M6, Y. enterocolitica pYV− Δinv pYL8, or Y. enterocolitica pYV− Δinv pEEV1 (Fig. 2C). Taken together, these data demonstrate that YadA, like Inv, can induce IL-8 secretion in epithelial cells. Moreover, conformational changes in the head domain of YadA affect collagen binding (36, 52), adhesion, and induction of IL-8 secretion.
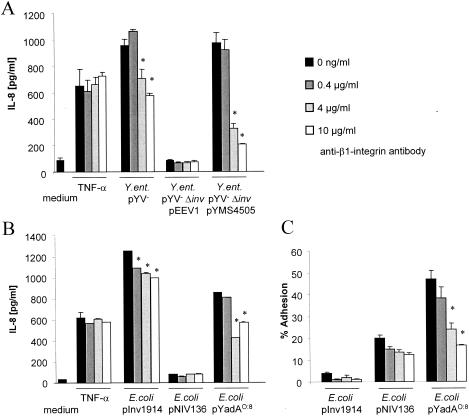
To investigate, whether IL-8 secretion mediated by YadA involves β1 integrins, the β1 integrin-specific blocking antibody Lia1/2 was added prior to infection of HeLa cells with Inv- or YadA-expressing Yersinia strains (Fig. 3A). Inv- and YadA-induced IL-8 secretion levels were significantly reduced (40 and 79%) by the addition of neutralizing anti-β1 integrin antibodies. The impact of anti-β1 integrin antibodies on IL-8 secretion mediated by YadA was even higher than that on Inv-induced IL-8 secretion. To exclude the possibility that the higher impact of anti-β1 integrin antibodies on IL-8 secretion mediated by YadA compared to that on IL-8 secretion mediated by Inv might be due to different expression levels of YadA and Inv, neutralization experiments were performed using E. coli strains expressing Inv and YadA in the same vector system under the control of the same promoter (IPTG-inducible lpp promoter) (Fig. 3B). Inv- and YadA-induced IL-8 secretion levels were significantly reduced (21 and 49%). To address whether reduced IL-8 secretion corresponds to reduced adhesion, adhesion assays were performed (Fig. 3C). Pretreatment with anti-β1 integrin antibodies significantly reduced adhesion of E. coli pInv1914 and E. coli pYadAO:8 (64 and 49%), indicating that reduced adhesion to β1 integrins corresponds to reduced IL-8 secretion.
FIG. 3.
YadA-mediated IL-8 secretion and adhesion by HeLa cells is β1 integrin dependent. Prior to infection of HeLa cells with Yersinia strains (A) or E. coli strains (B and C), the cells were incubated with the indicated amounts of blocking anti-β1 integrin antibody Lia1/2. IL-8 levels in culture supernatants were determined by ELISA 4 h postinfection (A and B) or adhesion experiments were performed as indicated in Materials and Methods (C). The experiments are representative of three further independent experiments. The error bars indicate standard deviations.
YadA induces IL-8 expression by engagement of Rho GTPases, MAP kinase cascade, and NF-κB activation.
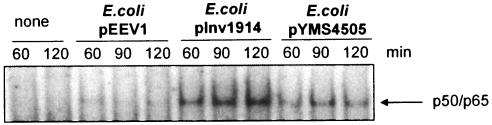
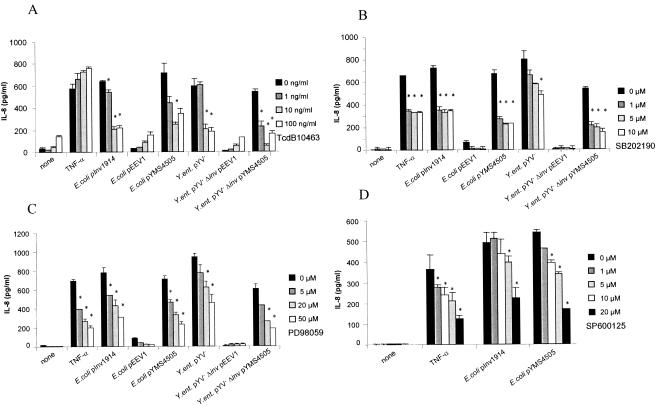
To investigate whether signaling events activated by YadA involve the same pathways as that elicited by Inv, we studied whether YadA leads to NF-κB activation. HeLa cells were infected with E. coli expressing either YadA or Inv, and subsequently, nuclear extracts were tested by EMSA for binding activity to an NF-κB consensus sequence (Fig. 4). Indeed, like Inv but to a lesser extent, YadA induced binding of NF-κB to the NF-κB consensus sequence. The involvement of Rho GTPases was tested by treating the cells with C. difficile toxin TcdB10463, which inhibits the Rho GTPases Rac, Rho, and Cdc42, prior to infection with YadA- or Inv-expressing E. coli (Fig. 5A). TcdB10463 inhibits Inv- and YadA-mediated IL-8 secretion by >60%, indicating the involvement of one or more of these Rho GTPases in IL-8 secretion. The involvement of MAP kinases was tested by using a specific inhibitor of p38 (SB202190), MEK1 (PD98059), or JNK (SP600125). The addition of SB202190, PD98059, or SP600125 reduced YadA-induced IL-8 secretion up to 70, 67, or 69%, respectively (Fig. 5B to D). All three MAP kinase inhibitors, SB202190, PD98059, and SP600125, reduced YadA- and Inv-mediated IL-8 secretion in a dose-dependent manner. Taken together, these data show that the signaling cascade leading to IL-8 secretion mediated by YadA involves β1 integrins; Rho GTPases; the MAP kinases MEK1, JNK, and p38; and NF-κB activation.
FIG. 4.
Determination by EMSA of NF-κB activation in nuclear extracts of HeLa cells after stimulation with Inv- or YadA-expressing E. coli. Sixty, 90, or 120 min postinfection, nuclear extracts were prepared and analyzed in gel shift experiments with 32P-labeled NF-κB consensus probes. The experiments are representative of three further independent experiments.
FIG. 5.
Modulation of YadA- and Inv-induced IL-8 secretion by inhibitory drugs. HeLa cells were treated with (A) C. difficile toxin TcdB10463 (an inhibitor of Rac, Rho, and Cdc42) 24 h prior to infection or (B) SB202190 (a p38 inhibitor), (C) PD98059 (a MEK1 inhibitor), or (D) SP600125 (a JNK inhibitor) 30 min prior to infection with Inv- or YadA-expressing bacteria or stimulation with tumor necrosis factor alpha (TNF-α) (50 ng/ml). IL-8 production was determined by ELISA in supernatants collected 4 h after infection. The experiments are representative of three further independent experiments. The asterisks indicate a significant difference between IL-8 secretion of infected or stimulated cells treated with inhibitors compared with that of untreated cells (P < 0.05). The error bars indicate standard deviations.
DISCUSSION
Infection of epithelial cells with Y. enterocolitica triggers the expression of a high number of genes, including genes encoding proinflammatory molecules, such as IL-8 (4). Inv was shown to be a major inductor of IL-8 expression and secretion. Plasmid-encoded factors, such as YopP, are able to counteract this response (4). The induction of a proinflammatory response by Inv is known to be dependent on adhesion to β1 integrins. Taking into account that β1 integrins are also involved in adhesion via YadA, we hypothesized that YadA may also be involved in triggering a proinflammatory host response. Experiments in this study confirmed this hypothesis and demonstrated that YadA can mediate IL-8 secretion. Moreover, we showed that, like Inv, β1 integrins are involved in this process. Furthermore, the downstream signaling events triggered by YadA involve the same components as in Inv-mediated signaling, namely, Rho GTPases and the MAP kinases MEK1, JNK, and p38.
YadA was expressed either in E. coli strains or in plasmid-cured Yersinia strains. These strains do not express any of the virulence factors (e.g., YopP) that are known to counteract a proinflammatory response induced by Y. enterocolitica in vitro. In this setting, YadA and Inv are similarly effective in mediating adhesion to GD25-β1A fibroblasts and even more effective in mediating adhesion to HeLa cells, as proven with E. coli overexpressing YadA and Inv by the same vector system. The different efficacies of YadA and Inv in adhering to HeLa and GD25-β1A cells may be due to cell-type-specific differences.
By using the fibroblast cell line GD25 (18), which lacks β1 integrin expression in comparison with the fibroblast cell line GD25-β1A overexpressing β1 integrins (57), we demonstrated that β1 integrins are crucial for YadA-mediated adhesion of Yersinia to cells. The data presented here are in line with previous reports (3, 16) that β1 integrins are required for YadA-mediated binding to cells. Eitel and Dersch (16) showed that YadA of Y. pseudotuberculosis can mediate highly efficient uptake into epithelial cells when overexpressed in E. coli or when Y. pseudotuberculosis is grown under specific growth conditions. This process can be blocked using neutralizing antibodies against fibronectin and β1 integrins, which led to the suggestion that internalization mediated by YadA occurs via extracellular-matrix-dependent bridging between YadA and the host cell β1 integrin receptors (16).
In line with previous data (23) and in contrast to YadA of Y. pseudotuberculosis, even overexpression of Y. enterocolitica YadA can only weakly mediate uptake of Y. enterocolitica into epithelial cells. Thus, YadA of Y. enterocolitica is much less efficient (18-fold) in mediating uptake than Inv of Y. enterocolitica. The reason why YadA of Y. enterocolitica may be less efficient in mediating uptake than Y. pseudotuberulosis is unclear. Although the amino acid sequence of YadA is highly homologous between the two species, there are two additional amino acid sequences in the head domain of Y. pseudotuberculosis and one additional amino acid sequence in the stalk domain of Y. enterocolitica. One can speculate that these sequences could be responsible for the different ability to invade cells mediated by YadA. Further studies will be necessary to address this question.
The interaction between the Yersinia Inv protein and the β1 integrin receptor permits bacterial attachment to the host cell and triggers internalization (27). Previous studies uncovered a role for Inv-mediated adhesion in the production of IL-8 in epithelial cells infected with Y. enterocolitica (30, 45, 49). In this study, we demonstrate that YadA expressed in E. coli in the same vector system as Inv also induces IL-8 secretion. Since both YadA and Inv mediate adhesion but only Inv efficiently mediates uptake, these data support previous studies showing that adhesion, but not internalization, of Yersinia is essential for triggering IL-8 secretion via β1 integrins (4, 49).
Tahir et al. (52) reported that NSVAIG-S motifs in the N-terminal half of YadA are required for collagen binding. A recent study revealed that mutations of these putative collagen binding domains are located in the center of the YadA trimers and therefore cannot be directly involved in collagen binding. Mutation of these domains led to conformational changes of the head domain of YadA, which abolishes collagen binding. Interestingly, it was also shown that binding of Y. enterocolitica to fibronectin and laminin is not affected by these mutations (52). Using Y. enterocolitica pYV− Δinv-expressing YadA mutants in which different NSVAIG-S motifs were mutated, we demonstrated that these mutations were sufficient to abolish YadA-mediated adhesion and subsequently IL-8 secretion. This is in keeping with the idea that collagen binding could be involved in adhesion and thus in subsequent IL-8 secretion.
Adhesion mediated by YadA and Inv is a prerequisite for IL-8 secretion. At least for HeLa cells, YadA mediates stronger adhesion than Inv. In contrast, the time delay of IL-8 secretion mediated by YadA compared to that mediated by Inv indicates that YadA is less effective in mediating IL-8 secretion than Inv. Moreover, YadA-mediated adhesion to and IL-8 secretion by HeLa cells can be blocked more efficiently by anti-β1 integrin antibodies than Inv-mediated adhesion and IL-8 secretion. Previously obtained results showed high-affinity binding of Inv to integrins (13), which may explain why blocking of adhesion and IL-8 secretion with antibodies is not effective. In contrast, there is evidence that the binding of YadA to β1 integrins may be an indirect effect via bridging to extracellular matrix proteins, such as fibronectin (16). Low-affinity binding to β1 integrins via extracellular matrix proteins may explain the more efficient adhesion and the delayed onset of IL-8 secretion, as well as the higher blocking capacities of anti-β1 integrin antibodies.
The involvement of β1 integrins in Inv- and YadA-triggered IL-8 secretion also indicated a common signaling pathway for YadA- and Inv-triggered proinflammatory responses. By cross-linking β1 integrins with antibodies, it was clearly shown that β1 integrins are directly involved in a signal cascade leading to IL-8 secretion via Rac1 and MAP kinase-p38 signaling pathways (32). Similarly, for Inv-mediated IL-8 secretion, it was shown that Rac1 is required for activation of NF-κB (20, 46) and that Inv activates the MAP kinases p38, MEK1, and JNK (20). The comparison of Inv- and YadA-mediated signaling presented here confirmed that the factors involved in Inv-mediated signaling leading to IL-8 secretion are also required for YadA-mediated signaling.
Taking these data together, we can conclude that YadA, in addition to a number of other biological properties, such as serum resistance (2, 7, 17), arithrogenicity (22), and epithelial-cell adhesion (23), contributes to the proinflammatory response of the host to infection with Y. enterocolitica. Our results and recent observations suggest that factors of Yersinia which can induce a proinflammatory response involving NF-κB activation seem to be very redundant. Thus, Viboud et al. (55) demonstrated for Y. pseudotuberculosis that YopB can also induce IL-8 by activation of the small GTPase Ras and NF-κB. However, the actual contributions of these various factors, like Inv, YadA, and YopB, as well as lipopolysaccharide, to triggering a proinflammatory response in vivo in different cell types in concert with the mechanisms of Yersinia which counteract the proinflammatory response in vivo are still unclear and will have to be addressed in future studies.
Acknowledgments
We thank Reinhard Faessler for kindly providing us with the cell lines GD25-β1A and GD25 and Christoph von Eichel-Streiber for kindly providing us with C. difficile toxins. We thank Birgit Manncke for perfect technical assistance.
This work was supported by grant DFG 102/10-2 from the Deutsche Forschungsgemeinschaft.
Editor: J. B. Bliska
REFERENCES
- 1.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defence mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balligand, G., Y. Laroche, and G. Cornelis. 1985. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect. Immun. 48:782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn, E., S. Muller, J. Lauber, R. Geffers, N. Speer, C. Spieth, J. Krejci, B. Manncke, J. Buer, A. Zell, and I. B. Autenrieth. 2004. Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica. Cell Microbiol. 6:129-141. [DOI] [PubMed] [Google Scholar]
- 5.Bottone, E. J. 1977. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. Crit. Rev. Microbiol. 5:211-241. [DOI] [PubMed] [Google Scholar]
- 6.Chaves-Olarte, E., M. Weidmann, C. Eichel-Streiber, and M. Thelestam. 1997. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. Investig. 100:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.China, B., M. P. Sory, B. T. N′Guyen, M. de Bruyere, and G. R. Cornelis. 1993. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect. Immun. 61:3129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis, G. R. 1994. Yersinia pathogenicity factors. Curr. Top. Microbiol. Immunol. 192:243-263. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cover, T. L., and R. C. Aber. 1989. Yersinia enterocolitica. N. Engl. J. Med. 321:16-24. [DOI] [PubMed] [Google Scholar]
- 13.Dersch, P., and R. R. Isberg. 1999. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 18:1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Genaro, M. S., M. Waidmann, U. Kramer, N. Hitziger, E. Bohn, and I. B. Autenrieth. 2003. Attenuated Yersinia enterocolitica mutant strains exhibit differential virulence in cytokine-deficient mice: implications for the development of novel live carrier vaccines. Infect. Immun. 71:1804-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1995. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 3:118-120. [DOI] [PubMed] [Google Scholar]
- 16.Eitel, J., and P. Dersch. 2002. The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect. Immun. 70:4880-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 18.Fassler, R., M. Pfaff, J. Murphy, A. A. Noegel, S. Johansson, R. Timpl, and R. Albrecht. 1995. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128:979-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauconnier, A., A. Van Elsen, and A. Bollen. 1998. Construction and sequencing of new derivatives of the pIN-III-ompA secretion vector. Genet. Anal. 14:129-131. [DOI] [PubMed] [Google Scholar]
- 20.Grassl, G. A., E. Bohn, Y. Muller, O. T. Buhler, and I. B. Autenrieth. 2003. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 293:41-54. [DOI] [PubMed] [Google Scholar]
- 21.Grassl, G. A., M. Kracht, A. Wiedemann, E. Hoffmann, M. Aepfelbacher, C. Eichel-Streiber, E. Bohn, and I. B. Autenrieth. 2003. Activation of NF-κB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell Microbiol. 5:957-971. [DOI] [PubMed] [Google Scholar]
- 22.Gripenberg-Lerche, C., M. Skurnik, L. Zhang, K.-O. Söderström, and P. Toivanen. 1994. Role of YadA in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect. Immun. 62:5568-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heesemann, J., and L. Grüter. 1987. Genetic evidence that the outer membrane protein Yop1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol. Lett. 40:37-41. [Google Scholar]
- 24.Heesemann, J., and R. Laufs. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 98:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isberg, R. R., and P. Barnes. 2001. Subversion of integrins by enteropathogenic Yersinia. J. Cell Sci. 114:21-28. [DOI] [PubMed] [Google Scholar]
- 28.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Immunity 60:861-871. [DOI] [PubMed] [Google Scholar]
- 29.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampik, D., R. Schulte, and I. B. Autenrieth. 2000. Yersinia enterocolitica invasin protein triggers differential production of interleukin-1, interleukin-8, monocyte chemoattractant protein 1, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha in epithelial cells: implications for understanding the early cytokine network in Yersinia infections. Infect. Immun. 68:2484-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong, J. M., R. S. Fournier, and R. R. Isberg. 1990. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 9:1979-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mainiero, F., A. Soriani, R. Strippoli, J. Jacobelli, A. Gismondi, M. Piccoli, L. Frati, and A. Santoni. 2000. RAC1/P38 MAPK signaling pathway controls β1 integrin-induced interleukin-8 production in human natural killer cells. Immunity 12:7-16. [DOI] [PubMed] [Google Scholar]
- 33.McCormick, B. A., A. Nusrat, C. A. Parkos, L. D'Andrea, P. M. Hofman, D. Carnes, T. W. Liang, and J. L. Madara. 1997. Unmasking of intestinal epithelial lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect. Immun. 65:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 173:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moos, M., and C. Eichel-Streiber. 2000. Purification and evaluation of large clostridial cytotoxins that inhibit small GTPases of Rho and Ras subfamilies. Methods Enzymol. 325:114-125. [DOI] [PubMed] [Google Scholar]
- 36.Nummelin, H., M. C. Merckel, J. C. Leo, H. Lankinen, M. Skurnik, and A. Goldman. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J. 23:701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 63:4837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepe, J. C., J. L. Badger, and V. L. Miller. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 11:123-135. [DOI] [PubMed] [Google Scholar]
- 39.Pepe, J. C., and V. L. Miller. 1993. The biological role of invasin during a Yersinia enterocolitica infection. Infect. Agents Dis. 2:236-241. [PubMed] [Google Scholar]
- 40.Pepe, J. C., and V. L. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruckdeschel, K., A. Roggenkamp, S. Schubert, and J. Heesemann. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ′mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulte, R., and I. B. Autenrieth. 1998. Yersinia enterocolitica-induced interleukin-8 secretion by human intestinal epithelial cells depends on cell differentiation. Infect. Immun. 66:1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulte, R., G. A. Grassl, S. Preger, S. Fessele, C. A. Jacobi, M. Schaller, P. J. Nelson, and I. B. Autenrieth. 2000. Yersinia enterocolitica invasin protein triggers IL-8 production in epithelial cells via activation of Rel p65-p65 homodimers. FASEB J. 14:1471-1484. [DOI] [PubMed] [Google Scholar]
- 47.Schulte, R., S. Kerneis, S. Klinke, H. Bartels, S. Preger, J. P. Kraehenbuhl, E. Pringault, and I. B. Autenrieth. 2000. Translocation of Yersinia enterocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to β1 integrins apically expressed on M-like cells. Cell Microbiol. 2:173-185. [DOI] [PubMed] [Google Scholar]
- 48.Schulte, R., P. Wattiau, E. L. Hartland, R. M. Robins Browne, and G. R. Cornelis. 1996. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulte, R., R. Zumbihl, D. Kampik, A. Fauconnier, and I. B. Autenrieth. 1998. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. 187:53-60. [DOI] [PubMed] [Google Scholar]
- 50.Schulze Koops, H., H. Burkhardt, J. Heesemann, T. Kirsch, B. Swoboda, C. Bull, S. Goodman, and F. Emmrich. 1993. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect. Immun. 61:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snellings, N. J., M. Popek, and L. E. Lindler. 2001. Complete DNA sequence of Yersinia enterocolitica serotype O:8 low-calcium-response plasmid reveals a new virulence plasmid-associated replicon. Infect. Immun. 69:4627-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tahir, Y. E., P. Kuusela, and M. Skurnik. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification Of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol. Microbiol. 37:192-206. [DOI] [PubMed] [Google Scholar]
- 53.Tamm, A., A.-M. Tarkkanen, P. K. Korhonen, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10:995-1011. [DOI] [PubMed] [Google Scholar]
- 54.Tertti, R., M. Skurnik, T. Vartio, and P. Kuusela. 1992. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 60:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viboud, G. I., S. S. So, M. B. Ryndak, and J. B. Bliska. 2003. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol. Microbiol. 47:1305-1315. [DOI] [PubMed] [Google Scholar]
- 56.Visser, L. G., P. S. Hiemstra, M. T. van den Barselaar, P. A. Ballieux, and R. van Furth. 1996. Role of YadA in resistance to killing of Yersinia enterocolitica by antimicrobial polypeptides of human granulocytes. Infect. Immun. 64:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wennerberg, K., L. Lohikangas, D. Gullberg, M. Pfaff, S. Johansson, and R. Fassler. 1996. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 132:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiedemann, A., S. Linder, G. Grassl, M. Albert, I. Autenrieth, and M. Aepfelbacher. 2001. Yersinia enterocolitica invasin triggers phagocytosis via β1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol. 3:693-702. [DOI] [PubMed] [Google Scholar]