Abstract
Shiga toxin 2 (Stx2) from the foodborne pathogen Escherichia coli O157:H7 is encoded on a temperate bacteriophage. Toxin-encoding phages from C600::933W and from six clinical E. coli O157:H7 isolates were characterized for PCR polymorphisms, phage morphology, toxin production, and lytic and lysogenic infection profiles on O157 and non-O157 serotype E. coli. The phages were found to be highly variable, and even phages isolated from strains with identical pulsed-field gel electrophoresis profiles differed. Examination of cross-plaquing and lysogeny profiles further substantiated that each phage is distinct; reciprocal patterns of susceptibility and resistance were not observed and it was not possible to define immunity groups. The interaction between Shiga toxin-encoding phage and intestinal E. coli was examined. Lytic infection was assessed by examining Shiga toxin production following overnight incubation with phage. While not common, lytic infection was observed, with a more-than-1,000-fold increase in Stx2 seen in one case, demonstrating that commensal E. coli cells can amplify Shiga toxin if they are susceptible to infection by the Shiga toxin-encoding phages. Antibiotic-resistant derivatives of the Stx2-encoding phages were used to examine lysogeny. Different phages were found to lysogenize different strains of intestinal E. coli. Lysogeny was found to occur more commonly than lytic infection. The presence of a diverse population of Shiga toxin-encoding phages may increase the pathogenic fitness of E. coli O157:H7.
The foodborne pathogen Escherichia coli O157:H7 is responsible for a reported 73,000 cases of illness per year in the United States (16). Disease can range from watery and bloody diarrhea to the life-threatening hemolytic uremic syndrome. A major virulence factor responsible for disease is the production of Shiga toxin (Stx). In Stx-producing E. coli (STEC) such as E. coli O157:H7, Stx can be present in two forms: Stx1, which is essentially identical to Stx from Shigella dysenteriae, or Stx2, which is about 55% homologous to Stx1 at the amino acid level (reviewed in reference 20). A STEC strain can produce either Stx1 or Stx2 or both; however, severe disease has been epidemiologically linked to the presence of Stx2 (3, 24).
The biology of Stx production is unusual. The genes for Stx are encoded in the late gene region of lysogenic phage (21, 28). Late phage genes are expressed only when the phage are engaged in the replicative or lytic cycle (reviewed in reference 11). For E. coli O157:H7, the toxin genes are silent during lysogeny; however, if the phage are induced to enter the lytic cycle, phage and toxin production will occur (37). Induction to the lytic cycle can occur after exposure of the bacteria to DNA damaging agents, such as UV light or mitomycin C (18), or to antibiotics, such as the quinolones (41), which stall DNA synthesis. In addition, neutrophil activation products such as hydrogen peroxide have been shown to induce Stx production (35).
Previous studies indicated that Stx2-encoding phages are highly variable (10, 17, 19, 34, 36). Wagner et al. (36) demonstrated that Stx2 phages from STEC clinical isolates displayed different host ranges and differed in toxin production, suggesting that phage heterogeneity could play a role in disease outcome. Indeed, Muniesa et al. (19) have shown that E. coli O157:H7 isolates from a single outbreak were lysogenized with different toxin-encoding phages. Furthermore, the severity of disease symptoms observed in different patients correlated with in vitro toxin production by their O157:H7 isolate. These studies suggest that phage variability can influence pathogenic fitness.
Shiga toxin-encoding phage can infect non-O157 E. coli. Phage infection can have two outcomes. In some instances, infection of non-toxin-producing E. coli will result in lysogeny, where the phage genome becomes incorporated into the bacterial chromosome (11). Lysogeny has been demonstrated to occur in vivo in a mouse model of disease (1, 7). Lysogeny with toxin-encoding phage has important implications for the evolution of new pathogenic strains. It has been shown that Shigella sonnei can become lysogenized with Stx-encoding phage, which has the potential to convert them to a more pathogenic form (32).
The other outcome of phage infection is lytic infection. Lytic infection of non-O157 E. coli can lead to production of phage and Stx. Our investigators have shown that lytic infection of non-toxin-producing E. coli with Stx-encoding phage in vitro can increase Stx production by more than 1,000-fold (7). Furthermore, in a mouse model of disease, intestinal Stx2 production was dramatically increased in some cases when the toxin-encoding phage were able to infect non-toxin-producing E. coli (7).
Epidemiological studies of E. coli O157:H7 suggest that following exposure, many individuals develop intestinal symptoms; however, only a small percentage of patients go on to develop severe, life-threatening systemic sequelae, such as hemorrhagic colitis or hemolytic uremic syndrome (16). Certain combinations of toxin-encoding phage and host intestinal flora could result in highly elevated levels of intestinal Stx production, and these individuals may be at increased risk for developing severe systemic disease. Human intestinal flora is highly variable and, furthermore, the host range of Stx-encoding phages is highly variable (2, 9, 22). It is currently unknown how often individuals possess intestinal flora that is susceptible to the toxin-encoding phages. In this study, we examined phage characteristics and immunity profiles of six Stx2-encoding phages from highly related clinical E. coli O157:H7 isolates from the Cincinnati area, with an emphasis on assessing their ability to undergo lysogenic integration and/or lytic infection in a variety of E. coli strains. The Stx2-encoding phages were found to be highly variable, even when the E. coli O157:H7 isolates were identical by pulsed-field gel electrophoresis (PFGE) analysis, consistent with the hypothesis that variations in phage host range may play a role in pathogenic fitness.
MATERIALS AND METHODS
Bacterial strains.
Luria-Bertani (LB) agar (Difco, Detroit, Mich.) or LB broth was used for routine bacterial propagation. For phage induction and infection experiments, LB agar and broth were supplemented with 10 mM CaCl2 (LB-modified). When indicated, chloramphenicol was added to the medium at a concentration of 15 μg/ml.
The E. coli O157:H7 and other E. coli strains used in this study are listed in Table 1. The clinical E. coli O157:H7 isolates were obtained from The Cincinnati Children's Hospital Medical Center culture collection. The strains from the ECOR collection were obtained from the STEC Center at Michigan State University (http://foodsafe.msu.edu/whittam/ecor/index.html).
TABLE 1.
Bacterial strains and phages used in this study
| Bacterial strain or phage | Relevant characteristics | Reference |
|---|---|---|
| C600 | E. coli K-12 | 22 |
| E. coli isolates | ||
| ECOR set | 72 diverse E. coli isolates of animal and human origin | 23 |
| Fecal Isolates | ||
| FI set | 29 E. coli isolates from healthy volunteers in the Cincinnati area | 7 |
| PT set | 12 E. coli isolates from patients at a Cincinnati hospital | This study |
| E. coli O157:H7a | ||
| PT22 | Isolated in 2000; stx2+stx1+ | This study |
| PT27 | Isolated in 2001; stx2+stx1+ | This study |
| PT32 | Isolated in July 1999; stx2+stx1+ | 7 |
| PT38a | Isolated in September 1999; stx2+stx1+ | This study |
| PT38b | Isolated in September 1999; stx2+stx1+ | This study |
| PT39a | Isolated in September 1999; stx2+stx1+ | This study |
| Phages | ||
| φ933W | Stx2 converting phage | 22 |
| φH19B | Stx1 converting phage | 22 |
| φ933WΔtox | φ933W, Stx2 deleted; Cmr, GFP; previously called Δtox | 7 |
| φPT22Δtox | From PT22, Stx2 deleted; Cmr, GFP | This study |
| φPT27Δtox | From PT27, Stx2 deleted; Cmr, GFP | This study |
| φPT32Δtox | From PT32, Stx2 deleted; Cmr, GFP | This study |
| φPT38aΔtox | From PT38a, Stx2 deleted; Cmr, GFP | This study |
| φPT38bΔtox | From PT38b, Stx2 deleted; Cmr, GFP | This study |
| φPT39aΔtox | From PT39a, Stx2 deleted; Cmr, GFP | This study |
Isolated at Cincinnati Children's Hospital Medical Center.
To obtain human intestinal strains, fecal samples were plated on MacConkey agar (Difco) and incubated at 37°C overnight. Species assignments were made using the BBL Enterotube II kit (Beckton Dickinson and Co., Sparks, Md.). In addition to E. coli, the following Enterobacteriaceae were isolated: Citrobacter freundii, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella ozaenae, Enterobacter aerogenes, Enterobacter amnigenes, Serratia marcescens, Acinetobacter lwolfii, and Edwardsiella spp.
Determination of biotype.
The phylogenetic grouping of the fecal E. coli isolates was determined by PCR using the primers of Clermont et al. (5).
PFGE.
E. coli O157:H7 strains were grown overnight on LB plates at 37°C, and colonies were suspended in 2 ml of Tris (100 mM)-EDTA (100 mM) buffer. Cells were prepared and lysed according to the method of Gautom (8). Bacterial DNA was digested with XbaI. DNA fragments were resolved on a 1% agarose (SeaKem gold; FMC Bioproducts, Philadelphia, Pa.) gel by using a CHEF PFGE apparatus (CHEF-DR II; Bio-Rad Laboratories, Hercules, Calif.) ramped from 5 to 50 s for 22 h. A lambda ladder (48.5-kb increments; Bio-Rad) was used for size determinations.
Construction of phage with stx2 phage.
Derivatives of E. coli O157:H7 phages with stx2 deleted are listed in Table 1. Our group previously described the construction of phage Δtox, in which the stx2 genes in phage 933W (referred to as φ933W) were replaced with a chloramphenicol resistance gene expressed from its own promoter and a green fluorescent protein (GFP) gene expressed from the Stx2 phage late gene promoter (7). In this report, we refer to φ933W with stx2 deleted as φ933WΔtox to distinguish it from the other phages with stx2 deleted that were constructed in this study. We used the same strategy to produce phages with the toxin deleted from clinical E. coli O157:H7 isolates. Briefly, E. coli O157:H7 isolates were transformed with the temperature-sensitive, kanamycin-resistant suicide vector pSG039 (7), a derivative of pPIR-K which has an insert that includes chloramphenicol resistance and GFP genes flanked by the sequences upstream and downstream from Stx2. Growth at 37°C on LB plates with chloramphenicol selected for recombinants where the Stx2 genes were replaced with chloramphenicol resistance and GFP genes. Deletion of Stx2 genes was confirmed by PCR (7). Phage isolated from the recombinant E. coli O157:H7 strains were incubated with E. coli strain C600, and chloramphenicol-resistant colonies were selected. Lysogeny was confirmed by PCR of GFP, and lack of Stx2 production was confirmed by enzyme-linked immunosorbent assay (ELISA).
Phage induction.
Phage from E. coli O157:H7 or from lysogenized strains of C600 were induced with either ciprofloxacin or mitomycin C. For ciprofloxacin inductions, overnight broth cultures were adjusted to an optical density (OD) at 600 nm of 0.08 in LB-modified broth, ciprofloxacin (30 ng/ml) was added, and the culture was incubated at 37°C with shaking for about 16 h. For mitomycin C inductions, overnight broth cultures were adjusted to an OD of about 0.1 in LB-modified broth, the cultures were incubated at 37°C for 2 h with shaking, mitomycin C (0.5 μg/ml) was added, and cultures were incubated at 37°C for about 16 h with shaking. The OD was used to monitor lysis. The induced cultures were centrifuged (5,000 × g, 10 min), and the supernatants were filter sterilized.
Determination of Stx concentrations.
Stx concentrations were determined either by ELISA (Premier EHEC ELISA; Meridian Bioscience, Inc., Cincinnati, Ohio) or by a Vero cell assay, as indicated. For the Vero cell assay, twofold serial dilutions of filter-sterilized culture supernatants were made in 25 μl of phosphate-buffered saline in a 96-well plate. Dilutions were overlaid with 100 μl of 105 Vero cells/ml, and plates were incubated at 37°C, 5% CO2 for 3 days. The cells were stained with Giemsa, and the reciprocal of the dilution at which 50% of the Vero cells were dead was determined. The amount of Stx in the samples was determined by comparison to a standard curve with purified Stx2 (Toxin Technology, Inc., Sarasota, Fla.).
Determination of GFP production.
GFP in supernatants from uninduced and induced cultures of the C600::Δtox lysogens was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 8 to 16% precast Tris-glycine gels (Cambrex Bioscience Rockland Inc., Rockland, Maine). Bands were transferred to polyvinylidene difluoride (PVDF) membranes by wet transfer with the use of a Trans-Blot cell (Bio-Rad Laboratories). GFP was detected by probing membranes with anti-GFP antibody (1:500 dilution; BD Biosciences Clontech, Palo Alto, Calif.) followed by goat anti-rabbit secondary antibody (1:37,500; Cappel, West Chester, Pa.). Bands were visualized with the Western Lightning chemiluminescence reagent plus kit (Perkin-Elmer Life Sciences, Boston, Mass.), and the relative intensity of the signals was determined with ImageQuant (version 5.1; Molecular Dynamics, Amersham Biosciences, Piscataway, N.J.).
Phage purification.
Phage were isolated from ciprofloxacin-induced cultures (200 ml) by polyethylene glycol precipitation and purified by cesium chloride centrifugation (15, 39). Morphology was assessed by electron microscopy with 2% phosphotungstic acid staining of phage particles (21, 28). Phage protein profiles were determined by SDS-PAGE with a 10% Tris-glycine precast gel (Cambrex Bioscience Rockland Inc.), and bands were visualized with Coomassie stain.
Southern analysis.
Phage DNA was isolated from ciprofloxacin-induced cultures of the parent O157 strains and the C600 lysogens by using polyethylene glycol precipitation and phenol-chloroform extraction (29). Chromosomal DNA was isolated with the DNeasy tissue kit (QIAGEN, Valencia, Calif.). Phage and chromosomal DNAs were digested with EcoRI for 3 h at 37°C, and bands were resolved by electrophoresis on a 0.7% agarose gel (SeaKem GTG agarose; Biowhittaker Molecular Applications, Rockland, Maine). The digoxigenin-11-dUTP (DIG)-labeled DNA molecular weight marker VII (Roche Diagnostics Corporation, Indianapolis, Ind.) was run as a standard. DNA bands were transferred by upward capillary transfer (29) to nylon membranes (Immobilon-NY+; Millipore Corporation, Bedford, Mass.).
DIG-labeled probes were prepared using the PCR DIG probe synthesis kit (Roche Diagnostics). The primers use to generate the stx2 probe (19) and the GFP probe (7) were described previously. After hybridization of the probes to the membranes (65°C; DIG Easy Hyb; Roche Diagnostics), bands were detected with the DIG wash and block buffer set (Roche Diagnostics) and the DIG luminescent detection kit (Roche Diagnostics).
Determination of phage immunity profiles.
C600 lysogens were induced with ciprofloxacin as described above and centrifuged (5,000 × g, 10 min), and the phage-containing supernatants were filter sterilized. Five microliters of supernatant was spotted onto LB-modified agar overlaid with LB-modified soft agar (0.7%) containing the test strain. Following overnight incubation at 37°C, plates were examined for the formation of plaques.
Susceptibility of E. coli strains to lysogeny.
Phages with stx2 deleted and encoding chloramphenicol resistance were used to examine the ability of the phage to lysogenize different E. coli isolates. Approximately 106 to108 phage were mixed with 7 ml of overnight cultures of the E. coli strains (approximately 109 CFU/ml). The samples were poured onto LB-modified agar plates and incubated overnight at 37°C as static cultures, and 100 μl was plated onto LB agar supplemented with chloramphenicol. Two or three chloramphenicol-resistant colonies were streaked for isolation, and the presence of the phage genome was confirmed by PCR with the primers to GFP as previously described (7).
Susceptibility of E. coli to lytic infection.
The stx2 genes are under control of the phage late gene promoter (28) and, therefore, Stx2 is produced and released during lytic phage infection. Elevated production of Stx2 was used to assess lytic infection. Phage were isolated from E. coli O157:H7 induced with 30 ng of ciprofloxacin/ml for 16 h at 37°C. The amount of Stx in the supernatants was determined by ELISA. Phage preparations were diluted in phosphate-buffered saline such that the amount of toxin added to the non-O157 E. coli strains was less than 50 ng/ml. Inoculated cultures were overlaid onto LB-modified agar and incubated at 37°C overnight as a static culture. The cultures were centrifuged (5,000 × g, 10 min), and the supernatants were filter sterilized. Stx2 production was assessed in the Vero cell assay.
RESULTS
Twenty-three clinical E. coli O157:H7 isolates from the Cincinnati area between 1999 and 2001 were characterized for differences in the Stx-encoding phage. The isolates fell into 11 groups based on different polymorphisms by PCR using previously published primers for Stx1 and Stx2 and surrounding phage late genes (34). Six of the E. coli O157:H7 isolates, which possessed different polymorphisms, and φ933W were chosen for further examination.
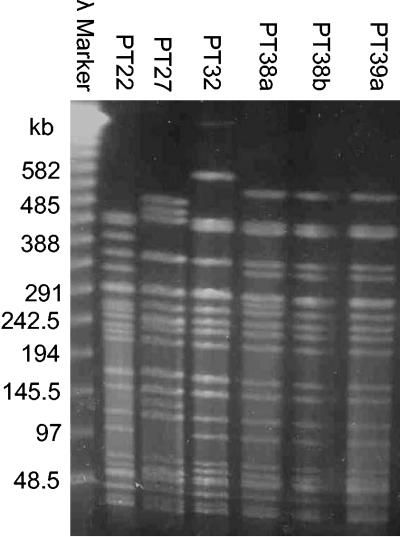
Strain PT22 was isolated in 2000, and strain PT27 was isolated in 2001. Cincinnati experienced an outbreak of E. coli O157:H7 in 1999. Strain PT32 was isolated in July 1999, and strains PT38a, PT38b, and PT39a were each isolated in the same week in September 1999. PT38b and PT39a had identical PFGE profiles after restriction of total DNA with XbaI (Fig. 1). PT38a had the same profile as the other isolates from September 1999, with an additional band at about 290 kb (Fig. 1). The XbaI profile of strain PT32 had a two-band difference from PT38b and PT39a (Fig. 1). PT32, PT38a, PT38b, and PT39a would be considered to be closely related (33). However, the XbaI digestion profiles of PT22 and PT27 had multiple different bands compared to each other and to the 1999 strains, and these strains would be considered to be different isolates (33).
FIG. 1.
PFGE of E. coli O157:H7 isolates. E. coli O157:H7 strains were digested with XbaI overnight and run on a 1% agarose gel for 22 h.
Stx2-encoding phage characteristics from E. coli O157:H7 and from C600 lysogens.
Toxin production from the E. coli O157:H7 strains was assessed by ELISA (Table 2). Under normal growth conditions, the strains produced similar amounts of Shiga toxin (about 20 μg/ml). Shiga toxin production was also assessed following treatments that cause chromosomal alterations and induce the phage to enter the lytic cycle. Treatment with ciprofloxacin resulted in about 10-fold more toxin production than in uninduced cultures, while mitomycin C resulted in about 20- to 60-fold more toxin (Table 2). To assess bacterial lysis following phage induction, the OD was determined after 16 h (Table 2). Induction of C600::933W typically results in an initial increase in OD as the culture grows to an OD of about 0.6, followed by a decrease in the OD as phage are produced and bacteria are lysed (7). In contrast to the laboratory strain, lysis of the E. coli O157:H7 strains was found to be variable (Table 2), even though Stx production was similar for all of the phages. These results differ from those of Muniesa et al. (19), who reported a strong correlation between lysis and toxin production.
TABLE 2.
Characteristics of phages from clinical E. coli O157:H7 isolates
| Strain and phage | Uninduceda | Ciprofloxacin inductionb
|
Mitomycin C inductionc
|
||
|---|---|---|---|---|---|
| Lysis (OD)d | Stx or GFP concn | Lysis (OD) | Stx or GFP concn | ||
| E. coli O157:H7 | |||||
| PT22 | 27e | 0.238 | 347e | 0.507 | 975e |
| PT27 | 23 | 0.409 | 354 | 0.340 | 588 |
| PT32 | 24 | 0.430 | 426 | 0.534 | 886 |
| PT38a | 18 | 0.395 | 355 | 0.618 | 942 |
| PT38b | 19 | 0.376 | 352 | 0.520 | 1127 |
| PT39a | 24 | 0.469 | 364 | 0.558 | 1119 |
| C600 lysogens (Stx2 deleted) | |||||
| φPT22Δtox | 0.05f | 0.226 | 41f | 0.845 | 59f |
| φPT27Δtox | NDg | 0.209 | 51 | 0.690 | 49 |
| φPT32Δtox | ND | 0.243 | 33 | 0.845 | 67 |
| φPT38aΔtox | ND | 0.430 | 49 | 0.570 | 51 |
| φPT38bΔtox | ND | 0.825 | 79 | 0.855 | 21 |
| φPT39aΔtox | ND | 0.430 | 72 | 1.460 | 28 |
Strains were grown overnight in LB broth at 37°C with shaking.
Strains were induced with 30 ng of ciprofloxacin/ml for 16 h at 37°C with shaking.
Strains were induced with 0.5 μg of mitomycin C/ml for 16 h at 37°C with shaking.
Lysis was assessed by measuring the OD600 after 16 h induction at 37°C with shaking, in the presence of ciprofloxacin or mitomycin C. Results in this trial are representative of the trends observed in at least one other trial.
Stx concentration (in micrograms per milliliter) from the E. coli O157:H7 strains was measured by ELISA.
GFP production from the C600 lysogens was determined by Western analysis, and bands within a strain were compared using ImageQuant. Results for uninduced, ciprofloxacin-induced, and mitomycin C-induced samples are reported as the percentage of the total volume of the bands for each strain.
ND, not detected.
The ELISA detects both Stx1 and Stx2, and the E. coli O157:H7 isolates characterized in this study possess the genes for both toxins. Furthermore, strains can harbor more than one lysogenic phage (2). To examine the induction profile of an isolated Stx2-encoding phage, the phage-encoded stx2 genes were replaced with two markers, chloramphenicol resistance and GFP, using allelic exchange as previously described (7). Phage with stx2 deleted were lysogenized into E. coli strain C600 to yield the Δtox phage described in Table 1. In the Δtox constructs, GFP expression is under the control of the late phage gene promoter (7), and the production of GFP with and without induction was used to monitor phage-mediated release of Stx2. GFP in the culture supernatant was determined by quantitative Western analysis (Table 2). Little to no GFP was detected in the uninduced supernatants (Table 2). Increased levels of GFP were detected after induction for all strains but were varied among the lysogens with respect to the inducing agent. As observed with the parental strain, lysis was highly variable and the amount of GFP produced after induction did not correlate well with the amount of lysis.
Southern analysis to detect stx2 and GFP genes.
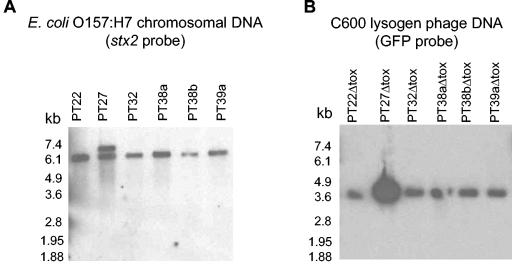
E. coli O157:H7 can harbor multiple genes for Stx2. We used Southern analysis to examine the Stx2-encoding genes in the E. coli O157:H7 isolates. The stx2 probe hybridized to a 6.5-kb fragment present in the chromosomal DNA from all of the E. coli O157:H7 isolates; however, in PT27 the stx2 probe also hybridized to a 7-kb band (Fig. 2A). For the Δtox phage derivatives, a single 3.6-kb band hybridized with the GFP probe for both phage DNA (Fig. 2B) and chromosomal DNA (data not shown) for all of the C600 lysogens. These results suggest that PT27 contains two copies of the stx2 gene and Δtox recombined into the copy of stx2 encoded in the 6.5-kb EcoRI fragment.
FIG. 2.
Southern analyses of stx2 and GFP genes in phage and chromosomal DNA digested with EcoRI. (A) Chromosomal DNA from E. coli O157:H7 strains probed with DIG-labeled Stx2. (B) Phage DNA from C600 lysogens of phage with the toxin deleted that was probed with DIG-labeled GFP.
Phage production and plaquing profiles.
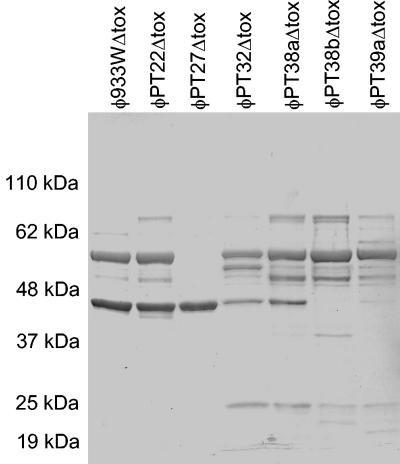
Phage from E. coli O157:H7 often produce very small plaques. In initial studies following induction, only supernatants from the C600::933W control, PT22, PT27, and PT32 were able to form visible plaques on the C600 indicator strain, suggesting that the other strains of E. coli O157:H7 might be defective for phage production. However, our ability to lysogenize C600 with all the Δtox derivatives of E. coli O157:H7 strains suggests these strains produce viable phage. To further examine phage production, phage were purified from the C600 Δtox lysogens and C600::H19B, an Stx1-encoding phage (22). The phages were examined by electron microscopy. All six phages from the clinical isolates and φ933WΔtox were similar in shape (hexagonal heads with short tails) and head size (55 to 65 nm), consistent with published images of 933W (21, 28), the parental phage for φ933WΔtox. In contrast, the Stx1-encoding phage, H19B, had a larger, elongated head and long tail (data not shown). Proteins from the purified phages were separated by SDS-PAGE (Fig. 3). Each phage displayed a unique protein profile, suggesting each phage was distinct.
FIG. 3.
Phage protein profiles. Phage were isolated by polyethylene glycol precipitation and cesium chloride centrifugation. Phage proteins were separated by SDS-PAGE. Bands were visualized by Coomassie staining.
Immunity profiles of phage from C600 lysogens.
Bacteriophage lambda lysogens are resistant to lytic infection following superinfection by phage expressing the same immunity protein (11). To determine if the different Stx2-encoding phages in the present study possessed similar immunity genes, phage from C600 lysogenized with the Δtox phage were spotted onto C600. φ933WΔtox, φPT22Δtox, φPT27Δtox, and φPT32Δtox produced distinct zones of clearing on the C600 indicator (Table 3), while no plaquing was observed when C600 was infected with φPT38aΔtox, φPT38bΔtox, or φPT39aΔtox. These results are identical to those seen when phage were prepared from the parental O157:H7 strain. Since C600 was successfully lysogenized by these phage, these results suggest that these phages are defective in lytic infection, but not lysogeny. This is further supported by the observation that certain phages were able to form plaques only on other lysogens, suggesting genes from the resident phage could complement the lysis defect in the infecting phage. For example, both φPT38aΔtox and φPT38bΔtox formed plaques on C600::φPT32Δtox.
TABLE 3.
Phage immunity profiles determined by cross-plaquing
| Phage | Result with C600 lysogen indicator
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C600 | φ933W | φPT22Δtox | φPT27Δtox | φPT32Δtox | φPT38aΔtox | φPT38bΔtox | φPT39aΔtox | |
| φ933WΔtox | +++a | − | − | − | − | − | − | ++ |
| φPT22Δtox | +++ | − | − | − | − | − | + | + |
| φPT27Δtox | +++ | +++ | +++ | − | − | ++ | +++ | + |
| φPT32Δtox | +++ | ++ | − | + | − | +++ | +++ | ++ |
| φPT38aΔtox | − | − | − | − | + | − | − | − |
| φPT38bΔtox | − | − | − | − | + | ++ | − | + |
| φPT39aΔtox | − | − | − | − | − | − | − | − |
−, no plaques observed; +, single plaques; ++, hazy zone; +++, distinct clearing.
Cross-plaquing on lysogenic strains was examined. Consistent with lambda immunity to superinfection, all of the lysogens were resistant to lytic infection with their phage. However, highly variable results were obtained when the phages were incubated with the nonhomologous C600 lysogens. No two phages had the same cross-plaquing profile. Furthermore, no clear evidence of reciprocal cross-susceptibility was apparent. For example, both φPT22Δtox and φPT27Δtox produced phage capable of lytic infection, as evidenced by clear plaques on the C600 indicator. φPT27Δtox produced zones of clearing on C600:φPT22Δtox; however, φPT22Δtox did not produce plaques on C600:φPT27Δtox.
Susceptibility of E. coli O157:H7 strains to lysogeny.
The ability of the Δtox phage derivatives to infect or lysogenize E. coli O157:H7 was also examined. None of the phages formed plaques on the E. coli O157:H7 strains. To examine lysogeny, overnight cultures of E. coli O157:H7 were incubated with the Δtox phage. Chloramphenicol-resistant colonies were selected, and lysogeny was confirmed by a positive PCR for the GFP gene. None of the phages lysogenized the E. coli O157:H7 strain from which it was derived (Table 4). E. coli O157:H7 strains PT22 and PT32 were not lysogenized with any of the phages with the toxin deleted, and the other O157:H7 isolates each had a different lysogenic profile. Together the results for lytic and lysogenic infections suggest that all of the Stx2-encoding phages belong to different immunity groups and some of the phages were defective for lytic infection.
TABLE 4.
Susceptibility of E. coli O157:H7 isolates to lysogeny by phages with the toxin deleted
| Phage | Result with clinical E. coli O157:H7 recipient
|
|||||
|---|---|---|---|---|---|---|
| PT22 | PT27 | PT32 | PT38a | PT38b | PT39a | |
| φ933WΔtox | − | − | − | + | + | − |
| φPT22Δtox | − | − | − | + | − | − |
| φPT27Δtox | − | − | − | + | − | + |
| φPT32Δtox | − | − | − | + | + | + |
| φPT38aΔtox | − | + | − | − | − | − |
| φPT38bΔtox | − | − | − | − | − | − |
| φPT39aΔtox | − | − | − | − | − | − |
Susceptibility of human intestinal E. coli to lysogeny.
In initial studies, the ability of φ933WΔtox, a derivative of φ933W (7), to lysogenize C600 and C600::933W was examined. Following overnight incubation, about 106 φ933WΔtox chloramphenicol-resistant lysogens were obtained for C600 and 104 chloramphenicol-resistant lysogens were obtained for C600::933W. While previous studies have shown that C600::933W is resistant to lytic infection by φ933WΔtox (7), these results suggest integration of the phage does not appear to be blocked by the resident φ933W genome, a result that was not observed for the E. coli O157:H7 isolates (Table 4). The C600::933W lysogens were characterized for the presence of Stx2 to determine if chloramphenicol resistance was due to insertion of a second phage genome or if allelic exchange replaced the stx2 genes with the GFP or chloramphenicol cassette. Six of 12 chloramphenicol-resistant C600::933W isolates lacked the stx2 genes, suggesting allelic exchange had occurred. The other six chloramphenicol-resistant lysogens of C600::933W possessed the stx2 genes in addition to the chloramphenicol and GFP genes, suggesting they represented double lysogens.
This same procedure was used to determine the ability of φ933WΔtox to establish lysogeny in natural isolates of E. coli. In preliminary studies we used the ECOR strain collection (23). The ECOR collection consists of 72 isolates of E. coli from human and animal sources that are thought to be representative of the genetic diversity of the species. These strains have been extensively characterized for many traits. Lysogeny with φ933WΔtox was detected for 31 of the 72 isolates (43%). Forty of the ECOR strains were isolated from humans and 35%, or 14 of 40, of the human isolates were sensitive to lysogeny. Phage susceptibility did not correlate with lipopolysaccharide (LPS) core or O-antigen type. Results are reported only for the positive strains of the 40 ECOR isolates of human origin (Table 5).
TABLE 5.
Human E. coli isolates susceptible to lysogenic and/or lytic infection by Stx phages
| Strain | Phylogenetic group | φ933WΔtox or φ933W
|
φPT32Δtox or φPT32
|
||
|---|---|---|---|---|---|
| Lysogenya | Stx amplificationb | Lysogeny | Stx amplification | ||
| Human ECORc (O:H/core)d | |||||
| ECOR-1 (O144:H4/R2) | A | + | − | − | − |
| ECOR-4 (OR:H?/R4) | A | + | 256× | + | − |
| ECOR-6 (O173:H?/R3) | A | + | 8× | + | 32× |
| ECOR-9 (O167:H−/R3) | A | + | − | + | − |
| ECOR-10 (O6:H10/R2) | A | + | − | − | − |
| ECOR-12 (O?:H32/R2) | A | + | − | − | − |
| ECOR-13 (OR:H25/K-12) | A | + | 512× | + | 1,024× |
| ECOR-15 (O25:H30/R1) | A | + | − | − | − |
| ECOR-71 (OR:H19/R1) | B1 | + | − | − | − |
| ECOR-72 (O8:H30/R1) | B1 | + | − | − | − |
| ECOR-61 (O2:H4/R1) | B2 | + | − | − | − |
| ECOR-51 (O25:H1/R1) | B2 | − | − | − | 4× |
| ECOR-39 (O7:H−/R1) | D | + | − | − | − |
| ECOR-42 (O87:H26/R1) | E | + | − | − | − |
| ECOR-43 (O?:H18/R4) | E | + | − | − | − |
| Fecal isolatese | |||||
| FI-13 | A | + | − | − | − |
| FI-8 | B2 | + | − | − | − |
| FI-9 | B2 | − | − | + | − |
| FI-15 | B2 | + | − | − | 8× |
| FI-16 | B2 | + | − | − | − |
| FI-31 | B2 | − | − | − | 10× |
| FI-37 | B2 | − | − | − | 9× |
| PT-3 | A | + | − | − | − |
| PT-1 | B1 | + | − | + | − |
| PT-8 | B1 | + | − | − | − |
| PT-2 | B2 | + | − | − | − |
| PT-10a | B2 | + | − | − | − |
| PT-6 | D | − | − | + | − |
| PT-12 | D | + | − | − | − |
Lysogeny was assessed by acquisition of GFP DNA from colonies selected for chloramphenicol resistance following infection with φ933WΔtox or φPT32Δtox. +, lysogens detected; −, no lysogens detected.
Stx, a late gene product, was monitored as evidence of lytic infection following incubation with phage φ933W or φPT32. Fold increase over Stx present in initial phage inoculum is reported when significantly elevated over control values (P < 0.05). −, toxin amplification not detected.
Fifteen of 40 human ECOR isolates tested positive for lysogeny or lytic infection (negative strains or results are not reported).
Serotyping and determination of LPS core type as reported by Amor et al. (2a). OR, rough LPS; ?, unidentifiable.
Seven of 29 FI isolates and 7 of 12 PT isolates tested positive for lysogeny or lytic infection.
Studies examining the lysogeny of the human ECOR isolates were repeated with φPT32Δtox. Only 10% (4 of 40) of the human ECOR strains were lysogenized by φPT32Δtox, compared to 35% of the strains by φ933WΔtox, and these four strains were lysogenized by both φ933WΔtox and φPT32Δtox.
In addition to testing the ECOR set of E. coli for lysogeny by φ933WΔtox and φPT32Δtox, a number of human E. coli isolates from the Cincinnati area were also examined. Twenty-nine E. coli isolates were obtained from healthy volunteers, and 12 E. coli isolates were obtained from stool cultures from patients treated with antibiotics but not infected with E. coli O157:H7. Four of 29 (14%) fecal E. coli isolates from healthy volunteers and 6 of 12 (50%) fecal E. coli isolates from patients were sensitive to lysogeny by φ933WΔtox (Table 5). Only 1 out of 29 E. coli isolates (3%) from healthy volunteers and 2 out of 12 isolates (17%) from patients were susceptible to lysogeny by φPT32Δtox (Table 5). These studies indicate that φ933WΔtox and φPT32Δtox have different host ranges with respect to lysogeny.
Phylogenetic studies have divided E. coli into four main groups: A, B1, B2, and D (31). Virulent strains primarily belong to groups B2 and D, characterized by a large genome size (4). Human commensal intestinal isolates have been reported to primarily belong to groups A and B1, which are characterized by a small genome size (4); however, recent studies suggest this may not be true for all populations (40). For the fecal isolates examined in this study, most (23 of 29, or 79%) of the E. coli isolates from the healthy individuals belonged to the larger genome-size groups, group B2 and group D. However, distribution of the 12 E. coli isolates from patients was more even, with 5 of 12 (42%) in group A or B1 and 7 of 12 (58%) in groups B2 and D.
Susceptibility to lysogeny by phage 933WΔtox appeared to be somewhat dependent on phylogenetic group. When the results for all the human E. coli isolates were compiled, lysogeny by phage 933WΔtox was more prevalent in strains belonging to the phylogenic groups with the smaller genome size. For example, 59% (19 of 32) of the group A isolates and 48% (10 of 21) of the group B1 isolates were susceptible to lysogeny by phage 933WΔtox. Lysogeny was observed less often in the larger genome-size groups; only 17% (7 of 40) of the group B2 isolates and 19% (3 of 16) of the group D isolates were susceptible to lysogeny by phage 933WΔtox. The relationship between phylogenetic group and susceptibility to lysogeny is less apparent for φPT32Δtox, primarily due to the low number of lysogens. However, four of the seven strains capable of lysogeny were in phylogenetic group A.
Several non-Escherichia fecal isolates were also characterized for susceptibility to lysogeny with φ933WΔtox. These include five isolates of C. freundii, two isolates of K. pneumoniae, and a single isolate of K. oxytoca, K. ozaenae, E. amnigenes, E. aerogenes, S. marcescens, A. lwolfii, and Edwardsiella spp. and a strain that was not identifiable by the Enterotube system. No lysogens were detected with these isolates.
Shiga toxin production by fecal E. coli following lytic infection.
We have previously shown that lytic infection of E. coli by φ933W can result in highly elevated levels of Stx2 (7). The ability of phage to promote lytic infection was monitored by assessing the ability of bacteria incubated with about 102 phage to produce elevated levels of Shiga toxin. Amplified Stx2 following infection with φ933W was detected for three strains, ECOR-4, ECOR-6, and ECOR-13, all of which were capable of being lysogenized with the Δtox derivative of φ933W (Table 5).
The ability of phages from the six Cincinnati E. coli O157:H7 isolates to promote lytic infection and amplify Shiga toxin production was also monitored (Table 5). Only the phage from clinical isolate PT32 amplified toxin in any of the non-toxin-producing E. coli strains tested. Susceptibility profiles to φ933W and φPT32 were different. ECOR-6 and ECOR-13 were susceptible to lysogenic and lytic infection by φPT32 as well as φ933W (Table 5). ECOR-51 was susceptible to lytic infection by φPT32 but not φ933W; however, lysogeny of this strain with either phage was not detected. Overall, the non-O157 isolates were highly variable in their susceptibility to lytic or lysogenic infection with the different phages. Interestingly, while φ933W was much more likely to lysogenize the non-O157 isolates than φPT32, infection with φPT32 was much more likely to result in amplified Shiga toxin expression.
DISCUSSION
A number of phage-related factors have been described that influence the severity of disease caused by Shiga toxin-encoding E. coli. Toxin production is regulated by phage late gene expression. Antibiotic treatment has been shown to induce late gene expression and increase toxin expression in vitro (12, 13, 41). Furthermore, epidemiological studies have suggested that the use of antibiotics may exacerbate the disease process in humans (12, 38). This aspect of phage biology has seriously limited the treatment options for E. coli O157:H7.
In addition, Muniesa et al. (19) showed that E. coli O157:H7 isolates from different patients in a single outbreak were highly variable with respect to their ability to produce phage and Shiga toxin in vitro. Toxin production was genetically linked to the phage type expressed by the strain, and isolates capable of producing high levels of Shiga toxin were more likely to be isolated from patients with severe systemic disease. The inherent ability of the phage to produce high levels of Stx is likely to be a significant factor in influencing whether a patient develops self-limiting disease or life-threatening complications, such as hemolytic uremic syndrome.
In this study we examined the diversity of Stx2-encoding phage isolated from highly related strains of E. coli O157:H7. In contrast to the study by Muniesa et al. (19), all of the E. coli O157:H7 isolates characterized in this study produced similar, high levels of toxin in the presence or absence of treatments which induce the phage lytic cycle. This difference could be due to the fact that the E. coli O157:H7 strains characterized in this study were all obtained from Cincinnati Children's Hospital and may represent isolates likely to be highly virulent. Livny and Friedman (14) demonstrated that Stx-encoding phages were more readily induced than non-Stx-encoding phages and suggested that there is selective pressure on Stx-encoding phages to enter the lytic cycle and release Stx as a competitive advantage for pathogenic bacteria in the intestine. The isolates used in our studies may have demonstrated particular fitness in patients due, in part, to the high levels of Stx released.
Electron microscopy revealed that all of the phages had morphological characteristics similar to those reported for other Stx2-encoding phages (2, 28) but different from Stx1-encoding phages from E. coli O26 strain H19 (22). In contrast to published reports (2) the protein profiles for the phages used in this study differed. The Stx2 phages were also found to be remarkably different with regard to host range for both lysogeny and lytic infection, despite being isolated, in some cases, from otherwise indistinguishable E. coli O157:H7 strains. The sequence of E. coli O157:H7 strain EDL933 revealed that this strain possessed only a single Stx2-encoding phage, but multiple defective phage genomes were present (26). Recombination between phage genomes could generate diversity, even between highly related strains, as observed in this study. Phage diversity and changes in either lysogenic or lytic host range within an otherwise clonal population of E. coli O157:H7 have implications for disease. Future studies that include epidemiological data of the isolates will add valuable information to the role of phage variability and host range in an outbreak.
Lysogeny with Stx2-encoding phage is thought to be a driving force for evolution of new pathogens. For example, lysogeny of S. sonnei with Stx-encoding phage (32) is a very disturbing development. We examined a small sample of non-E. coli commensal Enterobacteriaceae and did not detect lysogeny of φ933WΔtox. Stx-related disease has been documented that was reportedly due to strains of C. freundii (30) and Enterobacter spp. (25), and it is possible that a larger sampling of Enterobacteriaceae or a broader range of Stx-encoding phages would yield lysogens.
While lysogeny may influence evolution of pathogens, lytic infection may directly influence disease outcome. Two Stx2-encoding phages were able to infect normal E. coli cells and amplify Shiga toxin production, and lytic infection increased toxin production by more than 1,000-fold in one case (Table 5). The susceptible E. coli isolates varied with respect to O-antigen and LPS core types, suggesting that factors other than LPS influence susceptibly. E. coli isolates from all phylogenetic groups were susceptible to lysogeny by Shiga toxin-encoding phage; however, E. coli isolates belonging to the phylogenetic groups with smaller genome sizes, groups A and B1, were more likely to be susceptible to phage. Initial reports on subjects from around the world have suggested that E. coli strains in groups A, B1, and D are the predominant colonizers of the intestine (6, 27, 40). A recent study from the United States (40) and our examination of fecal isolates from the Cincinnati area indicate that in some populations the group B2 E. coli, usually associated with extraintestinal disease, predominate. The composition of the intestinal flora could influence the susceptibility of individuals and populations to lytic infection by Shiga toxin-encoding phages. Having a diverse host range may confer a selective advantage for toxin-encoding phages by allowing them to infect intestinal E. coli.
Similar to previous studies, the phages examined in this study were found to be highly variable, despite the relatedness of the E. coli O157:H7 strains from which they originated. Phage diversity with regard to toxin production can directly influence the ability to cause disease. Variations in phage host range with regard to lysogeny can influence the evolution of new pathogenic strains. We suggest that variation in phage host range may also confer a selective advantage for toxin-encoding phage by increasing the probability that infected intestinal E. coli will produce toxin. The ability of toxin-encoding phage to influence disease outcome underscores the importance for understanding the diversity of these phages.
Acknowledgments
We thank Melanie Cushion and Sandy Rebholz for assistance with the PFGE analysis, Joel Mortensen and the Cincinnati Children's Hospital Medical Center culture collection for the E. coli O157:H7 strains, and Judith Rhodes for patient isolates.
This work was supported by grant R21-AI-02-008 to A.A.W. S.D.G. was supported by T32-AI055406.
Editor: A. D. O'Brien
REFERENCES
- 1.Acheson, D. W., J. Reidl, X. Zhang, G. T. Keusch, J. J. Mekalanos, and M. K. Waldor. 1998. In vivo transduction with Shiga toxin 1-encoding phage. Infect. Immun. 66:4496-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, H. E., M. J. Sergeant, C. E. James, J. R. Saunders, D. L. Smith, R. J. Sharp, T. S. Marks, and A. J. McCarthy. 2003. Immunity profiles of wild-type and recombinant Shiga-like toxin-encoding bacteriophages and characterization of novel double lysogens. Infect. Immun. 71:3409-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Amor, K., D. E. Heinrichs, E. Frirdich, K. Ziebell, R. P. Johnson, and C. Whitfield. 2000. Distribution of core oligosaccharides types in lipopolysaccharides from Escherichia coli. Infect. Immun. 68:1116-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, E. F., and D. L. Hartl. 1998. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J. Bacteriol. 180:1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duriez, P., O. Clermont, S. Bonacorsi, E. Bingen, A. Chaventre, J. Elion, B. Picard, and E. Denamur. 2001. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology 147:1671-1676. [DOI] [PubMed] [Google Scholar]
- 7.Gamage, S. D., J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect. Immun. 71:3107-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in one day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James, C. E., K. N. Stanley, H. E. Allison, H. J. Flint, C. S. Stewart, R. J. Sharp, J. R. Saunders, and A. J. McCarthy. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen, B. K., Y. Wasteson, P. E. Granum, and S. Brynestad. 2001. Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology 147:1929-1936. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, A. D., A. R. Poteete, G. Lauer, R. T. Sauer, G. K. Ackers, and M. Ptashne. 1981. λ repressor and cro-components of an efficient molecular switch. Nature 294:217-223. [DOI] [PubMed] [Google Scholar]
- 12.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 1999. Induction of type 2 Shiga toxin synthesis in Escherichia coli O157 by 4-quinolones. Lancet 353:1588-1589. [DOI] [PubMed] [Google Scholar]
- 13.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 2000. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livny, J., and D. I. Friedman. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol. Microbiol. 51:1691-1704. [DOI] [PubMed] [Google Scholar]
- 15.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto, H., W. Nakai, N. Yajima, A. Fujibayashi, T. Higuchi, K. Sato, and A. Matsushiro. 1999. Sequence analysis of Stx2-converting phage VT2-Sa shows a great divergence in early regulation and replication regions. DNA Res. 6:235-240. [DOI] [PubMed] [Google Scholar]
- 18.Muhldorfer, I., J. Hacker, G. T. Keusch, D. W. Acheson, H. Tschape, A. V. Kane, A. Ritter, T. Olschlager, and A. Donohue-Rolfe. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect. Immun. 64:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muniesa, M., M. de Simon, G. Prats, D. Ferrer, H. Panella, and J. Jofre. 2003. Shiga toxin 2-converting bacteriophages associated with clonal variability in Escherichia coli O157:H7 strains of human origin isolated from a single outbreak. Infect. Immun. 71:4554-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely, M. N., and D. I. Friedman. 1998. Arrangement and functional identification of genes in the regulatory region of lambdoid phage H-19B, a carrier of a Shiga-like toxin. Gene 223:105-113. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 23.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 25.Paton, A. W., and J. C. Paton. 1996. Enterobacter cloacae producing a Shiga-like toxin II-related cytotoxin associated with a case of hemolytic-uremic syndrome. J. Clin. Microbiol. 34:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 27.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Elion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plunkett, G., III, D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Schmidt, H., M. Montag, J. Bockemuhl, J. Heesemann, and H. Karch. 1993. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect. Immun. 61:534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selander, R. K., D. A. Caugant, and T. S. Whittam. 1987. Genetic structure and variation in natural populations of Escherichia coli, p. 1625-1648. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 32.Strauch, E., R. Lurz, and L. Beutin. 2001. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 69:7588-7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unkmeir, A., and H. Schmidt. 2000. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect. Immun. 68:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner, P. L., D. W. Acheson, and M. K. Waldor. 2001. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect. Immun. 69:1934-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner, P. L., D. W. Acheson, and M. K. Waldor. 1999. Isogenic lysogens of diverse Shiga toxin 2-encoding bacteriophages produce markedly different amounts of Shiga toxin. Infect. Immun. 67:6710-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto, K. R., B. M. Alberts, R. Benzinger, L. Lawhorne, and G. Treiber. 1970. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40:734-744. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, L., B. Foxman, and C. Marrs. 2002. Both urinary and rectal Escherichia coli isolates are dominated by strains of phylogenetic group B2. J. Clin. Microbiol. 40:3951-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. Acheson. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]