Abstract
Yersinia enterocolitica evades innate immunity by expression of a variety of pathogenicity factors. Therefore, adaptive immunity including CD4+ T cells plays an important role in defense against Y. enterocolitica. We investigated whether Y. enterocolitica might target dendritic cells (DC) involved in adaptive T-cell responses. For this purpose, murine DC were infected with Y. enterocolitica wild-type and mutant strains prior to incubation with ovalbumin (OVA) as antigen and 5-(6)-carboxyfluorescein diacetate N-succinimidyl ester-labeled OVA-specific T cells from DO11.10 mice. While T-cell proliferation was partially affected by infection of DC with plasmid-cured and YopP-deficient Yersinia mutant strains, no T-cell proliferation occurred after infection of DC with wild-type Y. enterocolitica. Infection of DC with Y. enterocolitica wild type resulted in decreased up-regulation of major histocompatibility complex class II, CD54 (intercellular adhesion molecule 1), CD 80, and CD86 expression. Experiments with plasmid-cured Y. enterocolitica or a YopP-deficient mutant strain revealed that YopP accounts for inhibition of surface molecule expression. Wild-type Y. enterocolitica suppressed the release of KC, tumor necrosis factor alpha, interleukin-10 (IL-10), and IL-12 by DC, while infection of DC with plasmid-cured Y. enterocolitica or with the YopP-deficient mutant resulted in the production of these cytokines. Moreover, infection with wild-type Y. enterocolitica induced apoptosis in DC mediated by YopP. Apoptosis occurred despite translocation of NF-κB to the nucleus, as demonstrated by electromobility shift assays. Together, these data demonstrate that Y. enterocolitica targets functions of murine DC that are required for T-cell activation. This might contribute to evasion of adaptive immune responses by Y. enterocolitica.
Yersinia enterocolitica is a gram-negative bacterial pathogen in humans and rodents which causes diarrhea and systemic infections, including abscesses in the spleen and liver, as well as immunopathological sequelae, such as reactive arthritis. The action of several pathogenicity factors of Y. enterocolitica, encoded by the chromosome and the pYV plasmid, are required. The pYV plasmid, including the yop virulon, encodes a type III secretion system by which Yersinia outer proteins (Yops) are secreted and translocated into the cytosol of host cells. YopB and YopD are part of a needle by which effector Yops (YopE, YopH, YopM, YopO, YopP, and YopT) are injected into the cytoplasm of mammalian target cells (9). YopE, YopT, and YopO cause disruption of the actin microfilament structures, and YopH dephosphorylates host cell proteins, such as focal adhesion kinase, paxillin, and p130Cas (5, 22). By this means, Y. enterocolitica prevents uptake into host cells. In fact, electron microscopy studies demonstrated that Y. enterocolitica is predominantly extracellularly located in host tissues (16). YopP inhibits both NF-κB signaling pathways by targeting IκB kinase-β and mitogen-activated protein kinase cascades by interacting with various members of the mitogen-activated protein kinase kinase superfamily, resulting in inhibition of cytokine production and apoptosis in macrophages (20, 26).
There is substantial evidence that elimination of Y. enterocolitica from infected tissue is accomplished by T-cell-activated macrophages. Therefore, T-cell responses, in particular those of CD4+ T cells, as well as the production of gamma interferon (IFN-γ), are essential to limit yersiniosis (1, 6, 7). In fact, T-cell-deficient nude mice or mice deficient in interleukin-12 (IL-12), IL-18, TNFRp55, or IFN-γ receptor are highly susceptible to Y. enterocolitica and cannot eliminate the pathogen from infected tissues. Consequently, immunity against a lethal Y. enterocolitica infection can be adoptively transferred by Yersinia-specific T-cell clones into naïve mice.
T cells need to be stimulated by antigen-presenting cells (APC) in order to become activated. Dendritic cells (DC) are the most important APC, as indicated by observations that DC are able to stimulate T cells even in the presence of minute quantities of antigen and by their capacity to activate naïve T cells (12).
Previous studies demonstrated that Y. enterocolitica might also target DC (27). Those authors demonstrated that although infection of human DC with Y. enterocolitica did not result in DC death, a transient down-regulation of major histocompatibility complex (MHC) class II and CD80 molecules was observed and accompanied by a decreased T-cell activation capacity as indicated by mixed lymphoid reaction analyses (20). A detailed analysis of these events and the effect of Y. enterocolitica on murine DC, however, has not yet been examined. In addition to DC, macrophages may also serve as APC. The effect of Yersinia infection on murine macrophages was analyzed, and induction of apoptosis was found to be one of the most important features of impairment of macrophage function by Y. enterocolitica (10, 25).
Therefore, we analyzed whether Y. enterocolitica O:8 impedes the CD4 T-cell activation capacity of bone marrow-derivedmurine DC by means of 5-(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled ovalbumin (OVA)-specific T cells from DO11.10 mice. Various apoptosis assays were performed to examine whether induction of host cell death might be involved in decreased T-cell activation. Furthermore, expression patterns of molecules contributing to immunological synapse formation (MHC class II, CD80, CD86, and intercellular adhesion molecule 1 [ICAM-1]) and also secretion of cytokines (IL-12, IL-10, KC, and tumor necrosis factor alpha [TNF-α]) were determined.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Winkelmann (Borchen, Germany). DO11.10 mice (19) were maintained under specific-pathogen-free conditions. The majority (>95%) of T cells in the DO11.10 mice are CD4+ T cells which bear a T-cell receptor that recognizes an OVA-derived peptide, OVA323-339, which is presented by I-Ad. The experiments were performed with 6- to 12-week-old female mice.
Bacterial strains and growth conditions.
The following Y. enterocolitica strains were used in this study: Y. enterocolitica WA-314 of serotype O:8 wild type carrying the virulence plasmid pYV (designated as WA-pYV+), a YopP-deficient mutant (WA-pYV+YopP−; kindly provided by K. Ruckdeschel, Munich, Germany), and a plasmid-cured Y. enterocolitica strain (WA-pYV−). All strains of Y. enterocolitica were grown overnight in Luria-Bertani broth at 27°C supplemented with chloramphenicol (20 μg/ml), nalidixic acid (10 μg/ml; ICN Biomedicals, Irvine, Calif.), spectinomycin (100 μg/ml), or kanamycin (25 μg/ml; Sigma, Deisenhofen, Germany). A 1:20 dilution of the overnight Yersinia culture was incubated for an additional 1.5 h at 37°C. The bacteria were washed once in phosphate-buffered saline (PBS; Invitrogen, Karlsruhe, Germany), and the optical density at 600 nm was determined. Heat-killed WA-pYV+ cells were generated by heating the bacteria at 60°C for 4 h. The bacteria were added at a multiplicity of infection (MOI) of 10 to the bone marrow-derived DC.
Cell cultures and infection.
DC and T cells were grown in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (Sigma), 2 mM l-glutamine (Invitrogen), 100 U of penicillin/ml, 100 μg of streptomycin (Biochrom)/ml, 50 μM β-mercaptoethanol (Sigma), 1% (vol/vol) nonessential amino acids (Biochrom), and 1 mM sodium pyruvate (Biochrom). DC were prepared according to the protocol of Lutz et al. (17). Briefly, 2 × 106 bone marrow cells, flushed from the femurs and tibias of BALB/c mice, were seeded in 100-mm dishes in 10 ml of medium containing 200 U of granulocyte-macrophage colony-stimulating factor (GM-CSF; produced by mouse myeloma strain P3X63)/ml. After 3 days, an additional 10 ml of fresh medium containing 200 U of GM-CSF/ml was added to the cultures. After 6 and 8 days, half of the culture supernatant was collected and centrifuged, and the cell pellet was resuspended in 10 ml of fresh medium containing GM-CSF and given back to the original plate. At days 8 and 9, the slightly attached cells were used for the experiments described herein. More than 90% of these cultures expressed CD11c as assessed by flow cytometric analysis, and characteristic clustering of DC was observed by light microscopy.
For infection of DC, 4 × 105 cells (apoptosis assays), 106 cells (proliferation assays and enzyme-linked immunosorbent assay [ELISA]), or 5 × 106 cells (adhesion molecule expression analyses, electrophoretic mobility shift assay, and Western blotting) were seeded in 24- or 6-well plates in medium without antibiotics and infected with an MOI of 10 or stimulated with 1 μg of lipopolysaccharide (LPS; from Salmonella enterica serovar Typhimurium; Sigma)/ml or 1 μM staurosporine (Sigma). The bacteria were sedimented onto the cells at 400 × g for 5 min. After 1 h of infection, 0.1 mg of gentamicin (Sigma)/ml was added to kill the extracellular bacteria. Where indicated, the cells were cultured further for various experiments.
Flow cytometry analysis of adhesion molecules.
To examine the regulation of adhesion molecules of Yersinia-infected DC, 5 × 106 cells were infected as described above. At 3 h postinfection, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-I-A/I-E (2G9), anti-CD80 (16-10A1), anti-CD86 (GL1), or anti-CD54 (3E2) (BD PharMingen, Heidelberg, Germany) antibodies and propidium iodide (Calbiochem, La Jolla, Calif.). Samples were analyzed on a FACSCalibur flow cytometer (BD Immunocytometry Systems) with gating on the propidium iodide-negative cells. Data were analyzed with WINMDI version 2.3 software (J. Trotter, Scripps Institute, La Jolla, Calif.).
Determination of cytokine production by ELISA.
To study the effects of different Y. enterocolitica strains on cytokine release, murine DC (106) were infected as described above and the supernatants were collected after 6 h for KC ELISA (Duo set; R&D Systems, Wiesbaden, Germany) and after 24 h for IL-10, IL-12 (p70), and TNF-α ELISA (OptEIA ELISA set; BD PharMingen). Cytokine levels were measured according to the manufacturers' instructions.
Assessment of apoptosis. (i)Assessment of mitochondrial transmembrane potential.
DC (4 × 105) were infected with different Yersinia strains as described above. The mitochondrial transmembrane potential (ΔΨm) was analyzed using the ΔΨm-specific stain TMRE (Molecular Probes, Eugene, Oreg.). Briefly, TMRE (40 nM) was added at 3.75 h postinfection and incubated for a further 15 min at 37°C. Thereafter, the cells were additionally stained with propidium iodide (40 ng/ml) and analyzed by flow cytometry.
(ii) Assessment of DNA fragmentation: Nicoletti assay.
At 24 h after infection, the cells were transferred into fluorescence-activated cell sorter tubes and centrifuged at 400 × g for 5 min. The pellet was resuspended in 400 μl of Nicoletti reagent (50 μg of propidium iodide/ml, 0.1% trisodium citrate-2-hydrate, and 0.1% Triton X-100 dissolved in H2O), incubated on ice for 10 min, and analyzed by flow cytometry.
(iii) TUNEL assay.
Twenty-four hours after infection, cells were fixed and processed to detect fragmented genomic DNA in a terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labeling (TUNEL) assay (Roche, Mannheim, Germany) according to the manufacturer's instructions. Cells were analyzed by flow cytometry.
(iv) Assessment of phosphatidylserine residue translocation.
Translocation of phosphatidylserine residues from the inner to the outer leaflet of the cell membrane and the loss of cell membrane integrity were measured at 4 h postinfection by incubating the cells with Annexin-V-fluorescein and propidium iodide according to the manufacturer's instructions (Annexin-V-FLUOS staining kit; Roche). Cells were analyzed by flow cytometry.
Transmission electron microscopy.
Cell pellets were fixed for 24 h in 4% glutaraldehyde in a 0.05 M phosphate-buffered solution containing 0.15 M NaCl at pH 7.3 at room temperature. Postfixation was based on 1% osmium tetroxide containing 1% potassium dichromate in 0.85% NaCl at pH 7.3 for 45 min. After embedding in glycide ether, the blocks containing cells were cut using an ultramicrotome (Ultracut; Reichert, Vienna, Austria). Ultra-thin sections (80 nm) were stained with 0.5% uranyl acetate for 10 min at 30°C and with 2.7% lead citrate for 5 min (Ultrastainer; LKB, Uppsala, Sweden) at 20°C. Grids were examined using a Zeiss EM 902 transmission electron microscope (Zeiss, Oberkochen, Germany) operating at 80 kV and at magnifications between ×2,000 and ×50,000.
Purification of DO11.10 CD4+ T cells.
CD4+ T cells were purified from spleens of DO11.10 mice by preparing a single-cell suspension, followed by lysis of red blood cells (80 mM NH4Cl, 5 mM KHCO3, 5 mM Na2EDTA) and passage through a nylon wool column. Using the CD4-negative T-cell isolation kit and a magnetic-activated cell sorter system (Miltenyi Biotech, Bergisch-Gladbach, Germany), MHC class II+, B220+, and CD8+ cells were depleted. The resulting T-cell preparations, containing 97 to 99% CD4+ T cells, were used without further purification.
T-cell proliferation assay.
At 1 h postinfection, 0.1 mg of gentamicin/ml and 0.1 mg of OVA (Sigma)/ml were added and the cells were incubated at 37°C for a further 2 h. Freshly isolated DO11.10 T cells were labeled with CFSE (Molecular Probes) as previously described (15, 18) with minor modifications. Briefly, 5 × 107 T cells/ml were washed twice with ice-cold PBS and incubated with CFSE (5 μM) in PBS for 3 min at 37°C. The cells were then washed twice with ice-cold heat-inactivated fetal calf serum, resuspended in cell culture medium, and used for the experiments. CFSE-labeled T cells (4 × 105) were coincubated with the OVA-loaded DC (105) in 48-well plates. Following 4 days of coincubation, the proliferation rate was analyzed by flow cytometry with excitation at 488 nm and the FL1 detection channel.
Electrophoretic mobility shift assay.
DC (5 × 106) were infected in six-well plates as described above. At various intervals postinfection, nuclear extracts were prepared as previously described (28). Aliquots of the supernatants containing nuclear proteins were stored at −80°C. Protein concentrations were determined using the Bradford assay. Oligonucleotide probes were labeled with [γ-32P]ATP (Amersham Biosciences, Freiburg, Germany) using T4 polynucleotide kinase (New England Biolabs, Frankfurt, Germany) and purified on a NucTrap probe purification column (Stratagene, Amsterdam, The Netherlands). The following oligonucleotides were used: NF-κB wild type (NF-κBc; 5′-ATGTGAGGGGACTTTCCCAGGC-3′), NF-κB mutant (NF-κBm; 5′-ATGTGAGGcGACTTTCCCAGGC-3′ [the lowercase letter represents the mutation]; Santa Cruz, Heidelberg, Germany). Nuclear extracts (3 to 6 μg) were incubated with 30,000 cpm of the 32P-labeled oligonucleotide probe for 45 min on ice in a buffer containing 5% glycerol, 80 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA (pH 8.0), 10 mM Tris-HCl (pH 7.2), and 1 μg of dI-dC. Antibodies against p50 (sc-1190X) and p65 (sc-372X) (both from Santa Cruz) were included in the binding reaction mixture for supershift analysis. Samples were resolved on a 5% nondenaturing polyacrylamide gel using 0.5× TBE (25 mM Tris-HCl, 25 mM boric acid, 0.5 mM EDTA) as running buffer. Gels were transferred to Whatman 3M paper (Schleicher & Schüll, München, Germany) and dried under vacuum. Protein binding was assessed by autoradiography.
Statistics.
Data were analyzed by the unpaired two-tailed Student's t test. A P value of <0.05 was considered significant.
RESULTS
T-cell activation after infection of DC with Y. enterocolitica.
T-cell responses are required for elimination of Y. enterocolitica from infected tissues (2, 3). T-cell proliferation assays were performed to analyze whether Yersinia infection alters the T-cell activation capacity of DC. For this purpose, DC were infected with Y. enterocolitica wild-type and mutant strains. Then, OVA was added as an antigen as well as OVA-specific CFSE-labeled T cells from DO11.10 mice. After 4 days of incubation, cultures were analyzed by flow cytometry to assess T-cell proliferation.
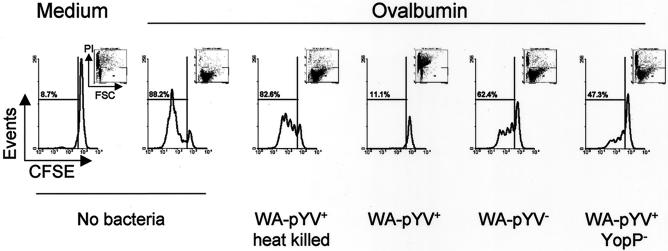
Strong T-cell proliferation was observed in uninfected control cultures. OVA-induced T-cell proliferation was completely inhibited after infection of DC with wild-type Yersinia (WA-pYV+), while T-cell proliferation was observed when DC were infected with heat-killed wild-type Yersinia or with viable plasmid-cured Y. enterocolitica (Fig. 1). Lower T-cell proliferation was observed upon exposure of DC to a YopP-deficient Yersinia mutant strain compared with plasmid-cured Y. enterocolitica. Together, these data suggest that gene products encoded by the virulence plasmid affect DC functions required to promote T-cell proliferation.
FIG. 1.
Proliferation of T cells after coculture with DC infected with Y. enterocolitica. DC were incubated with various strains of Y. enterocolitica, wild type (WA-pYV+), plasmid cured (WA-pYV−), YopP deficient (pYV+YopP−), and heat-killed wild type (WA-pYV+ heat killed), at an MOI of 10. After 1 h, gentamicin (100 μg/ml) and OVA (100 μg/ml) were added. DC were cocultured with CFSE-labeled OVA-specific T-cell receptor transgenic T cells from DO11.10 mice at a ratio of 4 (one DC to four T cells). T-cell proliferation was analyzed 4 days later by flow cytometry by gating on propidium iodide-negative cells (inserts). Results are representative of seven experiments.
Yersinia induces apoptosis in DC.
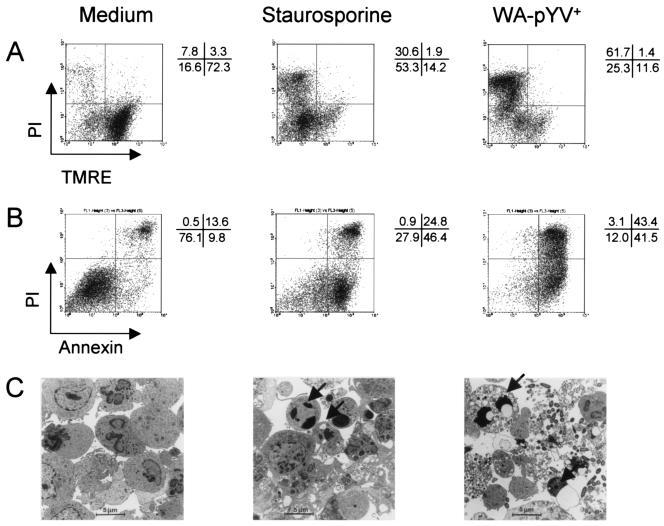
Previous studies demonstrated that Y. enterocolitica induces apoptosis in murine macrophages (10, 25) but not in human DC (27). To assess whether Y. enterocolitica induces death of murine DC and whether this might be involved in the ability of DC to stimulate T cells, apoptosis assays were performed with Yersinia-infected murine DC. Wild-type Y. enterocolitica (WA-pYV+) induced death of murine DC, as illustrated in Fig. 2. Four hours after Y. enterocolitica infection, DC were TMRE negative and propidium iodide positive, indicating the loss of mitochondrial potential and damage of the cellular membrane, respectively. In agreement with this finding, Annexin-V staining was also positive. Nuclear and DNA fragmentation were both obvious 24 h after infection as indicated by positive staining in Nicoletti and TUNEL assays (data not shown). Cellular fragmentation and nuclear condensation in apoptotic DC was confirmed by electron microscopy (Fig. 2). These data suggest that Y. enterocolitica-mediated cell death of murine DC is predominantly an apoptotic process.
FIG. 2.
Death of DC after infection with Y. enterocolitica. (A and B) DC were left untreated or incubated with staurosporine or wild-type Y. enterocolitica (WA-pYV+; MOI, 10). Four hours postinfection, DC were stained with TMRE and propidium iodide (PI) (A) or with FITC-conjugated Annexin-V and PI (B). Numbers indicate percentages of cells in each quadrant. Results are representative of 16 (A) and 9 (B) experiments. (C) Electron microscopy analysis of DC incubated with medium demonstrated normal morphology of the cells, while treatment with staurosporine or infection with wild-type Y. enterocolitica (4 h) induced numerous apoptotic bodies containing cytoplasmic and chromatin remnants (arrows).
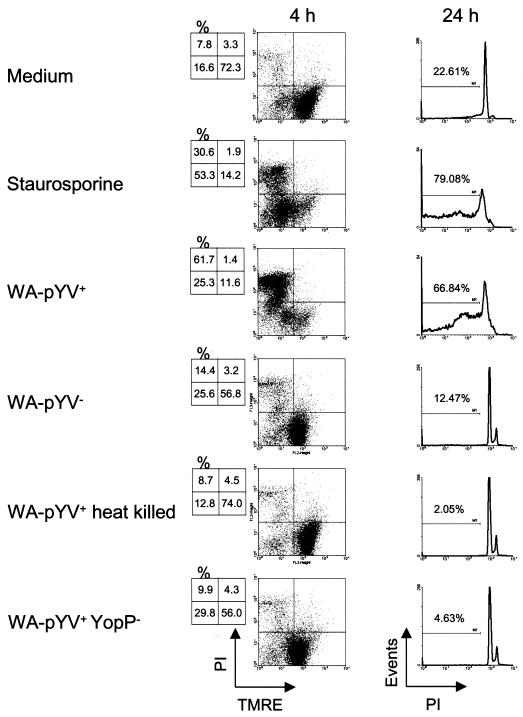
In contrast to infection with viable wild-type Y. enterocolitica (WA-pYV+), apoptosis was not induced by plasmid-cured (WA-pYV−), YopP deficient (WA-pYV+ YopP−), or heat-killed wild-type (WA-pYV+) Yersinia (Fig. 3). Therefore, YopP appears to be the most important factor involved in cell death caused by Yersinia.
FIG. 3.
Death of DC after infection with Y. enterocolitica. DC were left untreated or incubated with staurosporine or different strains of Y. enterocolitica: wild type (WA-pYV+), plasmid cured (WA-pYV−), YopP deficient (pYV+YopP−), and heat-killed wild type (WA-pYV+ heat killed). Four hours postinfection DC were stained with TMRE and propidium iodide (PI), and 24 h postinfection the Nicoletti procedure was performed. Results are representative of three experiments.
Surface molecule expression in DC after infection with Y. enterocolitica.
Maturation of DC is accompanied by changes in the expression of surface molecules. To examine whether Y. enterocolitica modulates maturation of DC, the expression of molecules which contribute to immunological synapse formation, including the expression of MHC class II and costimulatory molecules (ICAM-1, CD80, and CD86), was analyzed by flow cytometry.
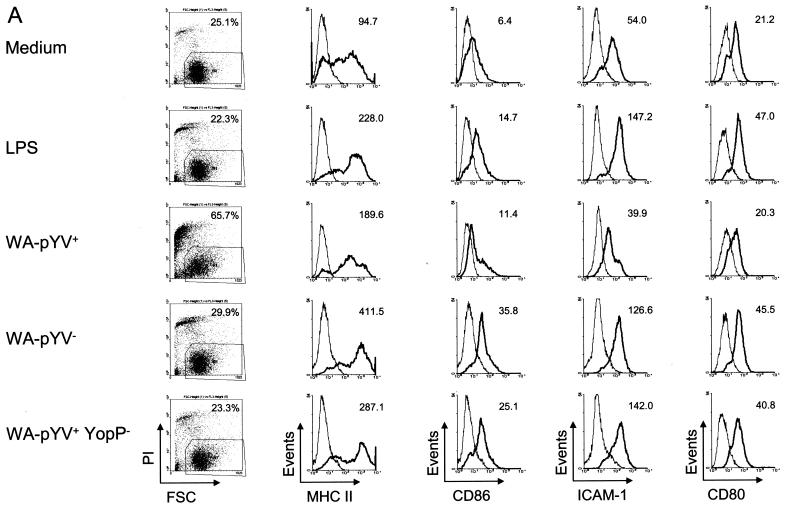
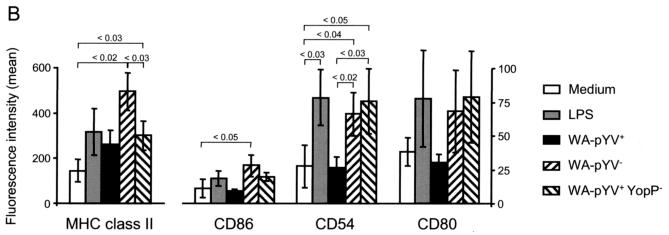
Three hours after incubation with either LPS, Y. enterocolitica WA-pYV−, or Y. enterocolitica WA-pYV+ YopP−, expression of MHC class II was markedly increased compared to that in untreated DC (Fig. 4). Increased MHC class II expression was also observed after infection with the Y. enterocolitica wild-type strain (WA-pYV+), although expression was less than after infection with Yersinia mutant strains deficient for YopP (WA-pYV+YopP−). The costimulatory molecules CD86, CD54 (ICAM-1), and CD80 were strongly expressed after infection of DC with Y. enterocolitica WA-pYV− or Y. enterocolitica WA-pYV+YopP−, whereas no increase in expression was observed after infection with wild-type (WA-pYV+) Y. enterocolitica. Together these results suggest that wild-type (WA-pYV+) Y. enterocolitica inhibits maturation of DC and that YopP accounts for this effect.
FIG. 4.
Expression of MHC class II and costimulatory molecules by DC after infection with Y. enterocolitica. (A) DC were incubated with various strains of Y. enterocolitica: wild type (WA-pYV+), plasmid cured (WA-pYV−), YopP deficient (pYV+YopP−), and heat-killed wild type (WA-pYV+ heat killed). Three hours after starting infection DC were stained (black lines) for MHC class II, CD86, CD54 (ICAM-1), and CD80 and analyzed by flow cytometry. By gating out propidium iodide-positive cells, 10,000 live cells were examined in each measurement. Gray lines, isotype-matched immunoglobulin G controls. The differences between the means of monoclonal antibodies to isotype controls are indicated in the top corner of each events graph. (B) Means of four independent experiments are shown. Numbers above graphs indicate P values.
Cytokine production after infection of DC with Y. enterocolitica.
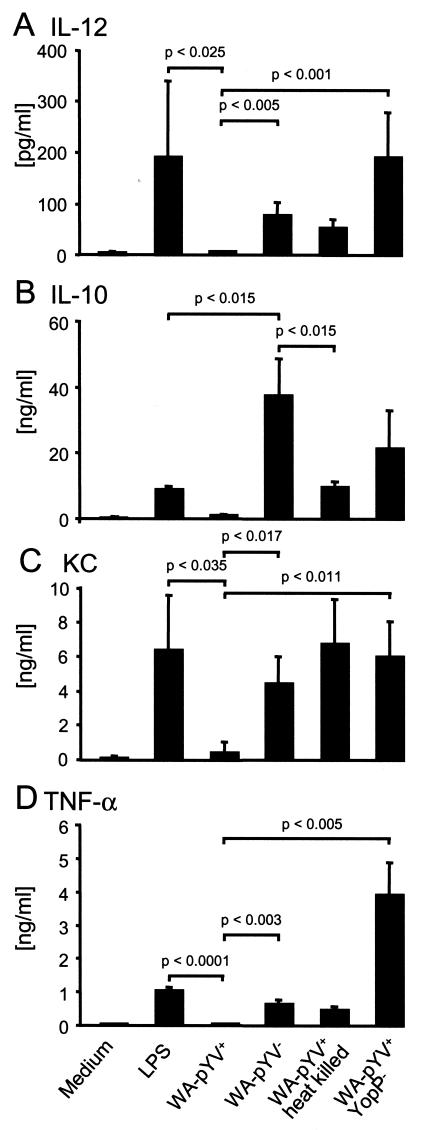
To analyze whether cytokine release by murine DC is altered after infection with Y. enterocolitica, DC were exposed to Y. enterocolitica strains and mutants as described above and the cytokines IL-12, IL-10, KC, and TNF-α were determined in culture supernatants by ELISA. Infection with wild-type (WA-pYV+) Yersinia did not result in IL-12 secretion. In contrast, IL-12 secretion was induced by LPS and by plasmid-cured (WA-pYV−) or YopP-deficient (WA-pYV+YopP−) Y. enterocolitica mutants (Fig. 5). While maximum IL-12 concentrations were observed after treatment of DC with LPS, the highest concentrations of IL-10 were detected after infection with plasmid-cured Yersinia, and these were significantly higher compared to incubation with LPS or heat-killed Yersinia (Fig. 5).
FIG. 5.
Production of IL-12 (A), IL-10 (B), KC (C), and TNF-α (D) by DC after infection with Y. enterocolitica. DC from BALB/c mice were incubated with LPS or with various strains of Y. enterocolitica: wild type (WA-pYV+), plasmid cured (WA-pYV−), YopP deficient (pYV+ YopP−), and heat-killed wild type (WA-pYV+ heat killed). Gentamicin (100 μg/ml) was added 1 h after infection. Three hours (KC) or 23 h (IL-12, IL-10, and TNF-α) later, supernatants were harvested and analyzed by ELISA. Data represent the means of results from three independent experiments.
IL-8 is known to attract neutrophil granulocytes and also to contribute to T-lymphocyte attraction (31). We analyzed whether Y. enterocolitica may also affect the ability of murine DC to secrete KC, the murine equivalent of IL-8. As shown in Fig. 5, incubation of DC with LPS or Yersinia mutant strains resulted in increased secretion of KC. In contrast, secretion of KC was not increased following infection with wild-type Y. enterocolitica (WA-pYV+). Similar results were observed regarding the release of TNF-α after Yersinia infection. Interestingly, maximum TNF-α was observed in DC treated with the YopP-deficient mutant strain (WA-pYV+YopP−) (Fig. 5). These results suggest that Y. enterocolitica suppresses cytokine production by DC predominantly via the action of YopP, and the results are consistent with previously published data for macrophages and epithelial cells (8, 21, 26, 29).
NF-κB activation after infection of DC with Y. enterocolitica.
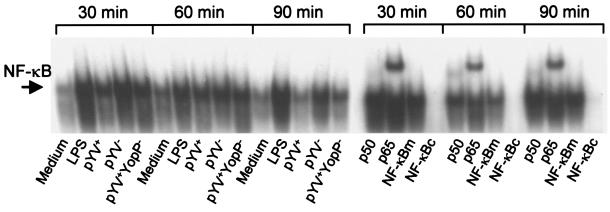
The transcription factor NF-κB promotes transcription of various immune response genes, including those encoding adhesion molecules (e.g., ICAM-1) and cytokines (e.g., TNF-α). While Y. enterocolitica invasin protein was found to activate NF-κB in epithelial cells, YopP inhibits the activation of NF-κB, and this, in concert with other effects, causes apoptosis of murine macrophages (24). Nuclear extracts showed low NF-κB binding activity in the control samples but increased binding activity in DC treated with LPS or plasmid-cured Y. enterocolitica WA-pYV−. Infection with YopP-deficient Y. enterocolitica (WA-pYV+YopP−) resulted in a moderate increase in the concentration of nuclear NF-κB. A moderate increase in the binding activity of NF-κB was also observed 30 and 60 min after infection with wild-type Y. enterocolitica (WA-pYV+); however, this binding activity decreased markedly at 90 min postinfection (Fig. 6).
FIG. 6.
Y. enterocolitica-induced activation of NF-κB in DC. DC were incubated with LPS or with various strains of Y. enterocolitica: wild type (WA-pYV+), plasmid cured (WA-pYV−), YopP deficient (pYV+YopP−), and heat-killed wild type (WA-pYV+ heat killed). After 30, 60, and 90 min, nuclear extracts were prepared and analyzed in gel shift experiments with 32P-labeled probes for NF-κB. (A) Time course of Yersinia-induced NF-κB activation. (B) Supershift analyses were performed by incubating nuclear extracts with antibodies against NF-κB subunits p50 or p65 and for competition experiments with a 100-fold excess of unlabeled NF-κB probe. NF-κBc, consensus sequence; NF-κBm, mutated sequences. Results are representative of three experiments.
DISCUSSION
The present study demonstrates that Y. enterocolitica may affect several important functions of murine DC, including maturation as well as cytokine production. In addition, Y. enterocolitica induces apoptosis in DC. These alterations of DC by Y. enterocolitica obviously contribute to decreased antigen-specific CD4 T-cell activation. Although these observations have been obtained in in vitro experiments, it is tempting to speculate that by targeting the aforementioned functions of DC Y. enterocolitica might at least partially subvert the adaptive immune defense mechanisms of the host which are required for elimination of this pathogen from infected tissue. However, further studies are now required in order to investigate whether the Yersinia-induced changes of DC observed herein can also be observed in vivo, e.g., upon infection of mice with pathogenic Y. enterocolitica strains.
The most prominent effect of Y. enterocolitica on DC was the induction of cell death. This observation is in contrast to a recent report that provided some evidence that human DC do not undergo cell death after infection with Y. enterocolitica (27). However, cell death is also observed in Yersinia-infected murine macrophages, and this effect is caused by YopP (10, 25). Consistent with these results we found that in contrast to wild-type Y. enterocolitica (WA-pYV+), a YopP-deficient (WA-pYV+YopP−) mutant strain did not induce cell death in DC. Moreover, it was found that LPS-tolerant macrophages that had been pretreated with LPS were much less susceptible to Yersinia-induced cell death by apoptosis (25). Likewise, we found that mature DC that had been prestimulated with LPS were less susceptible to Yersinia YopP-induced cell death than immature DC (data not shown). These data suggest that besides macrophages, DC may also undergo cell death upon interaction with pathogenic yersiniae, and YopP appears to be the most important factor for induction of DC death.
Exposure of DC to Y. enterocolitica caused a decrease in the mitochondrial potential, DNA fragmentation, and translocation of phosphatidylserine residues from the inner to the outer cellular membrane leaflet, strongly suggesting that Yersinia-mediated DC death is predominantly caused by apoptosis. In the present study the frequency of apoptotic DC 4 h after infection with Yersinia was similar to that observed in experiments with murine macrophages (25). While macrophages were infected with Yersinia at an MOI of 50 for 90 min (25), the DC used in this study were only infected at an MOI of 10 for 60 min. In our experiments higher MOIs caused a rapid and total disruption of DC, suggesting that murine DC are more susceptible to Yersinia-induced apoptosis than macrophages (data not shown). In fact, we infected macrophages (J774 murine macrophages) and DC with Y. enterocolitica in parallel, and 4 h later survival of macrophages was markedly higher (61%) than survival of DC (35%) (data not shown).
In macrophages, YopP-mediated inhibition of NF-κB activation appears to be involved in Yersinia-induced apoptosis (24). According to this finding, a biphasic NF-κB activation course was found in Yersinia-infected macrophages. NF-κB binding activity was observed after 30 min but was absent 90 min after Yersinia infection. In DC we observed a slight activation of NF-κB at 30 and 60 min, even after infection with wild-type Y. enterocolitica (WA-pYV+); however, this signal declined significantly after 90 min. The faster prevention of NF-κB signaling in macrophages might result from the higher Yersinia MOI used for infection in the experiments carried out by Ruckdeschel et al. (24). Despite the fact that nuclear NF-κB activity was obtained for longer periods in Yersinia-infected DC than in macrophages, DC turned out to be more susceptible to killing by Yersinia than macrophages. Therefore, at present it is not clear whether and to what extent prevention of NF-κB activation in DC triggers apoptosis or if other mechanisms contribute to the induction of apoptosis.
The temporary partial activation of DC observed after infection with wild-type Yersinia (WA-pYV+) might explain the difference between the up-regulation of MHC class II molecules and the lack of cytokine release. In contrast to cytokines, preformed MHC class II molecules are abundant in intracellular compartments of nonactivated DC (13). While limited DC activation might be sufficient to transmit preformed MHC class II molecules to the DC cell surface, de novo cytokine synthesis requires a prolonged period of cell activation.
Together, these results indicate that YopP is the most potent pathogenicity factor of Y. enterocolitica and prevents DC-mediated activation of CD4+ T cells, predominantly by induction of apoptosis. On the other hand, YopP-deficient (WA-pYV+YopP−) or plasmid-cured (WA-pYV−) Yersinia mutants were also able to decrease DC-mediated T-cell activation to some degree.
Exposure of DC to plasmid-cured (WA-pYV−) Yersinia induced moderate IL-12 levels but very high IL-10 levels. This cytokine production profile might contribute to the reduction of T-cell proliferation observed after infection with plasmid-cured Yersinia in vitro. In fact, IL-10 may cause a reduction of T-cell proliferation by at least two mechanisms. Firstly, the formation of regulatory T cells might be induced by IL-10, as reported for Bordetella pertussis infections (11). Secondly, IL-10 might induce genes of suppressor proteins, as discussed recently (4, 14, 30).
As the mechanisms of IL-10 and IL-12 production on T-cell proliferation in yersiniosis are actually unclear, further studies are required. Although it is unclear which compound of plasmid-cured (WA-pYV−) Yersinia contributes to stimulation of IL-10 production, we speculate that Yersinia LPS might be the responsible compound. In fact, although smooth LPS from Escherichia coli preferentially induces secretion of IL-12, LPS from Porphyromonas gingivalis induces IL-10 secretion (23). The semirough LPS from Yersinia might cause host responses similar to those from P. gingivalis. Based on these in vitro observations, it might be possible that plasmid-cured (WA-pYV−) Yersinia also mediates antiinflammatory properties in mice.
Although YopP was found to exert the most pronounced inhibitory effects on DC and T-cell activation, the use of a YopP-deficient (WA-pYV+YopP−) strain and a plasmid-cured (WA-pYV−) Yersinia mutant strain strongly suggests that in addition to YopP other factors exert inhibitory effects on DC-triggered T-cell proliferation. In keeping with this assumption, YopH was reported to suppress activation of T and B lymphocytes by inhibition of early phosphorylation events of the antigen receptor signaling complex of T cells (32). However, this was a direct effect of Yops on the T cells, an effect that can be excluded in the experimental system described herein. However, similar to our observations in DC, it was also found that in B cells Yersinia pseudotuberculosis inhibits up-regulation of surface expression of costimulatory molecules such as B7.2 (32).
In conclusion, our study demonstrates that Y. enterocolitica is able to impair the T-cell activation capacity of murine DC by various means. The most important factor might be YopP, which causes rapid apoptosis in DC. On the other hand, other yet-undefined Yops also impair T-cell activation.
Acknowledgments
We thank E. Kämpgen for providing the mouse myeloma cell line P3X63.
This work was supported by grants from the BMBF (IZKF Tübingen) and the Deutsche Forschungsgemeinschaft.
Editor: J. B. Bliska
REFERENCES
- 1.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., P. Hantschmann, B. Heymer, and J. Heesemann. 1993. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology 187:1-16. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., A. Tingle, A. Reske-Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlato, C., M. A. Cassatella, I. Kinjyo, L. Gatto, A. Yoshimura, and F. Bazzoni. 2002. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 168:6404-6411. [DOI] [PubMed] [Google Scholar]
- 5.Black, D. S., and J. B. Bliska. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohn, E., and I. B. Autenrieth. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458-1468. [PubMed] [Google Scholar]
- 7.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 8.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 11.Higgins, S. C., E. C. Lavelle, C. McCann, B. Keogh, E. McNeela, P. Byrne, B. O'Gorman, A. Jarnicki, P. McGuirk, and K. H. Mills. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171:3119-3127. [DOI] [PubMed] [Google Scholar]
- 12.Iezzi, G., K. Karjalainen, and A. Lanzavecchia. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8:89-95. [DOI] [PubMed] [Google Scholar]
- 13.Kleijmeer, M. J., M. A. Ossevoort, C. J. van Veen, J. J. van Hellemond, J. J. Neefjes, W. M. Kast, C. J. Melief, and H. J. Geuze. 1995. MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells. J. Immunol. 154:5715-5724. [PubMed] [Google Scholar]
- 14.Kobayashi, M., M. N. Kweon, H. Kuwata, R. D. Schreiber, H. Kiyono, K. Takeda, and S. Akira. 2003. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Investig. 111:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, W. T., and W. J. Pelletier. 1998. Visualizing memory phenotype development after in vitro stimulation of CD4+ T cells. Cell. Immunol. 188:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Lian, C. J., W. S. Hwang, and C. H. Pai. 1987. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect. Immun. 55:1176-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 18.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 20.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 22.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, D. T. Van, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyi, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 27.Schoppet, M., A. Bubert, and H. I. Huppertz. 2000. Dendritic cell function is perturbed by Yersinia enterocolitica infection in vitro. Clin. Exp. Immunol. 122:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulte, R., P. Wattiau, E. L. Hartland, R. M. Robins-Browne, and G. R. Cornelis. 1996. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda, K., B. E. Clausen, T. Kaisho, T. Tsujimura, N. Terada, I. Forster, and S. Akira. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10:39-49. [DOI] [PubMed] [Google Scholar]
- 31.Taub, D. D., M. Anver, J. J. Oppenheim, D. L. Longo, and W. J. Murphy. 1996. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Investig. 97:1931-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]