Abstract
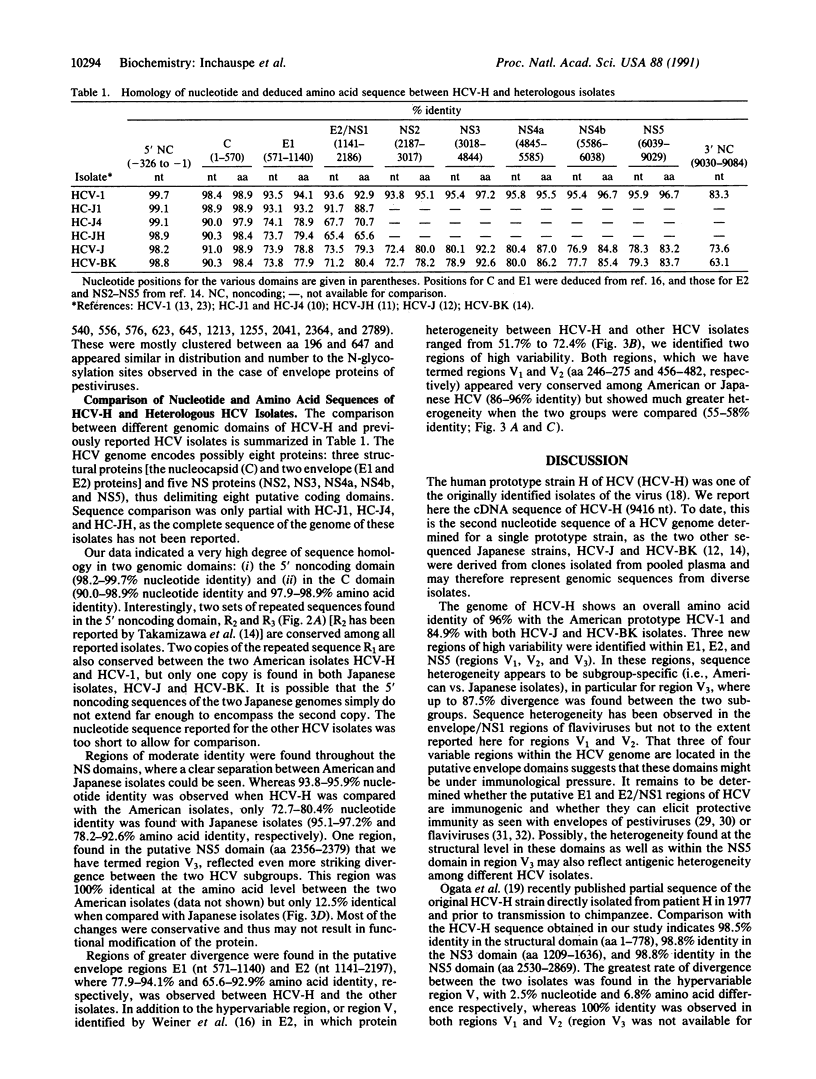
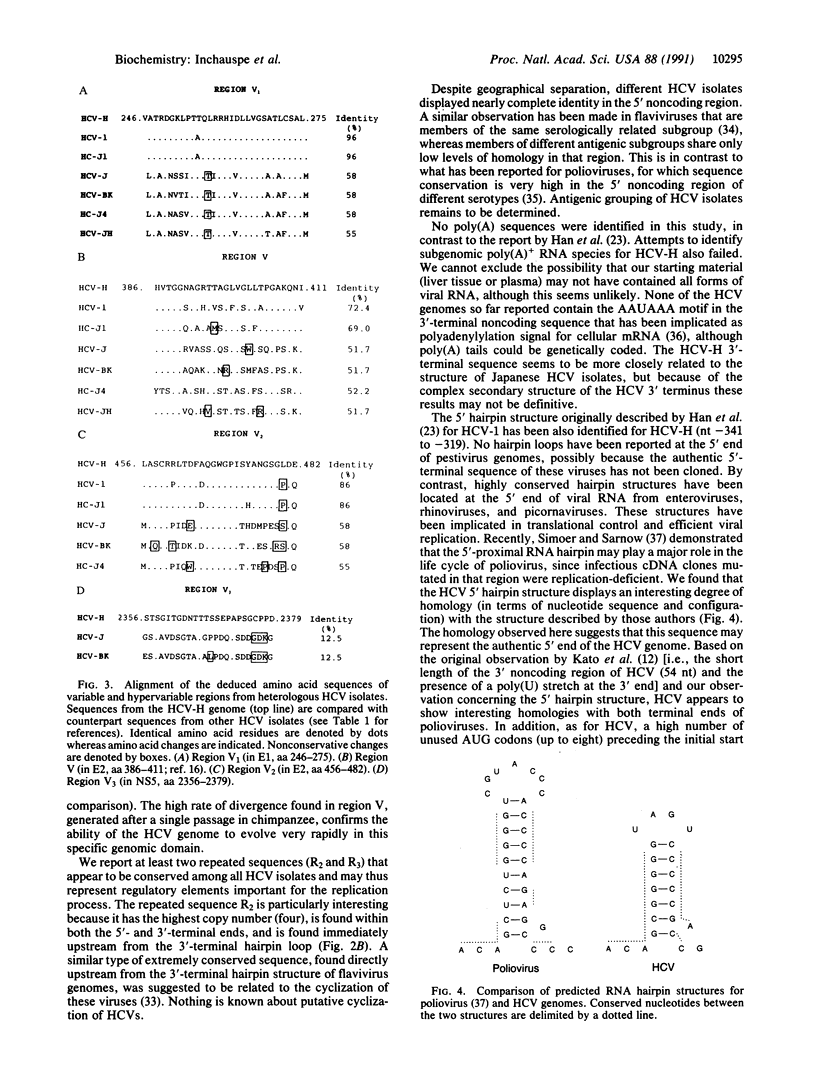
Genomic RNA from the human prototype strain H of the hepatitis C virus (HCV-H) has been molecularly cloned and sequenced. The HCV-H sequence reported consists of 9416 nucleotides including the 5' and 3' untranslated regions. HCV-H shows 96% amino acid identity with the American isolate HCV-1 but only 84.9% with the Japanese isolates HCV-J and HCV-BK. In addition to the hypervariable region (region V) previously identified in the putative E2 domain, three other variable domains were identified: region V1 (putative E1), region V2 (putative E2), and region V3 (putative NS5). These regions appear rather conserved (86-100%) among the American isolates (HCV-1 and HC-J1) or among various Japanese isolates (HCV-J, HCV-BK, HCV-JH, and HC-J4) but show striking heterogeneity when the two subgroups are compared (42-87.5% amino acid difference). A structural similarity between the 5'-terminal hairpin structure of HCV and of poliovirus was observed. This study further suggests the existence of at least two genomic subtypes of HCV and confirms a distant relationship between HCV and pestiviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Holland P. V., Morrow A. G., Purcell R. H., Feinstone S. M., Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975 Nov 1;2(7940):838–841. doi: 10.1016/s0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- Brinton M. A., Dispoto J. H. Sequence and secondary structure analysis of the 5'-terminal region of flavivirus genome RNA. Virology. 1988 Feb;162(2):290–299. doi: 10.1016/0042-6822(88)90468-0. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Koonin E. V., Blinov V. M. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 1989 May 25;17(10):3889–3897. doi: 10.1093/nar/17.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Hahn Y. S., Rice C. M., Lee E., Dalgarno L., Strauss E. G., Strauss J. H. Conserved elements in the 3' untranslated region of flavivirus RNAs and potential cyclization sequences. J Mol Biol. 1987 Nov 5;198(1):33–41. doi: 10.1016/0022-2836(87)90455-4. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Abe K., Zebedee S., Nasoff M., Prince A. M. Use of conserved sequences from hepatitis C virus for the detection of viral RNA in infected sera by polymerase chain reaction. Hepatology. 1991 Oct;14(4 Pt 1):595–600. doi: 10.1016/0270-9139(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Takeuchi K., Boonmar S., Katayama T., Choo Q. L., Kuo G., Weiner A. J., Bradley D. W., Houghton M., Saito I. A cDNA fragment of hepatitis C virus isolated from an implicated donor of post-transfusion non-A, non-B hepatitis in Japan. Nucleic Acids Res. 1989 Dec 25;17(24):10367–10372. doi: 10.1093/nar/17.24.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Maéno M., Kaminaka K., Sugimoto H., Esumi M., Hayashi N., Komatsu K., Abe K., Sekiguchi S., Yano M., Mizuno K. A cDNA clone closely associated with non-A, non-B hepatitis. Nucleic Acids Res. 1990 May 11;18(9):2685–2689. doi: 10.1093/nar/18.9.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A., Tomkins P. T., Maudsley D. J., Blackman M. Infection of cultured murine brain cells by Semliki Forest virus: effects of interferon-alpha beta on viral replication, viral antigen display, major histocompatibility complex antigen display and lysis by cytotoxic T lymphocytes. J Gen Virol. 1987 Jan;68(Pt 1):99–106. doi: 10.1099/0022-1317-68-1-99. [DOI] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Prince A. M., Brotman B., Grady G. F., Kuhns W. J., Hazzi C., Levine R. W., Millian S. J. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet. 1974 Aug 3;2(7875):241–246. doi: 10.1016/s0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Roggendorf M., Deinhardt F., Rasshofer R., Eberle J., Hopf U., Möller B., Zachoval R., Pape G., Schramm W., Rommel F. Antibodies to hepatitis C virus. Lancet. 1989 Aug 5;2(8658):324–325. doi: 10.1016/s0140-6736(89)90501-1. [DOI] [PubMed] [Google Scholar]
- Rümenapf T., Stark R., Meyers G., Thiel H. J. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol. 1991 Feb;65(2):589–597. doi: 10.1128/jvi.65.2.589-597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Cropp C. B., Monath T. P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986 Dec;60(3):1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. K., Feinstone S. M., Purcell R. H., Alter H. J., London W. T. Non-A, non-B hepatitis: ultrastructural evidence for two agents in experimentally infected chimpanzees. Science. 1979 Jul 13;205(4402):197–200. doi: 10.1126/science.451589. [DOI] [PubMed] [Google Scholar]
- Simoes E. A., Sarnow P. An RNA hairpin at the extreme 5' end of the poliovirus RNA genome modulates viral translation in human cells. J Virol. 1991 Feb;65(2):913–921. doi: 10.1128/jvi.65.2.913-921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor E., Gerety R. J., Drucker J. A., Seeff L. B., Hoofnagle J. H., Jackson D. R., April M., Barker L. F., Pineda-Tamondong G. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet. 1978 Mar 4;1(8062):463–466. doi: 10.1016/s0140-6736(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. Nucleotide sequence of core and envelope genes of the hepatitis C virus genome derived directly from human healthy carriers. Nucleic Acids Res. 1990 Aug 11;18(15):4626–4626. doi: 10.1093/nar/18.15.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Brauer M. J., Rosenblatt J., Richman K. H., Tung J., Crawford K., Bonino F., Saracco G., Choo Q. L., Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991 Feb;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- van Zijl M., Wensvoort G., de Kluyver E., Hulst M., van der Gulden H., Gielkens A., Berns A., Moormann R. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol. 1991 May;65(5):2761–2765. doi: 10.1128/jvi.65.5.2761-2765.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Lelie P. N., Leentvaar-Kuypers A., Choo Q. L., Kuo G., Houghton M. Anti-hepatitis C antibodies and non-A, non-B post-transfusion hepatitis in The Netherlands. Lancet. 1989 Aug 5;2(8658):297–298. doi: 10.1016/s0140-6736(89)90486-8. [DOI] [PubMed] [Google Scholar]





