Abstract
Th17 cells and their cytokines are linked to the pathogenesis of rheumatoid arthritis, a chronic autoimmune disease characterized by joint inflammation. Th17 development is initiated by combined signaling of TGF-β and IL-6 or IL-21, and can be reduced in the absence of either IL-6 or IL-21. The aim of this study was to assess whether combinatorial IL-6/IL-21 blockade would more potently inhibit Th17 development, and be more efficacious in treating arthritis than targeting either cytokine. We assessed in vitro Th17 differentiation efficacy in the absence of IL-6 and/or IL-21. To investigate in vivo effects of IL-6/IL-21 blockade on Th17 and arthritis development, antigen-induced arthritis (AIA) was induced in IL-6-/- x IL-21R-/- mice. The therapeutic potential of this combined blocking strategy was assessed by treating mice with collagen-induced arthritis (CIA) with anti-IL-6R antibodies and soluble (s)IL-21R.Fc. We demonstrated that combined IL-6/IL-21 blocking synergistically reduced in vitro Th17 differentiation. In mice with AIA, absence of IL-6 and IL-21 signaling more strongly reduced Th17 levels and resulted in stronger suppression of arthritis than the absence of either cytokine. Additionally, anti-IL-6/anti-IL-21 treatment of CIA mice during the arthritis induction phase reduced disease development more potent than IL-6 or IL-21 inhibition alone, as effective as anti-TNF treatment. Collectively, these results suggest dual IL-6/IL-21 inhibition may be a more efficacious therapeutic strategy compared to single cytokine blockade to suppress arthritis development.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects up to 1% of the population worldwide. It is characterized by chronic inflammation leading to destruction of bone and cartilage of synovial joints [1]. Although the use of biologicals blocking a specific pathogenic target considerably improved symptoms and prognosis of some RA patients, approximately 30% of the patients still fail to respond adequately to currently available treatment [2–5]. Interestingly, anti-TNFα non-responders were shown to have increased levels of T helper (Th)17 cells in their circulation [5–7], suggesting Th17 cells and Th17-derived cytokines might be interesting alternative therapeutic targets in RA. Th17 cells produce various pro-inflammatory cytokines like IL-17, IL-21, and IL-22, that have been linked to RA pathogenesis [8–14]. Arthritis animal models have further elucidated the role of these Th17 cytokines in the processes of joint inflammation and destruction of cartilage and bone [15,16]. These findings indicate that Th17 cells and their cytokines are important contributors to the pathogenesis of chronic and destructive joint inflammation during RA.
Differentiation of Th17 cells from naïve CD4+ T cells is mediated by the enhanced expression of the transcription factor RAR-related orphan nuclear receptor γt (RORγt), originally thought to be induced exclusively by the combined signaling of TGF-β and IL-6 [17,18]. However, later it was shown that signaling of TGF-β combined with the autocrine signaling of IL-21 could drive Th17 differentiation as well [19–21]. Both IL-6 and IL-21 induce upregulation of the IL-23 receptor (IL-23R) in naïve CD4+ T cells, making those cells responsive to IL-23. IL-23 can subsequently amplify the differentiation of Th17 cells [20,22]. When either the IL-6 or IL-21 signaling pathway is absent, differentiation of naïve T cells towards Th17 cells is reduced. However, to date it is not known whether Th17 differentiation can be further reduced when both IL-6 and IL-21 pathways are blocked. Therefore, the purpose of this study was to determine whether IL-6/IL-21 combinatorial pathway blockade has additional inhibitory effects on Th17 differentiation, and more effectively reduces development of T cell-dependent experimental arthritis. Firstly, we assessed the effects of complete IL-6/IL-21 pathway inhibition on Th17 differentiation in vitro. Secondly, we used IL-6-/- x IL-21R-/- mice to study the in vivo effects of cytokine pathway blockade on Th17 differentiation and development of experimental arthritis. Finally, we investigated the therapeutic potential of our combined blocking strategy by treating mice with collagen-induced arthritis (CIA) with anti-IL-6R antibodies and soluble (s)IL-21R.Fc.
Materials and methods
Mice
Wild type (WT) C57Bl6/J mice and DBA-1 mice were purchased from Janvier-Elevage (Le Genest Saint Isle, France), and breeding pairs of the IL-21R-deficient mice on a C57Bl6/J background were provided by Pfizer. Breeding pairs of the IL-6−/− mice, back-crossed eight times with C57Bl6/J, were a kind gift from Dr Manfred Kopf (Basel, Switzerland)[23]. Animals were used between 10 and 12 weeks of age and were housed in filter-top cages under specific pathogen-free conditions. Microbiome synchronization was promoted by housing purchased WT mice near the genetically modified mice for a period of at least one week, before initiating experiments. A standard diet and water were provided ad libitum. Before being sacrificed by cervical dislocation, mice were anesthetized using 2–3% isoflurane. All animal procedures were approved by the ethics committee of the Radboud University Nijmegen (approval numbers 2014–045, 2010–002, and 2012–207).
In vitro stimulation of CD4+ T cells
Naïve murine T helper cells were negatively selected from spleen, isolated from IL-21R-/- or WT C57Bl6/J mice using a mouse naïve CD4+ T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Naïve CD4+ T cells were cultured for four days in 6-wells plates at 37°C, 5% CO2, in RPMI-1640 medium containing 5% fetal calf serum (FCS), 50 μm β-mercaptoethanol, 50 μg/ml gentamycin, and 1% pyruvate. The following Th17 stimulation cocktail was added, with or without recombinant mouse (rm)IL-6 (50 ng/ml; eBioscience, San Diego, CA, USA): anti-CD3 (5 μg/ml; eBioscience; adsorbed to the wells over night at 4°C), anti-CD28 (2.5 μg/ml; eBioscience), anti-IL-2 (5 μg/ml; eBioscience), TGF-β (1 ng/ml; eBioscience), IL-1β (10 ng/ml; kind gift of Pfizer), and TNFα (10 ng/ml; eBioscience). Differentiation efficacy was determined with flow cytometry. Supernatant cytokine levels were determined using the Luminex multianalyte technology, in combination with Milliplex cytokine kits (Merck Millipore, Darmstadt, Germany).
In vivo study protocol I: Antigen-induced arthritis (AIA) in knockout mice
AIA was induced in WT, IL-21R-/-, IL-6-/-, and IL-21R-/- x IL-6-/- mice as previously described [24]. Inflammation of the knee joint was measured by 99mTechnetium (99mTc) pertechnetate uptake. Joint swelling was scored as the ratio of 99mTc uptake of the arthritic right (R) and control left (L) knee joint. Joint swelling of the right knee joint was indicated as R:L ratios >1.1. Mice were sacrificed by cervical dislocation either at day 2 or day 7 after arthritis induction, and serum and joints were collected for further analysis. Draining lymph nodes (dLN) of mice sacrificed two days after arthritis induction were isolated to determine Th17 levels.
In vivo study protocol II: Antibody treatment during collagen-induced arthritis (CIA)
CIA was induced as described previously [25]. Arthritis development was macroscopically scored on a scale of 0–2 per paw, according to changes in redness and/or swelling of the paws. IL-6 signaling was inhibited with a single bolus intraperitoneal (i.p.) injection with 8 mg of a rat anti-mouse IL-6 receptor antibody (MR16-1; kind gift of Chugai Pharmaceutical Co. Ltd). IL-21 was neutralized by three i.p. injections per week with 200 μg soluble (s)IL-21R.Fc (Pfizer, New York, NY, USA), from now on referred to as anti-IL-21 therapy [26,27]. Treatment was initiated at the day of immunization (day 0, early treatment), or at the day of booster injection (day 21, late treatment). Both treatments were administered as single and combination treatments. The TNFα inhibitor Enbrel (200 μg; Pfizer) and rat IgG1 (200 μg; Pfizer) were injected as positive and negative control respectively, both i.p. 3 times a week. Mice were terminally bled at day 35 to determine serum antibody levels. Subsequently ankle joints were isolated for X-ray and histological analysis, and draining popliteal and inguinal lymph nodes were isolated to determine T cell levels.
Histology
Isolated joints were fixed for at least four days in 4% formaldehyde, decalcified in 5% formic acid, dehydrated, and embedded in paraffin. Standard frontal sections of 7 μm were mounted on SuperFrost slides (Menzel-Gläser, Braunschweig, Germany), and stained with haematoxylin and eosin (H&E), or Safranin O (SO). Joint arthritis severity was scored on an arbitrary scale of 0–3, as previously described [28]. Histopathological changes were scored on three semiserial sections of the joint, in a blindfolded manner.
Flow cytometry
Cells were stimulated four hours with phorbol myristate acetate (PMA; 50 ng/ml; Sigma-Aldrich, Saint Louis, MO, USA), ionomycin (1 μg/ml; Sigma-Aldrich) and the Golgi-traffic inhibitor Brefeldin (1 μl/ml; BD Biosciences, Franklin Lakes, NJ, USA). Differentiated T cells or isolated LN cells were stained with anti-CD3-PE (BD Biosciences) and anti-CD4-APC (Biolegend, San Diego, CA, USA), fixed and permeabilized using BD Cytofix/Cytoperm (BD Biosciences), followed by intracellular staining with anti-IL-17-FITC (Biolegend), anti-IFNγ-FITC (BD Biosciences), or appropriate isotype-matched control antibodies (all from BD Biosciences). Cells were measured on a FACSCalibur using the CellQuest software (BD Biosciences), and analyzed using FlowJo software (version 7.6.5).
Measurement of anti-mBSA and anti-CII antibodies
Levels of anti-mBSA and anti-CII antibodies were determined in serum using enzyme-linked immunosorbent assay (ELISA). In short, 10 ng of mBSA or 100 ng of bovine type II collagen were coated onto 96-well plates overnight. Nonspecific binding sites were blocked with 1% BSA in PBS-Tween (0.05%) for anti-mBSA antibody detection, and with a 5% milk powder solution for anti-CII antibody detection. Serial dilutions of mouse sera were incubated for 1 hour, before adding isotype-specific horseradish peroxidase-labeled goat anti-mouse Ig (1:1000). After incubation of another hour, 5-aminosalicyclic acid was added as a substrate, absorbance was subsequently measured at 450 nm.
Statistics
To determine the level of statistical significance between means of experimental groups, the Kruskal-Wallis with a Dunns post-test, or a one-way ANOVA with a Bonferroni post-test was applied as indicated, using GraphPad Prism version 5. P values less than 0.05 are considered significant. Results are expressed as Tukey box plots.
Results
Combined blocking of IL-6 and IL-21 synergistically inhibits in vitro Th17 differentiation
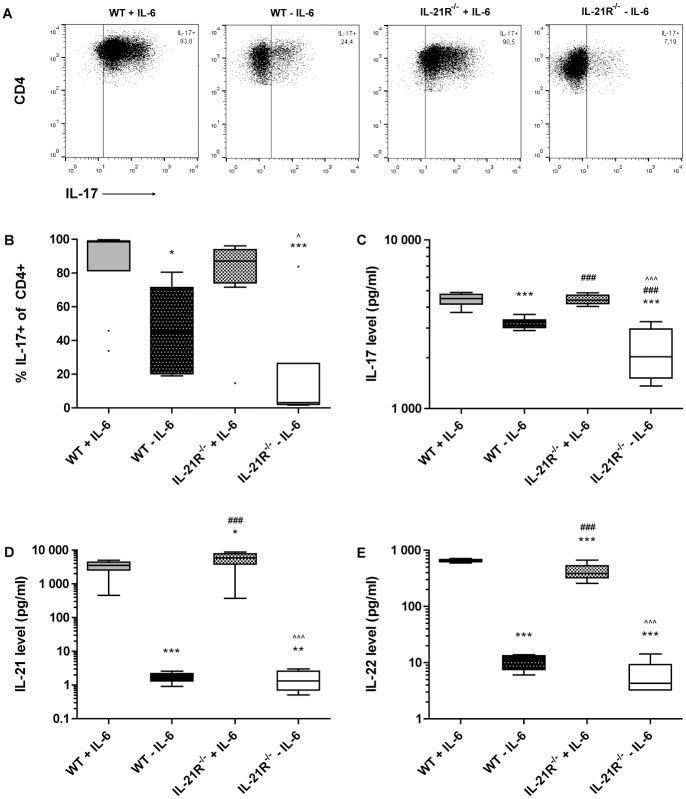
To investigate the exchangeability of IL-6 and IL-21 and potential additive or synergistic effects of these cytokines during Th17 differentiation, we first determined the differentiation efficacy of naïve T cells towards Th17 cells in the presence of both IL-6 and endogenous IL-21. After culturing, 86% of CD4+ cells were positive for IL-17 (Fig 1A and 1B), and produced high levels of IL-17 (Fig 1C). Subsequently, we determined Th17 differentiation efficacy in the absence of either IL-6 or IL-21 signaling. Without IL-6, naïve T cells reached only 46% of Th17 development upon stimulation, while Th17 differentiation efficacy of naïve IL-21R-/- T cells was similar to that observed after stimulation of WT cells (Fig 1A and 1B). Interestingly, when both IL-6 and IL-21 signaling pathways were absent, only 17% of CD4+ cells differentiated into Th17 cells after culturing (Fig 1A and 1B), and IL-17 secretion was reduced by 50% (Fig 1C). High endogenous IL-21 levels were detected in supernatants of cultures with IL-6 (Fig 1D), potentially contributing to Th17 development in an autocrine fashion [19–21]. Finally, secretion of IL-22 in supernatant of those cultures was confirmed (Fig 1E). This shows that by blocking IL-6 and IL-21 signaling pathways in vitro, Th17 differentiation is dramatically reduced, thereby providing excellent rationale for further in vivo studies.
Fig 1. Highly reduced Th17 differentiation in the absence of both IL-6 and IL-21 signaling.
WT and IL-21R-/- naïve T cells were stimulated for four days with a differentiation cocktail either with or without IL-6 as described in Methods. Representative flow cytometry dot plots reflecting the IL-17+ fraction of the CD4+ population. Gates were set at a maximum of 0.3% IL-17-positive cells in the ‘fluorescence minus one’ control per condition, without addition of IL-17A antibodies (A). Summary of the relative proportion of IL-17+ cells within the CD4+ population (B). Culture supernatant levels of IL-17 (C), IL-21 (D), and IL-22 (E). 6–10 mice/group; *p<0.05, **p<0.01, ***p<0.001 versus WT + IL-6; ###p<0.001 versus WT—IL-6; ^p<0.05, ^^^p<0.001 versus IL-21R-/- + IL-6; A—Kruskal-Wallis, B-D—One-way ANOVA.
Th17 differentiation and arthritis severity are potently reduced by combinatorial blockade of IL-6 and IL-21 signaling pathways
We next investigated the in vivo effects of IL-6 and IL-21 pathway blockade on Th17 differentiation and the development of experimental arthritis. The T cell-driven AIA model was induced in WT, IL-6-/-, IL-21R-/-, and IL-6-/- x IL-21R-/- mice, and the number of Th17 cells in draining LNs was assessed two days after arthritis onset. Expression of various T (CD3, CD4, CD8) and B (CD45R, CD19, CD22) cell markers was unaffected in spleens of IL-6-/- x IL-21R-/- mice as compared to WT, indicating normal lymphocyte development (data not shown). Among total measured cell population of WT mice, 0.3% was CD4+IL-17+ (Fig 2A). In line with our in vitro data, Th17 levels were reduced in mice lacking IL-6 signaling, and in mice in which both IL-6 and IL-21R were absent. Remarkably, in contrast to our in vitro data (Fig 1), IL-21R-/- mice also showed significantly reduced Th17 numbers in vivo (Fig 2A). In addition to its importance in T cell development, IL-21 has been implicated in B cell development and antibody production [29,30], processes relevant to RA and important in this experimental arthritis model as well. Hence, we were interested in determining the effect of a lack in IL-6 but especially in IL-21 on antibody production in AIA. Unexpectedly, we did not observe any effect on antibody titers in mice deficient only for the IL-21R (Fig 2B). However, mice lacking both IL-6 and IL-21R expression showed reduced antibody production as compared to WT controls (Fig 2B), suggesting that combined deficiency of IL-6 and IL-21 can significantly suppress the development of disease-relevant antibodies.
Fig 2. IL-6 and IL-21 play an important role during in vivo Th17 differentiation and antibody production.
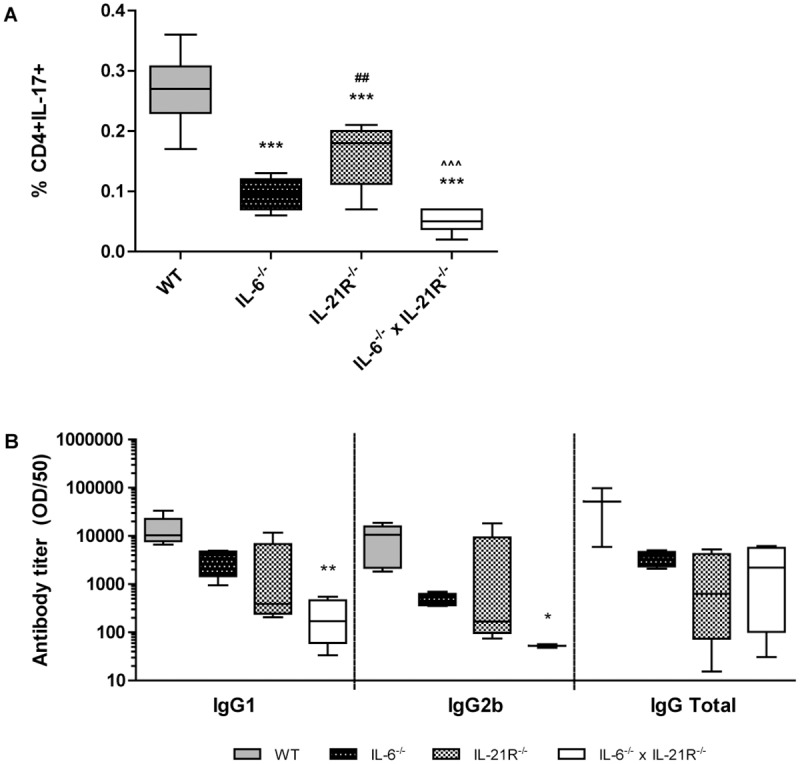
AIA was induced in WT, IL-6-/-, IL-21R-/-, and IL-6-/- x IL-21R-/- mice. Draining lymph node CD4+IL-17+ cell fraction two days after arthritis induction as measured using flow cytometry (A; n = 10/group). Serum IgG1, IgG2b, and total IgG levels as measured by ELISA (B; n = 5/group). *p<0.05, **p<0.01, ***p<0.001 versus WT; ##p<0.01 versus IL-6-/-; ^^^p<0.001 versus IL-21R-/-; A—One-way ANOVA, B—Kruskal-Wallis.
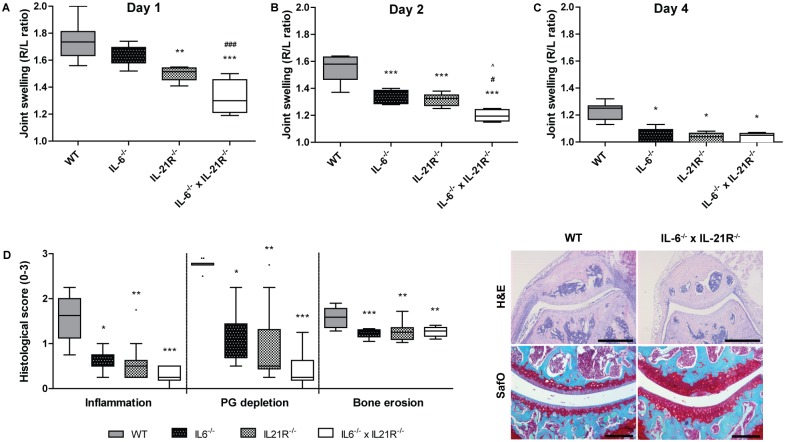
In line with lowered Th17 levels, reduced joint swelling was measured in the absence of IL-6 or IL-21R, as compared to WT controls (Fig 3A–3C). Interestingly, joint swelling was reduced more potently in IL-6-/- x IL-21R-/- mice compared to mice lacking either IL-6 or IL-21R expression early in disease development (Fig 3A and 3B). Even though joint swelling of WT mice started to decline four days after arthritis induction, joint swelling was still significantly lower in all knock out groups (Fig 3C). Importantly, when assessing histologically scored inflammation, cartilage proteoglycan (PG) depletion, and bone erosion at this time point, we observed significantly reduced damage in IL-6-/-, IL-21R-/-, and IL-6-/- x IL-21R-/- mice as compared to the WT controls for all parameters (Fig 3D).
Fig 3. Antigen-induced arthritis severity is potently reduced by combinatorial blockade of IL-6 and IL-21 signaling pathways.
Joint swelling of WT, IL-6-/-, IL-21R-/-, and IL-6-/- x IL-21R-/- mice at day 1 (A), day 2 (B), and day 4 (C) after arthritis induction, depicted as ratio between right and left knee joint, measured by 99mTechnetium pertechnetate uptake in the joint (n = 6/group). Histologically scored inflammation, bone erosion (both H&E staining, scale bar 500 μM), and cartilage proteoglycan depletion (SafO staining, scale bar 200 μM) (D; n = 10/group). *p<0.05, **p<0.01, ***p<0.001 versus WT; #p<0.05, ###p<0.001 versus IL-6-/-; ^p<0.05 versus IL-21R-/-; A+B One-way ANOVA, C+D Kruskal-Wallis.
Overall, this study confirmed the importance of IL-6 and IL-21 during Th17 development in vivo, and showed higher potency of blocking both cytokine pathways above blocking either one in reducing the severity of arthritis.
Combination therapy with anti-IL-6R antibodies and sIL-21R.Fc more potently suppresses disease development than targeting either cytokine during the induction phase of experimental arthritis
To determine the therapeutic potential of combined IL-6/IL-21 signaling pathway blockade during experimental arthritis, mice with CIA were treated with anti-IL-6R antibodies and/or sIL-21R.Fc starting either from the day of immunization (day 0, early treatment), or from the day of the booster injection (day 21, late treatment).
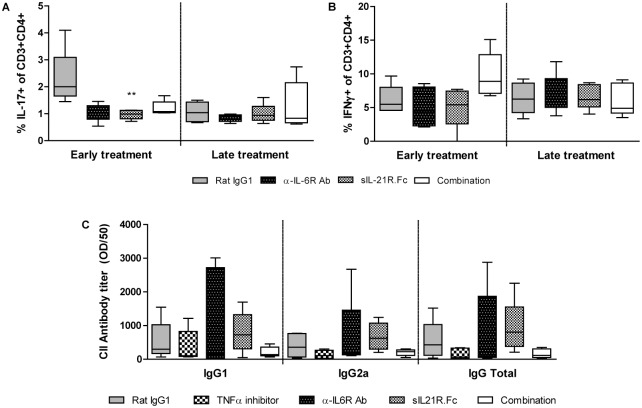
Surprisingly, mice receiving early anti-IL-6R antibody and/or anti-IL-21 treatment, only showed a minor trend towards lower Th17 levels in their LNs as compared to isotype control mice (Fig 4A). This trend in Th17 reduction was not present when treatment was initiated later in the disease process, i.e. at the day of booster injection. Th1 levels were largely unaffected, except for a minor trend towards an increase in mice receiving early combination treatment (Fig 4B). In contrast to observations in our AIA study using knockout mice (Fig 2B), we did not observe any changes in antibody profile when using this anti-IL-6/anti-IL-21 treatment strategy in mice with CIA (Fig 4C).
Fig 4. Effect of anti-IL-6 and/or anti-IL-21 treatment on Th17, Th1, and antibody development in CIA mice.
Th17 (A) and Th1 (B) levels in draining lymph nodes as measured using flow cytometry, and anti-CII antibody level in serum (C) as measured by ELISA of mice with CIA receiving anti-IL-6 and/or anti-IL-21 treatment. n = 5/group; **p<0.01 versus Rat IgG1; A+C—Kruskal-Wallis, B—One-way ANOVA.
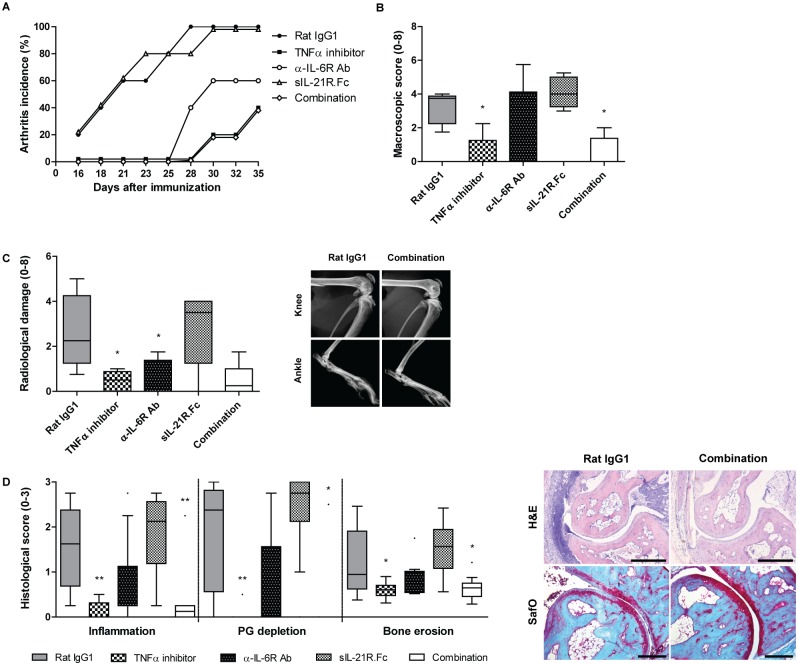
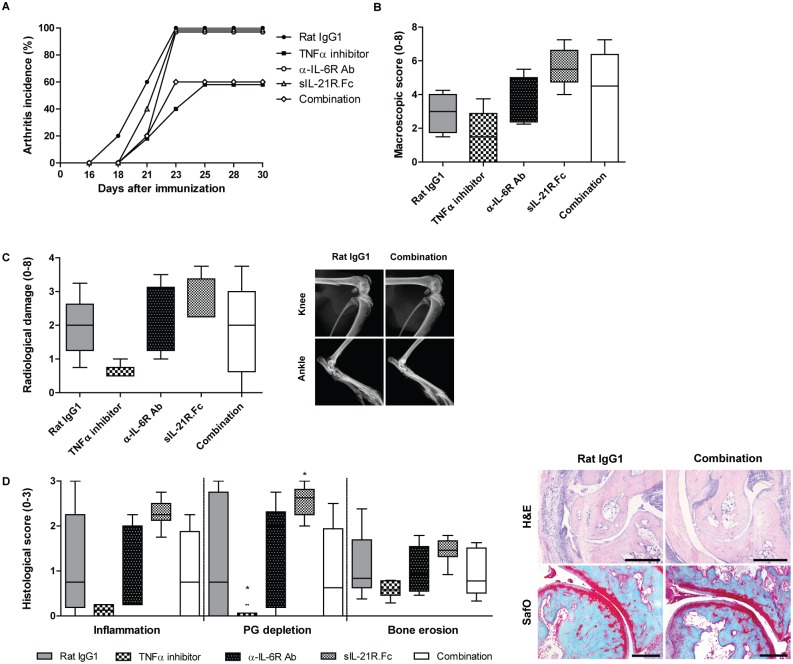
Even though the effect of cytokine neutralization on Th17 levels was minor, we observed potent clinical effects of this treatment strategy. Mice receiving early αIL-6R antibody treatment showed potently reduced disease incidence compared to isotype-treated mice, with only 60% of these mice having arthritis at day 35 (Fig 5A). Interestingly, arthritis incidence was further reduced when mice were treated with the anti-IL-6R/anti-IL-21 combination therapy, resulting in only 40% of mice developing arthritis. This greatly reduced disease incidence shows the potential of the early IL-6/IL-21 combination strategy over single cytokine inhibition in blocking arthritis development, with comparable efficacy as the positive control group receiving TNFα inhibitors. In contrast to early anti-IL-6R therapy, late treatment with anti-IL-6R antibodies could not inhibit arthritis; all mice developed arthritis already around day 23 (Fig 6A). Remarkably, mice treated with the late anti-IL-6R/anti-IL-21 combination therapy show a clear protective effect on arthritis incidence; only 60% of those mice developed arthritis before the end of the study (day 30). Unfortunately, none of the late experimental treatments had an effect on arthritis severity, as joint swelling (Fig 6B), radiological bone damage (Fig 6C), and histological damage (Fig 6D), were all comparable to levels measured in the isotype control group. In mice treated from the day of immunization with anti-IL-6 and anti-IL-21 treatment on the other hand, clinical arthritis severity based on swelling and redness of the four paws was greatly reduced in comparison with the isotype control group (Fig 5B). Unexpectedly, we found reduced radiological scored bone damage only to be significant in early anti-IL-6R antibody receiving mice, with no additional value of blocking IL-21 (Fig 5C). Interestingly, histological damage was reduced more potent in mice receiving anti-IL-6/IL-21 combinatorial treatment than in mice receiving single anti-IL-6 or anti-IL-21 treatment (Fig 5D), altogether suggesting higher efficacy of this combination treatment strategy over single cytokine blockade during the induction phase of the disease.
Fig 5. Arthritis incidence and severity of mice receiving anti-IL-6 and/or anti-IL-21 treatment during the induction phase of the disease.
Collagen-induced arthritis was initiated in DBA-1 mice, subsequently treated with anti-IL-6R antibodies and/or sIL-21R.Fc from the day of the immunization injection (d = 0; n = 5/group). Arthritis incidence (A) and severity (B) based on macroscopic scoring. Bone damage as measured using the Faxitron depicted as radiological damage (C). Histologically scored inflammation, bone erosion (both H&E staining, scale bar 500 μM), and cartilage proteoglycan depletion (SafO staining, scale bar 200 μM) (D). *p<0.05, **p<0.01 versus Rat IgG1; One-way ANOVA.
Fig 6. Arthritis incidence and severity of mice receiving anti-IL-6 and/or anti-IL-21 treatment late in disease development.
Collagen induced arthritis was initiated in DBA-1 mice, subsequently treated with anti-IL-6R antibodies and/or sIL-21R.Fc from the day of booster injection (d = 21; n = 5/group). Arthritis incidence (A) and severity (B) based on macroscopic scoring. Bone damage as measured using the Faxitron depicted as radiological damage (C). Histologically scored inflammation, bone erosion (both H&E staining, scale bar 500 μM), and cartilage proteoglycan depletion (SafO staining, scale bar 200 μM) (D). *p<0.05 versus Rat IgG1; One-way ANOVA.
Discussion
IL-6 and IL-21 are important mediators in the differentiation of Th17 cells [17–19]. The redundancy and possible additive or synergistic effects of the IL-6 and IL-21 pathways, as well as the therapeutic potential of combined pathway blockade in Th17-driven autoimmunity remain unclear. This study on the role of IL-6 and IL-21 during Th17 differentiation and the development of experimental arthritis revealed synergistic effects of IL-6 and IL-21 signaling pathways in driving Th17 differentiation in vitro, and indicated that the IL-6/IL-21 combination blocking strategy may be a highly effective way of treating patients with early rheumatoid arthritis.
In accordance with previously reported data on in vitro Th17-differentiation [17,18], we showed significantly lower levels of IL-17-producing CD4+ cells when differentiating naïve T cells in the absence of IL-6. However, we observed no reduction in Th17 differentiation when solely IL-21 signaling was blocked, indicating that IL-21 is not a critical factor during Th17 differentiation in vitro. Data from earlier studies on the role of IL-21 in in vitro Th17 differentiation are ambivalent. IL-21 is suggested to be essential for differentiation of Th17 cells [20], whereas others claim IL-21 is capable of, but not necessary for, driving Th17 development [31,32], the latter being in line with the current study. Although we cannot fully explain this discrepancy, the reason might lie within the concentrations of the various cytokines and antibodies used to drive T cell differentiation, or within the mouse strain used to isolate naïve T cells. Interestingly, when both IL-6 and IL-21 pathways were inactive, differentiation of naïve T cells towards Th17 cells was almost completely blocked, clearly showing the synergy between the two pathways in driving Th17 differentiation in vitro.
Accordingly, significantly reduced Th17 levels were present in draining lymph nodes of arthritic mice in which the IL-6 pathway was blocked, two days after induction of the AIA model. Moreover, blocking both IL-6 and IL-21 pathways resulted in almost undetectable Th17 levels, showing the importance of these two cytokines in driving Th17 differentiation in vivo. As IL-6 and IL-21 are both important in inducing IL-23 receptor expression in naïve CD4+ T cells, this combined blockade might reduce IL-23R expression levels, and thereby IL-23 signaling, to a minimum, possibly contributing to the major reduction in Th17 levels [20,22], In line with previously published data, arthritis severity was significantly reduced in IL-6-/- mice as compared to WT controls, two days after arthritis induction [33]. Earlier work of our group showed reduced arthritis severity in IL-21R-/- mice as compared to WT controls [24], the present study confirms these data. Additionally, both studies show reduced IL-17 production by CD4+ cells of mice lacking expression of the IL-21 receptor. Interestingly, as opposed to this T cell effect, previous studies showed the reducing effect of IL-21 blockade on arthritis development could be attributed to a defect in B cell development rather than in T cell development in the K/BxN and collagen-induced arthritis (CIA) murine arthritis models [34,35]. In contrast to our study, Th17 responses were unaffected in IL-21R-/- mice with CIA [35], as well as in IL-21R-/- mice with experimental autoimmune encephalitis (EAE) and myocarditis (EAM) [32]. The exact reason for this discrepancy in mechanism of disease reduction remains unclear, but might reflect the pleiotropic character of IL-21, with the genetic and inflammatory context influencing the functioning of the cytokine. Regardless of the mechanism of action, a block in IL-21 reduced experimental arthritis development in the majority of reported studies, including the present study. Of high interest, our double blocking strategy was most effective in reducing arthritis severity, which is in concordance with Th17 levels of those animals. These data hint towards therapeutic potential of using a combined IL-6/IL-21-blocking treatment strategy.
Indeed, when treating mice with both anti-IL-6R antibodies and sIL-21R.Fc either during the induction phase of arthritis (day 0) or in a later stage of the disease (day 21), arthritis incidence levels could potently be reduced, confirming the therapeutic potency of this strategy in a RA mouse model. Also in comparison with mice treated solely with anti-IL-6R antibodies, a compound currently used to effectively treat RA patients [36] and known as Tocilizumab (JW Pharmaceutical, Seoul, South Korea), the combination treatment proved to be much more potent in preventing arthritis development. This holds true when treatment was initiated early during arthritis development, but especially when first therapeutic injections were administered in a later stage of the disease, where the anti-IL-6R antibodies could not prevent disease onset. Tocilizumab is a humanized anti-IL-6R monoclonal antibody that has been tested in the clinic both as a monotherapy, as well as in combination with disease-modifying anti-rheumatic drugs (DMARDS), the first-line treatment for RA patients. Tocilizumab monotherapy potently inhibited structural joint damage, and led to increased percentages of patients achieving ACR20, 50 and 70 responses. Additionally, the number of patients achieving remission as defined by a Disease Activity Score in 28 Joints (DAS28) lower than 2.6, was increased in Tocilizumab-treated patients [37–40]. Samson et al. suggest that the successes of Tocilizumab may be attributed to a correction of the imbalance between the pathogenic Th17 cells and the protective regulatory T (Treg) cells that is found in part of the RA patients [41]. They showed decreased Th17 levels in peripheral blood of RA patients after Tocilizumab treatment, whereas Treg numbers were increased. As arthritis development in mice was more effectively reduced by our combination treatment than by the anti-IL-6R antibodies, a similar response may be expected when treating patients with Th17-driven joint pathologies, suggesting the potential of adding an anti-IL-21 treatment to the currently used Tocilizumab monotherapies.
In addition to reduced incidence after anti-IL-6R/anti-IL-21 treatment, disease severity of arthritic mice receiving early combination therapy was reduced. Interestingly, neither anti-IL-6R antibodies, nor anti-IL-21 therapy as single treatments could reduce arthritis severity in the severe and progressive collagen-induced arthritis model. A partial difference in the outcome of anti-IL-6R antibody treatment is present compared to earlier studies [27,42], which may be due to different study design. In line with our data, no protective effect was observed when this treatment was initiated later in disease development. Radiological bone damage of the knee and ankle joints was significantly reduced only in mice receiving early treatment with anti-IL-6R antibodies alone, a trend towards reduction was observed when combined with anti-IL-21 therapy, to levels comparable to that of anti-TNFα treated mice. Histological damage was only reduced by the combination treatment, again to levels comparable to those observed in mice treated with TNFα inhibitors.
Interestingly, a clear trend towards reduced Th17 levels in draining lymph nodes of mice receiving either early single or combination treatment was present, despite the late time point of analysis (day 35). In contrast, no reduced Th17 levels were observed when treatment was initiated later in disease development. Data of mice treated with anti-IL-6R antibodies initiated at both time points confirm previously published data [42]. Although our in vitro data demonstrated synergy between IL-6 and IL-21 in Th17 differentiation, this was not clearly reflected by Th17 levels in draining lymph nodes of arthritic mice during our in vivo studies. Determination of T cell levels in inflamed synovium may provide more information on local differences in T cell subsets.
Over the past few years, clinical trials have been performed testing various IL-17-targeting agents for a number of autoimmune diseases, amongst others Rheumatoid Arthritis. In RA patients who failed to respond to synthetic and/or biologic DMARDs, both ixekizumab and secukinumab, two agents targeting the IL-17A ligand, induced small but clinically relevant responses. Reported safety profiles were similar to that of other biological agents [43–48]. In contrast, no clinical response was observed when treating RA patients with the anti-IL-17-receptor antibody brodalumab [49,50], a clear explanation for the differences in efficacy of ligand versus receptor targeting is lacking. Even though these studies indicate targeted inhibition of the IL-17A ligand may have therapeutic value in the treatment of RA, pharmaceutical companies appear to shift the application of their anti-IL-17 therapies to other rheumatic diseases like psoriatic arthritis [51,52] and ankylosing spondylitis [53]. Most likely because obtained clinical effects of IL-17 blocking strategies in RA were below expectation, especially when comparing with the impressive therapy responses observed in patients suffering from plaque psoriasis [54].
As IL-17 and other Th17-derived cytokines are important contributors to RA pathology [8–16], identifying other ways to target this pathway remains a high priority. With this study we demonstrated that full inhibition of both IL-6 and IL-21 effectively abrogates Th17 differentiation, and that, despite the minor effect on Th17 development, a combination therapy neutralizing both cytokine pathways is more effective in treating early T cell-driven experimental arthritis than targeting either cytokine. This indicates that anti-IL-6/IL-21 combination therapy might be an interesting new strategy to treat early RA patients or to sustain RA remission.
Supporting information
Acknowledgments
We thank the Central Animal Laboratory, Radboudumc, Nijmegen, the Netherlands for good animal care.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
DR received funding from the Innovative Medicines Initiative Joint Undertaking funded project BTCure [http://btcure.eu/ grant number: 115142-2]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. 10.1038/nature01661 [DOI] [PubMed] [Google Scholar]
- 2.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594–1602. 10.1056/NEJM200011303432202 [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. 10.1056/NEJM199901283400401 [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- 5.Aerts NE, De Knop KJ, Leysen J, Ebo DG, Bridts CH, Weyler JJ, et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology. 2010;49:2264–2272. 10.1093/rheumatology/keq224 [DOI] [PubMed] [Google Scholar]
- 6.Alzabin S, Abraham SM, Taher TE, Palfreeman A, Hull D, McNamee K, et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann Rheum Dis. 2012;71:1741–1748. 10.1136/annrheumdis-2011-201024 [DOI] [PubMed] [Google Scholar]
- 7.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther. 2011;13:R126 10.1186/ar3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–1023. 10.1002/art.33446 [DOI] [PubMed] [Google Scholar]
- 10.Kwok SK, Cho ML, Park MK, Oh HJ, Park JS, Her YM, et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64:740–751. 10.1002/art.33390 [DOI] [PubMed] [Google Scholar]
- 11.Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1453–1457. 10.1136/ard.2011.152074 [DOI] [PubMed] [Google Scholar]
- 12.Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:R113 10.1186/ar2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roşu A, Mărgăritescu C, Stepan A, Muşetescu A, Ene M. IL-17 patterns in synovium, serum and synovial fluid from treatment-naïve, early rheumatoid arthritis patients. Rom J Morphol Embryol. 2012;53:73–80. [PubMed] [Google Scholar]
- 14.Zhao L, Jiang Z, Jiang Y, Ma N, Zhang Y, Feng L, et al. IL-22+CD4+ T cells in patients with rheumatoid arthritis. Int J Rheum Dis. 2013;16:518–526. 10.1111/1756-185X.12099 [DOI] [PubMed] [Google Scholar]
- 15.Koenders MI, van den Berg WB. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol Sci. 2015; 36:189–195. 10.1016/j.tips.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2015; 74:101–107. 10.1016/j.cyto.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. 10.1038/nature05970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. 10.1038/nature07021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. 10.1038/368339a0 [DOI] [PubMed] [Google Scholar]
- 24.Marijnissen RJ, Roeleveld DM, Young D, Nickerson-Nutter C, Abdollahi-Roodsaz S, Garcia de Aquino S, et al. Interleukin-21 receptor deficiency increases the initial toll-like receptor 2 response but protects against joint pathology by reducing Th1 and Th17 cells during streptococcal cell wall arthritis. Arthritis Rheum. 2014;66:886–895. [DOI] [PubMed] [Google Scholar]
- 25.Koenders MI, Marijnissen RJ, Devesa I, Lubberts E, Joosten LA, Roth J, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleuking-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011;63:2329–2339. 10.1002/art.30418 [DOI] [PubMed] [Google Scholar]
- 26.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. 10.1172/JCI27727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. [DOI] [PubMed] [Google Scholar]
- 28.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. 10.1002/art.20001 [DOI] [PubMed] [Google Scholar]
- 29.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naïve and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. [DOI] [PubMed] [Google Scholar]
- 30.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. [DOI] [PubMed] [Google Scholar]
- 31.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. [DOI] [PubMed] [Google Scholar]
- 32.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. 10.1002/eji.200838511 [DOI] [PubMed] [Google Scholar]
- 33.de Hooge AS, van de Loo FA, Arntz OJ, van den Berg WB. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol. 2000;157:2081–2091. 10.1016/S0002-9440(10)64846-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block KE, Huang H. The cellular source and target of IL-21 in K/BxN autoimmune arthritis. J Immunol. 2013;191:2948–2955. 10.4049/jimmunol.1301173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakuraba K, Ovamada A, Fujimura K, Spolski R, Iwamoto Y, Leonard WJ, et al. Interleukin-21 signaling in B cells, but not in T cells, is indispensable for the development of collagen-induced arthritis in mice. Arthritis Res Ther. 2016;18:188 10.1186/s13075-016-1086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song SN, Yoshizaki K. Tocilizumab for treating rheumatoid arthritis: an evaluation of pharmacokinetics/pharmacodynamics and clinical efficacy. Expert Opin Drug Metab Toxicol. 2015;11:307–316. 10.1517/17425255.2015.992779 [DOI] [PubMed] [Google Scholar]
- 37.Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. 10.1136/ard.2008.105197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maini RN, Taylor PC, Szechinski J, Pavelka K, Bröll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. 10.1002/art.22033 [DOI] [PubMed] [Google Scholar]
- 39.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. 10.1136/ard.2006.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19. 10.1007/s10165-008-0125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. 10.1002/art.34477 [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Hashizume M, Mihara M. IL-6 blockade preferentially inhibits Th17 differentiation in collagen-induced arthritis. Rheumatol Int. 2011;31:127–131. 10.1007/s00296-010-1552-9 [DOI] [PubMed] [Google Scholar]
- 43.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72 10.1126/scitranslmed.3001107 [DOI] [PubMed] [Google Scholar]
- 44.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. 10.1002/art.27334 [DOI] [PubMed] [Google Scholar]
- 45.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. 10.1136/annrheumdis-2012-201601 [DOI] [PubMed] [Google Scholar]
- 46.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion JA, et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol. 2014;41:414–421. 10.3899/jrheum.130637 [DOI] [PubMed] [Google Scholar]
- 47.Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naïve to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheum. 2014;66:1693–1704. [DOI] [PubMed] [Google Scholar]
- 48.Burmester GR, Durez P, Shestakova G, Genovese MC, Schulze-Koops H, Li Y, et al. Association of HLA-DRB1 alleles with clinical responses to the anti-interleukin-17A monoclonal antibody secukinumab in active rheumatoid arthritis.Rheumatology. 2016;55:49–55. 10.1093/rheumatology/kev258 [DOI] [PubMed] [Google Scholar]
- 49.Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164 10.1186/ar4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavelka K, Chon Y, Newmark R, Lin SL, Baumgartner S, Erondu N. A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J Rheumatol. 2015;42:912–919. 10.3899/jrheum.141271 [DOI] [PubMed] [Google Scholar]
- 51.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of Interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329–1339. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- 52.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis. Lancet. 2015;386:1137–1146. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 53.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an Interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 54.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorain G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.