Abstract
The goal of this research was to examine the role of cytotoxic T lymphocytes (CTL) in the control of Rhodococcus equi and specifically to determine if R. equi-specific CD8+ CTL occurred in the blood of immune horses. Equine peripheral blood mononuclear cells stimulated with antigen-presenting cells either infected with R. equi or exposed to soluble R. equi antigen lysed R. equi-infected target cells. Lysis was decreased to background by depletion of either CD2+ or CD3+ cells, indicating that the effector cell had a T-lymphocyte, but not NK cell, phenotype. Stimulation induced an increased percentage of CD8+ T cells in the effector population, and depletion of CD8+ T cells resulted in significantly decreased lysis of infected targets. Killing of R. equi-infected macrophages by effector cells was equally effective against autologous and equine leukocyte antigen A (classical major histocompatibility complex [MHC] class I) mismatched targets. To evaluate potential target antigens, target cells were infected with either virulent (80.6-kb plasmid-containing) or avirulent (plasmid-cured) R. equi. The degree of lysis was not altered by the presence of the plasmid, providing evidence that the virulence plasmid, which is required for survival within macrophages, was not necessary for recognition and killing of R. equi-infected cells. These data indicate that immunocompetent adult horses develop R. equi-specific CD8+ CTL, which may play a role in immunity to R. equi. The apparent lack of restriction via classical MHC class I molecules suggests a novel or nonclassical method of antigen processing and presentation, such as presentation by CD1 or other nonclassical MHC molecules.
Rhodococcus equi is a gram-positive, weakly acid-fast, facultative intracellular bacterium, closely related to Mycobacterium species (18). The cell wall structure and intracellular location during infection by these nocardioform actinomycetes are similar. Immunocompromised humans, notably AIDS patients, are susceptible to R. equi infection, which manifests as a severe pyogranulomatous pneumonia mimicking tuberculosis (7, 29). R. equi is also an important cause of mortality in 2- to 6-month-old horses, where it produces a similar pyogranulomatous lesion in the lungs (43). As R. equi is ubiquitous in the equine environment, virtually all horses are exposed early in life. Most horses ultimately develop protective immune responses which mediate clearance of the bacteria from the lung and provide lifelong immunity (16, 17).
Immunity to R. equi has been most extensively studied in murine systems. As with Mycobacterium tuberculosis, gamma interferon (IFN-γ) secretion by CD4+ Th1 lymphocytes appears to be critical for immune control (21, 23, 24). However, mice which lack major histocompatibility complex (MHC) class II-restricted CD4+ T lymphocytes also significantly curtail the pathogen load following challenge (22). These experiments and others suggest that both CD4+ and CD8+ T lymphocytes are involved in the clearance of R. equi (34, 39). In immune horses, studies of pulmonary recall responses have shown that clearance of a virulent pulmonary challenge is associated with antigen-specific proliferation by both CD4+ T lymphocytes and CD8+ T lymphocytes (16). Clearance of R. equi in immune horses also associates with increased numbers of IFN-γ-positive CD4+ and IFN-γ-positive CD8+ T lymphocytes at the site of pulmonary challenge (17).
CD8+ T lymphocytes clearly play a significant role in protective immune responses to M. tuberculosis (26, 40). Both CD8 knockout mice and β2-microglobulin knockout mice, which lack MHC class I-restricted CD8+ T lymphocytes, are more susceptible to infection than syngeneic controls (35). The effect may reflect, in part, the contribution of CD8+ T lymphocytes to IFN-γ secretion and, as a result, macrophage activation. However, human and murine CD8+ T-lymphocyte lines, such as Lyt-2+ murine T lymphocytes, have also been shown to lyse M. tuberculosis-infected macrophages in an MHC class I-restricted and antigen-specific fashion, thus supporting the role of CD8+ T-cell cytotoxicity in the control of M. tuberculosis infection (11). In addition, several MHC class I binding motifs have been characterized in M. tuberculosis proteins and shown to stimulate peptide-specific human CD8+ cytotoxic T lymphocytes (CTL) (50). However, CTL from infected humans and mice can also recognize and lyse M. tuberculosis-infected cells in an MHC-independent manner. These effector cells, which recognize M. tuberculosis lipid and glycolipid antigens presented via CD1 molecules, include CD8+ T lymphocytes and double-negative (CD4− CD8−) T lymphocytes (13, 41, 48). Presentation of these unique nonpeptide components of a pathogen by nonpolymorphic surface proteins dramatically expands the range of antigens recognized by T lymphocytes.
The present report provides the first evidence for R. equi-specific CTL. Importantly, this work was performed on horses, indicating that CTL are likely to play a role in controlling this pathogen in its natural host. We also examined the ability of CTL from immune horses to lyse target cells infected with R. equi that had been cured of the 80-kb virulence plasmid. This plasmid enables the organism to survive in host macrophages and is required to produce disease in young horses. Whereas virulent, plasmid-bearing strains of R. equi persist within macrophages, plasmid-negative strains are taken up by macrophages at comparable rates but are effectively eliminated (15). We hypothesized that due to either altered antigen presentation or a failure to express plasmid-encoded antigens recognized by effector cells, target cells infected with plasmid-negative R. equi would not be effectively recognized and lysed by CTL.
MATERIALS AND METHODS
Horses.
Nine healthy 3- to 19-year-old horses of various breeds, currently maintained in the Washington State University Veterinary Teaching Hospital herd, were randomly selected. Using previously described serologic reagents, equine lymphocyte alloantigen A (ELA-A) MHC class I haplotypes were determined by the microcytotoxicity assay (46) (Table 1). Mismatched horses were selected from the original nine based on ELA-A type. Venous blood was collected from the jugular vein of each horse using 500-ml evacuated containers (Baxter, Deerfield, Ill.) containing 80 ml of anticoagulant citrate dextrose (ACD)/420 ml of blood. Peripheral blood mononuclear cells (PBMC) were isolated from venous blood by using a Ficoll-Hypaque technique (33). PBMC were then used either as effector cells or for isolation of peripheral blood adherent cell (PBAC) targets (see below).
TABLE 1.
ELA-A typing of WSU-VTH horsesa
| Horse (haplotype) | Mismatch (haplotype) used in this study |
|---|---|
| H44 (A3/A10) | H71 (A5), H14 (A2/A9) |
| H55 (A10/W11) | H71 (A5), H81 (A3/A5) |
| H68 (A2/A10) | H71 (A5) |
| H81 (A3/A5) | H68 (A2/A10) |
| H71 (A5) | H68 (A2/A10) |
WSU-VTH, Washington State University Veterinary Teaching Hospital.
Bacteria.
The plasmid-bearing (PL+) virulent R. equi strain ATCC 33701, which contains the 80.6-kb virulence-associated plasmid and expresses the virulence-associated protein, VapA, was utilized in all experiments. An avirulent, plasmid-cured (PC) derivative of ATCC 33701 was used for comparison of plasmid-bearing versus plasmid-cured strains (6, 44). Both strains of bacteria were stored at −20°C as frozen stabilates and reconstituted prior to each experiment. Bacteria were initially grown on brain heart infusion (BHI) agar and then cultivated overnight in BHI broth at 37°C in a shaking incubator at 200 rpm. After washing twice with phosphate-buffered saline (PBS), the bacteria were adjusted to 107/ml in antibiotic-free complete medium, which consisted of RPMI-1640 supplemented with 2 mM l-glutamine, 2-mercaptoethanol, and 6.25 mM HEPES and 10% normal horse serum (NHS) (Gibco, Grand Island, N.Y.). The adjusted bacterial concentration was initially confirmed by plating serial dilutions on BHI plates and calculating numbers of CFU per milliliter.
Preparation of SRA.
To assess whether or not viability of R. equi was required to induce CTL stimulation, soluble R. equi antigen (SRA) was used to stimulate effectors. SRA has been previously used to induce R. equi-specific proliferation postchallenge in immune adult horses (16). Specifically, SRA induces proliferative responses similar to stimulation with native VapA (27). SRA was prepared as previously described (16, 27). Briefly, one colony of R. equi ATCC 33701 PL+ was inoculated into 10 ml of BHI broth and cultivated overnight at 37°C in a shaking incubator at 200 rpm. The inoculum was transferred to 1 liter of BHI broth and incubated with shaking for an additional 72 h. A previous study showed that 72-h cultures were in the late log phase of growth and associated with minimal autolysis of bacteria (25). The medium containing bacteria was poured into four 250-ml sterile centrifuge bottles and spun at 3,500 × g for 15 min at 4°C. The pellets were then washed one time in PBS and centrifuged at 3,500 × g for 15 min at 4°C. Two milliliters of bacterial pellet was suspended in 10 ml of PBS (pH 7.4) and transferred to a sterile 50-ml conical tube. The pellets were frozen at −20°C for a minimum of 2 h, thawed at room temperature, and sonicated on ice for 3 min. The bacterial homogenate was pelleted at 12,000 × g for 15 min at 4°C. The supernatant was set aside and the pellets were resuspended in 5 ml of PBS. The pellets were pooled in PBS, frozen at −20°C, and sonicated as above. The freeze-thaw cycles were repeated for a total of three times. All the supernatants were pooled, and the final pellet was discarded. The supernatant was centrifuged at 25,000 × g for 60 min at 4°C. The supernatant was collected as SRA. A bicinchoninic acid protein assay (Pierce, Rockford, Ill.) was performed to determine the protein concentration. The presence of specific antigens (e.g., VapA) was confirmed by immunoblotting as previously described (25).
Effector cell preparation and stimulation.
A total of 108 PBMC in antibiotic-free complete medium were plated in 75-cm2 flasks at 4 × 106 cells/ml. The cells were stimulated with one of the following: (i) 1 multiplicity of infection (MOI) of live R. equi 33701 PL+ per monocyte, (ii) 10 μg of SRA/ml, or (iii) medium alone (as a negative control). In the experiments comparing the avirulent and the virulent strain, effectors were stimulated with either 1 MOI of live R. equi 33701 PL+ (virulent) or 1 MOI of live R. equi 33701 PC (avirulent). Following a 1-h incubation at 37°C with 5% CO2, 50-μg/ml gentamicin was added to all cultures to kill extracellular bacteria. Culture flasks were then incubated for 5 days at 37°C with 5% CO2. For some experiments, including the depletion studies, the effector cells were then rested without antigenic stimulation prior to further use. Resting consisted of washing the cells twice with antibiotic-free complete medium, counting, and plating in fresh medium. These cultures were then rested for 2 to 10 days at 37°C with 5% CO2. Following stimulation and resting, cells were washed once, counted, and then characterized for surface marker expression by flow cytometry. Alternatively, the cells were used as effector cells in the CTL assay described below.
Target cell isolation.
Autologous and ELA-A mismatched PBACs were harvested from PBMC for use as target cells as previously described (38). Briefly, 150-cm2 petri dishes were coated with 2% sterile bovine collagen (6 ml per plate) by incubating the dishes for 2 h at 37°C with 5% CO2. The collagen solution was then removed and the dishes air dried for 30 min. The dishes were next coated with 6 ml of NHS by incubation for 30 min at 37°C with 5% CO2. Serum was removed and PBMC were plated on dishes coated with gelatin and serum at a density of 1.5 × 108 cells/dish in 20 ml of antibiotic-free complete medium. Cells were incubated for 15 to 19 h at 37°C with 5% CO2. Following incubation, nonadherent cells were resuspended in the culture medium by repeated pipetting (10 times) and then removed by washing each plate with 10 ml of PBS and repeated pipetting (10 times). Adherent cells were eluted with 20 ml of antibiotic-free complete medium containing 20% NHS mixed 1:1 (vol/vol) with 10 mM EDTA for 35 min at 37°C with 5% CO2. The cells were then collected by vigorously pipetting the eluting fluid 10 times, and subsequently each dish was washed twice with 10 ml of PBS per wash. Eluted cells were combined, washed once with antibiotic-free complete medium, counted, and added to 96-well plates at 105 cells/well in 100 μl of medium. Immediately prior to adding cells, 20 μl of commercial NHS was added to each well. In 11 trials using two horses, the adherent cells were stained with monoclonal antibody (MAb) DH59B, a PBMC-derived monocyte marker for anti-CD172A, and MAb HB88A, an anti-CD2 marker. The eluted population was 89% ± 4.6% (n = 6) or 89% ± 8.6% (n = 5) CD172a+ as well as 5% ± 4.3% (n = 6) or 5% ± 2.1% (n = 5) CD2+, with the remaining (approximately 6%) cells not reacting with these antibodies by fluorescence-activated cell sorter analysis. The cells were incubated for 1 h prior to use as target cells in the CTL assay (see below).
Cytotoxicity assay.
Each well of target cells was labeled with 50 μl of antibiotic-free complete medium containing 100 μCi of 51Cr per ml. Following 13 h of incubation, the targets were infected with live R. equi bacteria by adding 50 μl of antibiotic-free complete medium containing 107 live R. equi per ml. One hour postinfection, 20 μl of complete medium containing 1 mg of gentamicin sulfate/ml was added to each culture well to kill any extracellular bacteria. Following an additional 7.5 h of incubation, the cells were washed four times with complete medium containing 0.05 mg of gentamicin sulfate/ml to remove extracellular 51Cr. Effector cells were then added to target cells and incubated for an additional 4.5 h at 37°C with 5% CO2. Following incubation, the plates were centrifuged at 500 × g for 5 min to pellet cells. One hundred microliters of supernatant was removed and the amount of 51Cr was measured using a MicroBeta plate reader. The following formula for percent specific lysis was used: [(E − S)/(T − S)] × 100, where E is the mean of three test wells, S is the mean spontaneous release from three target cell wells without effector cells, and T is the mean total release from three target cell wells with 3% Triton X-100 (31). As previously reported, significant lysis was defined as 3 standard errors above the negative control value (31). In initial experiments, parallel cultures were also stained and examined microscopically to determine the percentage of infected cells.
Initial trials examined uptake and spontaneous release of 51Cr by PBACs. These trials supported the use of 100 μCi of 51Cr/ml of medium and incubation of the PBACs in the presence of chromium for a total of 27 h (data not shown). The spontaneous release of chromium by infected PBACs was affected by both the MOI and the time of infection. At 12 h postinfection using 1 MOI of the virulent strain of R. equi, the percent infected cells was 21 to 27% whereas the infection rate with 5 MOI was 78 to 79%. The percentage of infected PBACs plateaued at MOI greater than 5 bacteria per target cell (e.g., 84 to 90% at 10 MOI). With 5 MOI, the percent spontaneous release was less than 15%, whereas in five trials using 10 MOI the percent spontaneous release was consistently above 15% and often approached 75% (data not shown). To investigate the time of infection, the maximal release of 51Cr was measured following infection with 5 MOI of virulent bacteria. Total chromium counts per minute decreased when infection exceeded 14 h (data not shown). In contrast to stimulation of effectors, which was achieved with 1 MOI of bacteria, target cells in the CTL assay were infected with 5 MOI for a total of 11 to 12 h. This allowed for maximum presentation of antigen by targets with minimal effect on the viability of the cells. In greater than 50 subsequent trials using 10 horses, the spontaneous release of chromium from both uninfected and infected macrophages remained below 15%. With repeated trials (n = 5), there was no difference in the percentage of infected PBACs between infection with the virulent strain and the plasmid-cured strain of the bacterium.
Flow cytometry.
Before and after stimulation, and following depletion (see below), effector cells were labeled with 15 μg of either anti-equine CD8 MAb HT14A, anti-equine CD4 MAb HB61A, anti-equine CD2 MAb HB88A, anti-equine immunoglobulin G MAb 1.9/3.2 (B-cell marker), anti-equine CD172a MAb DH59B (1) (granulocyte marker), or isotype control antibody (COLIS69A) (control for nonspecific binding) (VMRD, Inc., Pullman, Wash.) per ml. For CD3 depletion assays, the cells were also labeled with 60 μg of anti-equine CD3 MAb F6-G (J. Stott, University of California, Davis) per ml. The mixtures were incubated on ice for 15 min at 4°C, washed three times, and pelleted at 4°C for 4 min at 500 × g. Cells were then suspended in 50 μl of 5-μg/ml fluorescein-conjugated anti-mouse immunoglobulin serum absorbed with normal human and horse sera. Cells were next incubated 15 min at 4°C in the dark, washed two times, and suspended in 200 μl of 2% formaldehyde in PBS (8, 33). Labeled cells were analyzed with a FACScan cytometer equipped with a Macintosh computer and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The effector cell population was analyzed before stimulation and immediately prior to testing in the CTL assay. The values were compared using a nonparametric t test with a P value of <0.05 considered significant.
Fractionation of effector cells.
In order to identify the subset responsible for cytotoxicity, antibody-coated Dynabeads (Dynal, Inc., Lake Success, N.Y.) were used to perform negative selection as previously described. Briefly, mouse anti-equine CD2 MAb HB88A (47), mouse anti-equine CD3 MAb F6-G (4, 28), mouse anti-equine CD8 MAb HT14A, or mouse anti-equine CD4 MAb HB61A was added to the washed beads at 0.1 μg/107 beads and incubated for 30 min at 2 to 8°C on a mixer. The beads were then washed to remove unbound antibody. Effector cells were diluted to 2.5 × 107 cells/ml in Hanks balanced saline solution and cooled to 2 to 8°C. Prepared antibody-coated beads at >2 × 107 beads/ml of sample were added to effector cells at 5 beads/targeted cell and incubated for 30 min at 2 to 8°C on a mixer. The cells were placed in the Dynal MPC magnet (Dynal, Inc.) for 2 to 3 min, and the supernatant was transferred to a fresh tube containing antibody-coated beads for a second depletion. The resultant populations of CD2-, CD3-, CD4-, or CD8-depleted cells were then used as effectors in cytotoxicity assays. The effectiveness of depletion was assessed by flow cytometry, as previously described.
RESULTS
Effector cell population.
Within a 2-year period prior to these experiments, horses H71, H44, H55, and H68 had been challenged intrabronchially with virulent R. equi ATCC 33701. Horse H81 had been pastured with horses H44 and H71 at the time of the latter horses' challenge. All challenged horses effectively cleared the bacterium and had a documented recall response to the infection (16). Ex vivo stimulation of effector cells from these horses was performed with antigen-presenting cells (APC) either infected with live, virulent R. equi or pulsed with SRA. As a negative control, effectors were cultured with medium alone (no antigen [NA]). Following stimulation, the percentage of CD8+ T lymphocytes in both of the antigen-stimulated populations (30% ± 3.9%) was significantly increased (P < 0.05) relative to both the PBMC prior to culture (9% ± 3.6%) and the control effector cells exposed to no antigen (16% ± 2.4%). In contrast, the percentage of CD4+ cells did not significantly change (stimulated, 41% ± 10.6%; PBMC, 46% ± 10.7%; NA, 55% ± 7.0%). Additionally, the percentages of CD2+ cells in stimulated cultures were not significantly different from PBMC populations or NA cultures (stimulated, 69% ± 14.4%; PBMC, 59% ± 16.1%; NA, 67% ± 27.7%).
Stimulated PBMC effectively lyse R. equi-infected targets.
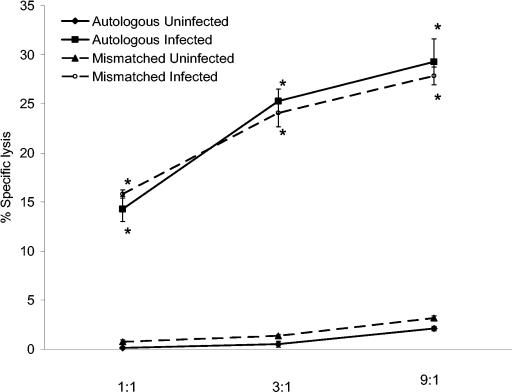
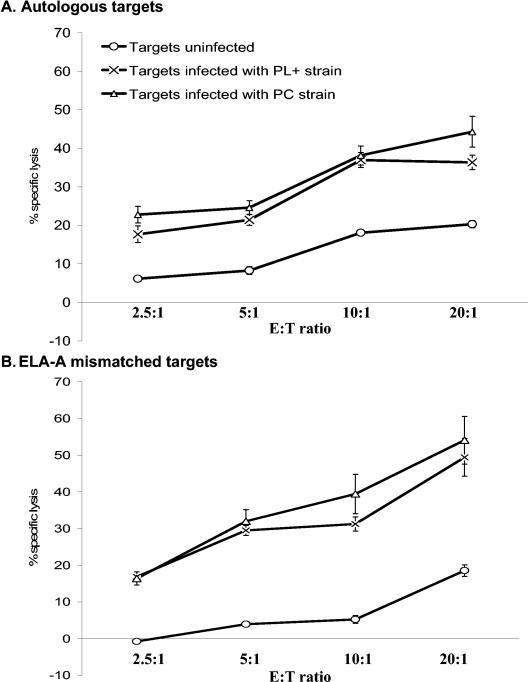
In an initial trial using horse H71, effector cells stimulated with live R. equi lysed autologous infected, but not uninfected, PBAC targets (Fig. 1). Increasing the number of effector cells to target cells (E:T ratio) in threefold increments, from 1:1 to 9:1, resulted in increased lysis of infected targets. However, lysis was not ELA-A (classical MHC class I) restricted, as evidenced by lysis of infected ELA-A mismatched targets (Fig. 1). As with autologous targets, uninfected ELA-A mismatched targets were not killed.
FIG. 1.
Specific lysis of PBAC targets increased as the E:T ratio increased. PBMC from H71 were stimulated in vitro for 5 days with virulent live R. equi and rested for 48 h. Stimulated effectors were then added, at increasing E:T ratios, to autologous and ELA-A mismatched infected and uninfected target cells. Lysis of infected targets was compared to uninfected negative controls. Increasing the number of effectors per infected target increased the percent specific lysis. An asterisk indicates significant increase compared to the corresponding uninfected control at the same E:T ratio.
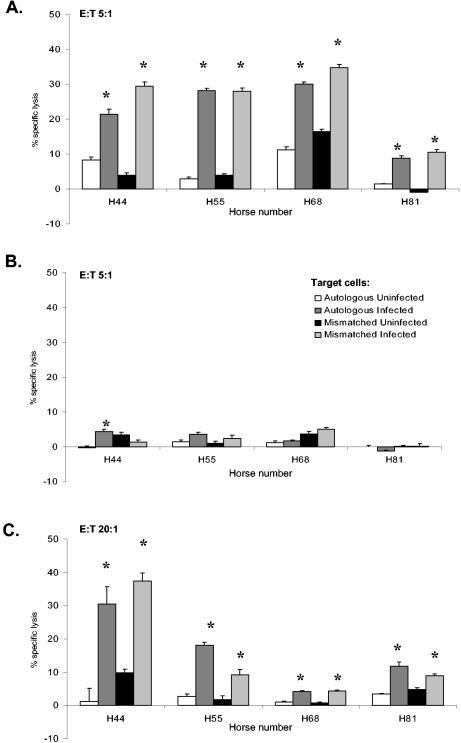
To confirm the initial observation and determine whether effector cells from other exposed adult horses could lyse R. equi-infected targets, eight additional horses representing seven ELA-A haplotypes were evaluated. Figure 2 depicts four representative horses from this group (see Table 1). Additional ELA-A haplotypes tested, not included in Table 1, included A9 and A6. At an E:T ratio of 5:1, effector cells stimulated by in vitro infection with live virulent R. equi caused significant killing, which again was not ELA-A restricted. Killing was, however, specific for infected PBAC targets and stimulation of effector cells with R. equi-infected APC was required (Fig. 2A). In all but one horse (H44), the killing of autologous and mismatched infected PBACs was not significantly different.
FIG. 2.
Classical MHC class I-unrestricted killing of R. equi-infected targets. PBMC from four horses were stimulated for 5 days with either (A) live virulent R. equi, (B) no antigen (medium alone), or (C) 10-μg/ml SRA. PBAC targets were infected with live virulent R. equi for 12 h or were uninfected. Four and a half hours prior to collection of the supernatants, effectors were added to targets at an E:T of 5:1 (A), 5:1 (B), or 20:1 (C). An asterisk indicates where lysis of infected targets is significantly increased (>3 standard errors compared to the corresponding uninfected control).
Control PBMC, grown under identical conditions but without antigenic stimulation, did not lyse infected or uninfected target cells (Fig. 2B). In the case of H44, lysis of autologous infected target, although significant, was minimal and readily explained by recent challenge of this animal (only horse to be infected within 3 months of experiments). However, significant lysis could also be induced by exposure of effector cells to SRA (Fig. 2C). As with R. equi-stimulated effectors, killing was not ELA-A restricted, as both autologous and mismatched infected targets were effectively lysed. The lysis of infected PBACs by PBMC stimulated with the soluble extract was significant but decreased compared to the lysis mediated by PBMC stimulated with live R. equi (compare Fig. 2A and 2C). To achieve similar levels of lysis (or in some cases statistically significant levels of lysis) it was necessary to increase the E:T ratio (e.g., from 5:1 to 20:1) when using SRA-stimulated effectors. Additional cytotoxicity experiments using cells from four additional horses (nine total) and stimulation with either live R. equi or SRA gave similar results.
Identification of the effector cell population.
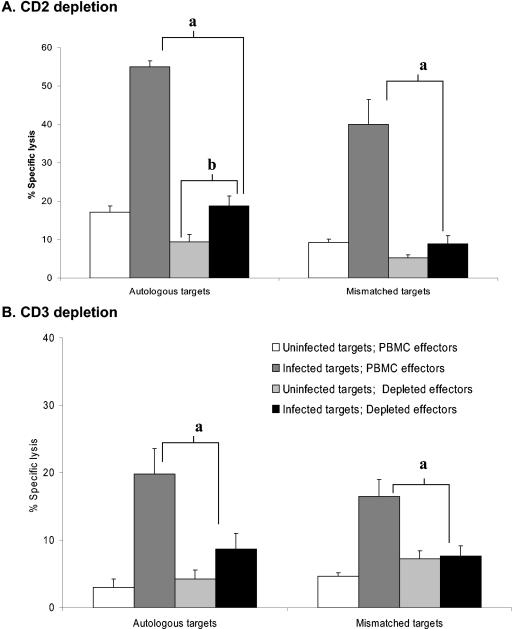
In order to determine the phenotype of the cells primarily responsible for lysis of R. equi-infected targets, negative depletion of the stimulated PBMC was performed. To differentiate T-cell (CD2+ CD3+) or natural killer (NK) cell (CD2+ CD3−) origin, stimulated PBMC were depleted of either CD2+ or CD3+ cells. Depletion resulted in CTL assay effector cells that were <1% CD3+ cells or <2.5% CD2+ cells. Figure 3A demonstrates that depletion of CD2+ cells significantly decreased killing by 83% ± 13.5% (P < 0.05, n = 7). Depletion of CD3+ cells (Fig. 3B) also resulted in a significant decrease in the percent specific lysis of R. equi-infected targets (83% ± 12.0%; P < 0.05, n = 2). With one exception, the specific lysis of infected targets by CD3- and CD2-depleted effectors was not significantly different from uninfected controls.
FIG. 3.
Depletion of CD2+ and CD3+ effectors resulted in decreased lysis of infected targets. PBMC from two horses were stimulated for 5 days ex vivo with live virulent R. equi and then rested for 48 h. Stimulated PBMC were then depleted of (A) CD2+ cells or (B) CD3+ cells and used as effectors. Autologous or ELA-A mismatched PBACs were infected for a total of 12 h with virulent R. equi 33701 and exposed to effectors for 4.5 h (E:T ratio of 10:1). Uninfected PBAC targets served as negative controls. Representative data are shown from H68. Similar results were obtained for H71. a, depletion results in a significant decrease in killing (>3 standard errors relative to the infected PBMC control). b, following depletion, lysis of infected targets was significantly different from the lysis of the uninfected controls in only one horse.
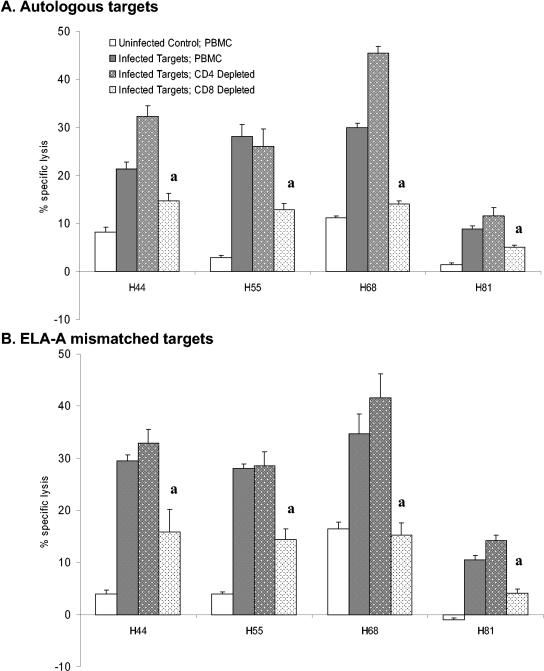
To further define the effector cell type, CD4+ or CD8+ cells were depleted to <1% CD4+ cells or <1% CD8+ cells. As shown in Fig. 4A, the percent specific lysis decreased significantly upon depletion of the CD8+ T lymphocytes (P < 0.05, killing decreased by 58% ± 20.7%, n = 8). In contrast, depletion of the CD4+ T lymphocytes resulted in increased lysis or no significant change in the percent specific lysis (18% ± 22.2% increased lysis compared to undepleted effectors, n = 8). These results are consistent with CD8+ T-lymphocyte-mediated lysis accounting for the majority of the lysis. Similarly, ELA-A mismatched infected targets were lysed by CD8+ T lymphocytes, as depletion had the same effect (Fig. 4B).
FIG. 4.
Depletion of CD8+ CTL decreases lysis of infected targets. PBMC from four horses were stimulated for 5 days with live virulent R. equi and then rested for 48 h. Stimulated PBMC were then depleted of CD4+ cells or CD8+ cells and used as effectors. (A) Autologous or (B) ELA-A mismatched PBACs infected with virulent R. equi 33701 (E:T ratio of 5:1). Uninfected PBAC targets served as negative controls. a, following CD8+ T-lymphocyte depletion of effectors, lysis significantly decreased (>3 standard errors) relative to the infected PBMC (undepleted) control.
Lysis of infected PBACs is not dependent on the presence of the virulence-associated plasmid.
Autologous and mismatched PBACs labeled with 51Cr were infected for 12 h with either virulent R. equi 33701 or an avirulent plasmid-cured derivative. The percentage of PBACs infected with 5 MOI of the plasmid-cured strain (71 to 89%) was not significantly different from the percentage infected with the virulent strain (78 to 79%). Infected targets were then exposed to effectors stimulated with virulent live bacteria. The lysis of autologous targets infected with either strain was not significantly different (Fig. 5A). Target cells infected with the avirulent, plasmid-cured strain were lysed to the same degree and in a similar ELA-A-unrestricted manner (Fig. 5B) compared to target cells infected with the virulent strain. Alternatively, effector cells were stimulated with either the avirulent or the virulent strain for 5 days, rested for 2 days, and then exposed to labeled targets infected with the virulent strain of the bacterium. Cells stimulated with either strain lysed infected targets to an equivalent degree (Fig. 6). Additionally, the percentage of CD8+ T lymphocytes in the effector cell population increased from 9% ± 2.0% to 38% ± 4.3% when stimulated with the virulent strain. With avirulent, plasmid-cured strain stimulation, the percentage of CD8+ T lymphocytes increased from 9% ± 2.0% to a similar 34% ± 0.03% (P = 0.45).
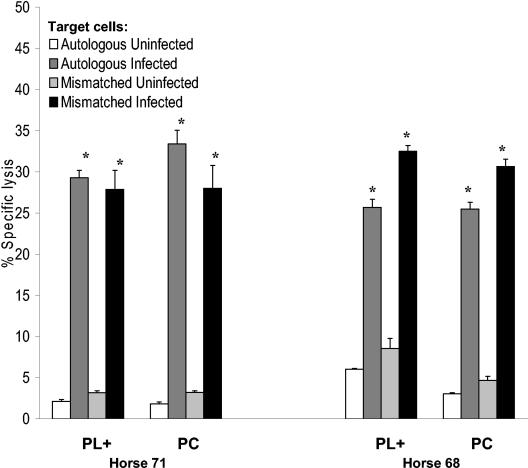
FIG. 5.
Equivalent lysis of targets infected with either the plasmid-cured (avirulent) or the plasmid-bearing (virulent) strain. PBMC effectors were stimulated in vitro for 5 days with live virulent R. equi. (A) Autologous and (B) ELA-A mismatched targets were infected with either virulent or avirulent (PC) R. equi. Uninfected PBACs served as controls. Representative data from one animal (H44) are presented in the figure. Similar results were obtained from H81. ELA-A-unrestricted lysis of PBAC targets infected with either plasmid-bearing R. equi 33701 or the PC derivative were not significantly different. However, in all cases, the lysis of infected targets was significantly different from that of uninfected targets at the same E:T ratio.
FIG. 6.
Equivalent lysis of infected targets was mediated by lymphocytes stimulated with either the plasmid-bearing (virulent) or the plasmid-cured (avirulent) strain. PBMC effectors were stimulated with either the PL+ or the PC strain of R. equi. Representative data from horses H71 and H68 at an E:T ratio of 9:1 are presented in the figure. ELA-A-unrestricted lysis of PBAC targets infected with the plasmid-bearing strain (virulent) of R. equi 33701 was equivalent for either stimulated lymphocyte population. The asterisks indicate significant killing compared to the corresponding uninfected control.
DISCUSSION
Rhodococcal pneumonia is an important disease of horses that has been estimated to account for up to 3% of all foal deaths (43). However, even on farms with endemic infection where natural exposure to virulent R. equi is high and a large percentage of foals develop pneumonia, adult horses do not manifest with disease. Previous research provides strong evidence that the resistance of adult horses to R. equi challenge reflects acquired immunity and their ability to mount effective recall responses (16, 17). Since foals are born into an environment containing R. equi, the goal of an immunization strategy will likely be to direct the immune response to this protective phenotype, rather than to induce sterile immunity. An improved understanding of the protective immune responses in adult horses is, therefore, central to efforts to prevent equine rhodococcal pneumonia by immunization.
Previous work, especially using mouse models, indicates that rhodococcal pneumonia is an immunopathologic disease. Animals that respond to infection with a Th2 response develop characteristic pulmonary lesions, whereas those that develop Th1 responses clear a virulent challenge (23). As with M. tuberculosis, a closely related pulmonary pathogen, the activation of macrophages via CD4+ T lymphocytes and IFN-γ production appears to be critical to immune clearance (22, 23, 36). These data, which are supported by work on horses, strengthen the hypothesis that directing the immune response of naive foals to the immune response seen in mature horses will be protective.
It is likely, however, that immune control of R. equi does not depend exclusively on CD4+ Th1 lymphocyte responses. A growing body of evidence suggests that CD8+ T lymphocytes also contribute to the protective immune response (16, 17, 34). For M. tuberculosis, CD8+ T lymphocytes that exhibit cytotoxic ability have been identified in mice, guinea pigs, and humans. Moreover, in humans the ex vivo cell-mediated lysis of M. tuberculosis-infected cells has been inversely correlated with the severity of tuberculosis (9). For example, PBMC from humans who had successfully contained M. tuberculosis infection in vivo demonstrated antigen-specific, HLA class I-restricted cytotoxicity to target cells pulsed with ESAT-6, a secreted mycobacterial antigen that has shown promise as an immunogen (37). T-cell lines from these individuals were then used to map peptide epitopes and enumerate ESAT-6-specific CD8+ T cells in blood.
The results reported here provide the first evidence for CTL directed against R. equi-infected targets. CTL were readily amplified in PBMC from immune adult horses by ex vivo stimulation with either live R. equi or a soluble extract of the bacterium. PBMC incubated without antigen stimulation (medium alone) did not produce significant specific lysis of R. equi-infected targets. Following antigenic stimulation, the percentage of CD8+ T lymphocytes in culture also increased significantly. This correlates well with the in vivo expansion of intrapulmonary CD8+ T lymphocytes that occurs in immune adult horses as part of a protective recall response to virulent R. equi (16, 17). The observation that stimulation with live bacteria amplified CTL from PBMC more effectively than stimulation with soluble antigen could reflect a difference in antigen presentation by infected versus antigen-pulsed APC. There could also be differences in the nature and/or amount of the antigen(s) available for presentation. Specifically, live R. equi may carry or actively produce antigen that may not be represented or that constitutes a small fraction of the soluble extract. A more exhaustive study of R. equi antigen capable of stimulating CTL, including nonprotein antigen (see below), is clearly warranted.
Importantly, the lysis observed using effector cells from immune horses was not restricted by classical MHC class I molecules. ELA-A-unrestricted killing, especially when considered across a variety of haplotypes and in conjunction with what is known about linkage to equine MHC class II alleles (12, 32), strongly suggests a novel or unconventional method of antigen presentation and/or recognition. One potential explanation for the unrestricted cytotoxicity was NK cell activity. In order to address natural cytotoxicity and further characterize the observed killing, effector cells were depleted of T lymphocytes or lymphocyte subsets prior to use in the CTL assay. In horses, like most species, T lymphocytes are CD2+ CD3+, whereas NK cells are CD2+ CD3− (49). More specific NK cell markers are not currently available. Depletion of CD2+ cells decreased specific lysis to background levels. However, depletion of CD3+ cells had the same effect, indicating that the primary effectors in this system are T lymphocytes or possibly NKT cells, rather than classical NK cells (13).
CD8+ T lymphocytes accounted for the majority of the cytotoxicity against R. equi-infected targets, as their removal from the effector cell population significantly decreased killing. However, although depletion resulted in a population of effector cells in which <1.0% expressed CD8, in most experiments it did not decrease killing to background levels, as was observed when CD2+ or CD3+ cells were removed. This suggests that horses also had a population of CD8-negative T lymphocytes, which contributed to the specific lysis of R. equi-infected targets. By comparison, depletion of CD4+ T lymphocytes resulted in no change in lysis or an increase in the percent specific lysis. The enhanced killing was likely a result of increasing the absolute number of effectors in the remaining cells, effectively increasing the E:T ratio. There was no evidence for ELA-A restriction when using fractioned PBMC as effectors. Depletion of CD8+ T lymphocytes decreased killing of both autologous and ELA-A mismatched targets to similar degrees. Likewise, depletion of CD4+ effector cells had no effect on ELA-A restriction, as remaining cells killed both autologous and mismatched R. equi-infected targets.
An important consideration for the ELA-A-unrestricted lysis of R. equi-infected target cells observed in these experiments is CD1-restricted cytotoxicity mediated by CD8+ and/or CD8− CD4− (double-negative) T lymphocytes. CD1 molecules are a minimally polymorphic family of MHC class I-like glycoproteins expressed on the surface of mammalian APC in association with β2-microglobulin (2, 5). Intracellularly, CD1 molecules are found in endocytic subcompartments where lipid or lipid-modified antigens derived from phagocytosed bacteria are known to traffic. In this manner, CD1 molecules appear to be well situated to monitor and sample phagosome-bound, intracellular bacteria. Recent research has shown that a significant portion of the T lymphocytes that recognize and lyse M. tuberculosis-infected cells utilize αβ T-cell receptors, or in some cases γδ receptors, to recognize lipids, glycolipids, or lipopeptides presented in the context of CD1 molecules (10, 20). A variety of nonpeptide mycobacterial antigens (especially from the cell wall) have been shown to be presented by the CD1 system, including mycolic acid and lipoarabinomannan (3). Importantly, these antigens are uniquely expressed by potential pathogens and not present in or on host cells.
To further investigate antigen presentation in our system, the initial focus was on the 80.6-kb R. equi virulence plasmid. Plasmid-bearing strains of R. equi have been shown to replicate within macrophages, whereas non-plasmid-bearing or plasmid-cured strains can survive for up to 48 h in macrophages but do not replicate and are ultimately destroyed (19). The plasmid is also required for pathogenicity in the natural host. R. equi isolates from the lungs of foals with rhodococcal pneumonia invariably carry the virulence plasmid (15). Moreover, foals challenged with PC strains rapidly clear the organism without developing clinical signs or lesions. In addition, avirulent PC strains do not induce IFN-γ responses in the lungs of immune horses, nor do they induce protective immune responses in naive, immunocompetent mice (17, 45). Even SCID mice, which lack both T lymphocytes and B lymphocytes and are unable to clear virulent strains of R. equi, effectively clear a pulmonary challenge with PC strains, apparently via innate immune responses (30). Based on the effective clearance both in vitro and in vivo (even in immunodeficient mice) and on the failure of PC strains to induce either primary immune responses in mice or memory responses in horses, we postulated that target cells infected with PC R. equi would not effectively present antigen to CTL. In contrast to our expectations, PBACs infected with PC R. equi were lysed at an equivalent level and in an identical ELA-A-unrestricted manner when compared to infection with the virulent strain. Additionally, PBMC stimulated with either the virulent or the avirulent strain lysed virulent strain-infected targets to the same degree. These results demonstrate that the virulence plasmid is not required for presentation of the antigens recognized by CTL in this system. Moreover, the antigens recognized by CTL are not encoded by the plasmid. Although there are several possible explanations for these observations, recognition of infected targets via CD1-restricted presentation of unique lipid or lipid-modified antigens is an important consideration. Notably, the nonpeptide, pathogen-specific antigens which constitute the R. equi cell wall (14, 42) should be present in both plasmid-bearing and PC strains and available in endocytic compartments to bind CD1 molecules (5).
Cytotoxic T lymphocytes have been shown to play an important role in the protective immune responses to actinomycete infection, specifically to human and murine tuberculosis. However, CTL have not been previously evaluated with reference to R. equi or other bacterial infections in the horse. The data presented here suggest that in the natural host, CD8+ T lymphocytes, and possibly double-negative CD8− CD4− T lymphocytes, curtail the pathogen load via lysis of infected cells. These cells may be directly involved in immune control of R. equi. Interestingly, the lack of classical MHC class I restriction suggests a novel form of antigen presentation and possibly the presentation of novel antigens, such as lipids. Future work will be directed towards understanding the nature of the antigens recognized and the method of antigen presentation, including assessment of the role of CD1 molecules. An interesting question will be whether relative deficiencies in this method of antigen presentation and in the development of R. equi-specific CTL in foals contribute to their unique susceptibility to rhodococcal pneumonia. If so, an immunization strategy that stimulates these responses early in life may be protective.
Acknowledgments
This work was supported by grant 5 K08 A1049391-03 from the National Institute of Allergy and Infectious Diseases and grant D0IEQ-42 from the Morris Animal Foundation.
We thank Melissa T. Hines and Wendy C. Brown and are grateful to Deb Alperin, Linda Norton, Ashley Alger, and Zack Joseph for their excellent technical assistance.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Alvarez, B., C. Sanchez, R. Bullido, A. Marina, J. Lunney, F. Alonso, A. Ezquerra, and J. Dominguez. 2000. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens 55:342-351. [DOI] [PubMed] [Google Scholar]
- 2.Batuwangala, T., D. Shepherd, S. D. Gadola, K. J. Gibson, N. R. Zaccai, A. R. Fersht, G. S. Besra, V. Cerundolo, and E. Y. Jones. 2004. The crystal structure of human CD1b with a bound bacterial glycolipid. J. Immunol. 172:2382-2388. [DOI] [PubMed] [Google Scholar]
- 3.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard-Channell, M., P. F. Moore, and J. L. Stott. 1994. Characterization of monoclonal antibodies specific for equine homologues of CD3 and CD5. Immunology 82:548-554. [PMC free article] [PubMed] [Google Scholar]
- 5.Brigl, M., and M. B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817-890. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, B. A., J. F. Prescott, G. H. Palmer, S. Takai, V. M. Nicholson, D. C. Alperin, and S. A. Hines. 2001. Virulence plasmid of Rhodococcus equi contains inducible gene family encoding secreted proteins. Infect. Immun. 69:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capdevila, J. A., S. Bujan, J. Gavalda, A. Ferrer, and A. Pahissa. 1997. Rhodococcus equi pneumonia in patients infected with the human immunodeficiency virus. Report of 2 cases and review of the literature. Scand. J. Infect. Dis. 29:535-541. [DOI] [PubMed] [Google Scholar]
- 8.Davis, W. C., J. E. Davis, and M. Hamilton. 1995. Use of monoclonal antibodies and flow cytometry to cluster and analyse leukocyte differentiation molecules, p. 149. In W. C. Davis (ed.), Monoclonal antibody protocols. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 9.De La Barrera, S. S., M. Finiasz, A. Frias, M. Aleman, P. Barrionuevo, S. Fink, M. C. Franco, E. Abbate, and M. D. C. Sasiain. 2003. Specific lytic activity against mycobacterial antigens is inversely correlated with the severity of tuberculosis. Clin. Exp. Immunol. 132:450-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Libero, G., A. Donda, L. Mori, V. Manolova, and A. Shamshiev. 2001. Immunity to glycolipid antigens in microbial infections. J. Biol. Regul. Homeost. Agents 15:249-256. [PubMed] [Google Scholar]
- 11.De Libero, G., I. Flesch, and S. H. Kaufmann. 1988. Mycobacteria-reactive Lyt-2+ T cell lines. Eur. J. Immunol. 18:59-66. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, D. G., and E. Bailey. 1996. Demonstration of three DRB loci in a domestic horse family. Immunogenetics 44:441-445. [DOI] [PubMed] [Google Scholar]
- 13.Gansert, J. L., V. Kiessler, M. Engele, F. Wittke, M. Rollinghoff, A. M. Krensky, S. A. Porcelli, R. L. Modlin, and S. Stenger. 2003. Human NKT cells express granulysin and exhibit antimycobacterial activity. J. Immunol. 170:3154-3161. [DOI] [PubMed] [Google Scholar]
- 14.Garton, N. J., M. Gilleron, T. Brando, H. H. Dan, S. Giguere, G. Puzo, J. F. Prescott, and I. C. Sutcliffe. 2002. A novel lipoarabinomannan from the equine pathogen Rhodococcus equi. Structure and effect on macrophage cytokine production. J. Biol. Chem. 277:31722-31733. [DOI] [PubMed] [Google Scholar]
- 15.Giguere, S., M. K. Hondalus, J. A. Yager, P. Darrah, D. M. Mosser, and J. F. Prescott. 1999. Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi. Infect. Immun. 67:3548-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hines, M. T., K. M. Paasch, D. C. Alperin, G. H. Palmer, N. C. Westhoff, and S. A. Hines. 2001. Immunity to Rhodococcus equi: antigen-specific recall responses in the lungs of adult horses. Vet. Immunol. Immunopathol. 79:101-114. [DOI] [PubMed] [Google Scholar]
- 17.Hines, S. A., D. M. Stone, M. T. Hines, D. C. Alperin, D. P. Knowles, L. K. Norton, M. J. Hamilton, W. C. Davis, and T. C. McGuire. 2003. Clearance of virulent but not avirulent Rhodococcus equi from the lungs of adult horses is associated with intracytoplasmic gamma interferon production by CD4+ and CD8+ T lymphocytes. Clin. Diagn. Lab. Immunol. 10:208-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh, D. C., and D. C. Zee. 1999. Veterinary microbiology. Blackwell Science, Inc., Malden, Mass.
- 19.Hondalus, M. K., and D. M. Mosser. 1994. Survival and replication of Rhodococcus equi in macrophages. Infect. Immun. 62:4167-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jullien, D., S. Stenger, W. A. Ernst, and R. L. Modlin. 1997. CD1 presentation of microbial nonpeptide antigens to T cells. J. Clin. Investig. 99:2071-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1995. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect. Immun. 63:3037-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1993. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect. Immun. 61:4929-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1996. Transfer of a CD4+ Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lung. Infect. Immun. 64:1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasuga-Aoki, H., S. Takai, Y. Sasaki, S. Tsubaki, H. Madarame, and A. Nakane. 1999. Tumour necrosis factor and interferon-gamma are required in host resistance against virulent Rhodococcus equi infection in mice: cytokine production depends on the virulence levels of R. equi. Immunology 96:122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler, A. K., D. M. Stone, M. T. Hines, B. A. Byrne, D. C. Alperin, L. K. Norton, and S. A. Hines. 2003. Rhodococcus equi secreted antigens are immunogenic and stimulate a type 1 recall response in the lungs of horses immune to R. equi infection. Infect. Immun. 71:6329-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarevic, V., and J. Flynn. 2002. CD8+ T cells in tuberculosis. Am. J. Respir. Crit. Care Med. 166:1116-1121. [DOI] [PubMed] [Google Scholar]
- 27.Lopez, A. M., M. T. Hines, G. H. Palmer, D. C. Alperin, and S. A. Hines. 2002. Identification of pulmonary T-lymphocyte and serum antibody isotype responses associated with protection against Rhodococcus equi. Clin. Diagn. Lab. Immunol. 9:1270-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunn, D. P., M. A. Holmes, D. F. Antczak, N. Agerwal, J. Baker, S. Bendali-Ahcene, M. Blanchard-Channell, K. M. Byrne, K. Cannizzo, W. Davis, M. J. Hamilton, D. Hannant, T. Kondo, J. H. Kydd, M. C. Monier, P. F. Moore, T. O'Neil, B. R. Schram, A. Sheoran, J. L. Stott, T. Sugiura, and K. E. Vagnoni. 1998. Report of the Second Equine Leucocyte Antigen Workshop, Squaw Valley, California, July 1995. Vet. Immunol. Immunopathol. 62:101-143. [DOI] [PubMed] [Google Scholar]
- 29.Macias, J., J. A. Pineda, F. Borderas, J. A. Gallardo, J. Delgado, M. Leal, A. Sanchez-Quijano, and E. Lissen. 1997. Rhodococcus or mycobacterium? An example of misdiagnosis in HIV infection. AIDS 11:253-254. [PubMed] [Google Scholar]
- 30.Madarame, H., S. Takai, C. Matsumoto, K. Minamiyama, Y. Sasaki, S. Tsubaki, Y. Hasegawa, and A. Nakane. 1997. Virulent and avirulent Rhodococcus equi infection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol. Med. Microbiol. 17:251-262. [DOI] [PubMed] [Google Scholar]
- 31.McGuire, T. C., D. G. Fraser, and R. H. Mealey. 2002. Cytotoxic T lymphocytes and neutralizing antibody in the control of equine infectious anemia virus. Viral Immunol. 15:521-531. [DOI] [PubMed] [Google Scholar]
- 32.McGuire, T. C., S. R. Leib, R. H. Mealey, D. G. Fraser, and D. J. Prieur. 2003. Presentation and binding affinity of equine infectious anemia virus CTL envelope and matrix protein epitopes by an expressed equine classical MHC class I molecule. J. Immunol. 171:1984-1993. [DOI] [PubMed] [Google Scholar]
- 33.McGuire, T. C., D. B. Tumas, K. M. Byrne, M. T. Hines, S. R. Leib, A. L. Brassfield, K. I. O'Rourke, and L. E. Perryman. 1994. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J. Virol. 68:1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordmann, P., E. Ronco, and C. Nauciel. 1992. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect. Immun. 60:2748-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottenhoff, T. H., and T. Mutis. 1995. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur. J. Clin. Investig. 25:371-377. [DOI] [PubMed] [Google Scholar]
- 36.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 37.Pathan, A. A., K. A. Wilkinson, R. J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A. V. Hill, and A. Lalvani. 2000. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur. J. Immunol. 30:2713-2721. [DOI] [PubMed] [Google Scholar]
- 38.Raabe, M. R., C. J. Issel, and R. C. Montelaro. 1998. Equine monocyte-derived macrophage cultures and their applications for infectivity and neutralization studies of equine infectious anemia virus. J. Virol. Methods 71:87-104. [DOI] [PubMed] [Google Scholar]
- 39.Ross, T. L., G. A. Balson, J. S. Miners, G. D. Smith, P. E. Shewen, J. F. Prescott, and J. A. Yager. 1996. Role of CD4+, CD8+ and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with Rhodococcus equi. Can. J. Vet. Res. 60:186-192. [PMC free article] [PubMed] [Google Scholar]
- 40.Serbina, N. V., and J. L. Flynn. 2001. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinkai, K., and R. M. Locksley. 2000. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J. Exp. Med. 191:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutcliffe, I. C. 1997. Macroamphiphilic cell envelope components of Rhodococcus equi and closely related bacteria. Vet. Microbiol. 56:287-299. [DOI] [PubMed] [Google Scholar]
- 43.Takai, S. 1997. Epidemiology of Rhodococcus equi infections: a review. Vet. Microbiol. 56:167-176. [DOI] [PubMed] [Google Scholar]
- 44.Takai, S., S. A. Hines, T. Sekizaki, V. M. Nicholson, D. A. Alperin, M. Osaki, D. Takamatsu, M. Nakamura, K. Suzuki, N. Ogino, T. Kakuda, H. Dan, and J. F. Prescott. 2000. DNA sequence and comparison of virulence plasmids from Rhodococcus equi ATCC 33701 and 103. Infect. Immun. 68:6840-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takai, S., C. Kobayashi, K. Murakami, Y. Sasaki, and S. Tsubaki. 1999. Live virulent Rhodococcus equi, rather than killed or avirulent, elicits protective immunity to R. equi infection in mice. FEMS Immunol. Med. Microbiol. 24:1-9. [DOI] [PubMed] [Google Scholar]
- 46.Terasaki, P., D. Bernoco, M. Park, G. Ozturk, and Y. Iwaki. 1978. Microdroplet testing for HLA-A, -B, -C and -D antigens. The Philip Levine Award Lecture. Am. J. Clin. Pathol. 69:103-120. [DOI] [PubMed] [Google Scholar]
- 47.Tumas, D. B., A. L. Brassfield, A. S. Travenor, M. T. Hines, W. C. Davis, and T. C. McGuire. 1994. Monoclonal antibodies to the equine CD2 T lymphocyte marker, to a pan-granulocyte/monocyte marker and to a unique pan-B lymphocyte marker. Immunobiology 192:48-64. [DOI] [PubMed] [Google Scholar]
- 48.Ulrichs, T., D. B. Moody, E. Grant, S. H. Kaufmann, and S. A. Porcelli. 2003. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect. Immun. 71:3076-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viveiros, M. M., and D. F. Antczak. 1999. Characterization of equine natural killer and IL-2 stimulated lymphokine activated killer cell populations. Dev. Comp. Immunol. 23:521-532. [DOI] [PubMed] [Google Scholar]
- 50.Vordermeier, H. M., X. Zhu, and D. P. Harris. 1997. Induction of CD8+ CTL recognizing mycobacterial peptides. Scand. J. Immunol. 45:521-526. [DOI] [PubMed] [Google Scholar]