Abstract
In this work, we describe synthesis of conjugates of betulinic acid with substituted triazoles prepared via Huisgen 1,3-cycloaddition. All compounds contain free 28-COOH group. Allylic bromination of protected betulinic acid by NBS gave corresponding 30-bromoderivatives, their substitution with sodium azides produced 30-azidoderivatives and these azides were subjected to CuI catalysed Huisgen 1,3-cycloaddition to give the final conjugates. Reactions had moderate to high yields. All new compounds were tested for their in vitro cytotoxic activities on eight cancer and two non-cancer cell lines. The most active compounds were conjugates of 3β-O-acetylbetulinic acid and among them, conjugate with triazole substituted by benzaldehyde 9b was the best with IC50 of 3.3 μM and therapeutic index of 9.1. Five compounds in this study had IC50 below 10 μM and inhibited DNA and RNA synthesis and caused block in G0/G1 cell cycle phase which is highly similar to actinomycin D. It is unusual that here prepared 3β-O-acetates were more active than compounds with the free 3-OH group and this suggests that this set may have common mechanism of action that is different from the mechanism of action of previously known 3β-O-acetoxybetulinic acid derivatives. Benzaldehyde type conjugate 9b is the best candidate for further drug development.
Introduction
Triterpenes are natural compounds that may be found in almost all living organisms and they are particularly prevalent in plants [1]. These compounds are not part of the main metabolic pathways, they are secondary metabolites. Interestingly, they have a variety of biological activities, which may be the reason why organisms produce them. Among the activities, we may find antitumor, antibacterial, anticariogenic, antiparasitic, antifungal and many others [2–11]. In our research group we are developing new derivatives of betulinic acid (1) in order to find compounds with higher cytotoxicity and better pharmacological properties than the parent compound. One of the possibilities explored was annealing of a heterocycle to the main terpenic skeleton, which resulted in a small library of about fifty new compounds [12–15]. Among them, four derivatives had IC50 in low micromolar range and currently belong to our most promising compounds in in vivo tests. All four active heterocycles are derivatives of betulinic acid (1). Recently, a number of new triterpenoid heterocycles were prepared and a number of them had high cytotoxic activity [16–20]. To improve pharmacological properties of triterpenes, especially their solubility in water, various modifications of triterpenes were done and some of them were successful, especially compounds with another (polar) molecule connected to them. Examples include esters with sugars, glycosides, esters with dicarboxylic acids, conjugates with polyethylene glycol, ammonium salts etc [21–29].
Recently, a number of new articles were published on connecting a terpene with another molecule of interest via Huisgen 1,3-cycloaddition reaction. The first approach used terpenes substituted with alkynes in the position 3 [30–34], second approach used propargylesters or amides prepared at 28-COOH group [35–45], and the third approach used 30-azidoderivatives prepared via 30-bromoderivatives [46;47]. Rarely, also position 2 is modified [48] or two position at once (3 and 30) in [49].
In this work, we decided to explore the third option and to connect betulinic acid (1) to other molecules of interest. Introduction of a rather polar triazole ring capable of forming hydrogen bonds was expected to improve solubility of the target molecules in water based media and bioavailability [50;51]. Possibly, the triazole ring may also become a part of the pharmacophore. On the other hand, introduction of a completely new moiety on the other side of the triazole ring (another aromatic rings—both heterocyclic and carbocyclic, aldehydes, amines etc.) could change the biological properties such as cytotoxicity or selectivity and the new compounds could act by different mechanism of action than the parent betulinic acid (1). Among the derivatives prepared by other research groups there are only few examples [46;47] containing both free 28-carboxylic group and free 3-hydroxy group. In most cases, cycloaddition reactions were done with acid 1 protected as methyl ester or as acetate and the final molecules were also tested with the protective group on. There are many examples in the literature [21;52;53] that betulinic acid (1) derivatives are highly cytotoxic when unprotected while methylesters and acetates are usually inactive. Therefore, the main aim of this work was to explore unprotected derivatives of betulinic acid (1) modified by cycloaddition reactions in the position 30 and to explore their cytotoxic activity and influence on cancer cells.
Results and discussion
Chemistry
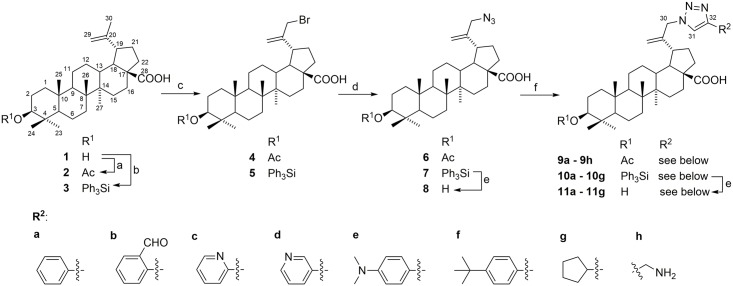
Bromination of betulinic acid (1) derivatives at the allylic position (C-30) is described in the literature [54–57] that mostly used NBS as the bromination agent, AIBN as a radical source and CCl4 as an inert solvent. In this work, we found that the method afforded only low yields when free acid 1 was used. All reagents have limited solubility in CCl4 and dibromoderivative starts forming before the full conversion of the starting betulinic acid (1) to 30-bromobetulinic acid. To increase the solubility of acid 1, we choose to protect it at the position 3 as acetate or triphenylsilylether. We decided to leave the 28-COOH group unprotected, since this neopentyl-type ester requires harsh conditions for its deprotection and the presence of free carboxylic group should not interfere with the following reactions. For protection of 3β-OH group, acetate was chosen as a stable protective group that would be cleavable in basic conditions, triphenylsilyl group was chosen as more labile protective group easily removable in acidic conditions or by the fluorine anion. This should allow for almost unlimited variability of the new substituents. Both acetate 2 and triphenylsilyl derivative 3 were synthesized by standard procedures. Bromination of 2 and 3 afforded good yields of pure bromoderivatives 4 and 5. The reaction of bromoderivatives 4 and 5 with sodium azide gave corresponding 30-azidoderivatives 7, 8, and to obtain the unprotected azide 6, silylether 8 was deprotected by TBAF in THF; Fig 1.
Fig 1. The preparation of all derivatives.
Reagents and conditions: (a) Ac2O, pyridine, r.t. 16 h; (b) Ph3SiCl, DMF, imidazole, r.t. 36 h; (c) NBS, AIBN, CCl4, 75°C for 1 h then 50°C for 3 h; (d) NaN3, DMSO, r.t., 36 h; (e) TBAF, THF, r.t., 18–32 h, or HCl, CH2Cl2, r.t., 5–11 h; (f) azides 6, 7 or 8, CuSO4·5H2O, sodium L-ascorbate, alkynes.
Final derivatives containing acetate or triphenylsilylether at the position 3 (compounds 9a–9h, 10a–10g) were prepared by Huisgen 1,3-cycloaddition from azides 6 and 7. Depending on the reactivity, one or two equivalents of alkyne was used and the reaction was performed either at r.t. or at 50°C. Yields were moderate to high with the only exception—reaction of each azide 6–8 with propargylamine that gave 10% yield of acetate 9h but no sililated nor free product was obtained. Reaction with FMOC or BOC protected propargylamine also did not yield the desired product. In all cases, a mixture of polar unseparable compounds was obtained and we were unable to isolate the desired product. The cycloaddion was catalyzed by CuI species which ensured that only the proposed 1,4-isomer formed. Traces of Cu were removed by treatment of the crude products with H2S before chromatography but the amount of remaining metal was not determined. This will be performed if any compound of this set will enter further biological tests. Free derivatives 11a–11g were obtained by the deprotection of their silylated analogues using TBAF in THF (all 11a–11g were prepared this way) with reasonable yields 58–81%. To find out if the compounds are stable during acidic deprotection procedure, derivatives 10a, 10c, 10d, and 10f were also deprotected by HCl in CHCl3 with comparable yields of 62–76%. Attempts to prepare compounds 11c and 11d directly from the unprotected azide 8 were also successful with average yields of 75%. We may conclude, that all three ways to the unprotected derivatives 11a–11g give similar results.
Biological assay
Cytotoxicity
Cytotoxic activity of all synthesized compounds was investigated in vitro against eight human cancer cell lines and two non-tumor fibroblasts using the standard MTS test (Table 1). The cancer cell lines were derived from T-lymphoblastic leukemia CCRF-CEM, leukemia K562 and their multiresistant counterparts expressing P-glycoprotein, MRP1 and LRP proteins (CEM-DNR, K562-TAX) [58], solid tumors including lung (A549) and colon (HCT116, HCT116p53-/-) carcinomas, osteosarcoma cell line (U2OS), and for comparison, tests were performed on two human non-cancer fibroblast cell lines (BJ, MRC-5).
Table 1. Cytotoxic activities of prepared derivatives on eight tumor (including resistant) and two normal fibroblast cell lines.
All other compounds prepared in this work were also tested but their activities on these 10 cell lines were higher than 50 μM which is considered inactive.
| Comp. | IC50 (μM/L)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCRF-CEM | CEM-DNR | K562 | K562- TAX | A549 | HCT116 | HCT116p53-/- | U2OS | BJ | MRC-5 | TIb | |
| 1 | 45.5 | 45.4 | 40.0 | 43.1 | 43.4 | 38.0 | >50.0 | >50.0 | 37.6 | 32.9 | 0.7 |
| 4 | 5.7 | 15.3 | 21.7 | 15.8 | 13.2 | 15.9 | 14.1 | 21.2 | 31.7 | 24.2 | 4.9 |
| 5 | >50.0 | 13.2 | >50.0 | 12.2 | >50.0 | 35.6 | 31.1 | 27.9 | >50.0 | >50.0 | - |
| 6 | 7.4 | 19.2 | 12.3 | 15.5 | 17.6 | 18.6 | 19.7 | 20.9 | 26.7 | 20.0 | 3.2 |
| 8 | 21.7 | 25.8 | 17.2 | 23.9 | 23.0 | 25.5 | 24.0 | 25.0 | 34.0 | 19.1 | 1.2 |
| 9a | 13.4 | 19.5 | 15.1 | 19.1 | 23.4 | 25.1 | 31.1 | 24.8 | >50.0 | 32.4 | >3.1 |
| 9b | 3.3 | 4.0 | 3.6 | 3.9 | 14.8 | 6.4 | 9.5 | 12.8 | 31.3 | 28.9 | 9.1 |
| 9c | 9.0 | 14.4 | 22.1 | 13.8 | 13.2 | 30.3 | 13.7 | 16.0 | 29.5 | 27.6 | 3.2 |
| 9d | 14.9 | 12.0 | 13.3 | 11.2 | 11.3 | 19.2 | 18.7 | 17.3 | 29.9 | 28.2 | 1.9 |
| 9e | 19.4 | 29.6 | 35.4 | 30.0 | 26.6 | 32.9 | 33.1 | 31.0 | 35.1 | 30.3 | 1.6 |
| 9f | 34.5 | 15.0 | 15.6 | 23.4 | 45.0 | 41.9 | 48.4 | 45.8 | >50.0 | >50.0 | >1.4 |
| 9g | 26.2 | 30.8 | 43.6 | 29.1 | >50.0 | 38.3 | 47.6 | 44.1 | >50.0 | >50.0 | >1.9 |
| 9h | 20.2 | 28.3 | >50.0 | >50.0 | 30.5 | 32.0 | >50.0 | 30.6 | >50.0 | >50.0 | >2.5 |
| 11a | 16.6 | 23.8 | >50.0 | 23.3 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >3.0 |
| 11b | 8.5 | 11.5 | >50.0 | 13.7 | >50.0 | 26.3 | 26.9 | >50.0 | >50.0 | >50.0 | >5.9 |
| 11c | 14.4 | 8.5 | >50.0 | 7.2 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >3.5 |
| 11f | >50.0 | 13.7 | >50.0 | 16.0 | >50.0 | 36.7 | >50.0 | >50.0 | >50.0 | >50.0 | - |
| 11g | 16.7 | 38.0 | >50.0 | 23.3 | >50.0 | 33.5 | >50.0 | >50.0 | >50.0 | >50.0 | >3.0 |
aThe lowest concentration that kills 50% of cells. The standard deviation in cytotoxicity assays is typically up to 15% of the average value. Compounds with IC50 > 50 μM are considered inactive.
bTherapeutic index is calculated for IC50 of CCRF-CEM line vs average of both fibroblasts.
All derivatives prepared within this study have free 28-COOH group that was expected to be essential for retaining of the biological activity. Cytotoxicity of the selected starting compounds and also compounds modified at C-30 by Huisgen 1,3-cycloadditions are in Table 1 (acetylated derivatives 9a–9h, silylated analogues 10a–10g, and fully deprotected derivatives 11a–11g that were expected to be the most active).
Among the starting material, bromide 4 and azide 6 had significant activity (IC50 5.7 and 7.4 μM) on multiple cancer cell lines with therapeutic index of 4.9 or 3.2 (calculated for the reference CCRF-CEM line). To our surprise, both compounds 4 and 6 are 3β-O-acetates, which is in contrast to our initial assumptions that acetates should be less active than compounds with the free 3β-hydroxy group. In addition, our results indicate, that acetates 9a–9h are often highly active (with IC50 in low micromolar ranges) on multiple cancer cell lines (parental and mutiresistant) and in most cases, they are more active than their non-acetylated analogues 11a–11g. The most active compound of this study is derivative 9b (IC50 3.3 μM on the reference CCRF-CEM cell line) which belongs among acetates and contains benzaldehyde connected to the position 30 through the triazole ring formed by the cycloaddition. The compound has reasonable therapeutic index 9.1 and seems the most promising derivative of this study. Its non-acetylated derivative is also active on the reference line (IC50 8.5 μM) and we see this trend throughout all of the prepared derivatives, compounds 9a–9g are more active than free compounds 11a–11g with only few exceptions.
In general, it seems that modified C-30 position, conjugated to a large triazole-aromatic substituent became an important part of the pharmacophore and is responsible for the cytotoxicity. In contrast, the functional group at C-3 probably influences the bioavailability of each molecule. Compounds with free both 28-COOH and 3β-OH groups contain two hydrophilic functional group on the opposite sides of their molecules and this may interfere with their permeability through cellular membranes. Small and lipophilic acetate on one side of the molecule can solve this problem.
Cell cycle analysis
We have observed that most cytotoxic derivatives from this study are 3O-acetylated 30-bromo, 30-azido derivatives 4 and 6, 3O-acetylated conjugates 9b and 9c, and one 3-hydroxyderivative 11b. All of them are inhibiting DNA and RNA synthesis. The inhibition of the cell cycle in G0/G1 was observed with highest accumulation after treatment with acetylated derivative 9b. The high percentage of apoptotic cells (sub G1) is observed at 5 × IC50 concentration, pointing on rapid induction of apoptosis (Table 2).
Table 2. Influence of compounds 4, 6, 9b, 9c, 11b on cell cycle, DNA and RNA synthesis at 1a × and 5b × IC50.
| Comp. | Used conc. (μM) | Sub G1 (%) | G0/G1 (%) | S (%) | G2/M (%) | pH3Ser10 (%) | DNA synthesis | RNA synthesis |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 2.2 | 38.4 | 42.4 | 19.3 | 2.1 | 37.5 | 42.1 |
| 4 | 5.7a | 9.4 | 47.3 | 35.9 | 16.8 | 1.34 | 38.6 | 56.3 |
| 4 | 28.5b | 39.7 | 33.7 | 43.0 | 23.2 | 2.35 | 5.80 | 24.8 |
| 6 | 7.4a | 10.7 | 49.3 | 30.4 | 20.3 | 1.80 | 33.0 | 14.1 |
| 6 | 37.0b | 70.0 | 41.4 | 34.8 | 23.8 | 2.68 | 1.00 | 1.70 |
| 9b | 3.3a | 10.8 | 58.6 | 21.9 | 19.5 | 1.19 | 22.6 | 15.8 |
| 9b | 16.5b | 51.9 | 35.7 | 46.2 | 18.1 | 0.37 | 0.55 | 0.08 |
| 9c | 9.0a | 7.2 | 48.9 | 30.9 | 20.3 | 1.47 | 20.3 | 25.4 |
| 9c | 45.0b | 65.6 | 40.5 | 38.5 | 21.0 | 0.89 | 2.90 | 0.40 |
| 11b | 8.5a | 65.6 | 59.0 | 25.3 | 15.7 | 0.88 | 20.0 | 48.3 |
| 11b | 42.5b | 65.6 | 45.5 | 35.4 | 19.1 | 1.19 | 6.40 | 38.1 |
aThe values were obtained at 1 × IC50.
bThe values were obtained at 5 × IC50.
Conclusions
Three sets of betulinic acid derivatives modified at C-30 were prepared by Huisgen 1,3-cycloaddition catalyzed by CuI species. All compounds have free 28-COOH and the first set are 3β-O-acetates 9a–9h, the second set are 3β-silylethers 10a–10g, and the third set are compounds with free 3β-OH group 11a–11g. All compounds were subjected to tests of cytotoxicity on 8 cancer cell lines and 2 non cancer fibroblasts. Several derivatives had IC50 in low micromolar ranges for parental and multiresistant cell lines, the best compound was aldehyde-acetate 9b which also had high therapeutic index and this makes the compound the most promising candidate for future in vivo tests and for studies of mechanism of action. In this work, unusual trend was found between the activities of 3β-O-acetates vs. free compounds. Acetates 9a–9h, were usually more active than free derivatives 11a–11g. This suggests that compounds prepared in this study may have mechanism of action that differs from acetylated betulinic derivatives known from the literature [21] where this trend is opposite. Moreover, the inhibition of DNA and RNA was observed even at 1 × IC50 concentrations together with G1 cell cycle block which is highly similar to actinomycin D behavior [59]. Thus, one may speculate that conjugation of the new triazole ring equipped with carbocyclic (or heterocyclic) ring forms a new type of pharmacophore. To prove it, however, the compound will have to be further transformed into a probe suitable for pull down assays [49] or into a fluorescent probe and more biological tests will have to be done.
Experimental
General experimental procedures
Materials and instruments
Melting points were determined using a Büchi B-545 apparatus and are uncorrected. Optical rotations were measured on an Autopol III (Rudolph Research, Flanders, USA) polarimeter in MeOH at 25°C unless otherwise stated and are in [10−1 deg cm2 g-1]. 1H and 13C NMR spectra were recorded on VarianUNITY Inova 400 (400 MHz for 1H) or VarianUNITY Inova 300 (300 MHz for 1H) or Jeol ECX-500SS (500 MHz for 1H) instruments, using CDCl3, D6-DMSO or CD3OD as solvents (25°C). Chemical shifts were eider referenced to the residual signal of the solvent (CDCl3, D6-DMSO) or to tetramethylsilane added as an internal standard. 13C NMR spectra were eider referenced to CDCl3 (77.00 ppm) or D6-DMSO (39.51 ppm) or to tetramethylsilane added as an internal standard. EI MS spectra were recorded on an INCOS 50 (Finigan MAT) spectrometer at 70 eV and an ion source temperature of 150°C. The samples were introduced from a direct exposure probe at a heating rate of 10 mA/s. Relative abundances stated are related to the most abundant ion in the region of m/z > 180. HRMS analysis was performed using LC-MS an Orbitrap high-resolution mass spectrometer (Dionex Ultimate 3000, Thermo Exactive plus, MA, USA) operating at positive full scan mode in the range of 100–1000 m/z. The settings for electrospray ionization were as follows: oven temperature of 150°C, source voltage of 3,6 kV. The acquired data were internally calibrated with phthalate as a contaminant in methanol (m/z 297.15909). Samples were diluted to a final concentration of 0.1 mg/mL in methanol. The samples were injected to mass spectrometer over autosampler after HPLC separation: precolumn phenomenex 2.6 μm C18. Mobile phase isokrat. CH3CN/IPA/amonium acetate 0.01M 80/10/10, flow 0,3 mL/min. IR spectra were recorded on a Nicolet Avatar 370 FTIR. DRIFT stands for Diffuse Reflectance Infrared Fourier Transform. TLC was carried out on Kieselgel 60 F254 plates (Merck) detected by spraying with 10% aqueous H2SO4 and heating to 150–200°C. Starting triterpenes—betulin (1), dihydrobetulonic acid (2b), and allobetulin (3a) were obtained from company Betulinines (www.betulinines.com). All other chemicals and solvents were obtained from Sigma-Aldrich.
Synthetic procedures
General procedure for Huisgen cycloaddition of triterpenic azides
Each azide was dissolved in DMF (4 mL/100 mg) and sodium L-ascorbate (0.5 equiv.) was added followed by CuSO4·5H2O. The reaction mixture was stirred until its color turned green which is the sign for CuI species being formed (usually 20 min). Then, each alkyne was added (1–2 equiv.) and the reaction mixture was stirred at room temperature (or 50°C) for various time, conditions are specified for each compound. The reaction was monitored using TLC in hexane/EtOAc in ratios 3: 1–1: 2 depending on substrates. After the reaction was completed, the mixture was poured on ice where the product precipitated. The precipitate was filtered on frit, washed with water and dried in desiccator, then it was dissolved in EtOAc, traces of copper ions were precipitated by H2S and filtered off. Product was then purified by column chromatography on silica gel (100 × weight of the terpene) in hexane/EtOAc or cyclohexane/EtOAc in various ratio. Analytical samples were purified on HPLC, crystallized or lyophilized. Specific conditions, such as reaction times, temperature, and mobile phase for TLC, CC or HPLC are specified in each experiment.
General procedures for the deprotection of silylated compounds
Procedure 1: each triazole (0.2 mmol) was dissolved in THF (5 mL), then TBAF (2 mL; 10 equiv.; 1M solution in THF) was added. The reaction mixture was stirred at various temperature until the reaction was completed (monitored by TLC with 5% MeOH in CHCl3 as mobile phase), the deprotection usually took 18–32 h. The reaction mixture was poured to water and the product was extracted to EtOAc. The organic phase was washed twice with 5% NaHCO3 and with water, dried over MgSO4 and evaporated. Crude product was chromatographed on silica gel (10–20 g) in gradient CHCl3 to 10% MeOH in CHCl3. Analytical samples and samples for biological tests were purified on reverse phase C-18 HPLC in isocratic mobile phase: 80% CH3CN, 20% buffer (0.1% NH4OAc in water). Reaction temperature and time is specified at each experiment.
Procedure 2: each triazole (0.2 mmol) was dissolved in CH2Cl2 (5 mL) and HCl (0.3 mL, 35% in water) was added. The reaction mixture was stirred at r.t. for 5–11 h while monitored on TLC with 5% MeOH in CHCl3 as mobile phase. After the reaction was completed, 5% NaHCO3 in water was added to adjust the pH to about 5. The mixture was stirred yet another 1 h, then poured to water, extracted to CHCl3, washed with water and dried over MgSO4. Organic solvents were evaporated in vacuo and the crude product was chromatographed on silica gel (10–20 g) in gradient CHCl3 to 10% MeOH in CHCl3. Analytical samples and samples for biological tests were purified on reverse phase C-18 HPLC in isocratic mobile phase: 80% CH3CN, 20% buffer (0.1% NH4OAc in water).
3β-Triphenylsililbetulinic acid 3
5 g (10.9 mmol) of betulinic acid 1 was dissolved in DMF (100 mL), then Ph3SiCl (5.9 g, 20 mmol) and imidazole (1.4 g, 21 mmol) was added. The reaction mixture was stirred at r.t. for 36 h while being monitored on TLC (hexane/EtOAc 4: 1). The crude reaction mixture was poured on ice while the product precipitated, the precipitate was filtered off on a frit, washed with water and dried in desiccator. Crude product was chromatographed on silica gel (200 g) in gradient of hexane/EtOAc from 5: 1 to 2: 1 and crystallized from hexane.
Compound 3 was obtained as white crystals, 6.6 g (85%): mp 147–148°C. IR (DRIFT): 2400–3400, 1720, 1692, 1642 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.88 (s, 3H); 0.92 (s, 3H); 0.93 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 1.68 (s, 3H, H-30); 2.16 (td, 1H, J1 = 12.9 Hz, J2 = 3.7 Hz); 2.27 (dt, 1H, J1 = 12.9 Hz, J2 = 3.2 Hz); 3.00 (td, 1H, J1 = 10.9 Hz, J2 = 4.6 Hz, H-19β); 3.34 (dd, 1H, J1 = 11.8 Hz, J2 = 4.6 Hz, H-3α); 4.60 (dd, 1H, J1 = 2.0 Hz, J2 = 1.5 Hz, H-29 pro-E), 4.73 (d, 1H, J = 2.0 Hz, H-29 pro-Z); 7.35–7.41 (m, 6H); 7.42–7.46 (m, 3H); 7.63–7.68 (m, 6H, 15 × H-Ph). 13C NMR (125 MHz, CDCl3): δ = 14.68; 15.99; 16.12; 16.33; 18.40; 19.35; 20.77; 25.43; 27.92; 28.45; 29.65; 30.65; 32.14; 34.26; 37.00; 38.39; 38.54; 39.57; 40.63; 42.39; 46.89; 49.24; 50.32; 55.24; 56.37; 77.20; 81.16; 109.64; 127.67; 129.69; 135.38; 135.55; 150.37; 182.06. MS (ESI-): m/z (%) = 713 (100, [M-H]-). HRMS (ESI-) m/z calcd for C48H63O3Si [M-H]- 713.4384, found 713.4373.
Bromination of the position C-30 in derivatives 2 and 3
Each derivative 2 and 3 (2.8 mmol) was dissolved in CCl4 (30 mL). Then, NBS (0.8 g, 4.5 mmol) and AIBN (0.14 mmol, 5%) was added and the reaction mixture was stirred at 75°C for 1h. Then, the reaction mixture was stirred at 50°C for 3 h and another several hours (TLC) at 5°C to finish the reaction completion. The reaction had to be frequently monitored by TLC in hexane/EtOAc (2: 1 for product 4 and 5: 1 for 5) because keeping the reaction mixture at elevated temperature for longer period than necessary leads to dibrominated sideproducts. After the completion, the reaction mixture was poured into water, extracted to EtOAc, 3 × washed with water, dried over MgSO4 and evaporated. Crude products were purified on silica gel in gradient of hexane/EtOAc 4: 1 to 1: 1 and crystallized from hexane.
Compound 4 (3β-O-Acetyl-30-bromobetulinic acid) was obtained as white crystals, 1.1 g (65%): mp 170–172°C. IR (DRIFT): 2600–3400, 1721, 1642 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.85 (s, 3H); 0.86 (s, 3H); 0.94 (s, 3H); 0.99 (s, 3H, H-23, 24, 25, 26, 27); 1.98 (q, 1H, J = 8.0 Hz); 2.05 (s, 3H, Ac); 2.20 (dt, 2H, J1 = 11.5 Hz, J2 = 2.9 Hz); 2.31 (dd, 1H, J1 = 12.9 Hz, J2 = 2.9 Hz); 3.04 (td, 1H, J1 = 11.2 Hz, J2 = 4.6 Hz, H-19β); 4.01 (AB-system, 2H, JGEM = 10.3 Hz, H-30); 4.48 (dd, 1H, J1 = 10.9 Hz, J2 = 5.7 Hz, H-3α); 5.05 (s, 1H, H-29 pro-E); 5.16 (s, 1H, H-29 pro-Z). 13C NMR (125 MHz, CDCl3): δ = 14.65; 16.08; 16.17; 16.45; 18.14; 20.95; 21.30; 23.67; 26.77; 27.94; 29.68; 32.03; 33.11; 34.25; 36.74; 37.12; 37.14; 37.79; 38.38; 38.41; 40.71; 42.37; 42.99; 50.35; 50.82; 55.40; 56.43; 80.91; 113.46; 151.20; 171.06; 181.89. MS (ESI-): m/z (%) = 575 (100, [M-H]-). HRMS (ESI-) m/z calcd for C32H49BrO4 [M-H]* 575.2730, found 575.2722 and 577.2710, found 577.2701.
Compound 5 (3β-O-Triphenylsilyl-30-bromobetulinic acid) was obtained as white crystals, 1.8 g (81%): mp 208–210°C. IR (DRIFT): 2600–3400, 1718, 1642 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.88 (s, 3H); 0.92 (s, 3H); 0.94 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 1.75 (t, 2H, J = 10.9 Hz); 1.97 (dd, 1H, J1 = 12.0 Hz, J2 = 4.6 Hz); 2.17 (m, 2H); 2.30 (d, 1H, J = 9.7 Hz); 3.03 (td, 1H, J1 = 11.5 Hz, J2 = 4.6 Hz, H-19β); 3.33 (dd, 1H, J1 = 12.0 Hz, J2 = 4.6 Hz, H-3α); 3.99 (s, 2H, H-30); 5.03 (s, 1H, H-29 pro-E), 5.14 (s, 1H, H-29 pro-Z); 7.37–7.45 (m, 9H); 7.64–7.65 (d, 6H, J = 6.3 Hz, 15 × H-Ph). 13C NMR (125 MHz, CDCl3): δ = 14.68; 16.04; 16.12; 16.32; 18.39; 20.87; 23.67; 26.75; 27.90; 28.45; 29.64; 32.02; 33.04; 34.28; 36.73; 37.00; 38.38; 38.53; 39.56; 40.64; 42.35; 43.04; 50.27; 50.77; 55.22; 56.39; 81.13; 113.44; 127.67; 129.69; 135.36; 135.54; 151.18; 181.78. MS (ESI-): m/z (%) = 791 (100, [M-H]-). HRMS (ESI-) m/z calcd for C48H61BrO3Si [M+H]+ 791.3495, found 791.3491 and 793.3475, found 793.3480.
Azides 6–8
Bromoderivative 4 (2 mmol) was dissolved in DMSO (40 mL) and NaN3 (260 mg, 2 equiv.) was added. The reaction mixture was stirred at r.t. for 36 h, the reaction was monitored on TLC in hexane/EtOAc 10: 1. After that, the reaction mixture was worked up in the usual manner and crude product was chromatographed on silica gel (100 g) in gradient hexane/EtOAc 10: 1 to hexane/EtOAc 2: 1.
Compound 6 (3β-O-Acetyl-30-azidobetulinic acid) was obtained as 700 mg (65%): mp 148–150°C (hexane). IR (DRIFT): 2600–3400, 2103, 1719, 1642 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.85 (s, 3H); 0.93 (s, 3H); 0.99 (s, 3H); 1.04 (s, 3H, H-23, 24, 25, 26, 27); 2.05 (s, 3H, Ac); 2.29 (d, 1H, J = 10.2 Hz); 2.94 (td, 1H, J1 = 10.9 Hz, J2 = 4.6 Hz, H-19β); 3.77 (AB-system, 2H, JGEM = 16.0 Hz, H-30); 4.47 (dd, 1H, J1 = 10.9 Hz, J2 = 5.2 Hz, H-3α); 4.99 (s, 1H, H-29 pro-E), 5.04 (s, 1H, H-29 pro-Z). 13C NMR (125 MHz, CDCl3): δ = 14.61; 16.03; 16.15; 16.44; 18.11; 20.92; 21.29; 23.64; 26.73; 27.92; 29.65; 31.97; 34.20; 36.72; 37.09; 37.10; 37.77; 38.31; 38.35; 40.68; 42.33; 43.41; 50.29; 55.36; 55.62; 55.63; 56.41; 80.89; 111.55; 148.85; 171.07; 182.21. MS (ESI-): m/z (%) = 538 (100, [M-H]-). HRMS (ESI-TOF) m/z calcd for C32H49N3O4 [M-H]- 538.3639, found 538.3632.
Compound 7 (3β-O-Triphenylsilyl-30-azidobetulinic acid) was prepared by the same procedure as compound 6 except CH3CN was used as a solvent because of low solubility of 5 in DMSO. The reaction gave 815 mg (54%): mp 171–175°C (hexane). IR (DRIFT): 2600–3400, 2106, 1723, 1490 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.88 (s, 3H); 0.91 (s, 3H); 0.94 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 1.97 (dd, 1H, J1 = 12.6 Hz, J2 = 8.0 Hz); 2.28 (d, 1H, J = 9.2 Hz); 2.93 (td, 1H, J1 = 11.5 Hz, J2 = 5.2 Hz, H-19β); 3.33 (dd, 1H, J1 = 12.0 Hz, J2 = 4.6 Hz, H-3α); 3.75 (AB-system, 2H, JGEM = 13.8 Hz, H-30); 4.96 (s, 1H, H-29 pro-E); 5.02 (s, 1H, H-29 pro-Z); 7.36–7.45 (m, 9H); 7.65 (d, 6H, J = 8.0 Hz, 15 × H-Ph). 13C NMR (125 MHz, CDCl3): δ = 14.11; 14.66; 16.00; 16.12; 16.32; 18.38; 20.87; 22.64; 26.72; 27.88; 28.44; 29.63; 31.91; 31.97; 34.26; 36.72; 37.00; 38.29; 38.53; 39.56; 40.63; 42.33; 43.46; 50.27; 55.21; 55.49; 56.37; 81.11; 127.67; 129.69; 135.35; 135.54; 148.84; 181.73. MS (ESI-): m/z (%) = 754 (100, [M-H]-). HRMS (ESI-TOF) m/z calcd for C48H61N3O3Si [M+H]+ 754.4398, found 754.4384.
1.5 g (2 mmol) of compound 7 was deprotected according to the general procedure 1 to give colourless crystals of 30-azidobetulinic acid (8) 833 mg (84%): mp 171–175°C (CHCl3/MeOH). IR (DRIFT): 2600–3400, 2115, 1714, 1640 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 3H); 0.83 (s, 3H); 0.94 (s, 3H); 0.97 (s, 3H); 0.99 (s, 3H, H-23, 24, 25, 26, 27); 1.97 (dd, 1H, J1 = 12.6 Hz, J2 = 7.7 Hz); 2.16 (m, 2H); 2.30 (d, 1H, J = 12.6 Hz); 2.95 (td, 1H, J1 = 11.2 Hz, J2 = 4.8 Hz, H-19β); 3.20 (dd, 1H, J1 = 11.5 Hz, J2 = 4.6 Hz, H-3α); 3.78 (AB-system, 2H JGEM = 14.3 Hz, H-30); 4.98 (s, 1H, H-29 pro-E), 5.04 (s, 1H, H-29 pro-Z). 13C NMR (125 MHz, CDCl3): δ = 14.67; 15.32; 16.04; 16.10; 18.24; 20.94; 26.78; 27.33; 27.97; 29.36; 31.94; 34.31; 36.74; 37.19; 38.32; 38.69; 38.85; 40.68; 42.37; 43.46; 50.30; 55.30; 55.54; 56.37; 78.98; 111.50; 113.98; 127.76; 148.88; 181.25. MS (ESI-): m/z (%) = 496 (100, [M-H]-). HRMS (ESI-) m/z calcd for C30H47N3O3 [M-H]- 496.3534, found 496.3525.
Acetylated compounds 9a–9h
Compound 9a was obtained from 150 mg (0.28 mmol) 6 by the general procedure using 1 equiv. of alkyne, at 50°C while reaction time was 16 h. The yield of white crystals was 169 mg (95%): mp 177–178°C (hexane). IR (DRIFT): 2600–3400, 1725, 1647 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.85 (s, 6H); 0.92 (s, 3H); 0.97 (s, 3H, H-23, 24, 25, 26, 27); 1.77 (t, 1H, J = 11,5 Hz); 1.95 (dd, 1H, J1 = 8.3 Hz, J2 = 4.6 Hz); 2.05 (m, 3H, Ac); 2.17 (td, 1H, J1 = 12.3 Hz, J2 = 3.4 Hz); 2.29 (m, 1H); 2.99 (td, 1H, J1 = 10.9 Hz, J2 = 4.6 Hz, H-19β); 4.48 (dd, 1H, J1 = 8.2 Hz, J2 = 6.0 Hz, H-3α); 4.75 (s, 1H, H-29 pro-E); 5.00 (AB-system, 2H, JGEM = 15.5 Hz, H-30); 5.10 (s, 1H, H-29 pro-Z); 7.34 (t, 1H, J = 7.2 Hz, H-36); 7.43 (t, 2H, J = 7.2 Hz, H-35, 37); 7.77 (s, 1H, H-31); 7.84 (d, 2H, J = 7.2 Hz, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.63; 16.02; 16.15; 16.44; 18.10; 20.90; 21.29; 23.64; 26.84; 27.92; 29.60; 29.66; 31.92; 34.18; 36.61; 37.07; 37.76; 38.29; 38.34; 40.66; 42.35; 43.29; 50.27; 50.40; 54.75; 55.34; 56.28; 80.88; 112.03; 120.02; 125.74; 128.17; 128.81; 130.53; 147.97; 149.48; 171.06; 181.39. MS (ESI+): m/z (%) = 642 (100, [M+H]+), 664 (75, [M+Na]+). MS (ESI-): m/z (%) = 640 (100, [M-H]-). HRMS (ESI-) m/z calcd for C40H55N3O4 [M-H]- 640.4109, found 640.4098.
Compound 9b was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 19 h. The yield of white crystals was 160 mg (86%): mp 161–165°C (hexane). IR (DRIFT): 2600–3400, 1720, 1450 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.82 (s, 3H); 0.84 (s, 6H); 0.91 (s, 3H); 0.97 (s, 3H, H-23, 24, 25, 26, 27); 1.77 (t, 1H, J = 11.4 Hz); 1.96 (dd, 1H, J1 = 8.0 Hz, J2 = 4.4 Hz); 2.05 (s, 3H, Ac); 2.15 (td, 1H, J1 = 12.2 Hz, J2 = 3.1 Hz); 2.29 (d, 1H, J = 12.5 Hz); 2.99 (td, 1H, J1 = 10.6 Hz, J2 = 4.2 Hz, H-19β); 4.47 (dd, 1H, J1 = 10.4 Hz, J2 = 4.9 Hz, H-3α); 4.75 (s, 1H, H-29 pro-E); 5.05 (t, 2H, J = 4.7 Hz, H-30); 5.12 (s, 1H, H-29 pro-Z); 7.54 (t, 1H, J = 8.0 Hz); 7.66 (t, 1H, J = 7.3 Hz); 7.72 (d, 1H, J = 7.8 Hz); 7.86 (s, 1H, H-31); 8.03 (d, 1H, J = 7.8 Hz, H-35, 36, 37, 38); 10.38 (s, 1H, H-39). 13C NMR (125 MHz, CDCl3): δ = 14.28; 15.67; 15.81; 16.10; 17.74; 20.54; 20.98; 23.29; 26.56; 27.57; 29.24; 31.55; 33.82; 36.24; 36.72; 37.41; 37.94; 37.99; 40.29; 40.90; 41.99; 42.81; 49.90; 50.07; 53.39; 54.97; 55.99; 56.26; 80.53; 111.95; 123.42; 128.34; 128.49; 129.74; 132.69; 133.44; 144.50; 148.97; 170.78; 181.08; 192.00. MS (ESI+): m/z (%) = 670 (100, [M+H]+), 692 (21, [M+Na]+). HRMS (ESI+) m/z calcd for C41H55N3O5 [M+H]+ 670.4214, found 670.4211.
Compound 9c was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 24 h. The yield of white crystals was 131 mg (73%): mp 193–196°C (hexane). IR (DRIFT): 2600–3400, 1721, 1461 cm-1. 1H NMR (500 MHz, CDCl3, referenced to TMS): δ 0.76 (d, 1H, J = 10.3 Hz, H-5); 0.80 (s, 3H, H-24); 0.82 (s, 3H, H-26); 0.83 (s, 3H, H-23); 0.90 (s, 3H, H-25); 0.94 (m, 1H, H-1a); 0.95 (s, 3H, H-27); 1.07 (d, 1H, J = 12.4 Hz, H-12); 1.18 (dt, 1H, J1 = 13.4 Hz, J2 = 2.9 Hz, H-21a); 1.23 (m, 1H, H-2a); 1.24 (m, 1H, H-9); 1.34, (m, 1H, H-6a); 1.36 (m, 2H, H-7); 1.36 (m, 1H, J = 13.0 Hz, H-15a); 1.41 (m, 1H, H-2b); 1.42 (m, 1H, 12.4 Hz, H-12b); 1.44 (dd, 1H, J1 = 12.6 Hz, J2 = 2.6, H-16a); 1.48 (m, 1H, H-6b); 1.51 (q, 1H, J = 13.2 Hz, H-22a); 1.53 (m, 1H, J = 12.5 Hz, H-11a); 1.53 (q, 1H, J = 13.4 Hz, H-21b); 1.62 (m, 1H, J = 12.5 Hz, H-11b); 1.63 (dd, 1H, J1 = 13.0 Hz, J2 = 3.5 Hz, H-1b); 1.73 (t, 1H, J = 11.4 Hz, H-18); 1.93 (m, 1H, J = 13.2 Hz, H-22b); 2.02 (s, 3H, Ac); 2.03 (m, 1H, ∑J = 13.0 Hz, H-15b); 2.18 (td, 1H, J1 = 12.0 Hz, J2 = 3.0 Hz, H-13); 2.28 (td, 1H, J1 = 12.6 Hz, J2 = 2.6, H-16b); 3.00 (td, 1H, J1 = 11.1 Hz, J2 = 4.5 Hz, H-19β); 4.45 (m, 1H, J1 = 11.0 Hz, J2 = 5.0 Hz, H-3α); 4.69 (s, 1H, H-29 pro-E); 4.96 (d, 1H, J = 15.6 Hz, H-30a); 5.04 (d, 1H, J = 15.6 Hz, H-30b); 5.07 (s, 1H, H-29 pro-Z); 7.24 (td, 1H, J1 = 6.2 Hz, J2 = 1.0 Hz, H-36); 7.79 (td, 1H, J1 = 7.8 Hz, J2 = 1.7 Hz, H-37); 8.20 (dt, 1H, J1 = 7.9 Hz, J2 = 1.0 Hz, H-38); 8.20 (s, 1H, H-31); 8.58 (dq, 1H, J1 = 4.9 Hz, J2 = 0.8 Hz, H-35). 13C NMR (125 MHz, CDCl3): δ = 14.73 (C27); 16.14 (C25); 16.25 (C26); 16.55 (C24); 18.22 (C6); 21.01 (C2); 21.39 (Ac, CH3); 23.76 (C11); 26.91 (C12); 28.02 (C23); 29.72 (C21); 32.04 (C15); 32.11 (C16); 34.32 (C7); 36.82 (C22); 37.19 (C10); 37.87 (C4); 38.39 (C13); 38.46 (C1); 40.78 (C8); 42.48 (C14); 43.66 (C19); 50.38 (C9); 50.63 (C18); 54.72 (C30); 55.45 (C5); 56.35 (C17); 80.98 (C3); 112.19 (C29); 120.59 (C38); 122.83 (C31); 123.09 (C36); 137.36 (C37); 148.23 (C32); 149.20 (C35); 149.39 (C33); 150.11 (C20); 171.17 (Ac, C = O); 180.72 (C28). MS (ESI+): m/z (%) = 643 (100, [M+H]+), 665 (15, [M+Na]+). HRMS (ESI+) m/z calcd for C39H54N4O4 [M+H]+ 643.4218, found 643.4220.
Compound 9d was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 28 h. The yield of white crystals was 135 mg (76%): mp 176–179°C (cyclohexane). IR (DRIFT): 2500–3400, 1723, 1451 cm-1. 1H NMR (500 MHz, CDCl3, referenced to TMS): δ 0.76 (d, 1H, J = 10.3 Hz, H-5); 0.80 (s, 3H, H-24); 0.81 (s, 3H, H-25); 0.82 (s, 3H, H-23); 0.90 (s, 3H, H-26); 0.95 (m, 1H, H-1a); 0.96 (s, 3H, H-27); 1.09 (dd, 1H, J1 = 12.4 Hz, J2 = 4.6 Hz, H-12a); 1.18 (dt, 1H, J1 = 13.4 Hz, J2 = 2.9 Hz, H-21a); 1.24 (m, 1H, H-6a); 1.25 (m, 1H, H-2a); 1.25 (m, 1H, H-9); 1.26 (m, 1H, H-15a); 1.36 (m, 2H, H-7); 1.36 (m, 1H, J = 12.4 Hz, H-12b); 1.38 (m, 1H, H-2b); 1.40 (dd, 1H, J1 = 12.5 Hz, J2 = 3.6 Hz, H-6b); 1.42 (m, 1H, H-16a); 1.49 (m, 1H, H-22a); 1.53 (m, 1H, H-21b); 1.54 (m, 1H, J = 12.5 Hz, H-11a); 1.62 (m, 1H, J = 12.5 Hz, H-11b); 1.64 (dd, 1H, J1 = 13.0 Hz, J2 = 3.5 Hz, H-1b); 1.75 (t, 1H, J = 11.4 Hz, H-18); 1.93 (m, 1H, J = 13.2 Hz, H-22b); 1.96 (m, 1H, H-15b); 2.02 (s, 3H, Ac); 2.22 (td, 1H, J1 = 12.0 Hz, J2 = 3.0 Hz, H-13); 2.30 (td, 1H, J1 = 12.6 Hz, J2 = 2.6 Hz, H-16; 2.97 (td, 1H, J1 = 11.1 Hz, J2 = 4.5 Hz, H-19β); 4.45 (dd, 1H, J1 = 10.5 Hz, J2 = 5.2 Hz, H-3α); 4.82 (s, 1H, H-29 pro-E); 5.00 (d, 1H, J = 15.6 Hz, H-30a); 5.04 (d, 1H, J = 15.6 Hz, H-29 pro-Z); 5.11 (s, 1H, H-29b); 7.39 (m, 1H, J = 7.8 Hz, H-37); 7.92 (s, 1H, H-31); 8.28 (dt, 1H, J1 = 8.0 Hz, J2 = 1.8 Hz, H-38); 8.55 (dd, 1H, J1 = 4.9 Hz, J2 = 1.0 Hz, H-36); 8.98 (d, 1H, J = 1.5 Hz, H-34). 13C NMR (125 MHz, CDCl3): δ = 14.73 (C27); 16.15 (C26); 16.27 (C25); 16.56 (C24); 18.23 (C6); 21.07 (C2); 21.39 (Ac, CH3); 23.76 (C11); 27.22 (C12); 28.02 (C23); 29.76 (C21); 32.21 (C16); 32.31 (C15); 34.34 (C7); 36.79 (C22); 37.20 (C10); 37.88 (C4); 38.32 (C13); 38.49 (C1); 40.80 (C8); 42.49 (C14); 43.11 (C19); 50.44 (C9); 50.84 (C18); 55.47 (C5); 55.59 (C30); 56.33 (C17); 80.97 (C3); 112.29 (C29); 120.71 (C31); 124.14 (C37); 127.24 (C33); 133.73 (C38); 144.64 (C32); 146.37 (C34); 148.48 (C36); 149.65 (C20); 171.14 (Ac, C = O); 180.15 (C28). MS (ESI+): m/z (%) = 643 (100, [M+H]+). HRMS (ESI+) m/z calcd for C39H54N4O4 [M+H]+ 643.4218, found 643.4221.
Compound 9e was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 20 h. The yield of white crystals was 144 mg (76%): mp 196–198°C (cyclohexane). IR (DRIFT): 2450–3400, 1731, 1651 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.85 (s, 6H); 0.92 (s, 3H); 0.97 (s, 3H, H-23, 24, 25, 26, 27); 1.76 (t, 1H, J = 11,5 Hz); 1.95 (dd, 1H, J1 = 12.6 Hz, J2 = 8.0 Hz); 2.05 (m, 3H, Ac); 2.18 (m, 1H); 2.29 (m, 1H); 2.99 (s, 7H, Me2N); 2.99 (m, 1H, H-19β); 4.48 (dd, 1H, J1 = 8.2 Hz, J2 = 6.0 Hz, H-3α); 4.74 (s, 1H, H-29 pro-E); 4.98 (AB-system, 2H, JGEM = 15.5 Hz, H-30); 5.08 (s, 1H, H-29 pro-Z); 6.77 (s, 1H, aniline); 6.79 (s, 1H, aniline); 7.63 (s, 1H, H-31); 7.70 (s, 1H, aniline); 7.71 (s, 1H, aniline). 13C NMR (125 MHz, CDCl3): δ = 14.62; 16.03; 16.15; 16.45; 18.11; 20.91; 21.29; 23.66; 26.80; 27.93; 29.61; 31.17; 31.87; 31.93; 34.18; 36.62; 37.09; 37.77; 38.27; 38.35; 40.53; 40.66; 42.36; 43.36; 50.28; 50.34; 54.58; 55.34; 56.26; 80.89; 111.87; 112.58; 118.58; 121.52; 126.69; 148.40; 149.60; 150.37; 171.02; 180.99. MS (ESI+): m/z (%) = 485 (100, [M+H]+). HRMS (ESI+) m/z calcd for C42H60N4O4 [M+H]+ 685.4687, found 685.4687.
Compound 9f was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 1 equiv. of alkyne at r.t. while reaction time was 20 h. The yield of white crystals was 165 mg (85%): mp 193–196°C (hexane). IR (DRIFT): 2600–3500, 1724, 1655 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.85 (s, 6H); 0.92 (s, 3H); 0.98 (s, 3H, H-23, 24, 25, 26, 27); 1.35 (s, 9H, H-t-Bu); 1.78 (t, 1H, J = 11.4 Hz); 2.05 (s, 3H, Ac); 2.17 (td, 1H, J1 = 12.5 Hz, J2 = 2.6 Hz); 2.30 (dt, 1H, J1 = 13.0 Hz, J2 = 3.1 Hz); 2.99 (td, 1H, J1 = 10.9 Hz, J2 4.2 Hz, H-19β); 4.48 (dd, 1H, J1 = 10.4 Hz, J2 = 4.7 Hz, H-3α); 4.74 (s, 1H, H-29 pro-E); 5.00 (AB-system, 2H, JGEM = 16.6 Hz, H-30); 5.09 (s, 1H, H-29 pro-Z); 7.46 (d, 2H, J = 8.8 Hz, H-35, 37); 7.74 (s, 1H, H-31); 7.78 (d, 2H, J = 8.3 Hz, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.62; 16.02; 16.14; 16.44; 18.10; 20.90; 21.31; 23.65; 26.83; 27.91; 29.60; 29.68; 31.26; 31.92; 34.16; 34.64; 36.59; 37.08; 37.76; 38.27; 38.34; 40.64; 42.34; 43.22; 50.25; 50.36; 54.76; 55.33; 56.27; 80.87; 111.86; 119.74; 125.47; 125.72; 127.70; 147.93; 149.53; 151.29; 171.06; 181.43. MS (ESI-): m/z (%) = 696 (100, [M-H]-). HRMS (ESI-) m/z calcd for C44H63N3O4 [M-H]- 696.4735, found 696.4723.
Compound 9g was obtained from 150 mg (0.28 mmol) of 6 by the general procedure using 1 equiv. of alkyne at 50°C while reaction time was 18 h. The yield of white crystals was 147 mg (83%): mp 158–159°C (hexane). IR (DRIFT): 2600–3400, 1726, 1661 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.85 (s, 6H); 0.91 (s, 3H); 0.94 (s, 3H, H-23, 24, 25, 26, 27); 1.67 (m, 8H, H-34, 35, 36, 37) 2.05 (s, 3H, Ac); 2.29 (d, 1H, J = 12.9 Hz); 2.95 (td, 1H, J1 = 10.9 Hz, J2 = 4.3 Hz, H-19β); 3.21 (t, 1H, J = 8.3 Hz, H-33); 4.48 (dd, 1H, J1 = 10.9 Hz, J2 = 5.2 Hz, H-3α); 4.69 (s, 1H, H-29 pro-E); 4.91 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.05 (s, 1H, H-29 pro-Z); 7.26 (s, 1H, H-31). 13C NMR (125 MHz, CDCl3): δ = 14.59; 14.62; 15.99; 16.03; 16.12; 16.16; 16.46; 18.10; 20.90; 21.28; 23.65; 25.11; 26.68; 27.93; 29.59; 29.66; 31.90; 33.26; 34.18; 36.61; 36.68; 37.07; 37.76; 38.24; 28.36; 40.64; 42.34; 43.26; 50.20; 54.47; 55.34; 56.27; 80.87; 111.89; 120.05; 149.61; 152.93; 171.04; 180.94. MS (ESI-): m/z (%) = 632 (100, [M-H]-). HRMS (ESI-) m/z calcd for C39H59N3O4 [M-H]- 632.4422, found 632.4414.
Compound 9h was obtained from 300 mg (0.56 mmol) of 6 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 30 h. The yield of white crystals was 33 mg (10%): mp 178–184°C (cyclohexane). IR (DRIFT): 3650, 2600–3500, 1726, 1643 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.85 (s, 6H); 0.93 (s, 3H); 0.97 (s, 3H, H-23, 24, 25, 26, 27); 2.05 (s, 3H, Ac); 2.30 (bd, 1H, J = 12.9 Hz); 2.95 (m, 1H, H-19β); 4.48 (dd, 1H, J1 = 10.9 Hz, J2 = 5.2 Hz, H-3α); 4.70 (s, 1H, H-29 pro-E); 4.85–5.20 (m, 2H, CH2-NH2); 5.03 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.13 (s, 1H, H-29 pro-Z); 8.13 (s, 1H, H-31); 10.17 (s, NH2). 13C NMR (125 MHz, CDCl3): δ = 14.61; 16.01; 16.17; 16.46; 18.12; 20.90; 21.29; 23.65; 27.05; 27.93; 29.58; 31.97; 34.22; 36.20; 37.10; 37.79; 38.23; 38.39; 40.69; 42.37; 43.11; 50.29; 50.84; 55.34; 55.38; 56.33; 64.17; 77.20; 80.82; 112.57; 125.64; 132.51; 148.84; 171.02; 185.11. MS (ESI+): m/z (%) = 595 (100, [M+H]+). HRMS (ESI+) m/z calcd for C35H54N4O4 [M+H]+ 595.4218, found 595.4220.
Silylated compounds 10a–10g
Compound 10a was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 1 equiv. of alkyne at 50°C while reaction time was 20 h. The yield of white crystals was 157 mg (92%): mp 164–166°C (cyclohexane). IR (DRIFT): 2600–3400, 1734, 1652 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.89 (s, 3H); 0.90 (s, 3H); 0.92 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 2.15 (dd, 1H, J1 = 12.5 Hz, J2 = 3.1 Hz); 2.29 (d, 1H, J = 12.5 Hz); 3.00 (td, J1 = 10.9 Hz, J2 = 4.4 Hz, 1H, H-19β); 3.34 (dd, 1H, J1 = 11.9 Hz, J2 = 4.2 Hz, H-3α); 4.70 (s, 1H, H-29 pro-E); 4.99 (AB-system, 2H, JGEM = 15.6 Hz, H-30); 5.07 (s, 1H, H-29 pro-Z); 7.31–7.46 (m, 12H, H-35, 36, 37, 3 × Ph); 7.66 (dd, 6H, J1 = 7.8 Hz, J2 = 1.3 Hz, 3 × Ph); 7.75 (s, 1H, H-31); 7.84 (d, 2H, J = 8.3 Hz, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.66; 15.98; 16.10; 16.32; 18.35; 20.82; 26.81; 27.88; 28.42; 29.56; 31.81; 31.90; 34.21; 36.59; 36.95; 38.27; 28.52; 39.54; 40.59; 42.33; 43.38; 50.20; 50.31; 54.55; 55.16; 56.25; 81.08; 111.91; 120.01; 125.73; 127.67; 128.15; 128.80; 129.70; 130.53; 135.32; 135.53; 147.95; 149.43; 181.32. MS (ESI-): m/z (%) = 856 (100, [M-H]-). HRMS (ESI-) m/z calcd for C56H67N3O3Si [M-H]- 856.4868, found 856.4849.
Compound 10b was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 22 h. The yield of white crystals was 148 mg (84%): mp 161–163°C (cyclohexane). IR (DRIFT): 2600–3400, 1724, 1642 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.83 (s, 3H); 0.88 (s, 3H); 0.90 (s, 3H); 0.92 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 2.14 (td, 1H, J1 = 12.6 Hz, J2 = 2.6 Hz); 2.29 (d, 1H, J = 12.6 Hz); 2.99 (td, J1 = 10.9 Hz, J2 = 4.3 Hz, 1H, H-19β); 3.34 (dd, 1H, J1 = 11.7 Hz, J2 = 4.3 Hz, H-3α); 4.75 (s, 1H, H-29 pro-E); 5.04 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.10 (s, 1H, H-29 pro-Z); 7.34–7.45 (m, 9H, 3 × Ph); 7.51 (t, 1H, J = 7.7 Hz, H-36); 7.62 (m, 1H, H-37); 7.66 (m, 6H, 3 × Ph); 7.72 (d, 1H, J = 8.6 Hz, H-38); 7.83 (s, 1H, H-31); 8.03 (d, 2H, J = 8.9 Hz, H-35); 10.38 (s, 1H, H-39). 13C NMR (125 MHz, CDCl3): δ = 14.67; 15.98; 16.11; 16.32; 18.36; 20.84; 22.63; 26.87; 27.88; 28.43; 29.56; 31.56; 31.89; 34.23; 36.58; 38.29; 38.54; 39.54; 40.60; 42.35; 43.28; 50.22; 54.37; 54.78; 55.18; 56.27; 81.08; 112.24; 119.89; 123.71; 127.67; 128.63; 128.83; 129.70; 130.07; 133.05; 133.72; 133.84; 135.34; 135.53; 144.83; 149.30; 192.27. MS (ESI+): m/z (%) = 886 (100, [M+H]+), 908 (8, [M+Na]+). HRMS (ESI+) m/z calcd for C57H67N3O4Si [M+H]+ 886.4974, found 886.4975.
Compound 10c was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 26 h. The yield of white crystals was 130 mg (75%): mp 197–200°C (cyclohexane). IR (DRIFT): 2600–3400, 1718, 1641 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.82 (s, 3H); 0.88 (s, 3H); 0.89 (s, 3H); 0.92 (s, 3H); 0.95 (s, 3H, H-23, 24, 25, 26, 27); 2.17 (t, 1H, J = 11.7 Hz); 2.28 (d, 1H, J = 11.7 Hz); 3.01 (td, 1H, J1 = 10.4 Hz, J2 = 6.5 Hz, H-19β); 3.33 (dd, 1H, J1 = 12.2 Hz, J2 = 3.6 Hz, H-3α); 4.66 (s, 1H, H- 29 pro-E); 5.01 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.06 (s, 1H, H-29 pro-Z); 7.24 (m, 1H, H-36); 7.36–7.45 (m, 9H, 3 × Ph); 7.65 (d, 6H, J = 6.5 Hz, 3 × Ph); 7.79 (t, 1H, J = 7.8 Hz, H-35); 8.19 (s, 1H, H-31); 8.21 (d, 1H, J = 8.8 Hz, H-37); 8.59 (d, 1H, J = 4.7 Hz, H-34). 13C NMR (125 MHz, CDCl3): δ = 14.65; 15.99; 16.10; 16.33; 18.36; 20.81; 26.79; 27.87; 28.42; 29.68; 31.56; 32.00; 34.23; 36.70; 36.95; 38.24; 38.49; 39.53; 40.59; 42.33; 43.54; 50.19; 50.43; 54.52; 55.16; 56.21; 81.09; 111.83; 119.88; 122.69; 127.50; 127.67; 129.70; 135.32; 136.30; 137.21; 142.18; 148.13; 149.09; 149.98, 181.13. MS (ESI+): m/z (%) = 859 (100, [M+H]+), 881 (12, [M+Na]+). HRMS (ESI+) m/z calcd for C55H66N4O3Si [M+H]+ 859.4977, found 859.4977.
Compound 10d was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 36 h. The yield of white crystals was 131 mg (77%): mp 202–204°C (cyclohexane). IR (DRIFT): 2650–3450, 1729, 1646 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.82 (s, 3H); 0.88 (s, 3H); 0.89 (s, 3H); 0.91 (s, 3H); 0.93 (s, 3H); 0.95 (s, 3H, H-23, 24, 25, 26, 27); 2.30 (d, 1H, J = 12.6 Hz); 2.98 (td, 1H, J1 = 10.6 Hz, J2 = 4.0 Hz, H-19β); 3.33 (dd, 1H, J1 = 11.7 Hz, J2 = 4.0 Hz, H-3α); 4.79 (s, 1H, H-29 pro-E); 5.01 (AB-system, 2H, JGEM = 16.6 Hz, H-30); 5.10 (s, 1H, H-29 pro-Z); 7.36–7.45 (m, 9H, 3 × Ph); 7.65 (d, 6H, 3 × Ph); 7.89 (s, 1H, H-31); 8.28 (d, 1H, J = 7.7 Hz, H-37); 8.57 (d, 1H, J = 3.7 Hz, H-35); 8.99 (s, 1H, H-34). 13C NMR (125 MHz, CDCl3): δ = 14.67; 16.01; 16.13; 16.34; 18.39; 20.91; 27.11; 27.89; 28.44; 29.62; 32.07; 32.13; 34.27; 36.66; 36.99; 38.21; 28.56; 39.55; 40.65; 42.38; 43.02; 50.27; 50.72; 55.20; 55.40; 56.19; 81.10; 112.12; 120.54; 123.97; 127.07; 127.68; 129.70; 133.51; 135.35; 135.54; 144.57; 146.40; 148.50; 149.52; 181.13. MS (ESI+): m/z (%) = 859 (100, [M+H]+), 881 (7, [M+Na]+). HRMS (ESI+) m/z calcd for C55H66N4O3Si [M+H]+ 859.4977, found 859.4974.
Compound 10e was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 2 equiv. of alkyne at r.t. while reaction time was 24 h. The yield of white crystals was 128 mg (72%): mp 194–196°C (cyclohexane). IR (DRIFT): 2600–3400, 1724, 1652 cm-1. 1H NMR (500 MHz, CDCl3, referenced to TMS): δ 0.53 (d, 1H, J = 10.3 Hz, H-5); 0.64 (td, 1H, J1 = 13.2 Hz, J2 = 3.5 Hz, H-1a); 0.82 (s, 3H, H-25); 0.86 (s, 3H, H-24); 0.88 (s, 3H, H-27); 0.92 (s, 3H, H-26); 0.94 (s, 3H, H-23); 1.02 (qd, 1H, J1 = 12.4 Hz, J2 = 3.8 Hz, H-12a); 1.16 (t, 1H, J = 12.5 Hz, H-9); 1.19 (dd, 1H, J1 = 13.4 Hz, J2 = 2.9 Hz, H-21a); 1.28 (m, 1H, J = 12.5 Hz, H-2a); 1.31 (m, 2H, H-7); 1.33 (m, 1H, H-6a); 1.38 (m, 1H, J = 12.4 Hz, H-12b); 1.41 (dd, 1H, J1 = 12.5 Hz, J2 = 2.2 Hz, H-2b); 1.44 (m, 1H, H-15a); 1.45 (m, 1H, J = 12.6 Hz, H-16a); 1.49 (m, 1H, J = 13.5 Hz, H-22a); 1.50 (mm, 4H, H-11a, H-1, 6, 21b); 1.74 (t, 1H, J = 11.4 Hz, H-18); 1.74 (m, 1H, H-11b); 1.92 (m, 1H, J = 13.5 Hz, H-22b); 1.99 (m, 1H, J = 13.0 Hz, H-15b); 2.13 (td, 1H, J1 = 12.0 Hz, J2 = 3.0 Hz, H-13); 2.26 (dt, 1H, J1 = 12.6 Hz, J2 = 2.6 Hz, H-16b); 2.95 (td, 1H, J1 = 11.1 Hz, J2 = 4.5 Hz, H-19β); 2.98 (s, 6H, H-40, 41); 3.32 (dd, 1H, J1 = 11.0 Hz, J2 = 5.0 Hz, H-3α); 4.67 (s, 1H, H-29 pro-E); 4.94 (m, 2H, H-30); 5.03 (s, 1H, H-29 pro-Z); 6.80 (br, 2H, H-35, 37); 7.36 (t, 6H, J = 7.2 Hz, H-3´,3´´a,b,c); 7.42 (tt, 3H, J1 = 7.2 Hz, J2 = 1.5 Hz, H-4´a,b,c); 7.60 (s, 1H, H-31); 7.63 (d, 6H, J = 7.2 Hz, H-2´,2´´a,b,c); 7.69 (d, 2H, J = 8.2 Hz, H-34,38). 13C NMR (125 MHz, CDCl3): δ = 14.79 (C27); 16.11 (C26); 16.22 (C25); 16.44 (C24); 18.46 (C6); 20.97 (C2); 26.92 (C12); 28.00 (C11); 28.54 (C23); 29.70 (C21); 31.96 (C15); 32.05 (C16); 34.36 (C7); 36.71 (C22); 37.10 (C10); 38.38 (C13); 38.66 (C1); 39.67 (C4); 40.74 (C8); 40.86 (C40,C41); 42.47 (C14); 43.41 (C19); 50.34 (C18); 50.43 (C9); 54.65 (C29); 56.30 (C5); 56.32 (C17); 91.22 (C3); 111.62 (C29); 112.95 (C35, 37); 118.76 (C31); 122.23 (C33); 126.84 (C34,C38); 127.77 (Ca,b,c3´,3´´); 129.81 (Ca,b,c4´); 135.46 (Ca,b,c1´,1´´); 135.64 (Ca,b,c2´,2´´); 148.42 (C32); 149.70 (C20); 150.21 (C36); 180.72 (C28). MS (ESI+): m/z (%) = 901 (100, [M+H]+), 923 (6, [M+Na]+). HRMS (ESI+) m/z calcd for C58H72N4O3Si [M+H]+ 901.5446, found 901.5441.
Compound 10f was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 1 equiv. of alkyne at r.t. while reaction time was 24 h. The yield of white crystals was 147 mg (81%): mp 195–197°C (cyclohexane). IR (DRIFT): 2600–3400, 1717, 1639 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.89 (s, 3H); 0.90 (s, 3H); 0.95 (s, 3H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 1.35 (s, 9H, H-40, 41, 42); 1.76 (t, 2H, J = 1.2 Hz); 2.15 (td, 1H, J1 = 12.6 Hz, J2 = 2.9 Hz); 2.29 (d, 1H, J = 12.9 Hz); 2.98 (td, 1H, J1 = 11.2 Hz, J2 = 4.9 Hz, H-19β); 3.35 (dd, 1H, J1 = 11.7 Hz, J2 = 4.3 Hz, H-3α); 4.69 (s, 1H, H-29 pro-E); 4.98 (s, 2H, H-30); 5.05 (s, 1H, H-29 pro-Z); 7.37–7.47 (m, 11H, H-35, 37, 3 × Ph); 7.66 (d, 6H, J = 6.6 Hz, 3 × Ph); 7.78 (d, 2H, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.68; 16.00; 16.11; 16.33; 18.36; 20.84; 26.82; 27.89; 28.43; 29.58; 29.67; 31.26; 31.93; 34.22; 34.63; 36.59; 36.98; 38.26; 38.52; 39.55; 40.60; 42.34; 43.22; 50.21; 50.34; 54.37; 54.67; 55.18; 56.24; 81.09; 111.63; 119.73; 125.46; 125.71; 127.67; 129.70; 135.34; 135.52; 147.91; 149.58; 151.27; 181.28. MS (ESI+): m/z (%) = 914 (100, [M+H]+), 937 (75, [M+Na]+). HRMS (ESI+) m/z calcd for C60H75N3O3Si [M+H]+ 914.5650, found 914.5650.
Compound 10g was obtained from 150 mg (0.20 mmol) of 7 by the general procedure using 1 equiv. of alkyne at 50°C while reaction time was 15 h. The yield of white crystals was 148 mg (88%): mp 146–148°C (cyclohexane). IR (DRIFT): 2650–3400, 1730, 1452 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.84 (s, 3H); 0.89 (s, 9H); 0.96 (s, 3H, H-23, 24, 25, 26, 27); 2.29 (d, 1H, J = 11.5 Hz); 2.97 (t, 1H, J = 12.9 Hz, H-33); 3.21 (td, 1H, J1 = 15.5 Hz, J2 = 7.7 Hz, H-19β); 3.34 (d, 1H, J = 10.9 Hz, H-3α); 4.65 (s, 1H, H-29 pro-E); 4.89 AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.03 (s, 1H, H-29 pro-Z); 7.25 (s, 1H, H-31); 7.37–7.45 (m, 9H, 3 × Ph); 7.66 (d, 6H, J = 8.0 Hz, 3 × Ph). 13C NMR (125 MHz, CDCl3): δ = 14.65; 15.99; 16.10; 16.32; 18.37; 20.82; 25.11; 26.66; 27.89; 28.42; 29.58; 29.66; 31.76; 31.93; 33.23; 34.23; 36.69; 36.97; 38.20; 38.53; 39.53; 40.58; 42.32; 43.47; 50.11; 50.22; 54.19; 55.20; 56.29; 81.11; 111.92; 120.00; 127.65; 129.68; 135.32; 135.50; 149.62; 152.89; 181.13. MS (ESI+): m/z (%) = 850 (100, [M+H]+). HRMS (ESI+) m/z calcd for C55H71N3O3Si [M+H]+ 850.5337, found 850.5334.
Unprotected compounds 11a–11g
Compound 11a was obtained from 150 mg (0.17 mmol) of 10a by the general deprotection procedure 1 at 60°C for 18 h. The yield of white crystals was 143 mg (79%): mp 137–138°C (CH2Cl2). IR (DRIFT): 2600–3400, 1724, 1650 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 3H); 0.82 (s, 3H); 0.92 (s, 3H); 0.98 (s, 6H, H-23, 24, 25, 26, 27); 2.18 (dd, 1H, J1 = 13.5 Hz, J2 = 3.4 Hz); 2.30 (d, 1H, J = 12.7 Hz); 3.00 (td, 1H, J1 = 11.2 Hz, J2 = 4.6 Hz, H-19β); 3.21 (dd, 1H, J1 = 11.2 Hz, J2 = 4.9 Hz, H-3α); 4.72 (s, 1H, H-29 pro-E); 5.00 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.10 (s, 1H, H-29 pro-Z); 7.34 (tt, 1H, J1 = 7.3 Hz, J2 = 1.3 Hz, H-36); 7.43 (t, 2H, J = 7.3 Hz, H-35, 37); 7.77 (s, 1H, H-31); 7.84 (d, 2H, J = 7.0 Hz, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.66; 15.32; 16.01; 16.08; 18.21; 20.89; 26.84; 27.29; 27.94; 29.60; 29.67; 31.86; 31.92; 34.25; 36.62; 37.14; 38.30; 38.66; 38.81; 40.63; 42.36; 43.39; 50.36; 54.59; 55.24; 56.25; 78.95; 111.95; 120.02; 125.74; 128.16; 128.81; 130.54; 147.97; 149.48; 180.97. MS (ESI+): m/z (%) = 600 (100, [M+H]+). HRMS (ESI+) m/z calcd for C38H53N3O3 [M+H]+ 600.4160, found 600.4162. Note: deprotection procedure 2 at r.t. for 4 h was also tried with the yield of 76%.
Compound 11b was obtained from 150 mg (0.17 mmol) of 10b by the general deprotection procedure 1 at r.t. for 32 h. The yield of white crystals was 117 mg (62%): mp 127–129°C (CH2Cl2). IR (DRIFT): 2600–3400, 1727, 1453 cm-1. 1H NMR (500 MHz, CDCl3, referenced to TMS): δ 0.66 (d, 1H, J = 9.0 Hz, H-5); 0.73 (s, 3H, H-25); 0.80 (s, 3H, H-24); 0.88 (m, 1H, J = 13.2 Hz, H-1a); 0.90 (s, 3H, H-26); 0.95 (s, 3H, H-23); 0.96, (s, 3H, H-27); 1.04 (dd, 1H, J1 = 11.7 Hz, J2 = 4.2 Hz, H-12a); 1.23 (m, 1H, H-9); 1.24 (mm, 2H, H-15, 21a); 1.25 (m, 1H, H-11a); 1.36 (mm, 2H, H-7); 1.36 (m, 1H, H-6a); 1.38 (m, 1H, H-12b); 1.44 (m, 1H, H-11b); 1.44 (m, 1H, J = 12.2 Hz, H-16a); 1.50 (m, 1H, H-15b); 1.52 (mm, 3H, H-2,6,21b); 1.52 (m, 1H, J = 13.1 Hz, H-22a); 1.61 (m, 1H, H-2b); 1.63 (m, 1H, J = 13.2 Hz, H-1b); 1.75 (t, 1H, J = 11.4 Hz, H-18); 1.95 (m, 1H, H-22b); 2.16 (td, 1H, J1 = 12.1 Hz, J2 = 3.3 Hz, H-13); 2.28 (dt, 1H, J1 = 12.2 Hz, J2 = 3.1 Hz, H-16b); 2.98 (td, 1H, J1 = 11.1 Hz, J2 = 4.3 Hz, H-19β); 3.18 (dd, 1H, J1 = 11.3 Hz, J2 = 4.7 Hz, H-3α); 4.75 (s, 1H, H-29); 5.00 (d, 1H, J = 15.6 Hz, H-30); 5.06 (d, 1H, J = 15.6 Hz, H-30b); 5.10 (s, 1H, H-29); 7.51 (tt, 1H, J1 = 7.5 Hz, J2 = 0.8 Hz, H-36); 7.64 (td, 1H, J1 = 7.5 Hz, J2 = 1.5 Hz, H-37); 7.71 (dd, 1H, J1 = 7.8 Hz, J2 = 1.1 Hz, H-38); 7.82 (s, 1H, H-31); 8.02 (dd, 1H, J1 = 7.8, J2 = 1.2 Hz, H-35); 10.36 (d, 1H, J = 0.5 Hz, H-39). 13C NMR (125 MHz, CDCl3): δ = 14.78 (C27); 15.43 (C25); 16.12 (C26); 16.20 (C24); 18.34 (C6); 21.02 (C11); 27.06 (C12); 27.44 (C2); 28.06 (C23); 29.71 (C21); 29.77 (C15); 32.02 (C16); 34.38 (C7); 36.71 (C22); 37.28 (C10); 38.41 (C13); 38.80 (C1); 38.94 (C4); 40.77 (C8); 42.49 (C14); 43.39 (C19); 50.50 (C18); 50.52 (C9); 54.93 (C30); 55.38 (C5); 56.35 (C17); 79.03 (C3); 112.37 (C29); 123.84 (C31); 128.76 (C36); 128.96 (C35); 130.20 (C38); 133.18 (C33); 133.83 (C37); 133.96 (C34); 144.93 (C32); 149.45 (C20); 180.36 (C28); 192.41 (C39). MS (ESI+): m/z (%) = 628 (100, [M+H]+). HRMS (ESI-TOF) m/z calcd for C39H53N3O4 [M+H]+ 628.4109, found 628.4111.
Compound 11c was obtained from 150 mg (0.30 mmol) of 10c by the general deprotection procedure 1 at r.t. for 28 h. The yield of white crystals was 105 mg (58%): mp 141–142°C (CH2Cl2). IR (DRIFT): 2500–3450, 1735, 1654 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 3H); 0.82 (s, 3H); 0.92 (s, 3H); 0.97 (s, 3H); 0.98 (s, 3H, H-23, 24, 25, 26, 27); 1.75 (t, 1H, J = 11.5 Hz); 1.97 (dd, 1H, J1 = 12.6 Hz, J2 = 8.3 Hz); 2.06 (m, 1H); 2.22 (td, 1H, J1 = 13.9 Hz, J2 = 3.5 Hz); 2.31 (dt, 1H, J1 = 12.5 Hz, J2 = 3.2 Hz); 3.04 (td, 1H, J1 = 11.2 Hz, J2 = 4.9 Hz, H-19β); 3.21 (dd, 1H, J1 = 11.6 Hz, J2 = 4.9 Hz, H-3α); 4.67 (s, 1H, H-29 pro-E); 5.04 (AB-system, 2H, JGEM = 15.7 Hz, H-30); 5.09 (s, 1H, H-29 pro-Z); 7.26 (m, 1H, H-pyridine); 7.81 (td, 1H, J1 = 7.7 Hz, J2 = 1.7 Hz, H-pyridine); 8.22 (s, 1H, H-31); 8.22 (m, 1H, H-pyridine); 8.61 (dq, J1 = 4.9 Hz, J2 = 0.8 Hz, H-pyridine). 13C NMR (125 MHz, CDCl3): δ = 14.66; 15.34; 16.10; 18.23; 20.90; 26.81; 27.31; 27.96; 29.62; 31.17; 31.95; 32.02; 34.29; 36.72; 37.16; 38.28; 38.72; 38.82; 40.66; 42.38; 43.59; 50.39; 50.46; 54.47; 55.27; 56.21; 78.93; 111.81; 120.49; 122.76; 122.97; 137.24; 148.11; 149.07; 149.35; 150.01; 180.18. MS (ESI-): m/z (%) = 599 (100, [M-H]-). HRMS (ESI-) m/z calcd for C37H52N4O3 [M-H]- 599.3956, found 599.3947. Note: deprotection procedure 2 was also tried with yield of 58% and the attempt to prepare compound 11c from free azide 8 by the general procedure for click reaction gave the best yield of 74%.
Compound 11d was obtained from 150 mg (0.30 mmol) of 10d by the general deprotection procedure 1 at r.t. for 28 h. The yield of white crystals was 112 mg (62%): mp 141–142°C (CH2Cl2). IR (DRIFT): 2600–3400, 1734, 1645 cm-1. 1H NMR (500 MHz, DMSO): δ 0.64 (s, 3H); 0.75 (s, 3H); 0.85 (s, 3H); 0.87 (s, 3H); 0.93 (s, 3H, H-23, 24, 25, 26, 27); 2.12 (m, 2H) 2.18 (m, 1H); 2.97 (td, 1H, J1 = 10.9 Hz, J2 = 4.3 Hz, H-19β); 3.21 (dd, 1H, J1 = 8.3 Hz, J2 = 3.7 Hz, H-3α); 4.56 (s, 1H, H-29 pro-E); 5.02 (s, 2H, H-30); 5.05 (s, 1H, H-29 pro-Z); 7.48 (m, 1H, H-37); 8.23 (td, 1H, J1 = 7.7 Hz, J2 = 2.0 Hz, H-36); 8.54 (dd, 1H, J1 = 4.9 Hz, J2 = 1.7 Hz, H-35); 8.71 (s, 1H, H-31); 9.06 (d, 1H, J = 2.0 Hz, H-34). 13C NMR (125 MHz, CDCl3): δ = 14.65; 15.35; 16.11; 18.24; 20.95; 26.13; 27.09; 27.96; 27.92; 29.05; 29.58; 32.05; 34.31; 36.68; 37.17; 38.11; 38.84; 40.63; 40.86; 42.39; 48.72; 50.43; 50.51; 51.43; 55.29; 56.12; 78.92; 110.77; 120.63; 128.42; 128.55; 132.13; 144.54; 146.36; 148.57; 149.58; 168.10; 178.88. MS (ESI-): m/z (%) = 599 (100, [M-H]-). HRMS (ESI-TOF) m/z calcd for C37H52N4O3 [M-H]- 599.3956, found 599.3945. Note: deprotection procedure 2 was also tried with yield of 60% and the attempt to prepare compound 11c from free azide 8 by the general procedure for click reaction gave the best yield of 76%.
Compound 11e was obtained from 150 mg (0.17 mmol) of 10e by the general deprotection procedure 1 at r.t. for 30 h. The yield of white crystals was 124 g (64%): mp 150–151°C (CH2Cl2). IR (DRIFT): 2600–3400, 1731, 1651 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 1H); 0.82 (s, 3H); 0.92 (s, 3H); 0.97 (s, 6H, H-23, 24, 25, 26, 27); 2.30 (d, 1H, J = 9.2 Hz); 2.99 (s, 6H, H-39, 40); 2.99 (m, 1H, H-19β) 3.20 (dd, 1H, J1 = 11.2 Hz, J2 = 4.9 Hz, H-3α); 4.71 (s, 1H, H-29 pro-E); 4.97 (AB-system, 2H, JGEM = 15.5 Hz, H-30); 5.07 (s, 1H, H-29 pro-Z); 6.67 (d, 2H, J = 9.1 Hz, H-34, 38); 7.62 (s, 1H, H-31); 7.70 (d, 2H, J = 8.7 Hz, H-35, 37). 13C NMR (125 MHz, CDCl3): δ = 14.67; 15.33; 16.04; 16.10; 18.23; 20.92; 26.83; 27.31; 27.96; 29.64; 31.85; 34.28; 36.55; 36.69; 37.18; 38.32; 38.68; 38.84; 40.48; 40.49; 40.66; 42.38; 43.53; 50.39; 54.48; 55.26; 78.96; 111.79; 112.52; 118.61; 118.86; 126.71; 126.90 148.43; 149.65; 150.44; 179.98. MS (ESI+): m/z (%) = 643 (100, [M+H]+). HRMS (ESI+) m/z calcd for C40H58N4O3 [M+H]+ 643.4582, found 643.4583.
Compound 11f was obtained from 150 mg (0.16 mmol) of 10f by the general deprotection procedure 1 at r.t. for 30 h. The yield of white crystals was 148 mg (75%): mp 141–142°C (EtOAc). IR (DRIFT): 2500–3450, 1729, 1647 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 3H); 0.82 (s, 3H); 0.92 (s, 3H); 0.97 (s, 6H, H-23, 24, 25, 26, 27); 2.18 (dd, 1H, J1 = 12.5 Hz, J2 = 2.9 Hz); 2.29 (d, 1H, J = 12.8 Hz); 3.02 (td, 1H, J1 = 10.9 Hz, J2 = 4.6 Hz, H-19β); 3.20 (dd, 1H, J1 = 11.2 Hz, J2 = 4.7 Hz, H-3α); 4.72 (s, 1H, H-29 pro-E); 5.00 (AB-system, 2H, JGEM = 15.8 Hz, H-30); 5.08 (s, 1H, H-29 pro-Z); 7.45 (d, 2H, J = 8.6 Hz, H-35, 37); 7.73 (s, 1H, H-31); 7.77 (d, 2H, J = 8.6 Hz, H-34, 38). 13C NMR (125 MHz, CDCl3): δ = 14.65; 15.33; 16.02; 16.09; 18.23; 20.92; 26.87; 27.31; 27.95; 29.63; 30.14; 31.27; 31.90; 32.00; 34.26; 34.64; 36.67; 37.15; 38.27; 38.67; 38.82; 40.63; 42.36; 43.31; 43.43; 50.37; 54.71; 55.24; 56.29; 78.95; 111.78; 119.76; 125.47; 125.72; 127.72; 147.93; 149.67; 151.29; 180.99. MS (ESI+): m/z (%) = 456 (100, [M+H]+), 478 (4, [M+Na]+). HRMS (ESI+) m/z calcd for C42H61N3O3 [M+H]+ 456.4786, found 456.4786. Note: deprotection procedure 2 at r.t. for 4 h was also tried with the yield of 73%.
Compound 11g was obtained from 150 mg (0.18 mmol) of 10g by the general deprotection procedure 1 at 50°C for 20 h. The yield of white crystals was 144 mg (81%): mp 127–129°C (CHCl3). IR (DRIFT): 2500–3400, 1724, 1652 cm-1. 1H NMR (500 MHz, CDCl3): δ 0.76 (s, 3H); 0.82 (s, 3H); 0.92 (s, 3H); 0.95 (s, 3H); 0.97 (s, 3H, H-23, 24, 25, 26, 27); 2.12 (m, 2H) 2.13 (m, 1H); 2.29 (d, 1H, J = 3.0 Hz); 2.97 (td, 1H, J1 = 10.9 Hz, J2 = 4.3 Hz, H-19β); 3.21 (dd, 1H, J1 = 8.3 Hz, J2 = 3.7 Hz, H-3α); 4.66 (s, 1H, H-29 pro-E); 4.90 (AB-system, 2H, JGEM = 15.5 Hz, H-30); 5.05 (s, 1H, H-29 pro-Z); 7.24 (s, 1H, H-31). 13C NMR (125 MHz, CDCl3): δ = 14.66; 15.33; 16.01; 16.09; 18.20; 20.87; 25.12; 26.67; 27.31; 27.95; 29.61; 31.92; 33.24; 34.28; 36.64; 36.74; 37.16; 38.26; 38.73; 38.82; 40.63; 40.66; 42.36; 43.45; 50.16; 50.37; 54.22; 55.28; 56.29; 78.96; 111.87; 120.02; 149.66; 152.98; 180.95. MS (ESI+): m/z (%) = 592 (100, [M+H]+), 614 (10, [M+Na]+). HRMS (ESI+) m/z calcd for C37H57N3O3 [M+H]+ 592.4473, found 592.4474.
General information about the biological assays
Cell lines
Biological assays were performed in concordance with our previous publications [29, 58; 60; 61]. All cells (if not indicated otherwise) were purchased from the American Tissue Culture Collection (ATCC). The CCRF-CEM line is derived from T lymphoblastic leukemia, evincing high chemosenzitivity, K562 represent cells from an acute myeloid leukemia patient sample with bcr-abl translocation, U2OS line is derived from osteosarcoma, HCT116 is colorectal tumor cell line and its p53 gene knock-down counterpart (HCT116p53-/-, Horizon Discovery Ltd, UK) is a model of human cancers with p53 mutation frequently associated with poor prognosis, A549 line is lung adenocarcinoma. The daunorubicin resistant subline of CCRF-CEM cells (CEM-DNR bulk) and paclitaxel resistant subline K562-TAX were selected in our laboratory by the cultivation of maternal cell lines in increasing concentrations of daunorubicine or paclitaxel, respectively. The CEM-DNR bulk cells overexpress MRP-1 and P-glycoprotein protein, while K562-TAX cells overexpress P-glycoprotein only. Both proteins belong to the family of ABC transporters and are involved in the primary and/or acquired multidrug resistance phenomenon [58]. MRC-5 and BJ cell lines were used as a non-tumor control and represent human fibroblasts. The cells were maintained in nunc/corning 80 cm2 plastic tissue culture flasks and cultured in cell culture medium according to ATCC or Horizon recommendations (DMEM/RPMI 1640 with 5 g/L glucose, 2 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, 10% fetal calf serum, and NaHCO3).
Cytotoxic MTS assay
MTS assay was performed at Institute of Molecular and Translational Medicine by robotic platform (HighResBiosolutions). Cell suspensions were prepared and diluted according to the particular cell type and the expected target cell density (25000–35000 cells/mL based on cell growth characteristics). Cells were added by automatic pipetor (30 μL) into 384 well microtiter plates. All tested compounds were dissolved in 100% DMSO and four-fold dilutions of the intended test concentration were added in 0.15 μL aliquots at time zero to the microtiter plate wells by the echoacustic non-contact liquid handler Echo550 (Labcyte). The experiments were performed in technical duplicates and three biological replicates at least. The cells were incubated with the tested compounds for 72 h at 37°C, in a 5% CO2 atmosphere at 100% humidity. At the end of the incubation period, the cells were assayed by using the MTS test. Aliquots (5 μL) of the MTS stock solution were pipetted into each well and incubated for additional 1–4 h. After this incubation period, the optical density (OD) was measured at 490 nm with an Envision reader (Perkin Elmer). Tumor cell survival (TCS) was calculated by using the following equation: TCS = (ODdrug-exposed well/mean ODcontrol wells) × 100%. The IC50 value, the drug concentration that is lethal to 50% of the tumor cells, was calculated from the appropriate dose-response curves in Dotmatics software.
Cell cycle and apoptosis analysis
Suspension of CCRF-CEM cells, seeded at a density of 1.106 cells/mL in 6-well panels, were cultivated with the 1 or 5 × IC50 of tested compound in a humidified CO2 incubator at 37°C in RPMI 1640 cell culture medium containing 10% fetal calf serum, 10 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Together with the treated cells, control sample containing vehicle was harvested at the same time point after 24 h. After another 24 hours, cells were then washed with cold PBS and fixed in 70% ethanol added dropwise and stored overnight at -20°C. Afterwards, cells were washed in hypotonic citrate buffer, treated with RNAse (50 μg/mL) and stained with propidium iodide. Flow cytometer using a 488 nm single beam laser (Becton Dickinson) was used for measurement. Cell cycle was analyzed in the program ModFitLT (Verity), and apoptosis was measured in logarithmic model expressing percentage of the particles with propidium content lower than cells in G0/G1 phase (<G1) of the cell cycle. Half of the sample was used for pH3Ser10 antibody (Sigma) labeling and subsequent flow cytometry analysis of mitotic cells [61].
BrDU incorporation analysis (DNA synthesis)
For this analysis, the same procedure of cultivation as previously was used. Before harvesting, 10 μM 5-bromo-2-deoxyuridine (BrDU), was added to the cells for puls-labeling for 30 min. Cells were fixed with ice-cold 70% ethanol and stored overnight. Before the analysis, cellswere washed with PBS, and resuspended in 2 M HCl for 30 min at room temperature to denature their DNA. Following neutralization with 0.1 M Na2B4O7 (Borax), cells were washed with PBS containing 0.5% Tween-20 and 1% BSA. Staining with primary anti-BrDU antibody (Exbio) for 30 min at room temperature in the dark followed. Cells were than washed with PBS and stained with secondary antimouse-FITC antibody (Sigma). Cells were then washed with PBS again and incubated with propidium iodide (0.1 mg/mL) and RNAse A (0.5 mg/mL) for 1 h at room temperature in the dark and afterwards analyzed by flow cytometry using a 488 nm single beam laser (FACSCalibur, Becton Dickinson) [61].
BrU incorporation analysis (RNA synthesis)
Cells were cultured and treated as above. Before harvesting, pulse-labeling with 1 mM 5-bromouridine (BrU) for 30 min followed. The cells were then fixed in 1% buffered paraformaldehyde with 0.05% of NP-40 in room temperature for 15 min, and then stored in 4°C overnight. Before measurement, they werewashed in PBS with 1% glycin, washed in PBS again, and stained by primary anti-BrDU antibody crossreacting to BrU (Exbio) for 30 min at room temperature in the dark. After another washing step in PBS cells were stained by secondary antimouse-FITC antibody (Sigma). Following the staining, cells were washed with PBS and fixed with 1% PBS buffered paraformaldehyde with 0.05% of NP-40 for 1 hour. Cells were washed by PBS, incubated with propidium iodide (0.1 mg/mL) and RNAse A (0.5 mg/mL) for 1 h at room temperature in the dark, and finally analyzed by flow cytometry using a 488 nm single beam laser (FACS Calibur, Becton Dickinson) [61].
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
All material, chemicals, and small equipment for both chemistry and biology was paid by Czech Science Foundation (15-05620S); stipendia to students were paid by internal grants of Palacky University IGA-PrF-2016-020, IGA-LF-2016-19. Starting terpenes were paid from the Technology Agency of the Czech Republic (TE01020028). The infrastructural part (Institute of Molecular and Translational Medicine) is supported by the National Sustainability Programme (LO1304). We are grateful to Tereza Volna for measurement of HRMS.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
All material, chemicals, and small equipment for both chemistry and biology was paid by Czech Science Foundation (15-05620S); stipendia to students were paid by internal grants of Palacky University IGA_PrF_2016_020, IGA_LF_2016_19. Starting terpenes were paid from the Technology Agency of the Czech Republic (TE01020028). The infrastructural part (Institute of Molecular and Translational Medicine) is supported by the National Sustainability Programme (LO1304). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hill RA, Connolly JD. Triterpenoids. Nat Prod Rep. 2015; 32(2): 273–327. 10.1039/c4np00101j [DOI] [PubMed] [Google Scholar]
- 2.Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, et al. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep. 2006; 23(3): 394–411. 10.1039/b515312n [DOI] [PubMed] [Google Scholar]
- 3.Sarek J, Kvasnica M, Vlk M, Biedermann D. Semisynthetic lupane triterpenes with cytotoxic activity In: Salvador Jorge A.R., editor. Pentacyclic triterpenes as promising agents in cancer. Hauppauge, N.Y.: Nova Science Publishers; 2010. p. 159–89. [Google Scholar]
- 4.Dang Z, Ho P, Zhu L, Qian K, Lee KH, Huang L, et al. New Betulinic Acid Derivatives for Bevirimat-Resistant Human Immunodeficiency Virus Type-1. J Med Chem. 2013. March 14; 56(5): 2029–37. 10.1021/jm3016969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo WJ, Dai HF, Chen J, Chen HQ, Zhao YX, Mei WL, et al. Triterpenes and Triterpenoid Saponins from the Leaves of Ilex kudincha. Planta Med. 2011; 77(16): 1835–40. 10.1055/s-0030-1271164 [DOI] [PubMed] [Google Scholar]
- 6.Innocente A, Casanova BB, Klein F, Lana AD, Pereira D, Muniz MN, et al. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem Biol Drug Des. 2014. March; 83(3): 344–9. 10.1111/cbdd.12251 [DOI] [PubMed] [Google Scholar]
- 7.Chianese G, Yerbanga SR, Lucantoni L, Habluetzel A, Basilico N, Taramelli D, et al. Antiplasmodial Triterpenoids from the Fruits of Neem, Azadirachta indica. J Nat Prod. 2010. August 27; 73(8): 1448–52. 10.1021/np100325q [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Zhou E, Wei Z, Liang D, Wang W, Wang T, et al. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014. June; 281(11): 2543–57. 10.1111/febs.12801 [DOI] [PubMed] [Google Scholar]
- 9.Yano S, Harada M, Watanabe K, Nakamaru K, Hatakeyama Y, Shibata S, et al. Antiulcer Activities of Glycyrrhetinic Acid Derivatives in Experimenta Glastric Lesion Models. Chem Pharm Bull. 1989; 37(9): 2500–4. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa T, Ninomiya K, Imura K, Yamaguchi T, Akagi Y, Yoshikawa M, et al. Hepatoprotective triterpenes from traditional Tibetan medicine Potentilla anserina. Phytochemistry. 2014. June; 102: 169–81. 10.1016/j.phytochem.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Quesada C, Lopez-Biedma A, Warleta F, Campos M, Beltran G, Gaforio JJ. Bioactive Properties of the Main Triterpenes Found in Olives, Virgin Olive Oil, and Leaves of Olea europaea. J Agric Food Chem. 2013. December 18; 61(50): 12173–82. 10.1021/jf403154e [DOI] [PubMed] [Google Scholar]
- 12.Urban M, Sarek J, Kvasnica M, Tislerova I, Hajduch M. Triterpenoid Pyrazines and Benzopyrazines with Cytotoxic Activity. J Nat Prod. 2007. April 1; 70(4): 526–32. 10.1021/np060436d [DOI] [PubMed] [Google Scholar]
- 13.Urban M, Vlk M, Dzubak P, Hajduch M, Sarek J. Cytotoxic heterocyclic triterpenoids derived from betulin and betulinic acid. Bioorg Med Chem. 2012. June 1; 20(11): 3666–74. 10.1016/j.bmc.2012.03.066 [DOI] [PubMed] [Google Scholar]
- 14.Vlk M, Micolova P, Urban M, Kvasnica M, Saman D, Sarek J. 15N-labelled pyrazines of triterpenic acids. J Radioanal Nucl Chem. 2016; 308(2): 733–9. [Google Scholar]
- 15.Urban M, Klinot J, Tislerova I, Biedermann D, Hajduch M, Cisarova I, et al. Reactions of Activated Lupane Oxo-Compounds with Diazomethane: An Approach to New Derivatives of Cytotoxic Triterpenes. Synthesis. 2006; 2006(23): 3979–86. [Google Scholar]
- 16.Kvasnica M, Urban M, Dickinson NJ, Sarek J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: synthesis and medicinal significance. Nat Prod Rep. 2015; 32(9): 1303–30. 10.1039/c5np00015g [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Li Z, Luo J, Yang F, Liu T, Liu M, et al. Synthesis and Biological Evaluation of Heterocyclic Ring-Fused Betulinic Acid Derivatives as Novel Inhibitors of Osteoclast Differentiation and Bone Resorption. J Med Chem. 2012. April 12; 55(7): 3122–34. 10.1021/jm201540h [DOI] [PubMed] [Google Scholar]
- 18.Csuk R, Stark S, Nitsche C, Barthel A, Siewert B. Alkylidene branched lupane derivatives: Synthesis and antitumor activity. Eur J Med Chem. 2012. July; 53: 337–45. 10.1016/j.ejmech.2012.04.023 [DOI] [PubMed] [Google Scholar]
- 19.Dinh Ngoc T, Moons N, Kim Y, De Borggraeve W, Mashentseva A, Andrei G, et al. Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities. Bioorg Med Chem. 2014. July 1; 22(13): 3292–300. 10.1016/j.bmc.2014.04.061 [DOI] [PubMed] [Google Scholar]
- 20.Ghosh P, Rasul MdG, Chakraborty M, Mandal A, Saha A. Microwave assisted one-pot synthesis of pyrazine derivatives of pentacyclic triterpenoids and their biological activity. Indian J Chem. Sect B-Org Chem Incl Med Chem. 2011; 50B(10): 1519–23. [Google Scholar]
- 21.Urban M, Kvasnica M, Dickinson NJ, Sarek J. Biologically Active Triterpenoids Usable As Prodrugs In: Bates AR, editor. Terpenoids and Squalene: Biosynthesis, Function and Health Implications. Hauppauge, N.Y.: Nova Science Publishers; 2015. p. 25–50. [Google Scholar]
- 22.Kommera H, Kaluderovic GN, Kalbitz J, Drager B, Paschke R. Small structural changes of pentacyclic lupane type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur J Med Chem. 2010. August; 45(8): 3346–53. 10.1016/j.ejmech.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 23.Willmann M, Wacheck V, Buckley J, Nagy K, Thalhammer J, Paschke R, et al. Characterization of NVX-207, a novel betulinic acid-derived anti-cancer compound. Eur J Clin Invest. 2009. May; 39(5): 384–94. 10.1111/j.1365-2362.2009.02105.x [DOI] [PubMed] [Google Scholar]
- 24.Gauthier C, Legault J, Lavoie S, Rondeau S, Tremblay S, Pichette A. Synthesis and Cytotoxicity of Bidesmosidic Betulin and Betulinic Acid Saponins. J Nat Prod. 2009. January 23; 72(1): 72–81. 10.1021/np800579x [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Li X, Gu G, Liu S, Cui M, Lou HX. Facile synthesis of triterpenoid saponins bearing β-Glu/Gal-(1→3)-β-GluA methyl ester and their cytotoxic activities. Bioorg Med Chem Lett. 2012. April 1; 22(7): 2396–400. 10.1016/j.bmcl.2012.02.032 [DOI] [PubMed] [Google Scholar]
- 26.Borkova L, Jasikova L, Rehulka J, Frisonsova K, Urban M, Frydrych I, et al. Synthesis of cytotoxic 2,2-difluoroderivatives of dihydrobetulinic acid and allobetulin and study of their impact on cancer cells. Eur J Med Chem. 2015. May 26; 96: 482–90. 10.1016/j.ejmech.2015.03.068 [DOI] [PubMed] [Google Scholar]
- 27.Flekhter OB, Karachurina LT, Poroikov VV, Nigmatullina LP, Baltina LA, Zarudii FS, et al. The synthesis and hepatoprotective activity of esters of the lupane group triterpenoids. Russ J Bioorg Chem. 2000; 26(3): 192–200. [PubMed] [Google Scholar]
- 28.Leunis JC, Couche E, inventors; Betulonic and Betulinic Acid Derivatives. 8586569. 2013.
- 29.Borkova L, Gurska S, Dzubak P, Burianova R, Hajduch M, Sarek J, et al. Lupane and 18α-oleanane derivatives substituted in the position 2, their cytotoxicity and influence on cancer cells. Eur J Med Chem. 2016. October 4; 121: 120–31. 10.1016/j.ejmech.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Jia X, Dong J, Chen D, Liu J, Zhang L, et al. Synthesis and evaluation of novel oleanolic acid derivatives as potential antidiabetic agents. Chem Biol Drug Des. 2014. March; 83(3): 297–305. 10.1111/cbdd.12241 [DOI] [PubMed] [Google Scholar]
- 31.Pertino WM, Lopez C, Theoduloz C, Schmeda-Hirschmann G. 1,2,3-Triazole-Substituted Oleanolic Acid Derivatives: Synthesis and Antiproliferative Activity. Molecules. 2013. July 1; 18(7): 7661–7674. 10.3390/molecules18077661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty B, Dutta D, Mukherjee S, Das S, Maiti NC, Das P, et al. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur J Med Chem. 2015. September 18; 102: 93–105. 10.1016/j.ejmech.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 33.Majeed R, Sangwan PL, Chinthakindi PK, Khan I, Dangroo NA, Thota N, et al. Synthesis of 3-O-propargylated betulinic acid and its 1,2,3-triazoles as potential apoptotic agents. Eur J Med Chem. 2013. May; 63: 782–92. 10.1016/j.ejmech.2013.03.028 [DOI] [PubMed] [Google Scholar]
- 34.Govdi AI, Sokolova NV, Sorokina IV, Baev DS, Tolstikova TG, Mamatyuk VI, et al. Synthesis of new betulinic acid-peptide conjugates and in vivo and in silico studies of the influence of peptide moieties on the triterpenoid core activity. Med Chem Commun. 2015; 6(1): 230–8. [Google Scholar]
- 35.Khan I, Guru SK, Rath SK, Chinthakindi PK, Singh B, Koul S, et al. A novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur J Med Chem. 2016. January 27; 108: 104–16. 10.1016/j.ejmech.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 36.Csuk R, Barthel A, Sczepek R, Siewert B, Schwarz S. Synthesis, Encapsulation and Antitumor Activity of New Betulin Derivatives. Arch Pharm Chem Life Sci. 2011. January 1; 344(1): 37–49. [DOI] [PubMed] [Google Scholar]
- 37.Csuk R, Barthel A, Kluge R, Ströhl D. Synthesis, cytotoxicity and liposome preparation of 28-acetylenic betulin derivatives. Bioorg Med Chem. 2010. October 15; 18(20): 7252–9. 10.1016/j.bmc.2010.08.023 [DOI] [PubMed] [Google Scholar]
- 38.Vasilevsky SF, Govdi AI, Sorokina IV, Tolstikova TG, Baev DS, Tolstikov GA, et al. Rapid access to new bioconjugates of betulonic acid via click chemistry. Bioorg Med Chem Lett. 2011. January 1; 21(1): 62–5. 10.1016/j.bmcl.2010.11.072 [DOI] [PubMed] [Google Scholar]
- 39.Govdi AI, Vasilevsky SF, Nenajdenko VG, Sokolova NV, Tolstikov GA. 1,3-Cycloaddition synthesis of 1,2,3-triazole conjugates of betulonic acid with peptides. Russ Chem Bull. 2011; 60(11): 2401–5. [Google Scholar]
- 40.Dang Thi TA, Kim Tuyet NT, Pham The C, Thanh Nguyen H, Ba Thi C, Doan Duy T, et al. Synthesis and cytotoxic evaluation of novel ester-triazole-linked triterpenoid-AZT conjugates. Bioorg Med Chem Lett. 2014. November 15; 24(22): 5190–4. 10.1016/j.bmcl.2014.09.079 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Hernández D, Demuner AJ, Barbosa LC, Heller L, Csuk R. Novel hederagenin-triazolyl derivatives as potential anti-cancer agents. Eur J Med Chem. 2016. June 10; 115: 257–67. 10.1016/j.ejmech.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 42.Rashid S, Dar BA, Majeed R, Hamid A, Bhat BA. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur J Med Chem. 2013. August; 66: 238–45. 10.1016/j.ejmech.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Hernández D, Barbosa LC, Demuner AJ, de Almeida RM, Fujiwara RT, Ferreira SR. Highly potent anti-leishmanial derivatives of hederagenin, a triperpenoid from Sapindus saponaria L. Eur J Med Chem. 2016. November 29; 124: 153–159. 10.1016/j.ejmech.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 44.Gruza M, Jatczak K, Komor K, Świerk P, Szeja W, Grynkiewicz G. Synthesis of protoescigenin glycoconjugates with O-28 triazole linker. Acta Pol Pharm. 2014. Nov-Dec; 71(6): 959–65. [PubMed] [Google Scholar]
- 45.Wei G, Luan W, Wang S, Cui S, Li F, Liu Y, Liu Y, Cheng M. A library of 1,2,3-triazole-substituted oleanolic acid derivatives as anticancer agents: design, synthesis, and biological evaluation. Org Biomol Chem. 2015. February 7;13(5):1507–14. 10.1039/c4ob01605j [DOI] [PubMed] [Google Scholar]
- 46.Antimonova A, Petrenko N, Shakirov M, Rybalova T, Frolova T, Shul'ts E, et al. Synthesis and study of mutagenic properties of lupane triterpenoids containing 1,2,3-triazole fragments in the C-30 position. Chem Nat Compd. 2013. September; 49(4): 657–64. [Google Scholar]
- 47.Shi W, Tang N, Yan WD. Synthesis and cytotoxicity of triterpenoids derived from betulin and betulinic acid via click chemistry. J Asian Nat Prod Res. 2015; 17(2): 159–69. 10.1080/10286020.2014.979164 [DOI] [PubMed] [Google Scholar]
- 48.Spivak AY, Gubaidullin RR, Galimshina ZR, Nedopekina DA, Odinokov VN. Effective synthesis of novel C(2)-propargyl derivatives of betulinic and ursolic acids and their conjugation with β-d-glucopyranoside azides via click chemistry. Tetrahedron. 2016. March 3; 72(9): 1249–56. [Google Scholar]
- 49.Parida PK, Sau A, Ghosh T, Jana K, Biswas K, Raha S, Misra AK. Synthesis and evaluation of triazole linked glycosylated 18β-glycyrrhetinic acid derivatives as anticancer agents. Bioorg Med Chem Lett. 2014. August 15; 24(16): 3865–8. 10.1016/j.bmcl.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 50.Waring MJ. Lipophilicity in drug discovery. Expert Opin Drug Discov. 2010. March; 5(3): 235–48. 10.1517/17460441003605098 [DOI] [PubMed] [Google Scholar]
- 51.Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O'Donnell JP. Biotransformation reactions of five-membered aromatic heterocyclic rings. Chem Res Toxicol. 2002. March; 15(3): 269–99. [DOI] [PubMed] [Google Scholar]
- 52.Urban M, Sarek J, Tislerova I, Dzubak P, Hajduch M. Influence of esterification and modification of A-ring in a group of lupane acids on their cytotoxicity. Bioorg Med Chem. 2005. October 1; 13(19): 5527–35. 10.1016/j.bmc.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 53.Kim DSHL, Pezzuto JM, Pisha E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg Med Chem Lett. 1998. July 7; 8(13): 1707–12. [DOI] [PubMed] [Google Scholar]
- 54.Qian K, Kim SY, Hung HY, Huang L, Chen CH, Lee KH. New betulinic acid derivatives as potent proteasome inhibitors. Bioorg Med Chem Lett. 2011. October 1; 21(19): 5944–7. 10.1016/j.bmcl.2011.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uzenkova NV, Petrenko NI, Shakirov MM, Shul'ts EE, Tolstikov GA. Synthesis of 30-Amino Derivatives of Lupane Triterpenoids. Chem Nat Compd. 2005; 41(6): 692–700. [Google Scholar]
- 56.Srinivasan T, Srivastava GK, Pathak A, Batra S, Raj K, Singh K, et al. Solid-phase synthesis and bioevaluation of Lupeol-based libraries as antimalarial agents. Bioorg Med Chem Lett. 2002. October 21; 12(20): 2803–6. [DOI] [PubMed] [Google Scholar]
- 57.Soural M, Hodon J, Dickinson NJ, Sidova V, Gurska S, Dzubak P, et al. Preparation of Conjugates of Cytotoxic Lupane Triterpenes with Biotin. Bioconjugate Chem. 2015. December 16; 26(12): 2563–70. [DOI] [PubMed] [Google Scholar]
- 58.Noskova V, Dzubak P, Kuzmina G, Ludkova A, Stehlik D, Trojanec R, et al. In vitro chemoresistance profile and expression/function of MDR associated proteins in resistant cell lines derived from CCRF-CEM, K562, A549 and MDA MB 231 parental cells. Neoplasma. 2002; 49(6): 418–25. [PubMed] [Google Scholar]
- 59.Kim HK, Kong MY, Jeong MJ, Han DC, Choi JD, Kim HY, et al. Investigation of cell cycle arrest effects of actinomycin D at G1 phase using proteomic methods in B104-1-1 cells. Int J Biochem Cell Biol. 2005. September; 37(9): 1921–9. 10.1016/j.biocel.2005.04.015 [DOI] [PubMed] [Google Scholar]
- 60.Patro JN, Urban M, Kuchta RD. Interaction of Human DNA Polymerase alpha and DNA Polymerase I from Bacillus stearothermophilus with Hypoxanthine and 8-Oxoguanine Nucleotides Biochemistry 2009. September 1; 48(34): 8271–8. 10.1021/bi900777s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bourderioux A, Nauš P, Perlíková P, Pohl R, Pichová I, Votruba I, et al. Synthesis and Significant Cytostatic Activity of 7-Hetaryl-7-deazaadenosines. J Med Chem. 2011. August 11; 54(15): 5498–507. 10.1021/jm2005173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.