Abstract
This is a follow-up to our previous study using an intranasal vaccine composed of MrpH, the tip adhesin of the MR/P fimbria, and cholera toxin to prevent urinary tract infection by Proteus mirabilis (X. Li, C. V. Lockatell, D. E. Johnson, M. C. Lane, J. W. Warren, and H. L. Mobley, Infect. Immun. 72:66-75, 2004). Here, we have expressed a cholera toxin-like chimera in which the MrpH adhesin-binding domain (residues 23 to 157) replaces the cholera toxin A1 ADP-ribosyltransferase domain. This chimera, when administered intranasally without additional adjuvant, is sufficient to induce protective immunity in mice.
Proteus mirabilis is a common cause of urinary tract infection in patients with functional or structural abnormalities in the urinary tract and in patients with long-term urethral catheterization (10). The MR/P (for mannose-resistant/Proteus-like) fimbria is an important bladder colonization factor of P. mirabilis (8). In cochallenge experiments, a mutant unable to express MR/P fimbriae was out-competed by the wild-type strain in the bladder and the kidneys, whereas a mutant constitutively expressing MR/P fimbriae out-competed the wild-type strain in the bladder but not in the kidneys.
MrpH, the tip adhesin of MR/P fimbria, is composed of two functional domains: an N-terminal receptor-binding domain and a C-terminal pilin domain (6). In a previous study, the receptor-binding domain of MrpH (residues 23 to 157) was expressed as a C-terminal fusion to maltose-binding protein (MBP) (7). This recombinant fusion protein, designated as MHT (for maltose-binding protein fusion of MrpH truncate), was purified on an amylose column, covalently conjugated to cholera toxin (CT), and then used to immunize mice intranasally. Compared to naive mice, immunized mice were significantly protected from urinary tract infection following transurethral challenge by P. mirabilis (7).
Cholera toxin is a powerful mucosal adjuvant that induces immune responses to coadministered protein antigens after oral or intranasal immunization (1). The mechanism by which cholera toxin stimulates immune responses is not completely understood. Earlier studies suggested that the adjuvant effect of cholera toxin is associated with the ADP-ribosyltransferase activity of cholera toxin A subunit (9). Recent studies of nontoxic mutants of cholera toxin that lack ADP-ribosyltransferase activity and holotoxin-like chimeras, however, indicated that the toxicity of cholera toxin is not absolutely required for its adjuvant effect (1, 2, 12). Cholera toxin B subunit (CTB), the ganglioside GM1-binding moiety of cholera toxin, has significant mucosal adjuvanticity, especially when administered via the intranasal route (5, 11).
In this study, we investigated whether cholera toxin B subunit can be used as a mucosal adjuvant to stimulate a protective immune response against MHT. Taking the same chemical approach described in our previous study (7), we cross-linked MHT to CTB by using the heterobifunctional coupling reagent N-succinimidyl 3-(2-pyridyl dithio) propionate. CTB was purchased from Sigma, and N-succinimidyl 3-(2-pyridyl dithio) propionate was purchased from Amersham Pharmacia Biotech. Purification of MHT and conjugation of MHT to CTB were described previously (7).
Vaccination with MHT.
Two independent vaccination experiments were carried out to examine whether MHT conjugated with cholera toxin B subunit alone is sufficient to induce protective immunity when administered intranasally to mice (Fig. 1). Four groups of six mice each were used in each experiment: one group of naive mice and three groups of intranasally immunized mice. All immunized mice were given primary immunization on day 0 and two booster immunizations on days 14 and 24, at a dose of 10 μg of antigen per mouse each time. On day 34, mice were transurethrally challenged with 5 × 107 CFU of the wild-type P. mirabilis strain HI4320 in a volume of 50 μl. One week after challenge, the mice were sacrificed and bacteria in the bladder and kidneys were quantitatively cultured. The detection limit of our quantitative assay is 100 CFU/g of tissue. This murine model of ascending urinary tract infection has been described previously (7).
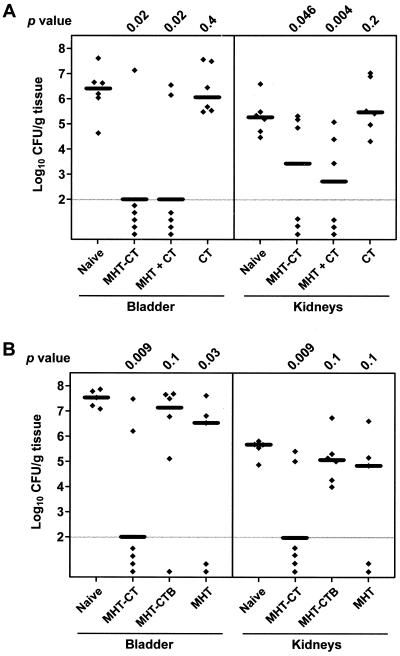
FIG. 1.
P. mirabilis colonization in bladders and kidneys of (A) naive mice and mice immunized with MHT-CT, MHT + CT, or CT and (B) naive mice and mice immunized with MHT-CT, MHT-CTB, or MHT. Each diamond represents the log10 CFU per gram of tissue from an individual mouse. Samples with undetectable colonization were given a value of 2 log10 CFU/g of tissue (the limit of detection). Horizontal bars represent the median log10 CFU per gram of tissue for each column. One-tailed P values were determined by the Mann-Whitney test, comparing the colonization levels in bladders and kidneys of the naive mice with those of the immunized mice.
In one experiment (Fig. 1A), mice were immunized with MHT covalently coupled to CT (MHT-CT), coadministered MHT and CT (MHT + CT), or administered CT alone (CT). Mice immunized with MHT-CT were colonized by significantly fewer bacteria in the bladder (median log10 CFU per gram of tissue, 6.43 for the naive mice versus 2.00 for the immunized mice; P = 0.02) and the kidneys (median log10 CFU per gram of tissue, 5.28 for the naive mice versus 3.43 for the immunized mice; P = 0.046). This result is consistent with our previous findings (7). It should be noted that our limit of detection was 100 CFU; thus, it is possible that sterile immunity resulted from immunization, but this was not determined. Compared to the naive mice, mice coadministered MHT and CT were also colonized by significantly fewer bacteria in the bladder (median log10 CFU per gram of tissue, 2.00; P = 0.02) and the kidneys (median log10 CFU per gram of tissue, 2.72; P = 0.004). Compared to the naive mice, mice administered CT alone were equally susceptible to bladder (median log10 CFU per gram of tissue, 6.08; P = 0.4) and kidney (median log10 CFU per gram of tissue, 5.48; P = 0.2) colonization by P. mirabilis.
In another experiment (Fig. 1B), mice were immunized with MHT-CT, MHT covalently coupled to CTB (MHT-CTB), or MHT alone (MHT). Following bacterial challenge, one of the naive mice and one of the mice immunized with MHT died before the day of sacrifice, likely due to severe blockage of urine flow caused by P. mirabilis infection-induced stone formation. Again, mice immunized with MHT-CT were colonized by significantly fewer bacteria in the bladder (median log10 CFU per gram of tissue, 7.53 for the naive mice versus 2.00 for the immunized mice; P = 0.009) and the kidneys (median log10 CFU per gram of tissue, 5.68 for the naive mice versus 2.00 for the immunized mice; P = 0.009). Mice immunized with MHT-CTB were susceptible to colonization by P. mirabilis (median log10 CFU per gram of tissue, 7.14 in bladder and 5.07 in kidneys). Compared to the naive mice, the mice immunized with MHT-CTB showed no statistically significant reduction in either bladder (P = 0.1) or kidney (P = 0.1) colonization by P. mirabilis. Furthermore, like the naive mice, all mice immunized with MHT-CTB were colonized by P. mirabilis in both kidneys. Mice immunized with MHT (no adjuvant), compared to the naive mice, were colonized by fewer bacteria in the bladder (median log10 CFU per gram of tissue, 6.53; P = 0.03) but not in the kidneys (median log10 CFU per gram of tissue, 4.84; P = 0.1). However, the biological significance of this modest reduction in bladder colonization was further diminished because one of the MHT-immunized mice died of P. mirabilis infection before the day of sacrifice.
The results of both experiments together show that when administered alone, neither MHT nor CT had any effect on protecting mice from urinary tract infection by P. mirabilis. However, in the form of either coadminstration or covalent coupling, immunization with MHT and CT together was shown to significantly protect mice from both bladder and kidney colonization by P. mirabilis. CTB, on the other hand, did not have the same effect as CT; mice immunized with MHT covalently coupled with CTB were still susceptible to urinary traction infection by P. mirabilis.
Expression of the MrpH23-157-holotoxin-like chimera (HA2-B).
We have also taken a genetic approach to antigen preparation by using a translational fusion system that results in assembly of a CT-like chimera. The CT-like chimera contains a microbial protein domain (in this case, residues 23 to 157 of MrpH), to be tested as a protective antigen, in place of the toxic A1 domain of wild-type CT. The receptor-binding activity of the wild-type pentameric CTB subunit facilitates highly efficient delivery of the entire CT-like chimera, including the MrpH truncate protective antigen, to the systemic and mucosal immune systems. Furthermore, the pentameric CTB subunit provides CTA1-independent adjuvant effects that contribute to the overall immunogenicity of the CT-like chimera. Advantages of CT-like chimeras as vaccines include their strong immunogenicity and their potential capacity to induce both mucosal and long-lasting systemic immune responses following mucosal administration. Lastly, unlike antigen preparations covalently attached to CT by biochemical cross-linking, the CT-like chimera described in this report is a defined homogenous structure.
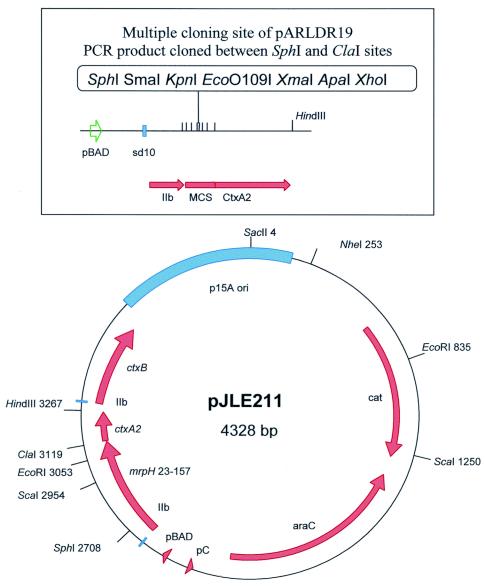
A DNA fragment encoding the receptor-binding domain of MrpH (amino acid residues 23 to 157) was PCR amplified and translationally fused at sequences encoding its N terminus to the signal peptide of the B subunit of Escherichia coli heat-labile enterotoxin LTIIb and at sequences encoding its C terminus to the nontoxic cholera toxin A2 domain (4). The DNA fragment encoding MrpH residues 23 to 157 was amplified by PCR from pXL5802 (6) by using primers MRPH23F (CAGCGCATGCGATGGCCTC) and MRPH157R (GCGTATCGATGAACTCACAG), which incorporate SphI and ClaI restrictions sites, respectively (underlined). This enabled cloning of the DNA encoding MrpH residues 23 to 157 in frame after the LTIIb B gene leader sequence and before the ctxA2 sequence in the vectors pLDR19 and pARLDR19 (unpublished data). Plasmids pLDR19 and pARLDR19 contain a multiple cloning site between the LTIIb B gene leader and ctxA2-coding sequences, followed by a second LTIIb B gene leader and mature ctxB gene sequences. Plasmid pLDR19 is IPTG (isopropyl-β-d-thiogalactopyranoside) inducible and kanamycin resistant, with a pMB1-based replication origin. Plasmid pARLDR19 (arabinose inducible, chloramphenicol resistant, p15a-based origin) has the multiple cloning site and ctxA2 sequences cloned in place of the sequence encoding mature CTA of pARCT2 (a precursor to the CT holotoxin-encoding vector pARCT4, which has been described previously [3]). The SphI-ClaI-digested PCR fragment encoding MrpH23-157 was cloned into similarly cut pLDR19 to create pJMS1. The same fragment was then subcloned into pARLDR19 to create pJLE211 (Fig. 2). In this clone the mrpH23-157::ctxA2 fusion and ctxB genes are expressed from the T7 gene 10 ribosome binding site (sd10), and the corresponding proteins are directed to the periplasm by the LTIIb B gene signal sequences (IIb). Expression of this operon is under control of the ara operon promoter (pBAD) and AraC.
FIG. 2.
Construction of pJLE211, carrying the mrpH23-157-ctxA2 translational fusion and ctxB. A PCR fragment carrying mrpH23-157 was cloned into the SphI and ClaI restriction sites within the multiple cloning site (MCS) that is immediately upstream of ctxA2 (see text for details). IIb, LTIIb B gene leader sequence; pBAD, inducible arabinose promoter; pC, araC promoter; cat, chloramphenicol acetyltransferase; p15A ori, origin of replication; sd10, T7 gene 10 ribosome binding site.
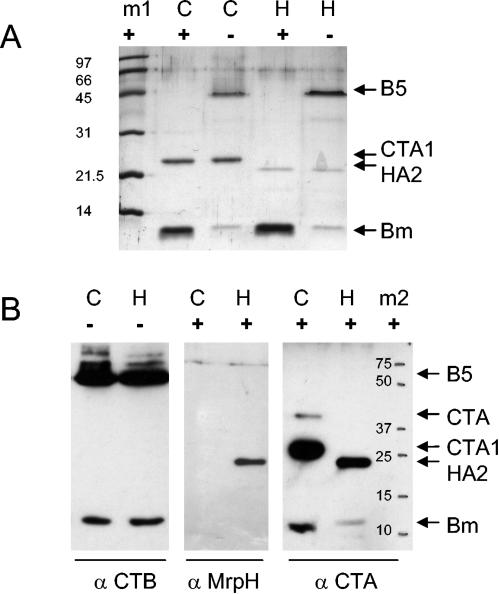
To evaluate the composition of the chimeric protein, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were performed with and without sample denaturation. Rabbit serum against MrpH was prepared previously (7). Rabbit anti-CTA serum (B09) was prepared from anti-cholera toxin serum by adsorption with pentameric CTB and retained weak reactivity with CTB monomer (3). Rabbit anti-CTB (B10) was raised by immunization with pentameric choleragenoid (3). On a Western blot (Fig. 3B), the majority of CTB in the preparation was pentameric, although a minor band of monomer was seen in unboiled CT holotoxin preparations, as is typically observed. Since the complex was purified by galactose affinity, which is specific for B, the presence of the extra band (HA2) is proof of assembly of a complex. Regarding stoichiometry, from the intensities of the A and B bands on the Coomassie blue-stained gel (Fig. 3A), comparing CT and HA2-B, it appears that the HA2-B complex still contains free CTB pentamer. The Western blot shows the presence of MrpH and CTA reactivity in the non-B band of the preparation, indicating the presence of the HA2 fusion protein in the complex. These observations are consistent with the assembly of a CT-like chimera in which the HA2 translational fusion is complexed to pentameric CTB.
FIG. 3.
Western blotting of vaccine preparations. (A) Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels of purified HA2-B chimera (H) or cholera toxin (C). Protein samples in Laemmli sample buffer with 5% 2-mercaptoethanol were boiled (+) or not boiled (−) prior to electrophoresis. Arrows indicate the positions of pentameric CTB (B5), CTA1, HA2, and CTB monomer (Bm). m1, Bio-Rad low-molecular-weight protein standards (in kilodaltons). (B) Western blots of HA2-B chimera (H) or cholera toxin (C), boiled (+) or not boiled (−), with rabbit antisera (α) specific for CTB, MrpH, or CTA subunits. Bound antibody was developed with horseradish peroxidase-conjugated goat anti-rabbit serum and detected by chemiluminescence. m2, positions of Bio-Rad Precision Plus markers (in kilodaltons).
Vaccination with the MrpH23-157-holotoxin-like chimera (HA2-B).
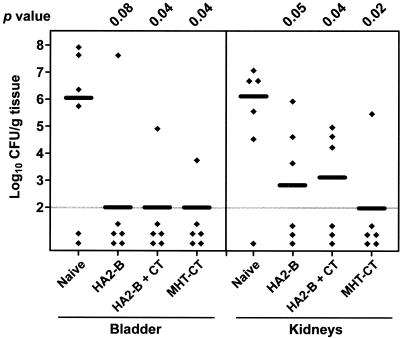
To test whether intranasal immunization with HA2-B can induce a protective immune response, four groups of nine mice each were used, including a group of naive mice and groups of mice intranasally immunized with HA2-B, HA2-B plus free cholera toxin (HA2-B + CT), or MHT-CT (Fig. 4). Immunization was carried out as described for the first set of experiments. Again, the group of mice immunized with MHT-CT was colonized by a significantly lower number of bacteria in the bladder (median log10 CFU per gram of tissue, 6.04 for the naive mice versus 2.00 for the immunized mice; P = 0.04) and the kidneys (median log10 CFU per gram of tissue, 5.04 for the naive mice versus 2.00 for the immunized mice; P = 0.004) (Fig. 4). Compared to the naive mice, the groups of mice immunized with HA2-B or HA2-B plus free cholera toxin were also colonized by fewer bacteria in the bladder and the kidneys (Fig. 4). Indeed, the colonization levels in the bladders and kidneys of all three groups of immunized mice were not statistically different from each other (P > 0.2). The CT-like chimera HA2-B was capable of inducing a protective immune response when administered intransally with or without addition of free cholera toxin. This suggests that the ADP-ribosyltransferase activity of the A subunit is not necessary for the adjuvant effect of cholera toxin.
FIG. 4.
P. mirabilis colonization in bladders and kidneys of naive mice and mice immunized with HA2-B, HA2-B + CT, or MHT-CT. Each diamond represents the log10 CFU per gram of tissue from an individual mouse. Samples with undetectable colonization were given a value of 2 log10 CFU/g of tissue (the limit of detection). Horizontal bars represent the median log10 CFU per gram of tissue for each column. One-tailed P values were determined by the Mann-Whitney test, comparing the colonization levels in bladders and kidneys of the naive mice with those of the immunized mice.
Clearly, the CT-like chimera HA2-B is much more effective than antigen chemically linked to CTB (MHT-CTB). In fact, mice immunized with MHT-CTB were not protected from bladder and kidney colonization by P. mirabilis. One obvious difference between these two constructs is how they were prepared, one through translational fusion and the other through chemical conjugation. The chemical conjugation process may compromise the adjuvanticity of CTB. Yet, the same process does not diminish the adjuvanticity of CT. Another difference between these two constructs is that unlike MHT-CTB, which is a mixture of various conjugates, HA2-B is homogeneous in terms of the A1B5 assembly. However, we do not understand how this structural difference between the two constructs could account for the difference in their ability to induce protective immunity.
In summary, we have constructed and expressed a stable CT-like chimera in which the cholera toxin A1 ADP-ribosyltransferase domain has been replaced by the MrpH adhesin-binding domain of MR/P fimbriae of P. mirabilis. This chimeric protein was purified and used to intranasally vaccinate mice, and it protected them from transurethral challenge with P. mirabilis HI4320. The vaccine protected with no additional adjuvant. The study confirms that ADP-ribosylating activity of cholera toxin is not required for adjuvanticity. We report the successful production of an antigen-adjuvant construct that expresses a single protein that can be purified and used directly as an effective intranasal vaccine to prevent infection of the urinary tract by P. mirabilis, a common cause of complicated urinary tract infections.
Acknowledgments
This work was supported by Public Health Service grants DK49720 (H.L.T.M.) and AI31940 (R.K.H.) from the National Institutes of Health.
Editor: A. D. O'Brien
REFERENCES
- 1.Eriksson, K., and J. Holmgren. 2002. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 14:666-672. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 3.Jobling, M. G., and R. K. Holmes. 2001. Biological and biochemical characterization of variant A subunits of cholera toxin constructed by site-directed mutagenesis. J. Bacteriol. 183:4024-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobling, M. G., and R. K. Holmes. 1992. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect. Immun. 60:4915-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, A., and M. Chen. 1994. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 62:3594-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, X., D. E. Johnson, and H. L. Mobley. 1999. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, X., C. V. Lockatell, D. E. Johnson, M. C. Lane, J. W. Warren, and H. L. Mobley. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect. Immun. 72:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, X., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol Microbiol. 45:865-874. [DOI] [PubMed] [Google Scholar]
- 9.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 10.Mobley, H. L., and R. Belas. 1995. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 3:280-284. [DOI] [PubMed] [Google Scholar]
- 11.Shen, X., T. Lagergard, Y. Yang, M. Lindblad, M. Fredriksson, and J. Holmgren. 2000. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine. Infect. Immun. 68:5749-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]