Abstract
There is a growing interest in identifying natural food ingredients that may serve to prevent dementia such as that due to Alzheimer disease (AD). Peptides derived from food proteins have been demonstrated to have various physiological activities such as a hypotensive action. Recent findings have indicated possible associations of hypertension with AD progression, and suggest that angiotensin converting enzyme (ACE) inhibitors with potential to pass through the blood brain barrier (BBB) may reduce the risk of AD. In this study, we investigated the effect of milk peptide (CH-3) on cognitive function in AD model mice. CH-3 contains a tripeptide (methionine-lysine-proline, MKP) that has been found to have a strong ACE inhibitory effect and the potential to pass through the BBB. Adult male ddY mice were used in this study, and an animal model of AD was induced by intracerebroventricular (ICV) injection of Aβ1–42. CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day) was orally administered every day starting 2 days before ICV injection. At 3 weeks after ICV injection, cognitive function was evaluated by the Morris water maze test. Brain samples were obtained after behavioral testing, and expression of inflammatory cytokines and NADPH oxidase subunits was measured by real-time quantitative RT-PCR. ICV injection of Aβ1–42 significantly impaired cognitive function compared with that in PBS-injected mice. Daily administration of CH-3 markedly attenuated this Aβ1-42-induced cognitive decline. Aβ1–42 injection significantly enhanced the expression of tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS) and p22phox in the mouse hippocampus compared with PBS injection, and showed a tendency to increase the expression of monocyte chemoattractant protein-1 (MCP-1), p47phox and gp91phox, whereas CH-3 treatment markedly reduced Aβ1-42-induced TNF-α, MCP-1, iNOS, p47phox and gp91phox expression. Finally, administration of MKP also attenuated Aβ1-42-induced cognitive impairment with an increase in cerebral blood flow. The present study demonstrated that repeated oral administration of CH-3 to AD model mice not only improved cognitive function but also suppressed the expression of inflammatory cytokines and production of oxidative stress, and suggests its therapeutic potential for preventing cognitive impairment in AD.
Introduction
Alzheimer disease (AD), an irreversible progressive neurodegenerative disorder, is one of the most prevalent neurodegenerative diseases in aging societies. It is associated with memory loss, and its typical symptoms are memory impairment and cognitive decline. It is becoming an increasing burden on not only patients but also their families. In the brain of AD patients, Aβ deposition is a crucial pathological event [1]. Aβ deposition in the brain has been suggested to cause oxidative damage and neuroinflammation, which are closely associated with progression of AD [2–4]. Once the disease becomes clinically obvious, neuronal loss might be too advanced for treatment, and it is becoming more important to prevent the onset of AD through improving lifestyle or diet. Therefore, there is growing interest in identifying possible natural food ingredients that can prevent AD onset and progression.
Many kinds of peptides derived from foods have been reported to have various physiological effects such as a hypotensive action [5,6]. Due to their beneficial health and safety properties, bioactive peptides derived from milk proteins have been well studied by many researchers [7]. We previously reported that bovine casein hydrolysate which is produced by 3 enzymes (“CH-3”) has an antihypertensive effect in vitro and in vivo [8,9]. CH-3 shows angiotensin-converting enzyme (ACE) inhibitory activity, and contains the tripeptide Met-Lys-Pro (MKP) which is the strongest ACE inhibitory peptide found in foods. MKP accounts for more than 30% of the ACE-inhibitory activity of CH-3 and plays a major role in the antihypertensive activity of CH-3 [9].
Recent findings have indicated an association between the brain renin-angiotensin system (RAS) and AD [10]. Angiotensin II (Ang II) is the principal substance of RAS and has a variety of physiological functions [11]. Ang II is generated by ACE, and interestingly the activity of ACE is elevated in the brain of AD patients [12]. The up-regulation of ACE activity may lead to an increase in Ang II level [12]. Brain Ang II is reported to induce reactive oxygen species through NADPH oxidase, and subsequently many transcription factors important for inflammation are activated [13]. Ang II may impair cognitive performance, lower acetylcholine release, and also block the induction of long-term potentiation (LTP) [14]. Some studies have shown beneficial effects of RAS inhibitors on cognitive decline both in AD model animals and AD patients [15–19]. Furthermore, many researchers have suggested that some ACE inhibitors and Ang II receptor blockers (ARBs) have a beneficial effect to prevent cognitive decline in AD model mice [15,17,19]. Ohrui et al. reported that long-term use of a centrally active ACE inhibitor slowed the rate of cognitive decline in patients with mild to moderate AD, and could have a protective effect against the development of AD [16]. Kehoe et al. [10] discussed brain RAS blockade as a possible new treatment option for AD disease.
As mentioned above, the milk peptide CH-3 has an ACE inhibitory effect, and an ACE inhibitor could have a protective effect against the development of AD. In this study, we investigated the possibility that administration of CH-3 could have a preventive effect on cognitive decline in a mouse model of AD. We also examined its potential effects on neuroinflammation and oxidative stress, focusing on the tripeptide MKP, which has a strong ACE inhibitory property.
Materials and methods
All procedures were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. The experimental protocol was reviewed and approved by the Animal Studies Committee of Ehime University.
Animals and treatment
Male 10-week-old ddY mice (SLC, Inc., Japan) were used in this study. Male 10-week-old SHRs (SHR/Hos, SPF) were purchased from Hoshino Laboratory Animals, Inc. (Ibaraki, Japan). These animals were housed in a room in which lighting was controlled (12 hours on and 12 hours off) and room temperature was kept at 25°C. ddY mice were given a MF diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. SHR rats were fed a MR stock diet (Nihon Nosan Kogyo Co., Ltd., Kanagawa, Japan) and had continuous access to tap water. CH-3 was prepared and provided by Morinaga Milk Industry Co., Ltd. as described previously [9]. Briefly, hydrolysis of lactic casein (Fonterra Co., Ltd., Auckland, New Zealand) was conducted by incubation for 8 hours at 50°C with three different enzymes: Bioprase (EC 3.4.21.62, accepted name: subtilisin, Nagase ChemteX Co., Ltd., Osaka, Japan), Protease N ‘‘Amano” (EC 3.4.24.28, accepted name: bacillolysin, Amano Enzyme Inc., Nagoya, Japan), and PTN6.0S (EC 3.4.4.4, Pancreatic Trypsin Novo, Novozymes Japan Co., Ltd., Chiba, Japan). The reaction was stopped by inactivating the enzymes at 80°C for 6 min. The hydrolysates were spray dried and used for further study. MKP content of CH-3 is 0.045%. The composition of CH-3 is shown in Table 1. Met-Lys-Pro (MKP) was prepared by solid-phase synthesis by Shimadzu Scientific Research Inc. (Kyoto, Japan). CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day) was orally administered every day starting 2 days before Aβ1–42 injection.
Table 1. Composition of CH-3.
| Component | Composition of CH (g/100g) |
|---|---|
| Protein | 85.0 |
| Fat | 0.0 |
| Carbohydrate | 4.0 |
| Ash | 7.0 |
| Moisture | 4.0 |
| Total | 100 |
Intracerebroventricular injection of Aβ1–42
Intracerebroventricular (ICV) injection was performed as described previously with some modification [19]. Briefly, each mouse was fixed in a stereotactic frame, anesthetized with Nembutal in saline, and a 28-gauge needle was inserted unilaterally 1 mm to the right of the midline, 0.2 mm posterior to the bregma and 2.5 mm deep to the skull surface. Aβ1–42 (Peptide Institute, Osaka, Japan) dissolved in PBS was injected intracerebroventricularly at 200 pmol in 3 μl PBS at rate of 1 μl/min using a syringe pump. After the injection, the needle was held in the original location for an additional 3 minutes and then withdrawn. For vehicle control mice, 3 μl PBS was injected. The body temperature was maintained with a heat lamp throughout the procedure and recovery. After they were completely alert, mice were returned to their home room, and normal food and water were given. General locomotor activity and diet volume of mice were checked daily and not changed significantly among all groups until sample preparation. Mice were weighed before and one week after ICV injection, and immediately before each analysis, and showed no significant difference at each time point in each group. In addition, we did not observe a significant change in systolic blood pressure among all groups during reagent administration.
Morris eater maze test
Spatial learning as a measure of cognitive function of mice at 3 weeks after injection of Aβ1–42 or PBS was evaluated by the Morris water maze test as described previously [19,20]. In brief, each mouse was trained 5 times a day at 20-minute intervals for 5 consecutive days. The test was performed blindly. In each trial, mice were given 120 seconds to find the platform. Swimming was video tracked (AnyMaze, Wood Dale, IL, USA), and latency, path length, swim speed, and cumulative distance from the platform were recorded. Mean swim latency for all of the trials on each day in each group was calculated.
Cerebral blood flow
After the Morris water maze test, cerebral blood flow (CBF) was determined by laser speckle flowmetry (Omegazone, laser speckle blood flow imager, Omegawave, Tokyo, Japan) as described previously [21]. Mice were anesthetized with Nembutal in saline, and a midline incision was made in the scalp. Anesthesia did not significantly modify blood pressure. The skull was exposed and wet with saline. A 780-nm laser semiconductor laser illuminated the whole skull surface. Mean CBF was measured on the skull surface. Light intensity was accumulated in a charge-coupled device camera and transferred to a computer for analysis. Image pixels were analyzed to produce average perfusion values.
Real-time quantitative RT-PCR
After CBF measurement, the mouse brain was removed after cardiac perfusion with ice-cold saline. The hippocampus were taken out and frozen in liquid nitrogen and stored at -80°C until use. Total RNA was extracted from the hippocampus with Sepasol reagent (Nacalai Tesque, Inc., Kyoto, Japan). Real-time quantitative RT-PCR was performed using SYBR Premix Ex Taq (Takara Bio Inc., Japan). The level of target gene expression was normalized against expression of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GADPH), in each sample. PCR primers were as follows: tumor necrosis factor-α (TNF-α), 5’-CGAGTGACAAGCCTGTAGCC-3’ (forward) and 5’GGTGAGGAGCACGATGTCG-3’ (reverse); monocyte chemotactic and activating factor-1 (MCP-1), 5’-TTAACGCCCCACTCACCTGCTG-3’ (forward) and 5’-GCTTCTTTGGGACACCTGCTGC-3’ (reverse); interleukin-6 (IL-6) 5’-CCACTTCACAAGTCGGAGGCTTA-3’ (forward) and 5’-GCAAGTGCATCATCGTTGTTCATAC-3’ (reverse); p22phox, 5’-TGGCTACTGCTGGACGTTTCAC-3’ (forward) and 5’-CTCCAGGAGACAGATGAGCACAC-3’ (reverse); p47phox, 5’-GTCCCTGCATCCTATCTGGA-3’ (forward) and 5’-GGGACATCTCGTCCTCTTCA-3’ (reverse); p67phox, 5’-CAGACCCAAAACCCCAGAAA-3’ (forward) and 5’-AGGGTGAATCCGAAGCTCAA-3’ (reverse); gp91phox, 5’-TGGGATCACAGGAATTGTCA-3’ (forward) and 5’-CTTCCAAACTCTCCGCAGTC-3’ (reverse); inducible nitric oxide synthase (iNOS), 5’-GTCACCTACCGCACCCGAG-3’ (forward) and 5’-GCCACTGACACTTCGCACAA-3’ (reverse); endothelial NOS (eNOS), 5’-GGCTCCCTCCTTCCGGCTG-3’ (forward) and 5’-TCCCGCAGCACGCCGAT-3’ (reverse); GAPDH, 5’-ATGTAGGCCATGAGGTCCAC-3’ (forward) and 5’-TGCGACTTCAACAGCAACTC-3’ (reverse).
Autoradiography of distribution of MKP
14C-labeled MKP, Met-[1-14C]Lys-Pro (14C-MKP), was purchased from GE Healthcare (Buckinghamshire, UK). Radiochemical purity assessed by high-performance liquid chromatography (HPLC) was 98.9%, and its specific radioactivity was 1.85 GBq/mmol. The test solution contained CH-3 (20 mg/ml) and 14C-MKP (0.25 mg/ml) dissolved in distilled water, and was prepared just before its administration. SHRs (n = 3) were starved for 16 h, and given the solution orally (5 ml/kg BW, 3.7 MBq/kg). At 15 minutes after administration, the rats were euthanized by isoflurane overdose, frozen in liquid nitrogen, and sliced with a microtome-cryostat (Leica). The sections were dried at −20°C and exposed to imaging plates (Fuji Photo Film Co., Ltd., Tokyo, Japan). After exposure, each autoradiographic image was analyzed using BAS2000 (Fuji Photo Film Co., Ltd.). Blood was collected at 15 min after administration, and radioactivity of the plasma samples was quantified with a liquid scintillation counter (LSC-1000; Aloka, Tokyo, Japan) as reported previously [8].
Statistical analysis
All values are expressed as mean ± S.D. in the text and figures. Statistical analyses were performed using PASW Statistics for Windows version 17 (SPSS Japan). Comparison of data among the Aβ1-42-injected groups was conducted using one-way ANOVA, and when a significant difference was detected, post hoc analysis was performed with Student’s t test or Welch’s t-test. Values of p < 0.05 were considered statistically significant.
Results
Effect of CH3 on cognitive function
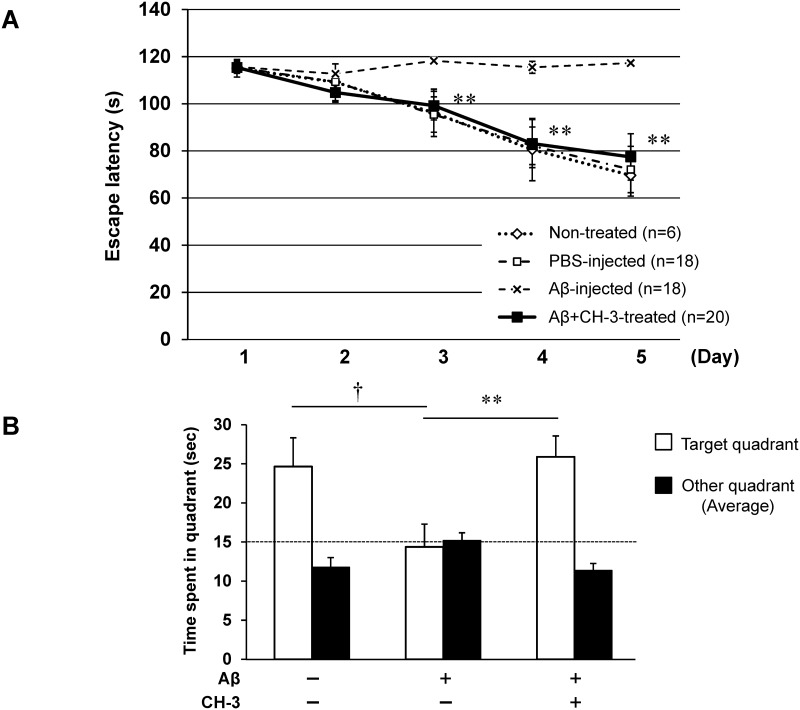
Mice with ICV injection of Aβ1–42 showed significant impairment of spatial learning ability evaluated by the Morris water maze test after 3 weeks, compared with PBS-injected mice (Fig 1A). Daily administration of CH-3 markedly attenuated such cognitive impairment, to the level in PBS-injected mice (Fig 1A). Time spent in the target quadrant including the former platform position was also impaired in Aβ1-42-injected mice compared with control mice; however, Aβ1-42-injected mice with CH-3 treatment exhibited similar ability to recognize the platform compared with PBS-injected mice (Fig 1B). Similarly to previous reports [15], we also confirmed that an ACE inhibitor, perindopril, reduced the cognitive decline in AD mice (S1 Fig).
Fig 1. Effect of CH-3 treatment on cognitive function in AD model mice evaluated by Morris water maze test.
(A) Swim latency in Morris water maze test. (B) Time spent in target quadrant including the former platform position. CH-3 was orally administered at 250 mg/kg/day to mice every day starting 2 days before Aβ1–42 injection. n = 18–20 mice in each group. *P<0.05 vs. Aβ1–42 (+) on day 3, day 4 and day 5, respectively. †P<0.05 vs. control, **P<0.01 vs. Aβ1–42 (+).
Brain inflammation and oxidative stress
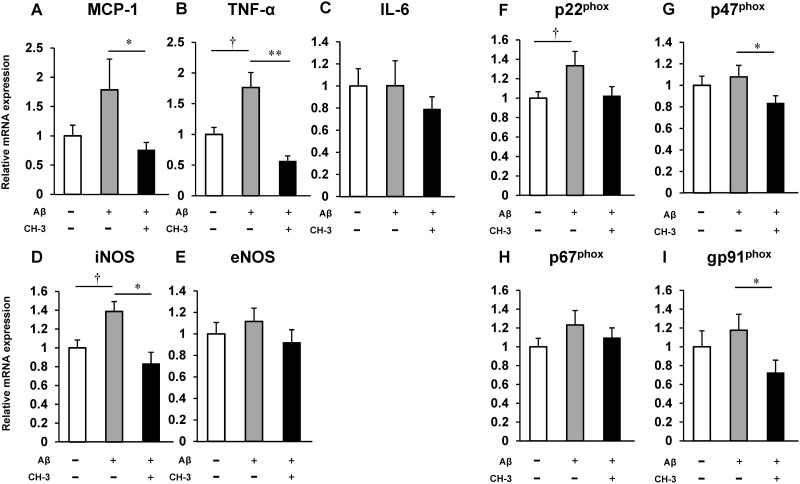
To investigate the possible involvement of inflammation and oxidative stress in the improvement of Aβ 1-42-induced cognitive decline by CH-3, the mouse hippocampus was obtained after behavioral testing, and the expression of inflammatory cytokines and NADPH oxidase subunits was assessed by real-time RT-PCR. Aβ1–42 injection significantly enhanced mRNA expression of TNF-α, iNOS and p22phox in the mouse hippocampus compared with PBS injection, and showed a tendency to increase mRNA expression of MCP-1, p47phox and gp91phox; whereas treatment with CH-3 markedly reduced Aβ 1-42-induced TNF-α, MCP-1, iNOS, p47phox and gp91phox expression (Fig 2).
Fig 2. Change in mRNA expression of inflammatory cytokines and NADPH oxidase subunits in hippocampus of AD model mice by CH-3 treatment.
mRNA expression of MCP-1 (A), TNF-α (B), IL-6 (C), iNOS (D), eNOS (E), p22phox (F), p47phox (G), p67phox (H) and gp91phox (I) in hippocampus. n = 8–12 mice in each group. †P<0.05 vs. control, *P<0.05 or **P<0.01 vs. Aβ1–42 (+).
Distribution of MKP after oral administration
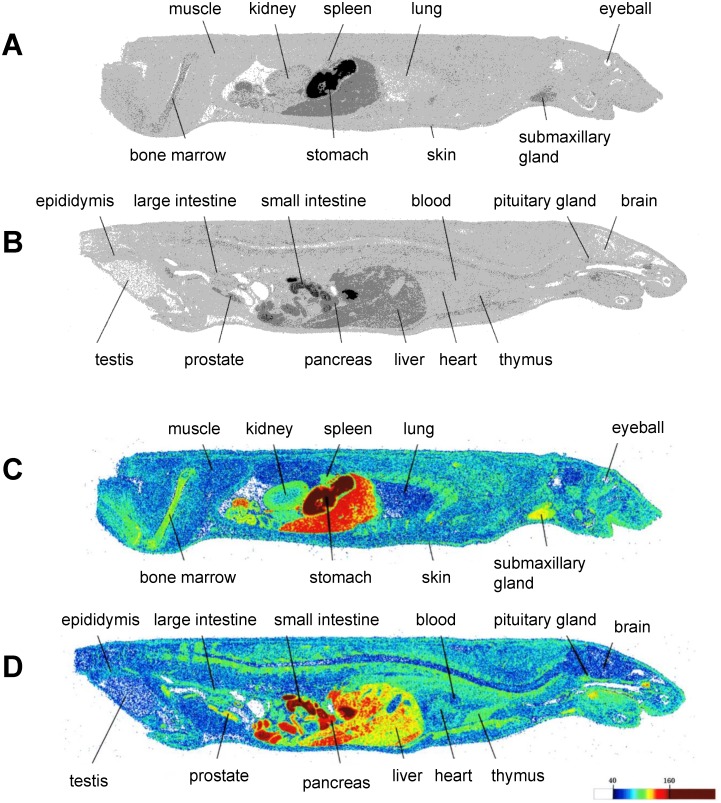
The distribution of radioactivity in tissues was detected by autoradiography after oral administration of 14C-MKP. Fig 3 shows the distribution of MKP 15 minutes after oral administration. The administered radioactivity was distributed in many organs and tissues. Of these tissues, radioactivity showed a relatively high concentration in the small intestine, pancreas and liver. It is noteworthy that the signal was also observed in brain tissue. Radioactivity in brain tissue was found to be 87,607 eq·dpm/g and that in plasma samples was 91,182±23,993 dpm/ml (mean±SE; n = 3). These data indicate that orally administered MKP was mainly distributed in the gastrointestinal system, but MKP could penetrate the blood brain barrier (BBB) and enter the brain.
Fig 3. Autoradiographic images of mice after oral administration of MKP labeled with 14C.
Radioactivity distribution at 15 min after oral administration of 14C-MKP and CH-3 to male mice. Dorso-ventral section including kidney (A, C: colored) and dorso-ventral mesion section (B, D: colored).
Effect of MKP on cognitive function
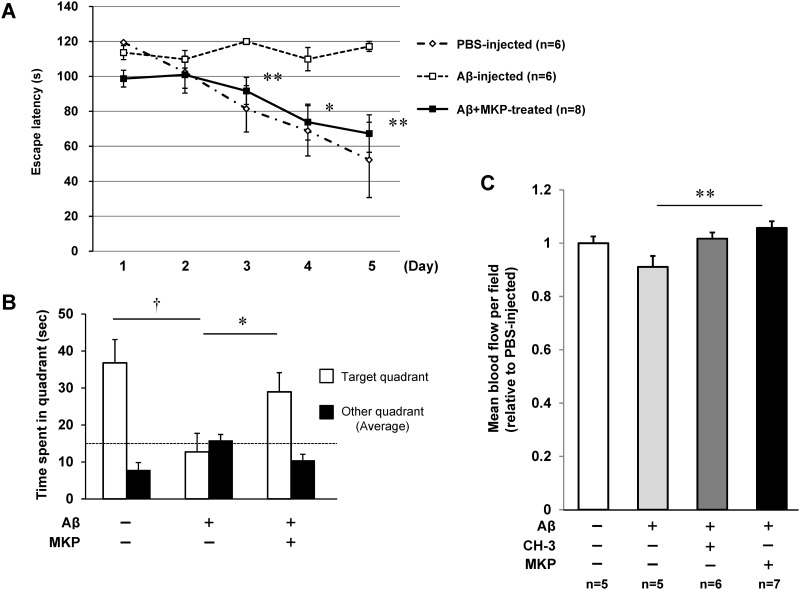
Finally, we investigated the effect of MKP, which accounts for the main ACE inhibitory effect of CH-3 and has the possibility to penetrate the BBB, on cognitive decline induced by ICV injection of Aβ1–42. Daily treatment with MKP attenuated Aβ 1-42-induced cognitive impairment such as escape latency and time spent in the target quadrant, to the level in PBS-injected mice (Fig 4A and 4B). Interestingly, cerebral blood flow in Aβ1-42-injected mice was significantly reversed by MKP treatment (Fig 4C). However, MKP treatment had no significant effect on cerebral blood flow in PBS-injected mice (S2 Fig). Peptide administration to PBS-injected mice had no effect on cognitive function (S3 Fig). Peptide administration had no effect on blood pressure in both PBS-injected and Aβ-injected mice (S4 Fig and S1 Text).
Fig 4. Improvement of cognitive decline by MKP treatment in AD model mice.
(A) Swim latency in Morris water maze test. (B) Time spent in target quadrant including the former platform position in Morris water maze test. (C) Cerebral blood flow measured by laser speckle flowmetry after Morris water maze test. MKP was orally administered to mice every day at 0.5 mg/kg/day starting 2 days before Aβ1–42 injection. n = 5–8 mice in each group. †P<0.05 vs. control, *P<0.05 or **P<0.01 vs. Aβ1–42 (+).
Discussion
Here, we focused on bovine casein-derived peptide CH-3, which has a strong ACE inhibitory effect, and demonstrated that administration of CH-3 prevented cognitive decline in AD model mice, with a reduction of inflammation and oxidative stress. In addition, we also confirmed that MKP, a tripeptide in CH-3 that has a strong ACE inhibitory effect with BBB penetration, showed a similar effect to CH-3 and thus may be one of the main players in the prevention of cognitive impairment by CH-3.
AD is the most common form of dementia, accounting for almost two thirds of all cases. Recently, interplay between hypertension and dementia, such as vascular dementia and AD, and neurovascular dysfunction via vascular degeneration induced by chronic hypertension, has been highlighted [22–25]. Epidemiological studies also suggest that midlife hypertension is a risk factor for dementia in the elderly [26]. The renin-angiotensin system (RAS) is a key player in maintenance of blood pressure, and thus, the RAS is a crucial target for preventing dementia. ACE activity is elevated in the brain of AD patients [12], and RAS-modulating medicine has possibility as a new treatment option for AD. In particular, BBB-penetrating RAS inhibitors are thought to have a beneficial effect in suppressing the development of AD. A clinical study, the Perindopril pROtection aGainst REcurrent Stroke Study (PROGRESS), demonstrated that treatment with perindopril, a centrally active ACE inhibitor, reduces the risk of severe cognitive decline and stroke-related dementia [27,28]. Ohrui et al. demonstrated a protective role of long-term use of ACE inhibitors against the development of Alzheimer disease [16]. Moreover, Yamada et al. and Dong et al. also demonstrated preventive effects of perindopril on cognitive impairment in an Alzheimer disease mouse model [15,29]. In fact, we found that perindopril could have a protective effect on AD mouse cognitive function (S1 Fig).
We previously reported that CH-3 has strong ACE inhibitory activity in vitro [8,9]. Orally administered MKP, which contributes 33% of the total ACE-inhibitory activity of CH-3, was rapidly absorbed into plasma, and exhibited an antihypertensive effect in spontaneously hypertensive rats, probably through its ACE inhibitory activity. Because a previous study showed that BBB penetration is crucial for the protective effect of a RAS inhibitor on AD, we measured MKP bioavailability such as absorption and distribution after oral CH-3 administration to determine the fate of MKP, using whole body autoradiography. To analyze MKP bioavailability under the condition in which MKP coexists with many other CH-3-containing peptides, we orally administered a mixed solution of CH-3 and 14C-MKP. The autoradiographic images revealed that radioactivity was detected throughout the body 15 min after administration (Fig 3). It is noteworthy that radioactivity was detected in the brain, and its concentrations in brain tissue was 87,607 eq·dpm/g and plasma radioactive intensity was 91,182 dpm/ml. Considering the cerebral blood volume in rats is around 5 ml/100 ml·brain tissue volume [30], the contribution on the signal intensity of brain radioactivity from blood in the cerebral circulation can be estimated to be less than 10%. Additionally, we reported that 15 min after 14C-MKP administration, nearly 90% of the radioactivity in plasma was attributable to intact 14C-MKP [8]. Therefore, we speculated that the radio-signal detected in the brain was mainly from intact 14C-MKP and not merely its metabolite, suggesting the possible ability of MKP to penetrate the BBB.
We hypothesized that CH-3 has a preventive effect on cognitive decline in AD, as have centrally active ACEIs. We investigated this possibility using Aβ1–42 ICV-injected mice, which are commonly used as an animal model of AD [15,19]. ICV infusion of Aβ in the rodent brain can mimic aspects of AD and this model has been used as an AD model mouse by many researchers. Takeda et al. validated the reliability of this model in detail based on three criteria (face validity, construct validity and predictive validity), which suggested that this model is useful to evaluate drugs targeting Aβ and their toxicity, as well as to investigate protective effects of pharmacological modulation of microglial signaling [31]. APP Tg mice, which overexpress APP in the brain, show age-related cognitive decline with Aβ deposition and neuroinflammation and are a useful model of AD. However, APP Tg model mice are based on transgenic overexpression of APP, and an extremely high level of APP might cause some undesirable side effects. For example, overexpression of APP results in increased production of Aβ1–40 and Aβ1–42, but also causes elevated levels of other APP fragments, which may induce an artificial phenotype [32]. When using an ICV injection model, it is possible to administer defined amounts of a specific Aβ species. Aβ1–40 and Aβ1–42 are the major components of senile plaques and are considered to have a causal role in the development and progression of AD. Aβ1–42 is thought to be more toxic than Aβ1–40, and our data confirmed that ICV injection of Aβ1–42 induced cognitive dysfunction evaluated by the Morris water maze test. Interestingly, the cognitive decline was significantly suppressed by daily administration of CH-3 (Fig 1).
Aβ is known to induce cerebral oxidative stress through activation of microglia and astrocytes and to enhance neuroinflammation, leading to neuronal injury and cognitive impairment [33]. This oxidative stress and neuroinflammation are also seen in AD patients. Oxidative damage is observed early in the progression of AD [34]. Paganelli et al. reported that the proinflammatory cytokine TNF-α level is increased in patients with severe AD compared to mild AD [35]. TNF-α in the AD brain is thought to induce overexpression of iNOS (also called NOS2) and peroxynitrite-mediated nitration of protein, leading to nitrosative stress in the AD brain [36]. A variety of agents targeting TNF-α is thought to be a therapeutic strategy for AD. Neuronal oxidative stress and damage seen in the brains of both AD patients and AD model mice are thought to be mainly attributable to microglial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) [37]. Therefore, to determine the detailed underlying mechanism of the beneficial effect of CH-3 on Aβ1-42-injected mice, we analyzed the hippocampal expression of TNF-α and NOX subunits. In this study, Aβ1–42 injection significantly enhanced hippocampal TNF-α expression and tended to induce some NOX subunit expression (Fig 2). CH-3 treatment suppressed TNF-α mRNA level and reduced gp91phox, p22phox and p47phox levels induced by Aβ injection. Our study also showed that iNOS expression in the brain was reduced to a normal expression level by the administration of CH-3, suggesting that CH-3 could suppress nitrosative stress induced by Aβ. From these data, we supposed that administration of CH-3 prevented cognitive decline in AD models through its suppressive effect on neuroinflammation or oxidative stress.
To verify the relationship between the ACE-inhibitory activity of MKP and the protective effects of CH-3 on AD mice, we administered MKP daily to Aβ-injected mice and evaluated cognitive function. Fig 4 demonstrates that administration of MKP also attenuated the cognitive dysfunction induced by Aβ1–42. We suggest that MKP is the main player in the beneficial activity of CH-3, through its ACE-inhibitory activity.
It is reported that Aβ induced up-regulation of ACE in neuroblastoma cells, and neuronal ACE activity was increased in the brain of both AD model mice and AD patients with Braak stage [12,15], implying that ICV injection of Aβ might induce excess Ang II production in the brain. Ang II in the brain was reported to induce oxidative stress via NADPH oxidase, leading to neuroinflammation, and to inhibit potassium-induced acetylcholine release in slices of rat entorhinal [38] and human temporal cortex [39]. Our data suggested that administration of CH-3 could reduce Ang II induced by Aβ through its ACE inhibitory property (mainly due to MKP), and suppress oxidative stress and neuroinflammation, leading to a preventive effect on cognitive dysfunction in AD model mice. A previous study confirmed that an ACE inhibitor or ARB could enhance cognition in AD model mice without affecting amyloid burden [15, 40]. The authors suggested that the beneficial effect of an ACEI or ARB on AD model mice could be due to suppression of Aβ-induced toxicity through its anti-inflammation and anti-oxidative properties. We assume that the prevention of cognitive impairment by CH-3 and MKP was attributable to the suppression of Aβ-induced toxicity rather than modulation of Aβ deposition. However, the involvement of other mechanisms cannot be excluded, and these should be examined in future studies.
Until now, no clinically successful therapeutic method or drug for the treatment of AD has been reported. In the disease progression of AD, Aβ production and accumulation start around the forties, and oxidative stress and neuroinflammation occur gradually in the brain. It may take more than 20 years for cognitive impairment to manifest. Disease progression is too advanced for treatment by the time AD is diagnosed. Therefore, therapy to reduce Aβ production or suppress oxidative damage and inflammation for several decades would be required. Thus, there is growing interest in identifying safe food ingredients that may serve to prevent AD. Although we didn’t investigate the possibility of an effect of CH-3 on progression of Aβ burden, we revealed its anti-oxidative stress and anti-inflammatory properties. We assumed that CH-3 would be a good candidate, because it has been taken by humans for a long time and is basically safe when administered in moderate doses.
Additional investigations are needed to clarify the effect of CH-3 on AD. First, this AD model utilizes ICV injection of Aβ1–42; therefore, the preventive effect of CH-3 on Aβ deposition in the brain is not well known. Furthermore, it is not well known whether CH-3 could suppress the progression of AD in humans, and how much CH-3 peptide would be needed per day, and it is necessary to compare the mode of treatment to determine whether CH-3 intake or administration of purified MKP would be better.
In conclusion, the present study demonstrated that oral administration of bovine milk peptide, CH-3, to AD model mice not only improved cognitive function but also suppressed the expression of inflammatory cytokines and oxidative stress-related proteins. The suppressive effect of CH-3 is mainly due to the tripeptide MKP. These results suggest therapeutic potential of CH-3 and MKP for preventing cognitive impairment in AD.
Supporting information
(A) Swim latency in Morris water maze test. (B) Time spent in target quadrant including the former platform position. Perindopril was orally administered at 1 mg/kg/day to mice every day starting 2 days before Aβ1–42 or PBS injection. n = 4–7 mice in each group. *P<0.05 vs. Aβ1–42 (+). †P<0.05 vs. control, **P<0.01 vs. Aβ1–42 (+).
(TIF)
Cerebral blood flow was measured by laser speckle flowmetry after Morris water maze test. MKP was orally administered to mice every day at 0.5 mg/kg/day starting 2 days before PBS injection. n = 4–5 mice in each group.
(TIF)
Swim latency in Morris water maze test. CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day), perindopril (1 mg/kg/day) was orally administered to mice every day starting 2 days before PBS injection. n = 4–6 mice in each group. The data of PBS-injected group mice (n = 4) are the same as the data of S1 Fig.
(TIF)
Blood pressure was measured by using a noninvasive computerized tail-cuff system. CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day), perindopril (1 mg/kg/day) was orally administered to mice every day starting 2 days before Aβ1–42 or PBS injection. n = 4 mice in each group.
(TIF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work. Morinaga Milk Industry Co., Ltd., provided support in the form of salaries for authors Y.K., A.Y., K.Y., F.A. and J.X., but did not have any role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are described in the ‘author contributions’ section. Moreover, this study was supported by JSPS KAKENHI [Grant Number 25293310 to M.H., 25462220 to M.M., 15K19974 to J.I., and 26860567 to L.-J.M.] and research grants from Morinaga Milk Industry Co., Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890. [DOI] [PubMed] [Google Scholar]
- 2.McNaull BB, Todd S, McGuinness B, Passmore AP (2010) Inflammation and anti-inflammatory strategies for Alzheimer's disease—a mini-review. Gerontology 56: 3–14. [DOI] [PubMed] [Google Scholar]
- 3.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, et al. (1996) Oxidative damage in Alzheimer's. Nature 382: 120–121. [DOI] [PubMed] [Google Scholar]
- 4.Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki T, Seki E, Osajima K, Yoshida M, Asada K, Matsui T, et al. (2000) Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J Hum Hypertens 14: 519–523. [DOI] [PubMed] [Google Scholar]
- 6.Tokunaga KH, Yoshida C, Suzuki KM, Maruyama H, Futamura Y, Araki Y, et al. (2004) Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol Pharm Bull 27: 189–192. [DOI] [PubMed] [Google Scholar]
- 7.Murray BA, FitzGerald RJ (2007) Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr Pharm Des 13: 773–791. [DOI] [PubMed] [Google Scholar]
- 8.Yamada A, Sakurai T, Ochi D, Mitsuyama E, Yamauchi K, Abe F (2015) Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem 172: 441–446. 10.1016/j.foodchem.2014.09.098 [DOI] [PubMed] [Google Scholar]
- 9.Yamada A, Sakurai T, Ochi D, Mitsuyama E, Yamauchi K, Abe F (2013) Novel angiotensin I-converting enzyme inhibitory peptide derived from bovine casein. Food Chem 141: 3781–3789. 10.1016/j.foodchem.2013.06.089 [DOI] [PubMed] [Google Scholar]
- 10.Kehoe PG, Wilcock GK (2007) Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol 6: 373–378. 10.1016/S1474-4422(07)70077-7 [DOI] [PubMed] [Google Scholar]
- 11.Mogi M, Iwanami J, Horiuchi M (2012) Roles of brain angiotensin II in cognitive function and dementia. Int J Hypertens 2012: 169649 10.1155/2012/169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miners S, Ashby E, Baig S, Harrison R, Tayler H, Speedy E, et al. (2009) Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res 1: 163–177. [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM (2013) Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120. 10.1089/ars.2012.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciobica A, Bild W, Hritcu L, Haulica I (2009) Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurol Belg 109: 171–180. [PubMed] [Google Scholar]
- 15.Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, et al. (2011) Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer's disease. FASEB J 25: 2911–2920. 10.1096/fj.11-182873 [DOI] [PubMed] [Google Scholar]
- 16.Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, et al. (2004) Angiotensin-converting enzyme inhibitors and incidence of Alzheimer's disease in Japan. J Am Geriatr Soc 52: 649–650. 10.1111/j.1532-5415.2004.52178_7.x [DOI] [PubMed] [Google Scholar]
- 17.Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, et al. (2014) Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer's disease model. Neurobiol Dis 68: 126–136. 10.1016/j.nbd.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 18.Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, et al. (2009) Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 169: 1195–1202. 10.1001/archinternmed.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, et al. (2009) Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension 54: 782–787. 10.1161/HYPERTENSIONAHA.109.136879 [DOI] [PubMed] [Google Scholar]
- 20.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Fujita T, et al. (2009) Sex-different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res 1300: 14–23. 10.1016/j.brainres.2009.08.068 [DOI] [PubMed] [Google Scholar]
- 21.Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, et al. (2008) Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension 51: 528–533. 10.1161/HYPERTENSIONAHA.107.101634 [DOI] [PubMed] [Google Scholar]
- 22.Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, et al. (2008) High-normal blood pressure is associated with poor cognitive performance. Hypertension 51: 663–668. 10.1161/HYPERTENSIONAHA.107.105577 [DOI] [PubMed] [Google Scholar]
- 23.Nagai M, Hoshide S, Kario K (2010) Hypertension and dementia. Am J Hypertens 23: 116–124. 10.1038/ajh.2009.212 [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, et al. (2011) Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension 58: 22–28. 10.1161/HYPERTENSIONAHA.110.163055 [DOI] [PubMed] [Google Scholar]
- 25.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10: 819–828. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers J, MacMahon S (2003) Perindopril pROtection aGainst REcurrent Stroke Study (PROGRESS): interpretation and implementation. J Hypertens Suppl 21: S9–14. [DOI] [PubMed] [Google Scholar]
- 28.PROGRESS Collaborative Group. (2001) Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358: 1033–1041. 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Uchida S, Takahashi S, Takayama M, Nagata Y, Suzuki N, et al. (2010) Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res 1352: 176–186. 10.1016/j.brainres.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 30.Watabe T, Shimosegawa E, Watabe H, Kanai Y, Hanaoka K, Ueguchi T, et al. (2013) Quantitative evaluation of cerebral blood flow and oxygen metabolism in normal anesthetized rats: 15O-labeled gas inhalation PET with MRI Fusion. J Nucl Med 54: 283–290. 10.2967/jnumed.112.109751 [DOI] [PubMed] [Google Scholar]
- 31.Takeda S, Sato N, Niisato K, Takeuchi D, Kurinami H, Shinohara M, et al. (2009) Validation of Abeta1-40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res 1280: 137–147. 10.1016/j.brainres.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 32.Van Dam D, De Deyn PP (2011) Animal models in the drug discovery pipeline for Alzheimer's disease. Br J Pharmacol 164: 1285–300. 10.1111/j.1476-5381.2011.01299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT (2010) Inflammation and Alzheimer's disease. Arch Pharm Res 33: 1539–1556. 10.1007/s12272-010-1006-7 [DOI] [PubMed] [Google Scholar]
- 34.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 21: 4183–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, et al. (2002) Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Exp Gerontol 37: 257–263. [DOI] [PubMed] [Google Scholar]
- 36.Alkam T, Nitta A, Mizoguchi H, Saito K, Seshima M, Itoh A, et al. (2008) Restraining tumor necrosis factor-alpha by thalidomide prevents the amyloid beta-induced impairment of recognition memory in mice. Behav Brain Res 189:100–6. 10.1016/j.bbr.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 37.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, et al. (2000) Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun 273: 5–9. 10.1006/bbrc.2000.2897 [DOI] [PubMed] [Google Scholar]
- 38.Barnes JM, Barnes NM, Costall B, Horovitz ZP, Naylor RJ (1989) Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res 491: 136–143. [DOI] [PubMed] [Google Scholar]
- 39.Barnes JM, Barnes NM, Costall B, Horovitz ZP, Ironside JW, Naylor RJ, et al. (1990) Angiotensin II inhibits acetylcholine release from human temporal cortex: implications for cognition. Brain Res 507: 341–343. [DOI] [PubMed] [Google Scholar]
- 40.Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, et al. (2014) Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer's disease model. Neurobiol Dis 68: 126–36. 10.1016/j.nbd.2014.04.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Swim latency in Morris water maze test. (B) Time spent in target quadrant including the former platform position. Perindopril was orally administered at 1 mg/kg/day to mice every day starting 2 days before Aβ1–42 or PBS injection. n = 4–7 mice in each group. *P<0.05 vs. Aβ1–42 (+). †P<0.05 vs. control, **P<0.01 vs. Aβ1–42 (+).
(TIF)
Cerebral blood flow was measured by laser speckle flowmetry after Morris water maze test. MKP was orally administered to mice every day at 0.5 mg/kg/day starting 2 days before PBS injection. n = 4–5 mice in each group.
(TIF)
Swim latency in Morris water maze test. CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day), perindopril (1 mg/kg/day) was orally administered to mice every day starting 2 days before PBS injection. n = 4–6 mice in each group. The data of PBS-injected group mice (n = 4) are the same as the data of S1 Fig.
(TIF)
Blood pressure was measured by using a noninvasive computerized tail-cuff system. CH-3 (250 mg/kg/day) or MKP (0.5 mg/kg/day), perindopril (1 mg/kg/day) was orally administered to mice every day starting 2 days before Aβ1–42 or PBS injection. n = 4 mice in each group.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.