Abstract
Background
Bioelectrical impedance analysis (BIA) is a convenient and child-friendly method for longitudinal analysis of changes in body composition. However, most validation studies of BIA have been performed on adult Caucasians. The present cross-sectional study investigated the validity of two portable BIA devices, the Inbody 230 (BIA8MF) and the Tanita BC-418 (BIA8SF), in healthy Taiwanese children.
Methods
Children aged 7–12 years (72 boys and 78 girls) were recruited. Body composition was measured by the BIA8SF and the BIA8MF. Dual X-ray absorptiometry (DXA) was used as the reference method.
Results
There were strong linear correlations in body composition measurements between the BIA8SF and DXA and between the BIA8MF and DXA. Both BIAs underestimated fat mass (FM) and percentage body fat (%BF) relative to DXA in both genders The degree of agreement in lean body mass (LBM), FM, and %BF estimates was higher between BIA8MF and DXA than between BIA8SF and DXA. The Lin’s concordance correlation coefficient (ρc) for LBM8MF met the criteria of substantial to perfect agreement whereas the ρc for FM8MF met the criteria of fair to substantial agreement. Bland-Altman analysis showed a clinically acceptable agreement between LBM measures by BIA8MF and DXA. The limit of agreement in %BF estimation by BIA and DXA were wide and the errors were clinically important. For the estimation of ALM, BIA8SF and BIA8MF both provided poor accuracy.
Conclusions
For all children, LBM measures were precise and accurate using the BIA8MF whereas clinically significant errors occurred in FM and %BF estimates. Both BIAs underestimated FM and %BF in children. Thus, the body composition results obtained using the inbuilt equations of the BIA8SF and BIA8MF should be interpreted with caution, and high quality validation studies for specific subgroups of children are required prior to field research.
Introduction
Growth monitoring is important for early detection of health and nutritional problems during child development. Growth charts of length-for-age, weight-for-age, and BMI-for-age are currently used to assess physical growth in children. These charts can provide a general clinical overview of the health and nutritional status of children. However, body composition undergoes dynamic changes throughout growth and development, and current growth charts only provide proxy measures for changes in body composition.
The techniques most commonly used to assess body composition in children are underwater weighing, isotope dilution, dual-energy X-ray absorptiometry (DXA), air-displaced plethysmography, and bioelectrical impedance analysis (BIA). Among these techniques, BIA employs portable equipment and is a safe, convenient, and child-friendly method that is suitable for measurement and tracking of body composition changes in children [1].
The two common BIA techniques are the whole-body and segmental modes, in which a current passes from hand-to-foot, foot-to-foot, or hand-to-hand, with subjects either in the supine position or standing [2]. Whole-body BIA employs four electrodes attached to different sides of the body for measurement of electrical resistance. Body composition parameters, such as fat free mass (FFM), lean body mass (LBM), fat mass (FM), and percentage body fat (%BF), are then calculated using specific equations based on recorded impedance, height, age, sex, anthropometric index, and other factors [3]. Multi-segmental BIA employs eight electrodes to calculate whole-body and regional body composition, and can provide information on the spatial distribution of different components of body composition and their changes over time [4]. Therefore, multi-segmental BIA is theoretically superior to classical BIA for studies of pediatric body composition. Moreover, multi-segmental BIA can provide an estimate of appendicular lean mass (ALM), which constitutes the majority of skeletal muscle mass (SM) and thus can be used as a proxy for SM [5, 6].
Multi-segmental BIA is available in single-frequency and multi-frequency modes. Single-frequency BIA generally employs a 50 kHz current that passes through extracellular and intracellular fluids for estimation of total body water [7]. The multi-frequency method uses multiple frequencies to differentiate intracellular from extracellular fluid, and, therefore, provides a better estimation of total body water than the single frequency method [7]. However, there is controversy concerning whether multi-frequency BIA provides more accurate estimates of body composition in children compared with the single frequency method [3, 8, 9].
Previous BIA validation studies were conducted predominantly in adult Caucasians [7]. Pietrobelli et al. [10] demonstrated that appendicular electrical resistance had a strong positive correlation with ALM in white healthy adults, and could be used to estimate the lean mass of the limbs. However, children are not simply “miniature adults”, thus, equations established for adults may not be applicable to children. Therefore, it is necessary to investigate the reliability and validity of different BIA devices before initiation of field studies on pediatric body composition.
This cross-sectional study of healthy Taiwanese children (age 7–12 years) examined the accuracy and validity of two portable multi-segmental BIA devices by comparing their results with those from DXA measurements.
Materials and methods
Study design
This cross-sectional study was approved by local Institutional Review Board of the Chang Gung Memorial Hospital (103-1027A3), and written informed consent was provided by the subjects and their parents. Subjects were recruited via hospital advertisements and word-of-mouth from February to December, 2015. All subjects were healthy Taiwanese children aged 7–12 years-old. None of the subjects were pregnant, had amputations, implants, or chronic illnesses, or were prescribed regular medication.
Participants were instructed to eat breakfast on the study day and then fasted completely for at least 2 h before reporting to the Chang Gung Memorial Hospital (Chiayi branch) between 8:30–11:00 am. Vigorous activities and alcohol were avoided for a minimum of 48 h before the study day. Girls were not given appointments during their menstrual cycle. On arrival, participants were asked to void and change into a hospital gown. All measurements including body weight, height, BIA, and DXA were completed on the same morning, with a total study time of approximately one hour. One measurement per subject was performed using each instrument. Body height (cm) and weight (kg) were measured with subjects wearing no shoes using a digital scale (Super-View, HW-3050, Taipei, Taiwan).
Bioelectrical impedance analysis (BIA)
All BIA measurements were made by trained research assistants. Subjects were measured wearing hospital gowns (< 0.2 kg) and weight adjustment for clothing was not applied. A single-frequency (50 kHz, 500 μA) BIA device (Tanita BC-418, Tanita Corp., Tokyo, Japan), referred to as BIA8SF, was used to estimate LBM8SF, ALM8SF, FM8SF, and %BF8SF [11]. This method allows bioelectricity impedance measurement of the whole body and each part (right leg, left leg, right arm and left arm). The age limits for the BIA8SF are 7–99 years. After the sex, age and height information had been entered into the BIA8SF, subjects were asked to stand in a stable position with bare feet. Their toes and heels were placed in contact with the anterior and posterior electrodes of the weighting platform, respectively. The measurements began when the grips were grasped by both hands. With BIA8SF, electric current was supplied from the toe tips of both feet and the fingertips of both hands, and the voltage was measured on the heel of both feet and the thenar area of both hands. Finally, the inbuilt equation was used to convert the input impedance to body composition estimates. Test-retest reliability for whole body LBM and %BF estimates by BIA8SF were both ≥ 0.99 (n = 5) using the intra-class correlation coefficient (ICC).
A multi-frequency (20 kHz and 100 kHz) BIA device using eight-point tactile electrode system (Inbody 230, Biospace Corp., Seoul, Korea), referred to as BIA8MF, was used to measure LBM8MF, ALM8MF, FM8MF, and %BF8MF [12]. The BIA8MF is suitable for individuals aged 3–99 years-old according to the manufacturer. The BIA8MF produces 10 impedance values by using two different frequencies to measurement the five segments of the body (right leg, left leg, right arm, left arm and the trunk). The measurement procedure for BIA8MF was similar to that for BIA8SF, except thumb should be placed on the electrode pad on the top surface of the handle for BIA8MF. Body composition estimates were calculated by using the manufacturer’s software (Lookin’Body 120, Biospace Corp., Seoul, Korea). Test-retest reliability for whole body LBM and %BF estimates by BIA8MF were both ≥ 0.99 (n = 5) using ICC.
Dual-energy X-ray absorptiometry (DXA)
DXA is the reference method for assessment of body composition. Whole body DXA was performed using a fan-beam system (Delphi A, QDR series, Hologic, Bedford, MA, USA) configured with software version 12.5. The scanner was equipped with switched pulse dual-energy x-ray tube, operating at 100 kVp and 140 kVp. The in vivo precision of the scanner for whole body measurement was 1.0%, according to the product specification. The scanner was calibrated daily with the Hologic spine and body composition step phantoms before scanning the subject. Then, subjects were instructed to lie supine on the scanning bed. The DXA operator manually assisted subjects to position within the scanning zone with their head, neck and torso parallel to the long-axis of the scanning bed; arms at their sides; palms down; legs internally rotated about 25° until the toes touched; and feet fixed together using strapping tape. Subjects were instructed to remain still and breathe normally during the scan. All DXA scans were analyzed by the same operator who followed the manufacturer’s instructions and used the pediatric mode and standardized cutoff for regional measurements [13]. The subregions were defined as the head, trunk, right arm, left arm, right leg, left leg. DXA measured regional and whole body composition, including LBMDXA, ALMDXA, FMDXA, and %BFDXA.
Statistical analysis
The statistical software package SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. All data are reported as means ± SDs. Analysis of variation (ANOVA) with Student’s independent t-test (two-sided) was applied for analysis of repeated measurements to compare the different testing methods. The statistical significance level was set at α = 0.05. Pearson’s product moment correlation and ordinary least products regression analysis were used to examine the relationship between the BIA and DXA and to determine the proportional bias and fixed bias [14]. The correlation coefficient (r) and determination coefficient (r2) from linear regression analysis were used to define the strength of linear association. The standard error of the estimate (SEE), a measure of the accuracy of predictions made with a linear regression, was used to assess the statistical conformity of the two BIA methods.
To assess the degree of agreement between BIA and DXA measurements, three statistical techniques were used: the ICC, Lin’s concordance correlation (CCC) and Bland-Altman plot. The ICC coefficient (r1) (with two-way random and single measure) was used to assess the agreement between BIA and DXA methods [15]. An r1 value ≥ 0.8 was considered a strong level of agreement. The CCC coefficient (ρc) was used to assess how close the data from BIA and DXA methods was about the line of best fit and also how far that line was from the 45-degree line through the origin [16]. The ρc and a concordance scale used including ratings of almost perfect: ρc > 0.99; substantial: 0.99 ≥ ρc > 0.95; fair: 0.95 ≥ ρc ≥ 0.9; poor: ρc < 0.9) were used to assess the concordance of the two BIA methods [17]. Bland-Altman plot with a regression analysis using ordinary least squares regression was used to display the difference between a pair of measurements against the mean of the pair [18]. Limits of agreement (LOA) were used to assess the agreement between two readings obtained by BIA and DXA on the same variable.
Results
A total of 150 children (72 boys and 78 girls) with a mean age of 9.3 ± 1.5 years were enrolled. Subject demographics and body composition estimates are shown in Table 1. There were no significant differences in age, height or weight between boys and girls. However, the boys had significantly higher BMI compared with the girls (18.3 ± 4.3 in boys and 17.1 ± 3.0 in girls, p = 0.038). Based on DXA results, FM and %BF showed no significant difference between boys and girls whereas the boys had significantly higher LBM and ALM than the girls. For both boys and girls, all body composition results by BIA8MF and BIA8SF were significantly different from the results by DXA (**P < 0.001, Table 1), except for LBM by BIA8MF.
Table 1. Anthropometric characteristics and body composition measurements of Taiwanese children (age 6 to 12 years) determined by DXA (reference method), BIA8MF, and BIA8MF.
| Boys (n = 72) | Girls (n = 78) | Total (n = 150) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 9.4 | 1.6 | 7.1–12.7 | 9.2 | 1.5 | 7.1–12.1 | 9.3 | 1.5 | 7.1–12.7 |
| Height (cm) | 138.0 | 11.0 | 114.7–164.9 | 137.5 | 11.3 | 112.2–159.1 | 137.7 | 11.1 | 112.2–164.9 |
| Weight (kg) | 35.6 | 11.9 | 19.2–73.1 | 33.0 | 9.5 | 19.3–60.4 | 34.2 | 10.8 | 19.2–73.1 |
| BMI | 18.3* | 4.3 | 13.4–30.0 | 17.1 | 3.0 | 12.3–26.6 | 17.7 | 3.7 | 12.2–30.0 |
| LBM (kg) | |||||||||
| DXA | 24.3 | 5.7 | 15.2–40.6 | 22.4 | 5.5 | 13.6–38.1 | 23.3 | 5.7 | 13.6–40.6 |
| BIA8MF | 24.1 | 5.7 | 14.9–39.0 | 22.8 | 5.6 | 13.9–38.6 | 23.4 | 5.7 | 13.9–39.0 |
| BIA8SF | 26.4** | 5.3 | 17.2–39.6 | 24.8** | 5.4 | 16.1–40.0 | 25.6** | 5.4 | 16.1–40.0 |
| FM (kg) | |||||||||
| DXA | 10.9 | 7.6 | 3.6–35.7 | 10.2 | 4.9 | 4.3–24.7 | 10.6 | 6.3 | 3.6–35.7 |
| BIA8MF | 9.6** | 7.2 | 2.8–34.6 | 8.5** | 4.4 | 3.0–21.8 | 9.1** | 5.9 | 2.8–34.6 |
| BIA8SF | 7.9** | 7.6 | 1.1–35.2 | 6.9** | 4.2 | 2.0–21.0 | 7.4** | 6.1 | 1.1–35.2 |
| %BF (%) | |||||||||
| DXA | 27.3 | 10.3 | 13.4–48.2 | 29.2 | 7.1 | 17.7–47.6 | 28.3 | 8.8 | 13.4–48.2 |
| BIA8MF | 24.3** | 10.5 | 11.6–47.2 | 24.7** | 7.1 | 14.4–42.9 | 24.5** | 8.8 | 11.6–47.2 |
| BIA8SF | 18.5** | 12.6 | 4.7–48.0 | 19.5** | 6.7 | 9.3–36.9 | 19.0** | 10.0 | 4.7–48.0 |
| ALM (kg) | |||||||||
| DXA | 10.4 | 2.9 | 5.3–19.0 | 9.4 | 2.6 | 5.3–16.5 | 9.9 | 2.8 | 5.3–19.0 |
| BIA8MF | 13.3** | 3.7 | 7.5–22.8 | 12.4** | 3.5 | 6.8–22.5 | 12.9** | 3.6 | 6.8–22.8 |
| BIA8SF | 12.3** | 3.5 | 6.9–22.3 | 10.8** | 2.5 | 7.0–18.4 | 11.5** | 3.1 | 6.9–22.3 |
Abbreviations: ALM, appendicular lean mass; BIA8SF, Tanita BC-418; BIA8MF, Inbody 230; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; FM, fat mass; LBM, lean body mass; SD, standard deviation; %BF: percent body fat.
*P < 0.05, by repeated-measures ANOVA with Student’s independent t-test;
**P < 0.01, by repeated-measures ANOVA with Student’s independent t-test
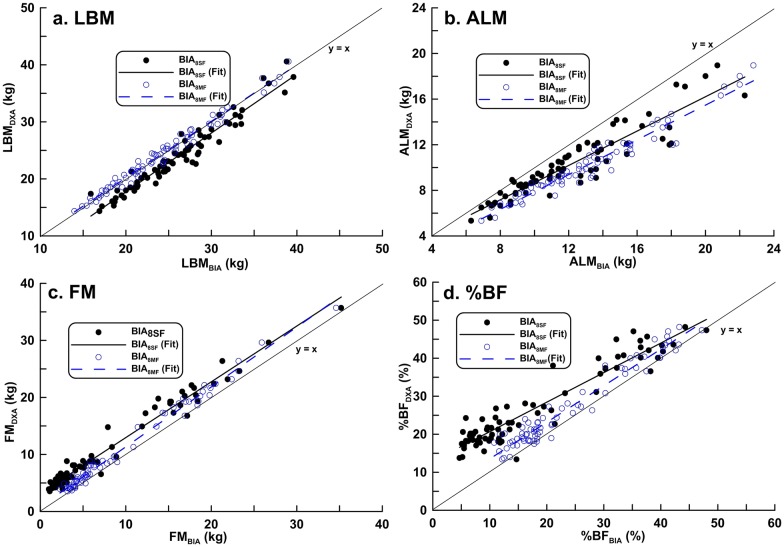
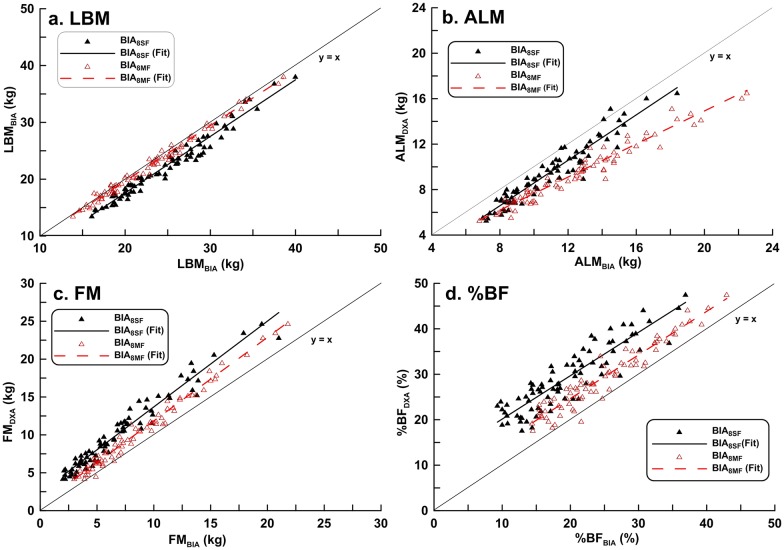
Table 2 shows the Pearson product moment correlations coefficient (r) and the regression equation used to predict DXA results from BIA readings. There were strong linear correlations between the two BIA methods and DXA in the measurement of LBM, ALM, FM, and BF% (r ≥ 0.9 for all comparisons). However, there was a proportional bias and/or a fixed bias for each BIA measurement, except for LBM8MF. The scatter plots of body composition data by BIA and DXA methods showed BIA underestimated FM and %FM relative to DXA in both genders (Figs 1 and 2).
Table 2. Correlation of body composition estimates using Pearson product moment correlation and ordinary least products regression.
| Method | r | a | 95% CI | b | 95% CI | Fixed bias | Proportional bias | SEE |
|---|---|---|---|---|---|---|---|---|
| Boys (n = 72) | ||||||||
| LBM8SF | 0.971 | -3.533 | -5.188, -1.877 | 1.053 | 0.991, 1.115 | Yes | No | 1.368 |
| LBM8MF | 0.989 | 0.354 | -0.509, 1.217 | 0.991 | 0.957, 1.026 | No | No | 0.839 |
| FM8SF | 0.986 | 3.248 | 2.813, 3.683 | 0.974 | 0.934, 1.014 | Yes | No | 1.283 |
| FM8MF | 0.993 | 0.854 | 0.508, 1.200 | 1.050 | 1.020, 1.078 | Yes | Yes | 0.876 |
| %BF8SF | 0.949 | 12.962 | 11.586, 14.339 | 0.776 | 0.715, 0.838 | Yes | Yes | 3.285 |
| %BF8MF | 0.976 | 3.880 | 2.530, 5.229 | 0.964 | 0.913, 1.014 | Yes | No | 2.256 |
| ALM8SF | 0.922 | 1.115 | 0.265, 2.178 | 0.748 | 0.673, 0.823 | Yes | Yes | 1.116 |
| ALM8MF | 0.970 | 0.287 | -0.337, 0.912 | 0.758 | 0.713, 0.804 | No | Yes | 0.698 |
| Girls (n = 78) | ||||||||
| LBM8SF | 0.982 | -2.354 | -3.469, -1.329 | 0.996 | 0.952, 1.042 | Yes | No | 1.043 |
| LBM8MF | 0.994 | 0.213 | -0.373, 0.800 | 0.972 | 0.947, 1.002 | No | No | 0.616 |
| FM8SF | 0.976 | 2.375 | 1.911, 2.840 | 1.132 | 1.074, 1.189 | Yes | Yes | 1.064 |
| FM8MF | 0.991 | 0.822 | 0.492, 1.153 | 1.102 | 1.068, 1.137 | Yes | Yes | 0.666 |
| %BF8SF | 0.897 | 10.609 | 8.407, 12.810 | 0.954 | 0.847, 1.061 | Yes | No | 3.141 |
| %BF8MF | 0.925 | 5.336 | 3.638, 7.434 | 0.984 | 0.892, 1.077 | Yes | No | 2.707 |
| ALM8SF | 0.956 | -1.408 | -2.190, -0.627 | 0.920 | 0.848, 0.989 | Yes | Yes | 0.783 |
| ALM8MF | 0.974 | 0.383 | -0.110, 0.876 | 0.727 | 0.668, 0.765 | No | Yes | 0.596 |
Abbreviations: r, Pearson product moment correlation coefficient; a, b, coefficients in ordinary least products regression model: E(A) = a + b(B); a, (y axis) intercept; b, slope; fixed bias, if 95% confidence interval (CI) for a does not include 0; proportional bias, if 95% confidence interval (CI) for b does not include 1; SEE, standard error of the estimate.
Fig 1. Correlation between dual-energy X-ray absorptiometry results and estimates of body composition in boys obtained with either BIA8SF or BIA8MF.
(a) LBM: BIA8SF: r2 = 0.940, BIA8MF: r2 = 0.979 (b) ALM: BIA8SF: r2 = 0.858, BIA8MF: r2 = 0.944 (c) FM: BIA8SF: r2 = 0.940, BIA8MF: r2 = 0.979 (d) %BF: BIA8SF: r2 = 0.898BIA8MF: r2 = 0.951.
Fig 2. Correlation between dual-energy X-ray absorptiometry results and estimates of body composition in girls obtained with BIA8SF or BIA8MF.
(a) LBM: BIA8SF: r2 = 0.964, BIA8MF: r2 = 0.987 (b) ALM: BIA8SF: r2 = 0.915, BIA8MF: r2 = 0.951 (c) FM: BIA8SF: r2 = 0.953, BIA8MF: r2 = 0.981 (d) %BF: BIA8SF: r2 = 0.802, BIA8MF: r2 = 0.964.
Pearson correlation was used to quantify the strength of linear association between two methods of measuring the same variable, and it should not be used to assess agreement between methods. Therefore, the agreement of BIA8SF and BIA8MF with DXA was further examined using three statistical techniques: ICC, CCC and Bland-Altman plot (Table 3). In general, an ICC value (r1) ≥ 0.8 is considered a strong level of agreement. This study showed that all BIA parameters had r1 ≥ 0.9 except for LBM8SF in boys, which was 0.887, indicating a strong agreement between the measures by BIA and DXA.
Table 3. Agreement between bioelectrical impedance analysis and dual-energy X-ray absorptiometry.
| Method | Bland-Altman Plot | CCC (ρc) | ICC (r1) | |||
|---|---|---|---|---|---|---|
| Bias | Limit of agreement | Function | p | |||
| Boys (n = 72) | ||||||
| LBM8SF | 2.12 | -0.65 to 4.90 | y = 0.082 x + 4.208 | 0.005 | 0.900 | 0.887 |
| LBM8MF | -0.15 | -1.82 to 1.52 | y = -0.002 x − 0.096 | 0.902 | 0.989 | 0.943 |
| FM8SF | -3.05 | -5.63 to -0.47 | y = 0.012 x − 3.156 | 0.571 | 0.911 | 0.973 |
| FM8MF | -1.35 | -3.21 to 0.55 | y = -0.055 x − 0.763 | < 0.0001 | 0.975 | 0.977 |
| %BF8SF | -8.82% | -17.46 to -0.19% | y = 0.205 x − 13.526 | < 0.0001 | 0.717 | 0.992 |
| %BF8MF | -3.00% | -7.54 to 1.55% | y = 0.013 x − 3.335 | 0.620 | 0.936 | 0.986 |
| ALM8SF | 1.87 | -0.97 to 4.71 | y = 0.216 x − 0.585 | < 0.0001 | 0.770 | 0.989 |
| ALM8MF | 2.93 | 0.69 to 5.17 | y = 0.248 x + 0.016 | < 0.0001 | 0.671 | 0.972 |
| Girls (n = 78) | ||||||
| LBM8SF | 2.44 | 0.37 to 4.52 | y = -0.015 x + 2.790 | 0.500 | 0.890 | 0.990 |
| LBM8MF | 0.37 | -0.88 to 1.63 | y = 0.018 x − 0.076 | 0.126 | 0.991 | 0.994 |
| FM8SF | -3.29 | -5.68 to -0.90 | y = -0.150 x − 2.008 | < 0.0001 | 0.763 | 0.992 |
| FM8MF | -1.70 | -3.30 to -0.10 | y = -0.107 x − 0.691 | < 0.0001 | 0.923 | 0.970 |
| %BF8SF | -9.72% | -15.99 to -3.45% | y = -0.065 x − 8.149 | 0.229 | 0.445 | 0.989 |
| %BF8MF | -4.48% | -8.50 to -0.46% | y = -0.001 x − 4.512 | 0.969 | 0.798 | 0.979 |
| ALM8SF | 1.42 | -0.14 to 2.97 | y = -0.045 x + 1.877 | 0.192 | 0.828 | 0.981 |
| ALM8MF | 3.01 | 0.74 to 5.28 | y = 0.295 x − 0.208 | < 0.0001 | 0.635 | 0.953 |
Abbreviation: CCC, Lin’s concordance correlation coefficient; ρc, CCC coefficient; ICC, intra-class correlation; r1, ICC coefficient
In general, the CCC values (ρc) for LBM, FM, and %BF were higher between BIA8MF and DXA than between BIA8SF and DXA (Table 3), indicating a better agreement between BIA8MF and DXA measures. In both sexes, the ρc values for LBM, FM, and %BF were ≥ 0.9 between BIA8MF and DXA, except for %BF8MF in girls (Table 2). The ρc for LBM8MF met the criteria for substantial to perfect agreement (ρc > 0.95) whereas the ρc for FM8MF met the criteria for fair to substantial agreement (0.99 > ρc ≥ 0.9). For the %BF estimations, only the ρc values obtained by BIA8MF in the boys (ρc = 0.936) met the criteria for fair agreement with DXA and the rest of the %BF estimations showed poor agreement (Table 3).
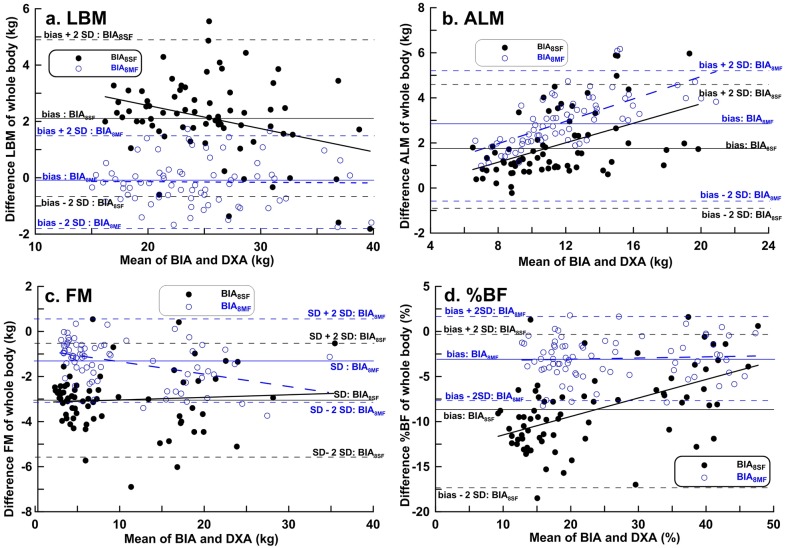
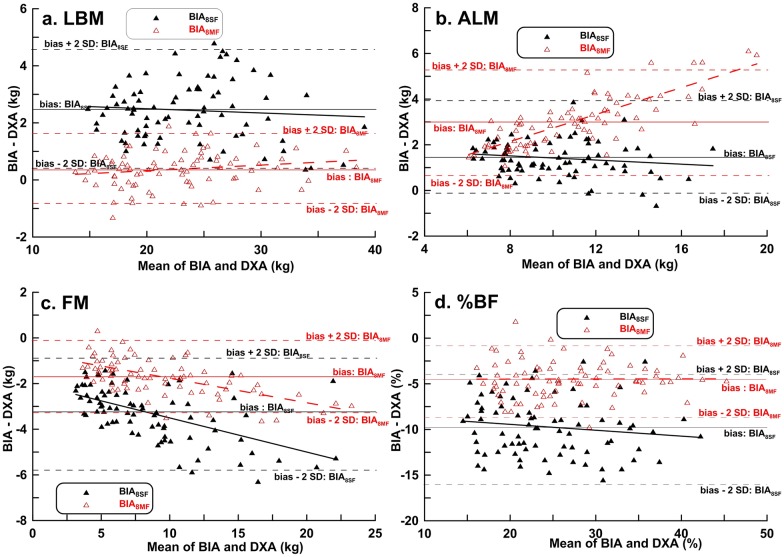
Bland-Altman plots were used to determine bias and LOA between BIA and DXA methods in boys (Fig 3) and girls (Fig 4). The LOAs were greater for the BIA8SF and DXA measurements than for the BIA8MF and DXA measurements, except for the ALM measures in girls (Table 3). Similar to the results by CCC, Bland-Altman analysis showed a good and clinically acceptable agreement between LBM measures by BIA8MF and DXA (LOA = -1.82 to 1.52 kg in boys and LOA = -0.88 to1.63 kg in girls, Table 3).
Fig 3. Bland-Altman plots with linear regression analysis of dual-energy X-ray absorptiometry results vs. BIA8SF and BIA8MF estimates of body composition in boys.
Fig 4. Bland-Altman plots with linear regression analysis of dual-energy X-ray absorptiometry results vs. BIA8SF and BIA8MF estimates of body composition in girls.
In the human body, the FM is the total body weight minus LBM. Indeed, the LOAs of FM measures by BIA8MF and DXA (-3.21 to 0.55 kg in boys and -3.30 to -0.10 kg in girls, Table 3) showed similar ranges to that of LBM but with different plus-minus sign (negative values in FM). In this study, the mean FM was about half of the LBM in children (Table 1) and thus, the degree of error was larger in FM estimation by BIA8MF and DXA compared with that in LBM.
Regarding %BF estimation, BIA8SF measurements underestimated %BF by 8.82% in boys and 9.72% in girls, whereas the BIA8MF measurements underestimated %BF by 3.00% in boys and 4.48% in girls (Figs 3d and 4d). The LOAs in %BF estimation between BIA8MF and DXA were clinically important. Even worse, there were larger LOAs in %BF estimation by BIA8SF and DXA (-17.46 to -0.19% in boys and -15.99 to -3.45% in girls, Table 3).
The ρc value for ALM estimated by BIA8SF was 0.770 in boys and 0.828 in girls, and the ρc value for ALM estimated by BIA8MF was 0.671 in boys and 0.635 in girls, all of which were considered poor agreement (Table 3). In agreement with CCC, Bland-Altman analysis showed a poor agreement with clinically importance between ALM estimations by BIA and DXA in both genders (Table 3).
Discussion
This study compared the estimates of body composition obtained from multi-segment BIA8SF and BIA8MF with DXA measurements in primary school children from Taiwan. Pearson product moment correlation was used to test the linear association whereas ICC, CCC and Bland-Altman Plot were used to test agreement between BIA and DXA results. So far, there is still a debate about which method is the best for assessing agreement between two instruments. The ICC and CCC are scaled agreement indices depending on the measurement range, and therefore they are easy to summarize but hard to interpret [19]. In contrast, bias and LOAs (Bland-Altman plot) are unscaled indices based on the original unit and interpretation of the agreement requires prior knowledge of the measurement variables [20]. Since these methods all have some disadvantages, we have used more than one statistical method to assess agreement between two instruments in this study.
The LBM estimates by BIA8MF and DXA were in high agreement for both genders using all statistical methods in this study. Therefore, BIA8MF and DXA were interchangeable test methods for the measurement of LBM in children. However, the FM estimates showed fair to substantial agreement between BIA8MF and DXA by CCC but clinically important differences by Bland-Altman plots. One possible explanation for the discrepancy in the degree of agreement may due to the fact that CCC was scaled relative to the between-subject variability and the large FM range in our subjects produced a relatively high ρc value. In contrast, Bland-Altman analysis was not dependent on between-subject variability such that it was easier to identify the error between the two methods.
Except for LBM estimates, the remainder of the BIA measurements showed strong linear correlated (but with clinically significant errors) with the gold standard method, DXA. Talma et al. [21] reported similar findings in a review article. Most previous BIA validation studies reported high precision using the BIA models but did not use a reference method to measure the accuracy of BIA estimates [22]. In addition to linear regression and ICC, we also performed Bland-Altman analysis and determined CCC to rigorously assess the statistical consistency of body composition estimates from BIA relative to DXA. Our results indicated clinically important errors in FM and %BF estimated by both BIA devices which may limit their applicability to body composition measurements at an individual level in children, even though the r and r1 values were high between both BIA methods and DXA. It is worth noting that although ICC is a popular test to compare the results between two methods, there is still a debate about the use of ICC in assessment agreement [23, 24].
We also compared both BIA8SF and BIA8MF models in children with a wide range of body fat composition, using DXA as the gold standard. Although the estimates from both BIA devices and DXA showed strong linear correlations, the correlation coefficients and agreements were higher for BIA8MF compared with BIA8SF. In general, the BIA devices (especially the BIA8SF) overestimated LBM and underestimated FM. In addition, the LOAs were larger and the biases were greater for BIA8SF measurements compared with BIA8MF measurements, except for ALM in girls. The CCC analysis also indicated better agreements in measurements of LBM, FM, and %BF for the BIA8MF in both sexes. These results confirm the findings of Kriemler et al. [25] that BIA8MF is superior to BIA8SF in pediatric body composition analysis.
In our study, both BIA8SF and BIA8MF underestimated FM and %BF in children who had large or small amounts of body fat. Additionally, BIA8SF had a fixed bias or proportional bias in all components of body composition. Talma et al. [21], in their systematic review, indicated that BIA provided inconsistent results, depending on the reference method used. A literature review of validation studies for the Tanita BC-418 system in children also showed inconsistent results similar to our findings, whereas other studies had results which contradicted our findings. For example, Pietrobelli et al. [26] showed a perfect linear correlation between body composition parameters measured by the Tanita BC-418 system and DXA in subjects aged 6–64 years. However, they did not perform agreement analysis, and had a small sample size and wide age range. Some studies showed that the Tanita BC-418 underestimated FM in obese children compared with other reference methods [27, 28]. Shaikh et al. [28] reported a strong linear correlation between FM determined by the Tanita BC-418MA and DXA in obese boys aged 11.0 ± 0.53 years; however, the BIA system underestimated %BF, and the LOA in %BF was -3.8 to 15.4%. Haroun et al. [27] examined obese subjects (between 5–22 years of age) and found that the Tanita BC-418 underestimated FM by 3.5 kg in males and 3.6 kg in females, compared with the isotope dilution method. In contrast, Prins et al. [29] showed the Tanita BC-418MA system overestimated %BF in normal-weight Gambian children aged 5–16 years relative to the isotope dilution method.
We found that LBM estimates between BIA (BIA8SF and BIA8MF) and DXA were in fair to substantial agreement whereas ALM estimates between BIA and DXA showed poor agreement. Few previous studies have used eight-electrode multi-frequency BIA devices (i.e. the Inbody-230) for estimates of body composition in children. Kriemler et al. [25] used a different BIA8MF device (Inbody 3.0, Biospace, Seoul, Korea) in 6 years-old and found no fixed bias or proportional bias in FFM or ALM relative to measurements from DXA. Jensky-Squires et al. [30] used the Inbody-320 (Biospace, Seoul, Korea) to estimate %BF in children between 10–17 years of age relative to underwater weighing, and found significant differences in girls but not boys. Lim et al. [31] used the Inbody 720 (Biospace, Seoul, Korea) to estimate FFM, FM, and %BF in healthy children between 6–18 years of age and reported a high precision relative to DXA results. In their study, the LOA in %BF was -2.2 ± 6.1%, which was far less than ours.
BIA is primarily designed to estimate FFM, and the FFM prediction equations were developed using a reference method, such as DXA and/or isotope dilution. Variables in the regression equations may include height, weight, age, sex, race, and other factors [7]. Therefore, the established FFM equations may not applicable to all pediatric populations such as our pediatric populations [32, 33]. Body hydration status can also influence FFM calculation from BIA measurements. Most BIA prediction equations assume that the FFM consists of 73% water. However, although the water content of FFM is about 73% in adults, it is greater in children [22]. Therefore, a BIA prediction equation developed for adults could overestimate FFM in children. Moreover, hydration status changes as a child develops [34]. Therefore, an equation developed for school-aged children may not be accurate for adolescents. These major limitations of the BIA method remain unresolved.
Conclusion
For all children, LBM measures using the BIA8MF were precise and accurate whereas clinically significant errors occurred in both FM and %BF estimates. The BIA8SF and BIA8MF both underestimated FM and %BF in children. For the estimates of ALM, both BIA devices showed poor agreement with DXA. Thus, the body composition results obtained using the inbuilt equations of the BIA8SF and BIA8MF should be interpreted with caution, and high quality validation studies in specific subgroups children are required prior to field research.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by a single institution, the Chang Gung Memorial Hospital (grant number CMRPG6D0353, CCC and CMRPG6C0052, LWL). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. One of the authors (KCH) is an employee of Charder Electronic Co, Ltd. This company did not provide KCH financial support in executing this study. Nor did the company have any additional role in the research funding, study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of the author are articulated in the Author Contributions section.
References
- 1.Wells JC, Fewtrell MS. Measuring body composition. Archives of disease in childhood. 2006;91(7):612–7. Epub 2006/06/23. 10.1136/adc.2005.085522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel). 2014;14(6):10895–928. Epub 2014/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. Journal of the American College of Nutrition. 1992;11(2):199–209. Epub 1992/04/01. [PubMed] [Google Scholar]
- 4.Organ LW, Bradham GB, Gore DT, Lozier SL. Segmental bioelectrical impedance analysis: theory and application of a new technique. J Appl Physiol (1985). 1994;77(1):98–112. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Shen W, Gallagher D, Jones A Jr., Wang Z, Wang J, et al. Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. The American journal of clinical nutrition. 2006;84(5):1014–20. Epub 2006/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. The American journal of clinical nutrition. 2002;76(2):378–83. Epub 2002/07/30. [DOI] [PubMed] [Google Scholar]
- 7.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–43. Epub 2004/09/24. 10.1016/j.clnu.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 8.Gudivaka R, Schoeller DA, Kushner RF, Bolt MJ. Single- and multifrequency models for bioelectrical impedance analysis of body water compartments. J Appl Physiol (1985). 1999;87(3):1087–96. Epub 1999/09/14. [DOI] [PubMed] [Google Scholar]
- 9.Dittmar M, Reber H. New equations for estimating body cell mass from bioimpedance parallel models in healthy older Germans. American journal of physiology Endocrinology and metabolism. 2001;281(5):E1005–14. Epub 2001/10/12. [DOI] [PubMed] [Google Scholar]
- 10.Pietrobelli A, Morini P, Battistini N, Chiumello G, Nunez C, Heymsfield SB. Appendicular skeletal muscle mass: prediction from multiple frequency segmental bioimpedance analysis. European journal of clinical nutrition. 1998;52(7):507–11. Epub 1998/07/31. [DOI] [PubMed] [Google Scholar]
- 11.Body Composition Analyzer BC-418 Instruction Manual: Tanita corporation; [cited 2017 Jan 9]. http://www.tanita.com/es/bc-418/.
- 12.InBody User's Manual: Biospace Co., Ltd; 1996 [cited 2017 Jan 9]. https://cdn.shopify.com/s/files/1/0832/8945/files/InBody230_User_sManual.pdf?7633250021353425932.
- 13.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Body Composition Procedures Manual 2013 [cited 2017 Jan 9]. https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Body_Composition_DXA.pdf.
- 14.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clinical and experimental pharmacology & physiology. 2002;29(7):527–36. Epub 2002/06/13. [DOI] [PubMed] [Google Scholar]
- 15.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin. 1979;86(2):420–8. Epub 1979/03/01. [DOI] [PubMed] [Google Scholar]
- 16.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–68. Epub 1989/03/01. [PubMed] [Google Scholar]
- 17.McBride GB. A proposal for strength-of-agreement criteria for Lin's Concordance Correlation Coefficient. 2005 NIWA Client Report: HAM2005-062.
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. Epub 1986/02/08. [PubMed] [Google Scholar]
- 19.Barnhart HX, Haber MJ, Lin LI. An overview on assessing agreement with continuous measurements. Journal of biopharmaceutical statistics. 2007;17(4):529–69. Epub 2007/07/07. 10.1080/10543400701376480 [DOI] [PubMed] [Google Scholar]
- 20.Giavarina D. Understanding Bland Altman analysis. Biochemia medica. 2015;25(2):141–51. Epub 2015/06/26. 10.11613/BM.2015.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talma H, Chinapaw MJ, Bakker B, HiraSing RA, Terwee CB, Altenburg TM. Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2013;14(11):895–905. Epub 2013/07/16. [DOI] [PubMed] [Google Scholar]
- 22.Silva AM, Fields DA, Sardinha LB. A PRISMA-driven systematic review of predictive equations for assessing fat and fat-free mass in healthy children and adolescents using multicomponent molecular models as the reference method. Journal of obesity. 2013;2013:148696 Epub 2013/07/12. 10.1155/2013/148696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. A note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Computers in biology and medicine. 1990;20(5):337–40. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 24.Zaki R, Bulgiba A, Ismail R, Ismail NA. Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison studies: a systematic review. PloS one. 2012;7(5):e37908 Epub 2012/06/05. 10.1371/journal.pone.0037908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriemler S, Puder J, Zahner L, Roth R, Braun-Fahrlander C, Bedogni G. Cross-validation of bioelectrical impedance analysis for the assessment of body composition in a representative sample of 6- to 13-year-old children. European journal of clinical nutrition. 2009;63(5):619–26. Epub 2008/02/21. 10.1038/ejcn.2008.19 [DOI] [PubMed] [Google Scholar]
- 26.Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. European journal of clinical nutrition. 2004;58(11):1479–84. Epub 2004/05/13. 10.1038/sj.ejcn.1601993 [DOI] [PubMed] [Google Scholar]
- 27.Haroun D, Croker H, Viner RM, Williams JE, Darch TS, Fewtrell MS, et al. Validation of BIA in obese children and adolescents and re-evaluation in a longitudinal study. Obesity (Silver Spring). 2009;17(12):2245–50. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh MG, Crabtree NJ, Shaw NJ, Kirk JM. Body fat estimation using bioelectrical impedance. Hormone research. 2007;68(1):8–10. Epub 2007/01/11. 10.1159/000098481 [DOI] [PubMed] [Google Scholar]
- 29.Prins M, Hawkesworth S, Wright A, Fulford AJ, Jarjou LM, Prentice AM, et al. Use of bioelectrical impedance analysis to assess body composition in rural Gambian children. European journal of clinical nutrition. 2008;62(9):1065–74. Epub 2007/07/12. 10.1038/sj.ejcn.1602830 [DOI] [PubMed] [Google Scholar]
- 30.Jensky-Squires NE, Dieli-Conwright CM, Rossuello A, Erceg DN, McCauley S, Schroeder ET. Validity and reliability of body composition analysers in children and adults. The British journal of nutrition. 2008;100(4):859–65. Epub 2008/03/19. 10.1017/S0007114508925460 [DOI] [PubMed] [Google Scholar]
- 31.Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Jeong JS, et al. Cross-calibration of multi-frequency bioelectrical impedance analysis with eight-point tactile electrodes and dual-energy X-ray absorptiometry for assessment of body composition in healthy children aged 6–18 years. Pediatrics international: official journal of the Japan Pediatric Society. 2009;51(2):263–8. Epub 2009/05/02. [DOI] [PubMed] [Google Scholar]
- 32.Lewy VD, Danadian K, Arslanian S. Determination of body composition in African-American children: validation of bioelectrical impedence with dual energy X-ray absorptiometry. Journal of pediatric endocrinology & metabolism: JPEM. 1999;12(3):443–8. Epub 2000/05/23. [DOI] [PubMed] [Google Scholar]
- 33.Clasey JL, Bradley KD, Bradley JW, Long DE, Griffith JR. A new BIA equation estimating the body composition of young children. Obesity (Silver Spring). 2011;19(9):1813–7. Epub 2011/06/18. [DOI] [PubMed] [Google Scholar]
- 34.Wells JC, Fewtrell MS, Davies PS, Williams JE, Coward WA, Cole TJ. Prediction of total body water in infants and children. Archives of disease in childhood. 2005;90(9):965–71. Epub 2005/08/23. 10.1136/adc.2004.067538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.