Abstract
Background
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that affects multiple organs, including the lungs, pancreas, liver and intestine. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) locus lead to defective proteins and reduced Cl- secretion and Na+ hyperabsorption in the affected organs. In addition, patients suffering from CF display chronic inflammation that contributes to the pathogenesis of CF. Recent work suggests that CF patients have a reduced capacity to biosynthesize specialized pro-resolving lipid mediators (SPMs), which contributes to the development and duration of the unwanted inflammation. Alterations in the metabolism of arachidonic acid (AA) and docosahexaenoic acid (DHA) to specialized pro-resolving mediators (SPMs), like lipoxins (LXs), maresins (MaRs), protectins (PDs) and resolvins (Rvs), may play a major role on clinical impact of airway inflammation in CF.
Methods
In this study, our aims were to detect and quantitate Resolvin D1 (RvD1) in sputum and plasma from patients with CF and compare levels of RvD1 with biomarkers of inflammation and lung function. We studied 27 CF patients aged 6 to 55 years (median 16 years) in a prospective approach.
Results
DHA can be found in the plasma of our CF patients in the milligram range and is decreased in comparison to a healthy control group. The DHA-derived pro-resolving mediator Resolvin D1 (RvD1) was also present in the plasma (286.4 ± 50 pg/ mL, mean ± SEM) and sputum (30.0 ± 2.6 pg/ mL, mean ± SEM) samples from our patients with CF and showed a positive correlation with sputum inflammatory markers. The plasma concentrations of RvD1 were ten times higher than sputum concentrations. Interestingly, sputum RvD1/ IL-8 levels showed a positive correlation with FEV1 (rs = 0.3962, p< 0.05).
Conclusions
SPMs, like RvD1, are well known to down-regulate inflammatory pathways. Our study shows that the bioactive lipid mediator RvD1, derived from DHA, was present in sputum and plasma of CF patients and may serve as a representative peripheral biomarker of the lung resolution program for CF patients.
Introduction
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that affects multiple organs, including the lungs, pancreas, liver, and intestine. Cystic fibrosis is caused by the mutation of the gene coding for the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), a cyclic AMP-dependent Cl- channel. The major clinical features of CF are chronic pulmonary disease, exocrine pancreatic insufficiency and male infertility [1, 2]. The lung disease is the main cause of morbidity and mortality in CF [3, 4]. The airway epithelium of patients with CF fails to transport Cl-, HCO3- and water, resulting in a reduced airway surface liquid (ASL) height and impaired mucociliary clearance. The hyperabsorption of electrolytes observed in the CF bronchial epithelium might further aggravate the dehydration of the ASL. Mutations in CFTR gene cause defective Cl- secretion and Na+ hyperabsorption by airway epithelia cells [5–7]. CFTR is also found on cells of the immune system, such as neutrophils [8], monocytes [9], and T cells [10], where loss of CFTR function leads to abnormal immune cellular function. Platelets from CF patients are affected by the molecular defect of CFTR and may play a role in the failure of resolution of inflammation in CF [11]. Mutations in CFTR lead to abnormally thick mucus [12] and aberrant immune responses [13, 14]. The net result is a propensity in CF patients for recurrent infections and over-exuberant, yet ineffective leukocyte recruitment, phagocytosis, killing, and clearance of pathogens. In comparison to other chronic inflammatory lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma, CF is the leading disease in terms of uncontrolled inflammation [15–18]. So far, with the exception of high-dose ibuprofen, there is no anti- inflammatory treatment available for patients. It is well known for more than a decade that patients with cystic fibrosis have altered levels of plasma fatty acids. Previous studies demonstrated that arachidonic acid (AA) levels are increased and docosahexaenoic acid (DHA) levels are decreased in affected tissues from cystic fibrosis [19]. Recently, it has become evident that resolution of inflammation is a biochemically active process regulated in part by endogenous polyunsaturated fatty acid (PUFA)-derived mediators [20]. Novel families of mediators including lipoxins, resolvins, protectins and maresins represent a new genus of specialized pro- resolving mediators (SPMs) [21]. It has been demonstrated that an active pro-resolving lipid mediator derived from the Ω-6 fatty acid AA, namely Lipoxin A4 (LXA4), was decreased in the airways in patients with CF [6, 22, 23]. Lipoxins are bioactive lipids derived from Ω-6 PUFAs and play important roles in various biological functions [24]. To our best knowledge, there is no data about clinical status and the role of active lipid mediators derived from the Ω-3 PUFA DHA, namely RvD1 in cystic fibrosis lung disease. The endogenous RvD1 (7S, 8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) is produced at inflammatory sites from the interaction of lipoxygenase activities of several cell types including activated neutrophils, platelets and epithelial cells. RvD1 is one member of the newly identified lipid molecules promoting resolution of inflammatory processes by modulating neutrophilic inflammation, clearing apoptotic PMN and inhibiting the production of pro-inflammatory cytokines/ chemokines such as IL-8 [25].
RvD1 levels in CF airways could be a contributing factor in chronic airway inflammation which characterises these patients. There is no data available about the role of RvD1 in the airways of CF patients. In this study, we have investigated the levels of PUFAs, and in particular the pro-resolving lipid mediator, RvD1, in plasma and sputum of CF patients in comparison to inflammatory biomarkers. Furthermore, we investigated RvD1 levels in relation to clinical consequences in terms of lung function and Pseudomonas infection status.
Materials and methods
2.1 Subjects and selection
Written informed consent was obtained from all participants. The study was approved by the local institutional ethics committee of the University Hospital Frankfurt. Patients were recruited from the Christiane Herzog CF- Center of the University Hospital Frankfurt, Germany. The population of this study consisted of 27 clinically stable patients with cystic fibrosis (8 were P. aeruginosa-infected) and 14 non-smoking healthy control subjects. Clinical stability was defined as absence of acute exacerbation of disease six weeks prior to inclusion. Acute exacerbation was defined by two of the following symptoms: Fever >38.0°C, increase of sputum, significant increase of C—reactive protein (CrP) and significant weight loss. Exclusion criteria were absence of current use of systemic antibiotic and steroid treatment, clinically relevant renal, cardiac, or hepatic (ALT/AST > 3 times of the upper normal limit, portal hypertension) dysfunction, chronic gastrointestinal disease not related to CF and pregnancy.
2.2 Sample collection and processing
Subjects first inhaled 200 μg Salbutamol and subsequent nebulised hypertonic saline at concentrations of 3%, 4% and 5% for every 7 minutes. This bronchial stimulus caused expectoration of sputum. Before inhalation of hypertonic saline, the mouth was cleaned by flushing with water. Sputum was processed within 2 hours of collection [26]. The selected sputum plugs were as far as possible without saliva, processed into a weighed Eppendorf tube and processed with 2× weight/volume of 0.1% Dithiothreitol (DTT). Afterwards 4× weight/volume of PBS was added. Samples were filtered through 48 μm mesh and centrifuged for 10 minutes at 790× g to remove the cells. Supernatants were stored at -80C° until further analysis.
2.3 Cytometric bead array
Concentrations of different cytokines/chemokines were determined in sputum samples using the BD™ CBA Flex Set System for the measurement of IL-1β and IL-8 (BD Bioscience-PharMingen, San Diego, CA, USA) levels. Each BD™ CBA Flex Set contained one bead population with distinct fluorescence intensity, as well as the appropriate phycoerythrin (PE) detection reagent and standard. The tests were performed according to the manufacturer’s advice, and samples were run in duplicate [27, 28]. For analyses of the cytokines/ chemokines, we added the same concentration of DTT (0.025%) as in the sputum supernatant to the standard curve and enzyme immunoassay buffer.
2.4 Biochemical analysis
DHA and AA measurement in blood plasma were performed by fatty acid methyl esters (FAME) analysis. Total lipids of plasma and cruor were extracted according to Bligh and Dyer [29]. For derivatization, plasma was dissolved in 1000 μL petroleum ether followed by addition of 50 μL sodium methoxide-solution in accordance with the method of Kohn [30]. The organic solvent was completely evaporated, overlaid with nitrogen and stored at -80°C until measurement. The samples were than analysed by capillary gas chromatography (CGC).
RvD1 concentrations were measured by ELISA technique using Greiner microtiter plates (Greiner Bio-One, Frickenhausen, Germany) according to the manufacturer´s instructions. Briefly, RvD1 was extracted from plasma and sputum via Sep-Pak C18 light cartridges (Waters, Eschborn, Germany). After equilibrating columns with methanol and washing with deionized water, samples diluted in 1M acetate buffer were applied to the column. Elution of RvD1 with ethyl acetate was conducted after second washing with deionized water. Solvent was evaporated under a gentle stream of nitrogen and the residue was immediately dissolved in sample buffer and subjected to ELISA.
2.5 Pulmonary function test
Pulmonary function tests were performed according to American Thoracic Society/ European Respiratory Society guidelines for performance and acceptance prior to sputum induction [31].
2.6 Statistical analysis
Data were analysed using the statistical program GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Differences between the groups were performed by the nonparametric Kruskal-Wallis test or a Mann- Whitney test depending on Gaussian distribution and the homogeneity of the variances. Correlations between biomarkers and clinical parameters were verified by using Spearman correlation coefficient (rs). A probability of p < 0.05 was regarded as significant. In figures data are shown as individual values and mean ± SEM.
Results
Pulmonary function is associated with sputum neutrophil count
The demographic and baseline clinical characteristics of the subjects with cystic fibrosis and the healthy control subjects are shown in Table 1. The distribution of genotypes in our study cohort closely reflected the genotype distribution in our CF clinic population. The majority of our patients were pancreatic insufficient (92.6%). The minority of our patients (29.6%) were Pseudomonas aeruginosa positive. Even though the FEV1 (% predicted) of our CF patients ranged from 49 to 119% (Fig 1a), most of our subjects had normal or mildly abnormal lung function (Fig 1a) with a broad distribution of the flows in the small airways represented by MEF25 (%predicted) ranging from 11.6 to 161%predicted (Fig 1c). Furthermore, we were able to demonstrate a negative correlation between pulmonary function data and sputum neutrophils (Fig 1b and 1d). This is shown most prominently by decreased air flow in the small airways (MEF25) in correlation with sputum PMNs (p < 0.01).
Table 1. Patient characteristics.
| Healthy Controls | CF | |
|---|---|---|
| Subjects | 14 | 27 |
| Sex [m/f] | 8/ 6 | 14/ 13 |
| Age | 18.2 ± 4.4 | 17.5 ± 11.5 |
| BMI [kg/ m2] | 22.1 ± 3.5 | 18.6 ± 3.4 |
| ΔF508 homozygous | - | 13 |
| Pancreatic insufficient | - | 25 |
| Ps. aeruginosa positive | - | 8 |
| FEV1 [%pred.] | 102.1 ± 9.4 | 88.1 ± 16.7 |
Fig 1. Baseline pulmonary function data of CF patients and healthy controls and correlation of pulmonary function in CF to sputum neutrophils.
Comparison of mean (a) FEV1 [%] and (c) MEF 25 [%] in CF patients and healthy controls. Correlation of (b) FEV1 [%] and (d) MEF25 [%] in CF patients with percentage of sputum neutrophils. Red dots represent Ps. a. positive patients. Values are represented as Mean ± SEM. The Spearman correlation coefficient (rs) and the associated p-value is given for each analysis. ** p< 0.01.
Plasma fatty acid level of docosahexaenoic acid (DHA) is decreased in CF
To investigate whether airway inflammation may also correlate with plasma fatty acid levels we determined fatty acid profiles in plasma. Indeed, DHA levels in plasma were decreased in our CF patients (37.2 ± 3.0 mg/dL, mean ± SEM) in comparison to the healthy control group (54.6 ± 7.3 mg/ dL, mean ± SEM, p< 0.05) (Fig 2a). No significant differences were measured for AA in plasma between both groups (Fig 2b).
Fig 2. Levels of PUFAs in plasma from CF patients and healthy controls.
Comparison of mean (a) DHA and (b) AA in plasma from CF patients and healthy controls. Values are represented as Mean ± SEM. * p< 0.05.
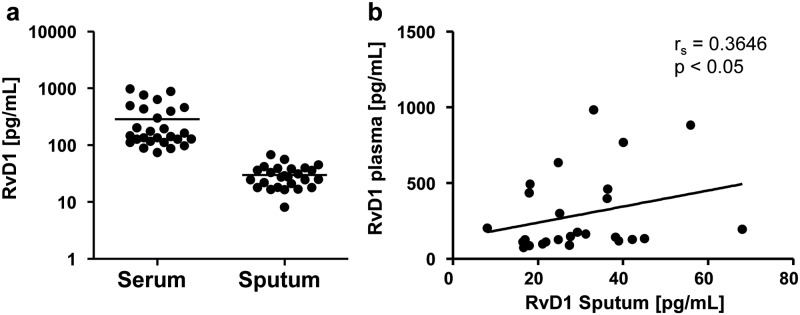
RvD1 is present in plasma and sputum of CF patients and levels of sputum RvD1 correlate with plasma RvD1 levels
To identify disturbances of pro-resolving mechanisms in CF resulting from differences in fatty acid levels, an active lipid mediator of DHA, namely RvD1, was measured in plasma and sputum of our CF patients. RvD1 was present in the picogram range in plasma (286.4 ± 50 pg/ mL, mean ± SEM) and sputum (30.0 ± 2.6 pg/ mL, mean ± SEM) (Fig 3a) of our CF patients and concentrations in plasma showed a positive correlation with concentrations in sputum (rs = 0.3646, p < 0.05) (Fig 3b).
Fig 3. Levels of RvD1 in plasma and sputum from CF patients.
Levels of (a) plasma and sputum RvD1 from CF patients and (b) correlation of plasma and sputum RvD1 levels. Values are represented as Mean ± SEM. The Spearman correlation coefficient (rs) and the associated p- value is given for each analysis.
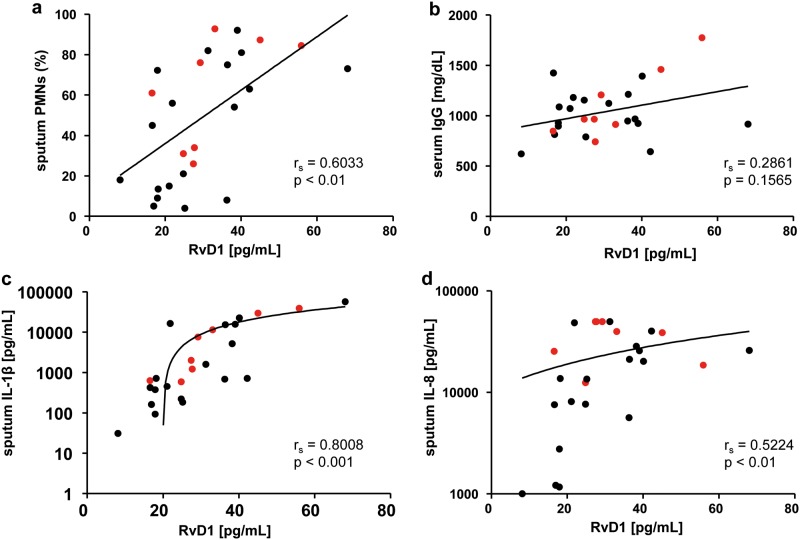
RvD1 in CF sputum correlates with sputum and serum inflammatory biomarkers
RvD1 is an anti-inflammatory and pro-resolving mediator, so correlation of RvD1 in sputum was performed with selected biomarkers of inflammation in sputum (Fig 4a, 4c and 4d) and serum (Fig 4b). Sputum RvD1 levels showed a significant positive correlation with sputum PMNs (rs = 0.6033, p< 0.01; Fig 4a), sputum IL-1β levels (rs = 0.8008, p< 0.001; Fig 4c) and sputum IL-8 levels (rs = 0.5224, p< 0.01; Fig 4d). In addition, sputum Resolvin D1 showed a tangential positive correlation with serum IgG levels (Fig 4b).
Fig 4. Correlation of pro-inflammatory sputum and serum biomarkers with sputum RvD1 levels.
Correlation of (a) sputum PMNs, (b) serum IgG, (c) sputum IL-1β and (d) sputum IL-8 with sputum RvD1 levels from CF patients. Red dots represent Ps. a. positive patients. The Spearman correlation coefficient (rs) and the associated p- value is given for each analysis.
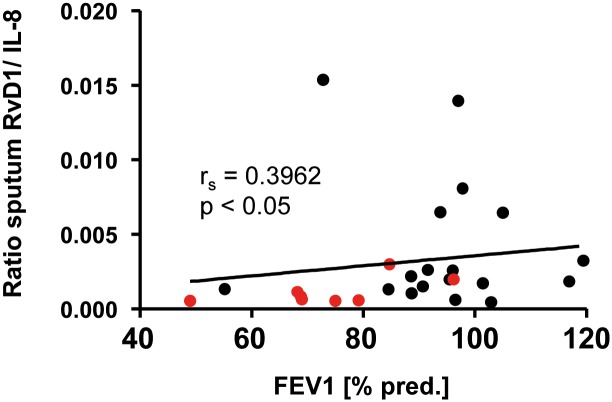
Increased RvD1/ IL-8 ratio in patients with CF is associated with a better pulmonary function
With both pro- inflammatory marker IL-8 and pro-resolution marker RvD1 present in CF sputum, we determined the impact of RvD1/ IL-8 ratio on lung function. There was a significant positive correlation of the RvD1/ IL-8 ratio with FEV1 (rs = 0.3962, p< 0.05) (Fig 5).
Fig 5. Correlation of sputum RvD1/ IL-8 ratio with FEV1.
Correlation of sputum RvD1/ IL-8 ratio with FEV1 [%]. Red dots represent Ps. a. positive patients. The Spearman correlation coefficient (rs) and the associated p- value is given for each analysis.
Discussion
Progressive pulmonary disease is the major cause of morbidity and mortality in CF patients. CF lungs are challenged by an immense burden with pathogenic bacteria resulting in chronic lung infiltration with neutrophils and release of neutrophil elastase and other damaging products. This contributes negatively to bronchiectasis and pulmonary fibrosis [13, 32–34]. Furthermore, additional abnormalities in innate immune responses have been identified that lead to chronic inflammation and contribute to the pathogenesis of CF. These abnormalities lead to a decrease in anti-inflammatory lipid mediator production [6, 35] and an increase in pro-inflammatory chemokines, such as IL-8 [36–38]. Anti-inflammatory and pro-resolution treatment strategies are a new way of combatting chronic inflammation in CF. The use of SPMs as part of humanized nano-pro-resolving medicines is an exciting concept for this purpose. Hence, it is of critical importance to define the SPM profile in CF patients in order to address specific unmet needs by pharmacological intervention [21]. In previous studies, we have analysed specific pro-inflammatory markers in chronic and severe inflammatory lung diseases in order to detect pathognomonic patterns for the underlying disease [18, 38–45]. The knowledge that chronic inflammation is harmful to the CF lung has resulted in strategies to reduce lung infection [46] and the damage associated with chronic inflammation [47].
Patients with cystic fibrosis (CF] show decreased serum and sputum levels of linoleic acid (LA) and docosahexaenoic acid (DHA). The potential benefits of dietary addition of polyunsaturated fatty acids (PUFAs) in CF are still uncertain [48]. Supplementation of high doses of omega-3 fatty acids was effective in terms of correction of the lipid imbalance, but data on improvement in lung function and other clinical aspects are not conclusive [49]. Further investigation is needed to determine if altered metabolism of arachidonic acid and DHA to specialized pro-resolving mediators, like lipoxins (LX), maresins (MaR), protectins (PD) and resolvins (Rv), may impact the efficacy of supplementation therapy. Of interest, and in contrast to administration of PUFAs, like DHA and eicosapentaenoic acid (EPA), in the range of milligram to gram doses in past and ongoing clinical trials, the pro-resolving mediators (Rvs) are bioactive in the nanogram to picogram range [21]. In this study, we have been able to confirm decreased DHA levels in plasma of our CF patients in comparison to healthy controls. Evidence suggests that reduced DHA plasma levels could be due to suboptimal intestinal absorption of fatty acids in CF patients [50, 51]. Freedman et al. showed that DHA therapy leads to fewer neutrophils in the lungs of CF knock-out mice [50]. We employed a standardized and validated sputum-induction protocol to noninvasively study the local inflammatory responses [26]. We were able to detect RvD1 as a representative of DHA derived SPMs in plasma as well as the sputum of our CF patients.
Markers of inflammatory activity are important for the assessment and management of chronic respiratory diseases like CF and COPD [52, 53]. Numerous studies have analysed inflammatory cytokines in sputum, in relation to clinical status [54–56]. Until today, there is no study which has investigated the relation of inflammatory status, and lung function to RvD1 in CF patients. We demonstrate a positive correlation between sputum RvD1/ IL-8 levels and lung function. We have also shown that there is an abnormally low ratio of this pro-resolution mediator in comparison to pro-inflammatory biomarkers that correlates to decreased lung function. The ratio of Resolvin D1 to pro-inflammatory chemokine IL-8 is low in sputum of CF patients, even in the absence of an acute infection or exacerbation, strongly suggesting that there is an important pathophysiological effect for Resolvin anti-inflammatory and pro- resolution activity in the CF lung. SPMs like Resolvins can enhance the release of anti- microbial peptides. Of note, 30% of our CF group consisted of Pseudomonas aeruginosa- infected patients. CF patients who have higher levels of SPMs, like Resolvin D1 in the lungs have more preservation of FEV1 and fewer Pseudomonas aeruginosa infections.
In conclusion, our study shows that the bioactive lipid mediator RvD1, derived from DHA, was present in sputum and serum of CF patients and may serve as a representative peripheral biomarker of the lung resolution program for CF patients. This is the first study confirming a correlation between RvD1 levels with biomarkers, such as IL-1β or IL-8, in induced sputum in CF patients. Moreover, this study strongly indicated a positive correlation of sputum RvD1/ IL-8 levels with lung function data suggesting an important role for Resolvin-mediated anti-inflammatory and pro- resolution activity in the CF lung.
Data Availability
Data may compromise the privacy of study participants and may not be shared publicly. Data are available upon request to the authors.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Elborn JS. Cystic fibrosis. Lancet (London, England) 2016:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. The European respiratory journal 2014; 44(4):1042–54. 10.1183/09031936.00228013 [DOI] [PubMed] [Google Scholar]
- 3.Plant BJ, Goss CH, Plant WD, Bell SC. Management of comorbidities in older patients with cystic fibrosis. The Lancet. Respiratory medicine 2013; 1(2):164–74. 10.1016/S2213-2600(13)70025-0 [DOI] [PubMed] [Google Scholar]
- 4.Doring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society 2012; 11(6):461–79. [DOI] [PubMed] [Google Scholar]
- 5.Knowles M, Gatzy J, Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. The Journal of clinical investigation 1983; 71(5):1410–7. 10.1172/JCI110894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B et al. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. The European respiratory journal 2014; 44(2):394–404. 10.1183/09031936.00106013 [DOI] [PubMed] [Google Scholar]
- 7.Verriere V, Higgins G, Al-Alawi M, Costello RW, McNally P, Chiron R et al. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS One 2012; 7(5):e37746 10.1371/journal.pone.0037746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Painter RG, Valentine VG, Lanson NA JR, Leidal K, Zhang Q, Lombard G et al. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006; 45(34):10260–9. 10.1021/bi060490t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettorre M, Verze G, Caldrer S, Johansson J, Calcaterra E, Assael BM et al. Electrophysiological evaluation of cystic fibrosis conductance transmembrane regulator (CFTR) expression in human monocytes. Biochimica et biophysica acta 2014; 1840(10):3088–95. 10.1016/j.bbagen.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 10.Shanshiashvili LV, Dabrundashvili N, Natsvlishvili N, Kvaratskhelia E, Zhuravliova E, Barbakadze T et al. mGluR1 interacts with cystic fibrosis transmembrane conductance regulator and modulates the secretion of IL-10 in cystic fibrosis peripheral lymphocytes. Molecular Immunology 2012; 51(3–4):310–5. 10.1016/j.molimm.2012.03.029 [DOI] [PubMed] [Google Scholar]
- 11.Mattoscio D, Evangelista V, Cristofaro de R, Recchiuti A, Pandolfi A, Di Silvestre S et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2010; 24(10):3970–80. [DOI] [PubMed] [Google Scholar]
- 12.Burgel P, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 2007; 62(2):153–61. 10.1136/thx.2006.062190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society 2015; 14(4):419–30. [DOI] [PubMed] [Google Scholar]
- 14.Ratner D, Mueller C. Immune responses in cystic fibrosis: are they intrinsically defective? American journal of respiratory cell and molecular biology 2012; 46(6):715–22. 10.1165/rcmb.2011-0399RT [DOI] [PubMed] [Google Scholar]
- 15.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax 2009; 64(1):81–8. 10.1136/thx.2007.082388 [DOI] [PubMed] [Google Scholar]
- 16.Bruscia EM, Bonfield TL. Innate and Adaptive Immunity in Cystic Fibrosis. Clinics in Chest Medicine 2016; 37(1):17–29. 10.1016/j.ccm.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Fahy JV, Dickey BF. Airway mucus function and dysfunction. The New England journal of medicine 2010; 363(23):2233–47. 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eickmeier O, Huebner M, Herrmann E, Zissler U, Rosewich M, Baer PC et al. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine 2010; 50(2):152–7. 10.1016/j.cyto.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. The New England journal of medicine 2004; 350(6):560–9. 10.1056/NEJMoa021218 [DOI] [PubMed] [Google Scholar]
- 20.Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. The Biochemical journal 2011; 437(2):185–97. 10.1042/BJ20110327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor perspectives in biology 2015; 7(2):a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature immunology 2004; 5(4):388–92. 10.1038/ni1056 [DOI] [PubMed] [Google Scholar]
- 23.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. The European respiratory journal 2006; 28(3):581–7. 10.1183/09031936.06.00071405 [DOI] [PubMed] [Google Scholar]
- 24.Haworth O, Levy BD. Endogenous lipid mediators in the resolution of airway inflammation. European Respiratory Journal 2007; 30(5):980–92. 10.1183/09031936.00005807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy BD, Serhan CN. Resolution of Acute Inflammation in the Lung. Annu. Rev. Physiol. 2014; 76(1):467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. The European respiratory journal. Supplement 2002; 37:19s [DOI] [PubMed] [Google Scholar]
- 27.Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem cells (Dayton, Ohio) 2007; 25(10):2638–47. [DOI] [PubMed] [Google Scholar]
- 28.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clinical immunology (Orlando, Fla.) 2004; 110(3):252–66. [DOI] [PubMed] [Google Scholar]
- 29.BLIGH EG, DYER WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology 1959; 37(8):911–7. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 30.Kohn G, van der Ploeg P., Möbius M, Sawatzki G. Influence of the derivatization procedure on the results of the gaschromatographic fatty acid analysis of human milk and infant formulae. Z Ernährungswiss 1996; 35:226–34. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R et al. Interpretative strategies for lung function tests. The European respiratory journal 2005; 26(5):948–68. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 32.Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. The European respiratory journal 2008; 32(3):783–95. 10.1183/09031936.00146807 [DOI] [PubMed] [Google Scholar]
- 33.Laval J, Ralhan A, Hartl D. Neutrophils in cystic fibrosis. Biological chemistry 2016; 397(6):485–96. 10.1515/hsz-2015-0271 [DOI] [PubMed] [Google Scholar]
- 34.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clinical microbiology reviews 2002; 15(2):194–222. 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Cystic fibrosis and lipoxins. Prostaglandins, leukotrienes, and essential fatty acids 2005; 73(3–4):263–70. 10.1016/j.plefa.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Beyer BA, Lewis C, Nadel JA. Normal CFTR inhibits epidermal growth factor receptor-dependent pro-inflammatory chemokine production in human airway epithelial cells. PLoS One 2013; 8(8):e72981 10.1371/journal.pone.0072981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojewodka G, De Sanctis Juan B, Bernier J, Bérubé J, Ahlgren HG, Gruber J et al. Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients. PLoS One 2014; 9(2):e88567 10.1371/journal.pone.0088567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckrich J, Zissler UM, Serve F, Leutz P, Smaczny C, Schmitt-Grohe S et al. Airway inflammation in mild cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society 2016. [DOI] [PubMed] [Google Scholar]
- 39.Hector A, Kormann MSD, Mack I, Latzin P, Casaulta C, Kieninger E et al. The chitinase-like protein YKL-40 modulates cystic fibrosis lung disease. PLoS One 2011; 6(9):e24399 10.1371/journal.pone.0024399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kormann, Michael S D, Hector A, Marcos V, Mays LE, Kappler M, Illig T et al. CXCR1 and CXCR2 haplotypes synergistically modulate cystic fibrosis lung disease. The European respiratory journal 2012; 39(6):1385–90. 10.1183/09031936.00130011 [DOI] [PubMed] [Google Scholar]
- 41.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M et al. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal immunology 2013; 6(2):256–66. 10.1038/mi.2012.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashi M, Sakata T, Murase T, Takagi Y, Nagai T, Sahashi M et al. Statistical evaluation of determinants of progression of superficial bladder cancer by proportional hazards model. Nihon Hinyokika Gakkai zasshi. The japanese journal of urology 1990; 81(1):116–21. [DOI] [PubMed] [Google Scholar]
- 43.Eickmeier O, Boom L v D, Schreiner F, Lentze MJ, NGampolo D, Schubert R et al. Transforming growth factor β1 genotypes in relation to TGFβ1, interleukin-8, and tumor necrosis factor alpha in induced sputum and blood in cystic fibrosis. Mediators of inflammation 2013; 2013:913135 10.1155/2013/913135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carevic M, Singh A, Rieber N, Eickmeier O, Griese M, Hector A et al. CXCR4+ granulocytes reflect fungal cystic fibrosis lung disease. The European respiratory journal 2015; 46(2):395–404. 10.1183/09031936.00173514 [DOI] [PubMed] [Google Scholar]
- 45.Rosewich M, Zissler UM, Kheiri T, Voss S, Eickmeier O, Schulze J et al. Airway inflammation in children and adolescents with bronchiolitis obliterans. Cytokine 2015; 73(1):156–62. 10.1016/j.cyto.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 46.Doring G, Worlitzsch D. Inflammation in cystic fibrosis and its management. Paediatric respiratory reviews 2000; 1(2):101–6. 10.1053/prrv.2000.0030 [DOI] [PubMed] [Google Scholar]
- 47.Blanchard J, Harvey S, Morgan WJ. Relationship between serum and saliva theophylline levels in patients with cystic fibrosis. Therapeutic drug monitoring 1992; 14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 48.Oliver C, Watson H. Omega-3 fatty acids for cystic fibrosis. The Cochrane database of systematic reviews 2016; (1):CD002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Turkmani MR, Freedman SD, Laposata M. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins, leukotrienes, and essential fatty acids 2007; 77(5–6):309–18. 10.1016/j.plefa.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 50.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr−/− mice. Proceedings of the National Academy of Sciences of the United States of America 1999; 96(24):13995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothman S. Pancreatic insufficiency in cystic fibrosis. Gastroenterology 1996; 111(3):833. [DOI] [PubMed] [Google Scholar]
- 52.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et biophysica acta 2015; 1851(4):397–413. 10.1016/j.bbalip.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC et al. Sputum proteomics in inflammatory and suppurative respiratory diseases. American journal of respiratory and critical care medicine 2008; 178(5):444–52. 10.1164/rccm.200703-409OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller U, Hentschel J, Janhsen WK, Hunniger K, Hipler U, Sonnemann J et al. Changes of Proteases, Antiproteases, and Pathogens in Cystic Fibrosis Patients' Upper and Lower Airways after IV-Antibiotic Therapy. Mediators of inflammation 2015; 2015:626530 10.1155/2015/626530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fantino E, Gangell CL, Hartl D, Sly PD. Airway, but not serum or urinary, levels of YKL-40 reflect inflammation in early cystic fibrosis lung disease. BMC pulmonary medicine 2014; 14:28 10.1186/1471-2466-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sagel SD. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Current opinion in pulmonary medicine 2003; 9(6):516–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may compromise the privacy of study participants and may not be shared publicly. Data are available upon request to the authors.