Abstract
Both innate and adaptive immunity play an important role in host resistance to Mycobacterium tuberculosis infection. Although several studies have suggested that the major histocompatibility complex (MHC) haplotype affects susceptibility to infection, it remains unclear whether the modulation of T-cell immunity by the MHC locus determines the host's susceptibility to tuberculosis. To determine whether allelic differences in the MHC locus affect the T-cell immune response after M. tuberculosis infection, we infected inbred and H-2 congenic mouse strains by the respiratory route. The H-2 locus has a profound effect on the antigen-specific CD4+-T-cell response after M. tuberculosis infection. CD4+ T cells from infected mice of the H-2b haplotype produced more gamma interferon (IFN-γ) after in vitro stimulation with mycobacterial antigens than mice of the H-2k haplotype. A higher level of IFN-γ was also detected in bronchoalveolar lavage fluid from infected mice of the H-2b haplotype. Furthermore, C3.SW-H2b/SnJ mice generate and recruit activated T cells to the lung after infection. Despite a robust immune response, C3.SW-H2b/SnJ mice succumbed to infection early and were similarly susceptible to infection as other C3H (H-2k) substrains. These results suggest that although the MHC haplotype has a profound impact on the T-cell recognition of M. tuberculosis antigens, the susceptibility of C3H mice to infection is MHC independent.
The public health threat caused by tuberculosis is worsening because of the spread of multidrug resistant strains of Mycobacterium tuberculosis, the continued human immunodeficiency virus epidemic, and the demise of the public health infrastructure in many areas of the world. T-cell-mediated immunity and the production of cytokines are critical for the control of infection in humans and in rodent models of the disease (13). Both CD4+ and CD8+ T cells participate in the immune response, and their production of cytokines, including gamma interferon (IFN-γ), is critical for the activation of macrophages, which ultimately kill intracellular bacteria (15, 34). A successful immune response can contain the infection, although a few mycobacteria may remain viable in a latent phase within the host's tissue for decades (16, 18, 36). Most individuals develop immunity to M. tuberculosis, and epidemiological studies indicate that only 5 to 10% of infected individuals will develop tuberculosis during their lifetime. Interestingly, studies comparing individuals that develop protective immunity to people who develop active disease have suggested that genetic factors are important for the development of host resistance to M. tuberculosis (6, 24).
The major histocompatibility complex (MHC) locus is known to play a key role in the control of susceptibility to infection. In humans, HLA-DR2 and -DR3 have been associated with susceptibility to pulmonary tuberculosis (38) and tuberculoid leprosy (12). The H-2 genes of mice control the clearance of many pathogens, such as retroviruses (25), protozoa (2, 21), and bacteria (35). Although various studies during the last 20 years have shown that the murine H-2 locus affects immune responsiveness (as measured by the antibody repertoire) or susceptibility (as measured by survival), it is not yet clear whether the modulation of immunity by the MHC locus determines the susceptibility of the host to tuberculosis. The purpose of this study was to determine how allelic differences in the MHC locus affect T-cell immunity to M. tuberculosis infection. Using congenic and inbred mouse strains, we show that the T-cell recognition of M. tuberculosis antigens is determined in part by the MHC haplotype; however, the susceptibility of C3H mice to M. tuberculosis is MHC independent. We found that both C57BL/6 (B6) mice and C3.SW-H2b/SnJ mice are able to generate and recruit activated T cells to the lung after infection. Despite robust IFN-γ production by C3.SW-H2b/SnJ mice in vitro and in vivo, these mice were as susceptible to infection as other C3H substrains and succumbed to infection early compared to B6 mice. These results indicate that while the MHC locus regulates the T-cell recognition of mycobacterial antigens, non-MHC-encoded genes make a critical contribution to the relative susceptibilities of mice to tuberculosis.
MATERIALS AND METHODS
Mice.
Age-matched female B10.BR-H2k H2-T18a/SgSnJ (B10.BR), B10.D2-Hc0 H2d H2-T18c/o2Sn (B10.D2), B10.A-H2a H2-T18a/SgSnJ (B10.A), C57BL/10J (B10/J), B10.A-H2h2/(2R) SgSnJ (2R), B10.A-H2h4/(4R) SgDvEg (4R), B10.A-H2i5 H2-T18a/(5R)SgSnJ (5R), C57BL/6 (B6), C3.SW-H2b/SnJ, C3H/HeJ, and C3H/HeSnJ (C3H/SnJ) mice were purchased from Jackson Laboratory (Bar Harbor, Maine) (Table 1). Mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions at the Animal Biohazard Containment Suite (Dana Farber Cancer Institute, Boston, Mass.) and were used in a protocol approved by the institution.
TABLE 1.
Mouse strains used for this study
| Strain name | Abbreviated name | Haplotypea | Class II MHC gene
|
|||
|---|---|---|---|---|---|---|
| Aβ | Aα | Eβ | Eα | |||
| B10.D2-Hc0 H2d H2-T18c/o2Sn | B10.D2 | d | d | d | d | d |
| B10.BR-H2k H2-T18a/SgSnJ | B10.BR | k | k | k | k | k |
| B10.A-H2a H2-T18a/SgSnJ | B10.A | a | k | k | k | k |
| B10.A-H2h2/(2R) SgSnJ | 2R | 2R | k | k | k | k |
| B10.A-H2h4/(4R) SgDvEg | 4R | 4R | k | k | k/bb | b |
| B10.A-H2i5 H2-T18a/(5R)SgSnJ | 5R | 5R | b | b | b/kb | k |
| C57BL/10J | B10/J | b | b | b | b | —c |
| C57BL/6 | B6 | b | b | b | b | —c |
| C3.SW-H2b/SnJ | C3.SW-H2b/SnJ | b | b | b | b | —c |
| C3H/HeSnJ | C3H/SnJ | k | k | k | k | k |
| C3H/HeJ | C3H/HeJ | k | k | k | k | k |
H-2 allele designation.
Recombination between alleles within I-Eβ gene locus.
—, I-Eα gene deleted in the H-2b haplotype.
Bacteria and intravenous and aerosol infections.
Virulent M. tuberculosis (Erdman strain) was prepared as previously described (8). Mice were infected via the aerosol route by use of a nose-only exposure unit (Intox Products, Albuquerque, N.Mex.) (7). Depending on the batch of bacteria used for experiments, the average lung inoculum for a given experiment was between 20 and 60 CFU or between 200 and 600 CFU per mouse, as determined 16 h after infection (data not shown). The standard deviation of the mean of the inoculum was generally between 30 and 45%. In some experiments, mice were infected intravenously (i.v.) with 106 live mycobacteria.
CFU determination.
After euthanasia by CO2 inhalation, the inferior vena cava of each mouse was severed and blood was purged from the lungs by perfusing them with RPMI 1640 through the right ventricle of the heart. The left lung and half of the spleen were aseptically removed and individually homogenized in 0.9% NaCl-0.02% Tween 80 with a Mini-Bead Beater 8 (Biospec Products, Bartlesville, Okla.). The numbers of CFU were quantified by plating 10-fold serial dilutions of organ homogenates onto 7H11 Mitchinson agar plates (Remel, Lenexa, Kans.). Colonies were counted after 3 weeks of incubation at 37°C.
Bronchoalveolar lavage.
Lavage was performed on euthanized mice by cannulating the surgically exposed trachea with a 19-gauge blunt needle. A 1-ml aliquot of sterile 0.005 M EDTA in phosphate-buffered saline was repeatedly injected into the lungs and aspirated a total of four times. The bronchoalveolar lavage fluid (BAL) was stored at −80°C prior to its use in enzyme-linked immunosorbent assays (ELISAs) to measure cytokines.
In vitro restimulation assays.
Splenocytes or lung mononuclear cells (MNC) were stimulated with an antigen in vitro as previously described (7). Briefly, single-cell suspensions were prepared from the lungs and spleens of infected mice by mechanical dissociation and a combination of collagenase digestion and mechanical dissociation, respectively. The red blood cells were lysed with lysis buffer (0.15 M NaCl, 1 mM KHCO3, 0.1 mM Na-EDTA; pH 7.3). All cells were washed and then resuspended in complete medium (RPMI 1640, 10% fetal calf serum, 2% HEPES, 1% l-glutamine, 1% penicillin-streptomycin, 0.1% β-mercaptoethanol). Lung mononuclear cells (3 × 106 cells/ml) were incubated in 2 ml of complete medium containing 1 μg of concanavalin A/ml and an M. tuberculosis H37Ra sonicate (3) (diluted 1:1,000, 1:5,000, or 1:25,000), culture filtrate protein (CFP), or antigen 85 (Ag85) (both obtained from Colorado State University) at 3.3 or 10 μg/ml or in medium alone for 48 h at 37°C. For some experiments, T cells were purified from infected B6, B10/J, B10.BR, C3.SW-H2b/SnJ, and C3H/SnJ mice by the use of CD90 beads (Miltenyi Biotech, Auburn, Calif.) and were cultured with mitomycin C-treated naïve splenocytes as antigen-presenting cells in the presence of mycobacterial antigens. Proliferation was measured by determining the [3H]thymidine uptake. Culture supernatants were assayed for cytokines by an ELISA using antibody pairs and cytokines from Pharmingen (San Diego, Calif.) (4).
Flow cytometry.
One million splenocytes and lung MNC were stained with antibodies to CD4, CD8, CD11a, CD22, CD69, and CD62L or an appropriate isotype control (all from Pharmingen). After fixation with 1% paraformaldehyde and storage at 4°C overnight, the cells were analyzed with a FACSort instrument (Beckton Dickinson, San Jose, Calif.). FlowJo software was used to analyze the data (Tree Star Inc., Stanford, Calif.).
Histological analysis.
Lung tissue was preserved in Z-fix (Anatech, Battle Creek, Mich.) and then embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin and eosin or used for acid-fast bacillus staining to confirm the bacterial load in the lung (3). Low-power images were obtained with a SprintScan 4000 digital film scanner (Polaroid Corporation, Cambridge, Mass.) in combination with a PathScan enabler (Meyer Instruments, Houston, Tex.). Image analysis was performed on a PC computer with the NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Statistics.
The Prism software program was used to perform all statistical analyses (Graphpad, San Diego, Calif.). CFU were log10 transformed before analysis. The statistical significance of three-, four-, and five-way comparisons was tested by one-way analysis of variance (ANOVA) with Tukey's multiple comparison test. The log rank test by the Kaplan and Meier method was used to compare differences in the survival rates of mouse strains.
RESULTS
The MHC modulates T-cell recognition of complex mycobacterial antigens.
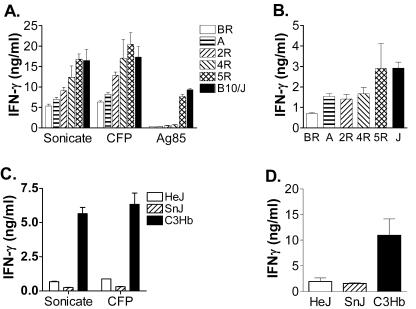
To examine how allelic differences in the MHC locus affect the T-cell recognition of mycobacterial antigens after infection, we infected B10 congenic mouse strains that had different H-2 haplotypes with virulent bacteria via the aerosol route. At 3 weeks postinfection, the antigen-specific T-cell response was determined by measuring IFN-γ production by immune splenocytes that were stimulated with mycobacterial antigens (7, 20). This assay primarily measures the activation of class II MHC-restricted CD4+ T cells (20). The MHC haplotype not only influenced the T-cell response to pure protein antigens such as Ag85, but it also affected T-cell recognition of the complex antigens M. tuberculosis sonicate and CFP (for a posttest for linear trends, P < 0.0001 by one-way ANOVA) (Fig. 1A). B10/J mice (H-2b) produced more IFN-γ than B10.BR (H-2k) or B10.A mice (H-2A). Splenocytes from B10/J and B10.D2 (H-2d) mice produced similar amounts of IFN-γ after stimulation (data not shown). A similar pattern was seen when H-2 recombinant B10 mice were studied. T-cell recognition was determined in large part by H-2 I-Ab since the pattern of IFN-γ production was 5R (I-Ab) > 4R (I-Ak) > 2R (I-Ak I-Ek). These results indicate that the MHC haplotype influences the T-cell response to mycobacterial antigens after infection. To determine the in vivo relevance of this finding, we measured IFN-γ levels in the BAL fluid of infected mice. The amounts of IFN-γ in the BAL fluid from B10 mouse strains had a pattern similar to that of the in vitro T-cell response: more IFN-γ was detected in the BAL fluid of B10 mice with the H-2b haplotype (for a posttest of linear trends, P < 0.0001 by one-way ANOVA) (Fig. 1B).
FIG. 1.
T-cell recognition of mycobacterial antigens is dependent on the MHC haplotype. (A) Splenocytes from infected B10.BR, B10.A, 2R, 4R, 5R, and B10/J mice were cultured with or without M. tuberculosis antigens, and the amounts of IFN-γ released in the supernatants after 48 h were measured by ELISA. Each group contained six mice, and the data represent means ± standard deviations. (B) Amounts of IFN-γ in the BAL fluid were measured by ELISA. Data represent the means of six mice per group ± standard deviations. (C) At 3 weeks postinfection, splenocytes from C3H/HeJ (HeJ), C3H/HeSnJ (SnJ), and C3.SW-H2b/SnJ (C3Hb) mice were cultured with or without mycobacterial antigens and then assayed as described for panel A. (D) Comparison of IFN-γ production in the BAL fluid of C3H substrains at week 3 postinfection.
The effect of the MHC haplotype on T cells obtained from infected C3H mice was also investigated. Similar to the finding with B10 congenic mice, mycobacterial antigens stimulated more IFN-γ production by T cells from infected C3.SW-H2b/SnJ (H-2b) mice than that by T cells from C3H/HeJ and C3H/SnJ mice (both H-2k) (P < 0.01) (Fig. 1C). The BAL fluid from C3.SW-H2b/SnJ mice also contained more IFN-γ than that from C3H/HeJ and C3H/SnJ mice (P < 0.05) (Fig. 1D). These data indicate that the MHC locus is a critical factor for regulating T-cell reactivity to mycobacterial antigens. Furthermore, the correlation between in vitro IFN-γ production and the IFN-γ concentration in BAL fluid suggests a possible relevance to immunity in vivo.
The MHC haplotype and other host genetic factors influence the in vitro T-cell recall response to mycobacterial antigens.
It was previously shown that resistant B6 mice (H-2b) initiate an immune response and recruit T cells to the lung more rapidly than susceptible C3H/HeJ mice (H-2k) (7, 9). The association between IFN-γ-producing pulmonary T cells and resistance to M. tuberculosis has led some investigators to suggest that antigen-stimulated IFN-γ production may be a correlate of immunity to M. tuberculosis (9, 26, 40, 42). Even for humans, the detection of IFN-γ-producing T cells is a sensitive indicator of M. tuberculosis infection (1, 27). However, given the significant differences in T-cell responsiveness between mice with the H-2b and H-2k haplotypes, we investigated whether IFN-γ production in MHC-matched resistant and susceptible mouse strains correlates with resistance to M. tuberculosis. Lung mononuclear cells (MNC) were isolated from infected B6 and C3.SW-H2b/SnJ mice and restimulated with a bacterial sonicate in vitro. Stimulated lung MNC from B6 mice produced more IFN-γ than those from C3.SW-H2b/SnJ mice at 2 weeks postinfection, which probably reflects an earlier initiation of immunity, as previously observed for B6 mice compared to C3H/HeJ mice (7). However, at later time points, lung MNC from C3.SW-H2b/SnJ mice produced far more IFN-γ than cells from B6 mice (P = 0.0007) (Fig. 2A). This was a consistent finding despite the fact that slightly more CD4+ and CD8+ T cells accumulated in the lungs of B6 mice (data not shown). Similar responses were also observed when splenocytes and pulmonary lymph node cells were stimulated in vitro (data not shown).
FIG. 2.
T-cell recall response to mycobacterial antigens is dependent upon MHC and non-MHC loci. (A) The in vitro production of IFN-γ by lung MNC in response to a mycobacterial sonicate (1/5,000 dilution) is displayed for C57BL/6 and C3.SW-H2b/SnJ (C3Hb) mice at various times after aerosol infection. (B) B10/J, B10.BR, B6, C3.SW-H2b/SnJ, and C3H/SnJ mice were infected i.v. with M. tuberculosis. After 4 weeks, splenic T cells were purified and cultured with uninfected irradiated syngeneic APC and mycobacterial antigens in vitro. IFN-γ production was measured by ELISA. Purified splenic T cells from infected B6 or C3.SW-H2b/SnJ mice were cultured with strain-matched or mismatched uninfected irradiated APC and mycobacterial antigens, and IFN-γ production (C) or [3H]thymidine uptake (D) was measured. For panels A and B, each group contained six mice per time point, and data represent means and standard deviations. For panels C and D, tissues were pooled before T-cell purification, and the assay was performed in triplicate, with data representing the means and standard deviations.
To better define the role of the MHC in the T-cell response, we compared the responses of purified splenic T cells from infected congenic mice after infection. The i.v. route of infection was used to minimize differences arising from variations in the kinetics of bacterial dissemination and initiation of the immune response. T cells were purified so that the variation in T-cell numbers between mouse strains after infection could be normalized. Purified T cells from mice of the H-2b haplotype (B6, B10, and C3.SW-H2b/SnJ) produced more IFN-γ than T cells from mice of the H-2k haplotype (B10.BR and C3H/SnJ) (Fig. 2B). For example, C3.SW-H2b/SnJ T cells produced more than twice the amount of IFN-γ than that produced by C3H/SnJ T cells (P < 0.001), revealing an important dependence on the MHC haplotype for T-cell immune responsiveness. An effect of non-MHC-encoded genes was also evident. C3H/SnJ T cells produced more IFN-γ than B10.BR T cells (both H-2k restricted; P < 0.05). Similarly, C3.SW-H2b/SnJ T cells produced more IFN-γ than B10 or B6 T cells (all H-2b restricted; P < 0.001). Thus, in vitro T-cell responses correlated with the MHC haplotype and the mouse strain but not with resistance, as measured by survival after infection (9; also see below).
T cells from infected B6 or C3.SW-H2b/SnJ mice were stimulated with mycobacterial antigens presented by strain-matched or mismatched uninfected irradiated splenocytes. The C3.SW-H2b/SnJ T cells produced more IFN-γ and proliferated more than B6 T cells, and these effects were independent of the source of the antigen-presenting cells (APC) (P < 0.001) (Fig. 2C and D). Thus, the larger response of C3.SW-H2b/SnJ T cells was not due to more efficient costimulation or antigen presentation. It is possible that C3H T cells have an enhanced ability to proliferate following activation, resulting in a higher frequency of antigen-specific cells, or that they have an intrinsic ability to produce more IFN-γ per cell. We concluded that the MHC haplotype affects the T-cell response to M. tuberculosis and that T cells from mice of the H-2b haplotype have the potential to make more IFN-γ than T cells from H-2k mice. Non-MHC-linked genes also contribute to increased T-cell responsiveness, and the C3H background was associated with a larger IFN-γ response than that in B6 or B10 mice.
Activated T cells are recruited to the lungs of both B6 and C3.SW-H2b/SnJ mice after infection.
We next asked whether T cells from B6 and C3.SW-H2b/SnJ mice become similarly activated after infection. Within 2 to 3 weeks after respiratory M. tuberculosis infection, T cells with an activated or memory phenotype accumulated in the lungs of both mouse strains. The activation marker CD69 was expressed by both CD4+ and CD8+ T cells in the lungs of B6 and C3.SW-H2b/SnJ mice after infection (Fig. 3). The percentage of pulmonary T cells expressing CD69 was modestly increased for C3.SW-H2b/SnJ mice compared to that for B6 mice. The percentage of CD4+ and CD8+ T cells expressing L-selectin (CD62L), a marker that is downregulated by naïve T cells after their activation in lymphoid tissue, rapidly declined, indicating that memory and effector T cells are recruited into the lungs of both susceptible and resistant mice after infection (Fig. 3). T cells uniformly express CD11a; however, memory T cells express higher levels of CD11a than naïve T cells. Consistent with the change in CD62L expression, the percentage of CD4+ CD11ahi T cells increased in the lung tissue of both B6 and C3.SW-H2b/SnJ mice within 3 weeks after infection (Fig. 3). Similarly, CD8+ CD11ahi cells were detected in the lungs of both mouse strains after infection (Fig. 3). These changes were accompanied by a reciprocal decline in the number of CD11amoderate T cells. Thus, activated CD4+ and CD8+ T cells are similarly recruited to the lungs of both B6 and C3.SW-H2b/SnJ mice after infection. The kinetics of this process correlate with the control of infection and the establishment of the plateau phase of infection in both B6 and C3H mice (7).
FIG. 3.
B6 and C3.SW-H2b/SnJ mice are able to recruit activated T cells to the lungs after aerosol infection with M. tuberculosis. The percentages of CD4+ or CD8+ T cells in the lungs of infected B6 or C3.SW-H2b/SnJ mice that expressed CD69+, CD62L+, or CD11a+ are shown for one experiment that was repeated three times with similar results. The expression of CD11a was divided into high and moderate expression. Filled symbols, B6 mice; open symbols, C3.SW-H2b/SnJ mice.
C3H/SnJ and H-2 congenic C3.SW-H2b/SnJ mice are similarly susceptible to respiratory infection with M. tuberculosis.
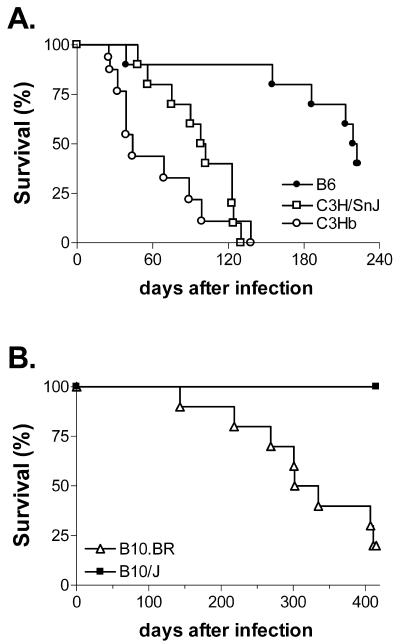
The survival rates of B6, C3.SW-H2b/SnJ, and C3H/SnJ mice were compared after aerosol infection with M. tuberculosis to determine whether the MHC affects susceptibility. Similar to the case for other C3H substrains, C3.SW-H2b/SnJ mice died earlier than B6 mice after respiratory infection with virulent M. tuberculosis. The mean survival time (MST) of C3.SW-H2b/SnJ mice was 44 days, whereas B6 mice had an MST of 220 days (P = 0.0003) (Fig. 4A). C3.SW-H2b/SnJ mice died slightly earlier than C3H/SnJ mice, and although this difference never achieved statistical significance, we observed this trend in three independent experiments. For more acute infections, the MST of C3.SW-H2b/SnJ mice was 25.5 days, while the MSTs of C3H/HeJ and C3H/OuJ mice (both H-2k) were 28 and 26.5 days, respectively (P = 0.76). Similarly, for chronic infections with a lung inoculum on day 1 of 38 ± 14 CFU, the MSTs of C3.SW-H2b/SnJ, C3H/SnJ, and B6 mice were 105, 168, and 192 days, respectively (no deaths had occurred among the B6 mice when the experiment was terminated on day 192; P = 0.16 for C3.SW-H2b/SnJ versus C3H/SnJ mice). Thus, similar to other substrains of C3H, C3.SW-H2b/SnJ is a susceptible mouse strain, and the presence of H-2b haplotypes did not confer resistance (20). In contrast, B10.J (H-2b) mice survived significantly longer than B10.BR mice (H-2k) (Fig. 4B). The latter result is similar to the observation by Medina and North that BALB.B mice (H-2b) are more resistant than BALB.K mice (H-2k) (30). Together, these results suggest that the interaction of non-MHC-encoded genes may be required for the modulating effect of the MHC on host resistance and survival.
FIG. 4.
Susceptibility of C3H mice to mycobacterial infection is independent of their H-2 haplotype. (A) Groups of 12 mice per strain of B6, C3H/SnJ, and C3.SW-H2b/SnJ (C3Hb) mice were infected by the respiratory route, and their survival was monitored. The results are representative of three experiments. (B) The survival of B10.BR and B10/J mice was monitored after infection by the respiratory route. One experiment was performed with 10 mice/group.
Bacterial burdens are similar in B6 and C3.SW-H2b/SnJ mice.
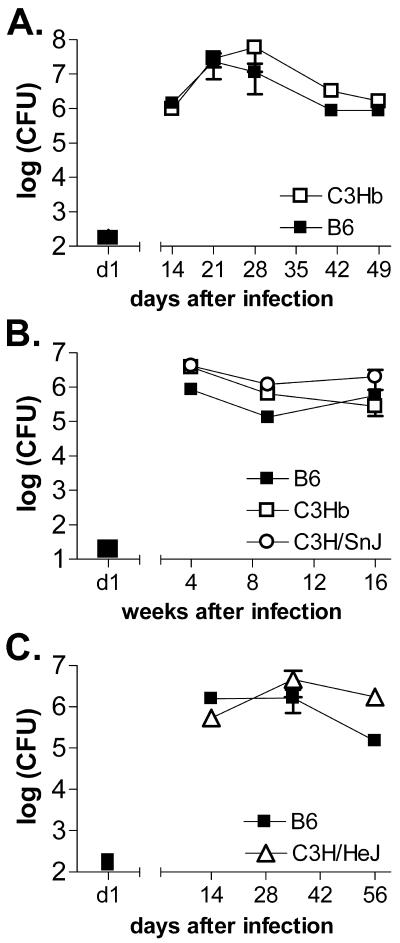
After a high-dose i.v. infection, bacterial replication proceeds essentially unchecked in C3H/HeJ mice and leads to their early death within 4 weeks (9). In contrast, bacterial growth is similar in B6 and C3H mice after aerosol infection for the first 3 weeks, and thereafter both mouse strains limit bacterial replication (7). There was little difference in lung CFU between B6 and C3.SW-H2b/SnJ mice either early (Fig. 5A) or late (Fig. 5B) during the course of infection. In fact, a statistically significant difference was not observed in two experiments that directly compared the bacterial burdens in the lungs of B6, C3.SW-H2b/SnJ, and C3H/SnJ mice 4, 9, or 16 weeks after infection (Fig. 5B). Other investigators have observed that the bacterial burden increases in susceptible mouse strains 2 to 3 months after respiratory infection (39). Such differences may not have been observed in the last two experiments because the inoculums were very low (28 and 35 CFU), leading to prolonged survival (MSTs of B6 and C3.SW-H2b/SnJ mice were 200 and 168 days, respectively). Thus, even 16 weeks may have been too early to see a significant loss of control of bacterial replication in C3H mice. On the other hand, we have occasionally observed significant differences in bacterial burdens in the lungs between resistant B6 and susceptible C3H mice (Fig. 5C; also see below).
FIG. 5.
Bacterial burden is independent of MHC haplotype. The numbers of CFU in the lungs of infected C57BL/6 (closed squares), C3.SW-H2b/SnJ (C3Hb) (open squares), C3H/SnJ (open circles), and C3H/HeJ (open triangles) mice were determined at various early (A and C) or late (B) time points after aerosol infection. The number of CFU deposited into the lungs 1 day after infection is indicated with a black bar. Panel A is representative of three experiments, and panels B and C are representative of two experiments. Data represent the means of six mice per group ± standard deviations.
Differences in pathology of lung lesions of susceptible mice are independent of MHC.
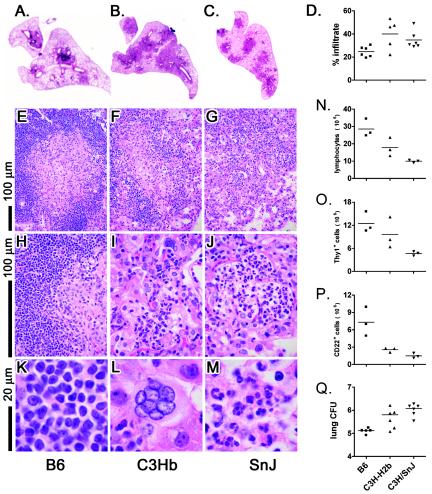
One of the main differences observed between infected B6 and C3H mice was the histological appearance of the lungs after infection. Lungs obtained 9 weeks after infection were analyzed both histologically and by flow cytometry. Under low-power magnification, we observed that the total lung area occupied by infiltrate was smaller in B6 mice than in C3.SW-H2b/SnJ or C3H/SnJ mice (Fig. 6A to C). C3.SW-H2b/SnJ mice had significantly more infiltrate than B6 mice at this time point (P < 0.05) (Fig. 6D). This difference was not always statistically significant at other time points, in part because of the scatter of the data. However, we do not believe that the total amount of infiltrate is necessarily indicative of a protective host response. For example, early during the development of granulomas, B6 mice have larger lung lesions because they develop an immune response more rapidly (7). More importantly, the structures of the granulomas were qualitatively different between mouse strains. B6 mice formed discrete granulomas that contained dense lymphocytic infiltrates (Fig. 6A and E). In contrast, the lung lesions in infected C3.SW-H2b/SnJ and C3H/SnJ mice were poorly organized, and although occasional areas of lymphocytic infiltrates could be identified, infiltrates of mixed cellularity dominated the lesions (Fig. 6F and G). At a higher magnification, the well-demarcated cuff of lymphocytes was typical of B6 granulomas (Fig. 6H). Areas of neutrophils, which are often associated with early signs of necrosis and bronchopneumonia, were present in both C3H mouse strains, as has been reported for other susceptible mouse strains (Fig. 6I and J) (also data not shown). At a still higher power, one can clearly appreciate the lymphoid character of the infiltrating cells in B6 mice (Fig. 6K). Both giant cells and neutrophils were more abundant in the lungs of C3.SW-H2b/SnJ and C3H/SnJ mice (Fig. 6L and M). The numbers of pulmonary lymphocytes in the lungs of these mice were determined by counting in combination with size gating by flow cytometry. More lymphocytes were found in the lungs of B6 mice than in those of C3.SW-H2b/SnJ and C3H/SnJ mice (P < 0.001), which corroborates the histological analysis (Fig. 6N). The numbers of T cells were increased in the lungs of B6 mice compared to C3H/SnJ mice (Fig. 6O). However, the number of pulmonary T cells in C3.SW-H2b/SnJ mice was intermediate and was not statistically different from those for the other two strains. Evaluations of other time points and other experiments did not clarify this question. Interestingly, the numbers of B cells were increased in the lungs of B6 mice compared to C3.SW-H2b/SnJ and C3H/SnJ mice (P < 0.001) (Fig. 6P).
FIG. 6.
B6 (A, E, H, and K), C3.SW-H2b/SnJ (C3Hb) (B, F, I, and L), or C3H/SnJ [SnJ] (C, G, J, and M) mice were infected by the aerosol route with M. tuberculosis, and lungs were removed 9 weeks after infection and fixed in formalin. Paraffin-embedded sections were stained with hematoxylin and eosin. Bar = 100 μm (E to J) or 20 μm (K to M). The histology and data are representative of five experiments each, with six mice per group and three to five time points per experiment. For each section, the lung area infiltrated by leukocytes was determined by image analysis (D). For the same experiment, lung tissues were obtained and pooled from pairs of mice. Single-cell suspensions were prepared, and flow cytometric analysis was used to determine the numbers of lymphocytes (N), T cells (O), and B cells (P) in the lung tissue. The left lung was used to determine the numbers of CFU (Q).
DISCUSSION
Host resistance to M. tuberculosis requires the coordination of both the innate and adaptive components of the immune system. Following initiation of the adaptive immune response, T cells are needed to produce IFN-γ to activate the bactericidal machinery of infected macrophages. Long-term cell-mediated immunity is required to prevent the replication of latent bacteria, which if unchecked can lead to reactivation tuberculosis (14). Why apparently healthy people with apparently adequate immunity go on to develop active disease remains unknown. The susceptibility of people to mycobacterial infections has been shown to be influenced by host genetic factors, and in particular, susceptibility to pulmonary tuberculosis has been linked to distinct HLA haplotypes (3, 38). Consequently, there has been a great deal of interest in determining genetic and immunological differences between individuals who have long-lived immunity and those who develop active disease.
Genetic differences also exist among inbred mouse strains that differ in their susceptibility to tuberculosis (8). Prior work has demonstrated that C3H/HeJ mice die more rapidly than B6 mice after infection with virulent M. tuberculosis by the i.v. route (9, 22, 30). This strain combination differs at several genetic loci that are known to affect resistance to microbial pathogens. For example, polymorphisms in the nramp1 gene appear to be critical for determining innate host resistance to a variety of intracellular pathogens, including Salmonella, Leishmania, Mycobacterium avium, and Mycobacterium bovis BCG (41). C3H mice are more resistant than B6 mice to these infections, in part because C3H mice express the nramp1Gly169 allele, which is associated with resistance to intracellular infection, while B6 mice express the nrampAsp169 allele, which is associated with susceptibility. However, these allelic differences in the nramp1 gene do not affect host resistance to virulent M. tuberculosis (28, 29, 31). Similarly, although Toll-like receptors are critical for activating the innate immune system, a defective tlr4 gene does not contribute to the susceptibility of C3H/HeJ mice to tuberculosis (9, 20). Recently, several genetic loci have been defined that affect the survival of mice after infection with M. tuberculosis, and one, the sst1 locus, is allelic between B6 and C3HeB/FeJ mice (22). These studies have also demonstrated that susceptibility to tuberculosis is a multigenic trait and that different loci may be associated with susceptibility in different inbred mouse strains (23, 32, 33, 37).
The H-2 locus represents another important genetic difference between B6 and C3H/HeJ mice, and allelic differences at this locus are known to modulate both the antibody response to mycobacterial proteins and host resistance to infection (5, 19). Few studies have examined the effect of the H-2 haplotype on the T-cell response after M. tuberculosis infection. Because we are ultimately interested in identifying immunological differences between resistant and susceptible mouse strains that could serve as surrogate markers of host resistance, we wanted to better understand the contribution of the MHC to the M. tuberculosis-specific T-cell response. Therefore, we compared the immune responses of B6 and C3.SW-H2b/SnJ mice, which are matched at the H-2 locus.
We found that the H-2 locus has a profound effect on antigen-specific CD4+-T-cell responses after M. tuberculosis infection. While there are many examples of antigens that are presented better by one particular class II MHC molecule than by another, one expects that such an allelic advantage will be offset when dealing with complex antigen mixtures. In other words, while an epitope from a single protein such as Ag85 may be presented by I-Ab but not I-Ak, we assume that other antigen epitopes will be presented by I-Ak but not I-Ab. Thus, for a bacterium such as M. tuberculosis with hundreds of different antigens, one expects that the numbers of epitopes presented by I-Ab and I-Ak will be similar.
However, this is not what we observed. More IFN-γ was produced by CD4+ T cells obtained from infected mice of the H-2b haplotype than by those of the H-2k haplotype after restimulation in vitro with mycobacterial antigens. This was unexpected, especially since C3H mice have two class II molecules (I-Ak and I-Ek) while B6 and B10 mice have only one (I-Ab). This bias in the favor of I-Ab may have stemmed from an abundance of Ag85 in our antigen preparations (CFP and sonicate), as Ag85 was clearly presented better by I-Ab than by I-Ak. However, IFN-γ levels in the BAL fluid, which may reflect T-cell activation in vivo, were higher in H-2b mice. Non-MHC-encoded genes also contribute to the ability of C3H T cells to produce more IFN-γ, since after in vitro stimulation, C3H T cells always produced more IFN-γ than T cells obtained from MHC-matched B10 mice. Despite a strong in vitro recall response to mycobacterial antigens, C3.SW-H2b/SnJ mice were still more susceptible to infection than B6 mice. C3H/SnJ and C3.SW-H2b/SnJ mice succumbed similarly to aerosol infection, indicating that their susceptibility to tuberculosis was independent of their H-2 haplotype (20). This result is consistent with a previous report that the H-2 haplotype does not alter the survival of C3H mice after i.v. infection (9). In contrast, we have confirmed that B10/J (H-2b) mice are more resistant than B10.BR (H-2k) mice (30). The protective effect of the H-2b allele on the B10 background, but not the C3H background, could be explained if alleles in the C3H genetic background were epistatic for (i.e., masking) the protective effect of the H-2 haplotype.
Other investigators have observed that allelic differences in the H-2 locus affect host resistance to mycobacterial infection. Curtis et al. showed that mice of the H-2k haplotype are more susceptible than mice of the H-2d or H-2b haplotype to subcutaneous infection with Mycobacterium lepraemurium (10, 11). This effect was more pronounced in the BALB/c background than the B10 genetic background, also suggesting the presence of epistatic effects. Brett et al. investigated the role of the H-2 genes in host resistance to intraperitoneal M. tuberculosis infection and found that the H-2k haplotype was associated with increased numbers of CFU in the lungs, although this effect was more pronounced in B10 congenic mice than in BALB/c mice (4, 5). Using survival as an end point after i.v. infection with M. tuberculosis, Medina and North showed that the H-2k haplotype contributed to the susceptibility of mice, although non-MHC genes were clearly more important (30).
Tremendous efforts have gone into developing new vaccines for M. tuberculosis. As these begin to enter clinical trials, one pressing issue is whether immunological surrogates of host resistance exist that could help to determine whether vaccines elicit protective immunity. Numerous labs have focused on the production of IFN-γ by T cells after stimulation with mycobacterial antigens because of the importance of IFN-γ in the host defense in both animal experiments and clinical studies. Although IFN-γ is absolutely required for optimum immunity in both mice and people, it remains unknown whether increasing the amount of IFN-γ at the site of disease improves immune control over M. tuberculosis.
Our results show that the in vitro T-cell response is dramatically modulated by the H-2 locus and, to a lesser extent, by non-H-2-encoded genes. However, despite the finding that C3.SW-H2b/SnJ mice produce more IFN-γ in vitro and in vivo than B6 mice after infection, these mice remained as susceptible as C3H/SnJ (H-2k) mice. Although we recognize that vaccination is a fundamentally different intervention than our genetic manipulations, it is clear that eliciting more IFN-γ production did not necessarily correlate with better protection against tuberculosis. What is particularly interesting is that the H-2k haplotype is clearly associated with susceptibility (compared to H-2b and H-2d) in other genetic backgrounds. As described above, this may be an example of genetic epistasis. For example, while T-cell-mediated immunity may be normal in C3H mice, there may be an underlying defect in the ability of their macrophages to respond optimally to IFN-γ. Our data are consistent with the idea that an impairment of innate immunity contributes to the susceptibility of C3H mice. Furthermore, our data suggest that an important factor that determines the outcome of infection may be the pathological response elicited by M. tuberculosis. In this regard, it is interesting that a fundamental difference between resistant and susceptible mouse strains is the qualitatively different pulmonary infiltrates (17, 39). While resistant strains have a prominent lymphocytic component, the infiltrates that develop in susceptible strains are dominated more by neutrophils and necrosis. Although it is unclear whether these differences are a manifestation of an underlying defect that impairs disease control or whether they develop secondary to progressive disease, it is interesting that some of these changes can be detected early during the course of infection. Future studies that map and identify susceptibility genes in the murine tuberculosis model followed by genomic analysis will be extremely useful for identifying their human homologues and to better understand the complexity of the immune response to tuberculosis infection. Elucidating the basis of host resistance and susceptibility will provide a rational basis for the development of new therapies and vaccines for tuberculosis.
Acknowledgments
We thank Steve Jean, Linda Callahan, and the staff of the Animal Biohazard Containment Suite at the Dana Farber Cancer Institute for their help in facilitating these experiments.
This work was supported by National Institutes of Health grants HL64540 and AI49093 and an award from the Potts Memorial Foundation to S.M.B. Materials were provided by Colorado State University through NIH/NIAID contract NO1-AI-75320, entitled “Tuberculosis Research Materials and Vaccine Testing.”
Editor: J. B. Bliska
REFERENCES
- 1.Barry, S. M., M. C. Lipman, B. Bannister, M. A. Johnson, and G. Janossy. 2003. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J. Infect. Dis. 187:243-250. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell, J. M. 1983. Leishmania donovani infection in heterozygous and recombinant H-2 haplotype mice. Immunogenetics 18:101-109. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley, G. H., J. S. Beck, G. M. Schreuder, J. D'Amaro, R. R. de Vries, T. Kardjito, and J. Ivanyi. 1989. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J. Infect. Dis. 159:549-555. [DOI] [PubMed] [Google Scholar]
- 4.Brett, S., J. M. Orrell, B. J. Swanson, and J. Ivanyi. 1992. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology 76:129-132. [PMC free article] [PubMed] [Google Scholar]
- 5.Brett, S. J., and J. Ivanyi. 1990. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology 71:113-119. [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, A. A., J. M. Alt, T. V. Perera, C. C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chackerian, A. A., and S. M. Behar. 2003. Susceptibility to Mycobacterium tuberculosis: lessons from inbred strains of mice. Tuberculosis 83:279-285. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis, J., H. O. Adu, and J. L. Turk. 1982. H-2 linkage control of resistance to subcutaneous infection with Mycobacterium lepraemurium. Infect. Immun. 38:434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis, J., H. Akuffo-Adu, and J. L. Turk. 1984. H-2-linked genes which modify resistance of C57BL/10 mice to subcutaneous infection with Mycobacterium lepraemurium. Infect. Immun. 46:635-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries, R. R., W. van Eden, and J. J. Van Rood. 1981. HLA-linked control of the course of M. leprae infections. Lepr. Rev. 52(Suppl. 1):109-119. [PubMed] [Google Scholar]
- 13.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L., and J. Chan. 2001. Tuberculosis: latency and reactivation. Infect. Immun. 69:4195-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruppo, V., O. C. Turner, I. M. Orme, and J. Turner. 2002. Reduced up-regulation of memory and adhesion/integrin molecules in susceptible mice and poor expression of immunity to pulmonary tuberculosis. Microbiology 148:2959-2966. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Pando, R., D. Aguilar, H. Orozco, M. Jeyanathan, G. A. W. Rook, G. Mengistu, M. Harboe, M. Harboe, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 19.Huygen, K., A. Drowart, M. Harboe, R. ten Berg, J. Cogniaux, and J. P. Van Vooren. 1993. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect. Immun. 61:2687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath, A. B., J. Alt, H. Debbabi, and S. M. Behar. 2003. Toll-like receptor 4-defective C3H/HeJ mice are not more susceptible than other C3H substrains to infection with Mycobacterium tuberculosis. Infect. Immun. 71:4112-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye, P. M., A. Cooke, T. Lund, M. Wattie, and J. M. Blackwell. 1992. Altered course of visceral leishmaniasis in mice expressing transgenic I-E molecules. Eur. J. Immunol. 22:357-364. [DOI] [PubMed] [Google Scholar]
- 22.Kramnik, I., W. F. Dietrich, P. Demant, and B. R. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavebratt, C., A. S. Apt, B. V. Nikonenko, M. Schalling, and E. Schurr. 1999. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J. Infect. Dis. 180:150-155. [DOI] [PubMed] [Google Scholar]
- 24.Levin, M., and M. Newport. 2000. Inherited predisposition to mycobacterial infection: historical considerations. Microbes Infect. 2:1549-1552. [DOI] [PubMed] [Google Scholar]
- 25.Lilly, F., M. L. Duran-Reynals, and W. P. Rowe. 1975. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J. Exp. Med. 141:882-889. [PMC free article] [PubMed] [Google Scholar]
- 26.Lyadova, I. V., E. B. Eruslanov, S. V. Khaidukov, V. V. Yeremeev, K. B. Majorov, A. V. Pichugin, B. V. Nikonenko, T. K. Kondratieva, and A. S. Apt. 2000. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J. Immunol. 165:5921-5931. [DOI] [PubMed] [Google Scholar]
- 27.Mazurek, G. H., P. A. LoBue, C. L. Daley, J. Bernardo, A. A. Lardizabal, W. R. Bishai, M. F. Iademarco, and J. S. Rothel. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740-1747. [DOI] [PubMed] [Google Scholar]
- 28.Medina, E., and R. J. North. 1996. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J. Exp. Med. 183:1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina, E., and R. J. North. 1996. Mice that carry the resistance allele of the Bcg gene (Bcgr) develop a superior capacity to stabilize bacille Calmette-Guerin (BCG) infection in their lungs and spleen over a protracted period in the absence of specific immunity. Clin. Exp. Immunol. 104:44-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina, E., B. J. Rogerson, and R. J. North. 1996. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology 88:479-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsos, L. M., L. R. Cardon, A. Fortin, L. Ryan, R. LaCourse, R. J. North, and P. Gros. 2000. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 1:467-477. [DOI] [PubMed] [Google Scholar]
- 33.Mitsos, L. M., L. R. Cardon, L. Ryan, R. LaCourse, R. J. North, and P. Gros. 2003. Susceptibility to tuberculosis: a locus on mouse chromosome 19 (Trl-4) regulates Mycobacterium tuberculosis replication in the lungs. Proc. Natl. Acad. Sci. USA 100:6610-6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogues, T., M. Goodrich, L. Ryan, R. LaCourse, and R. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauciel, C., E. Ronco, and M. Pla. 1990. Influence of different regions of the H-2 complex on the rate of clearance of Salmonella typhimurium. Infect. Immun. 58:573-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opie, E. L., and J. D. Aronson. 1927. Tubercle bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesions. Arch. Pathol. Lab. Med. 4:1-21. [Google Scholar]
- 37.Sanchez, F., T. V. Radaeva, B. V. Nikonenko, A. S. Persson, S. Sengul, M. Schalling, E. Schurr, A. S. Apt, and C. Lavebratt. 2003. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infect. Immun. 71:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh, S. P., N. K. Mehra, H. B. Dingley, J. N. Pande, and M. C. Vaidya. 1983. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J. Infect. Dis. 148:676-681. [DOI] [PubMed] [Google Scholar]
- 39.Turner, J., M. Gonzalez-Juarrero, B. M. Saunders, J. V. Brooks, P. Marietta, D. L. Ellis, A. A. Frank, A. M. Cooper, and I. M. Orme. 2001. Immunological basis for reactivation of tuberculosis in mice. Infect. Immun. 69:3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner, O. C., R. G. Keefe, I. Sugawara, H. Yamada, and I. M. Orme. 2003. SWR mice are highly susceptible to pulmonary infection with Mycobacterium tuberculosis. Infect. Immun. 71:5266-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidal, S., P. Gros, and E. Skamene. 1995. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J. Leukoc. Biol. 58:382-390. [DOI] [PubMed] [Google Scholar]
- 42.Wakeham, J., J. Wang, and Z. Xing. 2000. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect. Immun. 68:6946-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]