Abstract
Beginning with the sixth decade of life, the human immune system undergoes dramatic aging-related changes, which continuously progress to a state of immunosenescence. The aging immune system loses the ability to protect against infections and cancer and fails to support appropriate wound healing. Vaccine responses are typically impaired in older individuals. Conversely, inflammatory responses mediated by the innate immune system gain in intensity and duration, rendering older individuals susceptible to tissue-damaging immunity and inflammatory disease. Immune system aging functions as an accelerator for other age-related pathologies. It occurs prematurely in some clinical conditions, most prominently in patients with the autoimmune syndrome rheumatoid arthritis (RA); and such patients serve as an informative model system to study molecular mechanisms of immune aging. T cells from patients with RA are prone to differentiate into proinflammatory effector cells, sustaining chronic-persistent inflammatory lesions in the joints and many other organ systems. RA T cells have several hallmarks of cellular aging; most importantly, they accumulate damaged DNA. Because of deficiency of the DNA repair kinase ataxia telangiectasia mutated, RA T cells carry a higher burden of DNA double-strand breaks, triggering cell-indigenous stress signals that shift the cell’s survival potential and differentiation pattern. Immune aging in RA T cells is also associated with metabolic reprogramming; specifically, with reduced glycolytic flux and diminished ATP production. Chronic energy stress affects the longevity and the functional differentiation of older T cells. Altered metabolic patterns provide opportunities to therapeutically target the immune aging process through metabolic interference.
Keywords: immune aging, T cells, inflammation, DNA damage, glycolysis
Human survival is closely linked to a functional immune system, which protects the host against infections and malignancies, regulates wound healing, and ultimately separates “self” from surrounding organisms that compete for space and resources. The innate immune system provides fast and effective immune responses, but lacks discriminative power and long-term memory. The adaptive immune system functions by precise recognition of antigen, memory formation, and adaptive proliferation of those cells that provide antigen-specific immunity. T lymphocytes are the cell type with the highest proliferative potential in the body and with a survival span of several decades are subject to wear-and-tear damage.
At birth the immune system is equipped with an enormously diverse repertoire of antigen-reactive T and B cells, all of which are so infrequent that they cannot protect the host. Thus, as humans age and are exposed to infectious organisms and cancerous cells, antigen-specific lymphocytes need to expand massively in frequency and switch from a highly proliferative naive cell into a less proliferative effector and memory cell. On antigen reappearance such effector/memory cells take responsibility for host protection through secretory products (e.g., antibodies, cytokines) and cell-surface molecules (e.g., costimulatory and coinhibitory receptors and ligands). Massive clonal expansion and persistence of antigen-selected cells for decades impose enormous proliferative pressure on immune cells, rendering the immune system highly susceptible to the aging process.
Aging is associated with several morbidities that finally lead to organ failure and death. With progressive deterioration of protective immunity, older individuals become susceptible to cancers and infections (Table 1). Interestingly, aging is also associated with increased incidence of inflammatory disease, most notably cardiovascular disease (1). Many of the degenerative diseases of the elderly, such as Alzheimer’s disease, Parkinson’s disease, and osteoarthritis, have a strong component of tissue-damaging inflammation. Similarly, production of autoantibodies is much more likely to occur in older individuals (2). In essence, immune aging is associated with declining protective immunity combined with increasing incidence of inflammatory disease.
Table 1.
Cardinal features of immune system aging
| Weakened antimicrobial immunity |
| • Susceptibility to respiratory infections |
| • Reactivation of chronic viral infections (e.g., shingles) |
| Impaired antivaccine responses |
| Insufficient protection against malignancies |
| Predisposition for unopposed tissue inflammation |
| • Atherosclerotic disease |
| • Osteoarthritis |
| • Neurodegenerative disease |
| Failing wound repair mechanisms |
Vice versa, it has been proposed that chronic organ diseases, such as chronic obstructive pulmonary disease and chronic kidney disease, accelerate the aging process and thus lead to analogous phenotypes, such as muscle wasting, osteoporosis, and vascular aging (3). Acceleration of organismal aging due to failure of a major organ system, such as renal or respiratory impairment, has obvious implications for the assessment and care of patients affected by chronic debilitating diseases. Whether acceleration of aging in chronic obstructive pulmonary disease also has negative consequences for the immunocompetence of the host is not well understood (4, 5). However, predisposition to pulmonary infections is likely an accelerator for many chronic respiratory diseases, such as late-onset asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis, identifying immunosenescence as a critical risk factor for respiratory disease.
Immunosenescence in Humans: Rheumatoid Arthritis as a Model System
While the impact of aging on the innate immune system remains insufficiently understood, much progress has been made in defining and characterizing molecular processes underlying the aging of T lymphocytes (6–8). T-cell aging progresses considerably faster in patients with a diagnosis of rheumatoid arthritis (RA), and investigation of such patients has enabled the definition of molecular pathways underlying T-cell immunosenescence (9–11) (Table 2). Guiding observations were made almost 20 years ago, when T cells in the synovial lesions of patients with RA were found to have a unique phenotype, CD4+CD28null (12, 13). Loss of the costimulatory molecule CD28 is now recognized as a reliable aging marker on T cells (14). Subsequent studies revealed that RA T cells have shortening of telomeric sequences and that the telomeric loss affects naive, unprimed T cells as well as bone marrow precursor cells (15–19). Remarkably, healthy individuals typing HLA-DR4, a MHC class II haplotype associated with RA, share with patients the faster erosion of telomeres, already beginning during the second to third decades of life (16). These studies have eliminated systemic inflammation as the root cause of premature immune aging and gave rise to the hypothesis that aged T cells may not be a consequence of RA, but a critical effector cell in the chronic inflammatory process (20).
Table 2.
T-cell aging in rheumatoid arthritis
| Accumulation of clonal CD4 T-cell populations that have lost expression of CD28 (CD4+CD28null T cells) |
| Premature shortening of telomeres in |
| • Naive T cells |
| • Neutrophils |
| • CD34+ bone marrow precursor cells |
| Increased DNA breakage in CD4 T cells |
| Activation of the DNA repair response in CD4 T cells |
| Metabolic reprogramming of CD4 T cells |
| • Reduced glycolytic flux due to deficiency in PFKFB3 |
| • Reduced ATP production |
| • Enhanced shunting into the pentose phosphate pathway |
| • Excess reductive elements (NADPH) and lack of reactive oxygen species |
Definition of abbreviation: PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3.
Having RA is associated with the shortening of life expectancy, mostly due to acceleration of cardiovascular disease. CD4+CD28null T cells have also been isolated out of atherosclerotic plaque in patients with fatal myocardial infarction, implicating such cells in tissue inflammation (21, 22). CD4+CD28null T cells acquire cytotoxic functions, display cytolytic activity toward endothelial cells, and produce high amounts of the proinflammatory cytokine IFN-γ (23–26).
Overall, a diagnosis of RA is associated with profound remodeling of the immune system, including the accumulation of clonally expanded effector T cells that are proinflammatory and tissue destructive. While there remain unanswered questions about the “hen-and-egg” relationship of chronic autoimmune disease and immune aging (27), RA has emerged as an ideal model system to study the molecular characteristics of pre-aged T cells.
Much of the progress made is in understanding the molecular pathways underlying T-cell aging in CD4 T cells. Curiously, CD8 T cells appear to age faster; the age-related loss of naive T cells is much more pronounced in the CD8 compared with the CD4 compartment. Concomitantly, end-differentiated CD8 effector T cells expand to a higher degree than their counterparts in the CD4 T-cell compartment (28, 29). Shorter telomeric lengths among CD8 T cells compared with CD4 T cells support higher turnover in the CD8 compartment (30). Indeed, CD8+CD28neg T cells are now recognized as a typical aging-related population. So far, there is no evidence that fundamentally different processes are involved in aging CD4 and CD8 T cells. Several models have been proposed to explain why T cells in the same host may undergo differential aging. Such models include the speculation that CD4 and CD8 T cells live in distinct tissue niches, which are differentially susceptible to the impact of aging. An overall larger size of the CD4 compartment may be protective against the loss of naive cells. And, differences in cytokine responses maintaining homeostatic T-cell proliferation may ultimately impose higher proliferative pressure on CD8 T cells.
DNA Damage and Immune Aging
A hallmark of the aging process is the shortening of chromosomal ends, due to the loss of telomeric sequence repeats (31). Human CD4 T cells lose about 3,000 bp of telomeric sequences between age 20 and age 60, when telomeric length reaches a plateau at a total length of 5,000–6,000 bp. The curve correlating age and telomeric lengths in CD4 T cells is shifted toward higher age in patients with RA with a 1,500-bp length reduction that is already evident in early life (15). Human T cells do not reach telomeric crisis and those with telomeres shorter than 5–6 kb appear to be culled long before the chromosomal ends are critically reduced. The mechanisms leading to prematurity of telomeric erosion in RA T cells are not understood. One cause lies in the impaired induction of telomerase activity in RA T cells (32). Telomerase activity in CD4 T cells is directly related to their survival and with reduced telomerase activity RA T cells are apoptosis sensitive. However, telomerase deficiency is insufficient to provide an explanation for the premature uncapping of chromosomal ends in RA T cells. In vitro proliferation stress tests reveal that the loss of telomeric sequences is dependent on the differentiation status of T cells, with telomerase-high naive T cells shedding many more telomeric repeats than their memory counterparts. Thus, failure in telomerase-independent protection mechanisms may be more relevant for T-cell aging (33).
Short telomeres represent a special case of damaged DNA, and molecular studies in RA T cells have confirmed that DNA damage sensing and repair are fundamentally altered (Table 3). Specifically, measurements of DNA breakage by comet assay (measuring the leakage of broken DNA from the nucleus) have yielded important insights into genome stability. RA T cells, even in patients who are only in the third or fourth decade of life, have a high load of DNA double-strand breaks (34). This affects naive as well as memory CD4 T cells, is present in untreated patients, and is barely amenable to antiinflammatory therapy. Screening for DNA repair molecules has demonstrated that the serine/threonine protein kinase ataxia telangiectasia mutated (ATM) is insufficiently expressed in RA T cells. Protein levels are reduced to about 40–50%. Overexpression of ATM in RA T cells corrects the defect and normalizes the DNA breakage load.
Table 3.
DNA damage in aging T cells
| Increased load of DNA double-strand breaks |
| Reduction in the protein levels of the repair kinase ATM |
| Lacking activation of p53-dependent pathways |
| Chronic activation of the repair kinase DNA-PKcs |
| DNA-PKcs–dependent triggering of the stress kinase JNK |
| Inappropriate loss of telomeric ends |
Definition of abbreviations: ATM = ataxia telangiectasia mutated; DNA-PKcs = DNA-dependent protein kinase, catalytic subunit; JNK = c-Jun N-terminal kinase.
Interestingly, persistent DNA damage in such pre-aged T cells does not elicit induction of p53. On the contrary, RA T cells are distinctly low in the apoptosis inducer p53 (34, 35). The tumor-suppressive function of p53 relates to its ability to sense DNA damage and trigger protective measures, for example, cell cycle inhibition or promotion of apoptosis. Why RA T cells down-regulate p53 is not understood, but signals multifaceted abnormalities in the surveillance of genome stability in cell cycle regulation.
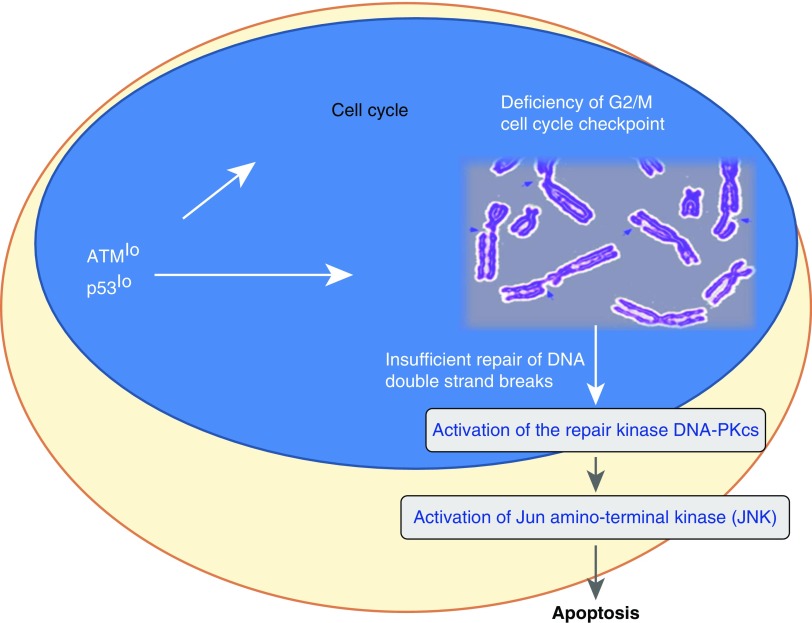
Pre-aged T cells are not mute to the fact that their DNA is no longer as stable as in young T cells. Evidence derived from studies of alternative DNA sensing and repair pathways indicates that older T cells are well aware of their precarious situation. Indeed, RA T cells up-regulate DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) (36), a member of the phosphatidylinositol-3-kinase–related kinase protein family and a close relative to ATM. DNA-PKcs is a principal component of the nonhomologous end-joining pathway of DNA repair. Mice born with mutated DNA-PKcs have a shorter life span and typically develop aging-related pathologies earlier in life (37). The prematurely aged T cells from patients with RA overexpress DNA-PKcs and its phosphorylated, active form. In a DNA-PKcs–dependent manner such T cells activate the stress kinase pathway via c-Jun N-terminal kinase (JNK). JNK has been implicated in regulating multiple key cellular functions, such as cell growth, differentiation, survival, and apoptosis (38). Molecules regulated by JNK include c-Jun, ATF2, SMAD4, HSF1, ELK1, STAT3, and NFAT, which in immune cells are responsible for modulation of cytokine-induced, mitogen-activated protein kinase (MAPK), and transforming growth factor (TGF)-β–dependent signaling pathways. In essence, the inability to properly repair DNA initiates chronic stress signaling in aging T cells that has implications for most of their functional activities (Figure 1).
Figure 1.
DNA damage in rheumatoid arthritis (RA) T cells. Deficiency of the cell cycle regulator and DNA repair kinase ataxia telangiectasia mutated (ATM) changes the fate of T cells in patients with RA. ATMlo T cells bypass the G2/M cell cycle checkpoint and insufficiently repair DNA double-strand breaks. One of the major consequences is excessive death of the T cells, possibly leading to lymphopenia, proliferative pressure to compensate for the loss, and thus premature aging. DNA-PKcs = DNA-dependent protein kinase, catalytic subunit.
Metabolic Reprogramming of Aging T Cells
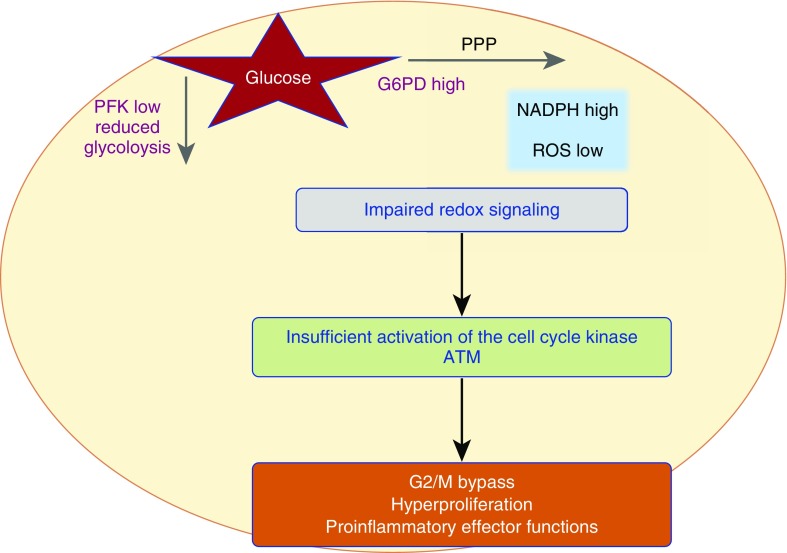
The need to massively proliferate and to survive for decades imposes high demands on cellular energy sources and requires access to biosynthetic molecules as cell building blocks. To fulfill these needs, T cells switch their metabolic program to increased glucose utilization, well known as the Warburg effect (39). Glucose-intensive metabolism allows visualizing inflammatory T cells by positron emission tomography (PET) imaging. The energy needs of naive, memory, and effector T cells are fundamentally different, and studies deciphering energy regulation and the connection to functional parameters must be carefully controlled for the composition of young and old T-cell populations. Studies have emphasized that the metabolism of aging T cells is fundamentally different from their young counterparts (Table 4). Naive CD4 T cells from patients with RA, pre-aged by about 20 years, generate lower amounts of lactate and ATP (40), whereas the accumulation of NADPH is increased (41). The underlying molecular defect has been mapped to the imbalance of two enzymes regulating glucose utilization: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and glucose-6-phosphate dehydrogenase (G6PD) (41, 42). With decreased activity of PFKFB3 and enhanced activity of G6PD, pyruvate production is disfavored. Such old T cells suffer from insufficient substrate to feed mitochondrial respiration and are energy deprived. Instead of breaking down glucose, they shunt it into the pentose phosphate pathway, promoting an anabolic state. One of the metabolic consequences is the accumulation of reductive elements, particularly NADPH and reduced glutathione, and the scavenging of reactive oxygen species. Overall, this shift in the metabolic program deprives old T cells from oxidant signaling. Multiple critical functions of proliferating and differentiating T cells are dependent on oxidant signaling, leaving individuals with prematurely aged T cells with a functionally reprogrammed immune system (Figure 2).
Table 4.
Metabolic reprogramming of aging T cells
| Energy deficiency caused by insufficient ATP production |
| Reduced production of lactate |
| Impaired glycolytic breakdown due to insufficiency of the glycolytic enzyme PFKFB3 (“anti-Warburg effect”) |
| Accumulation of reductive elements (NADPH) and reduced glutathione |
| Loss of activation-induced release of reactive oxygen species and weakening of oxidant signaling |
Definition of abbreviation: PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3.
Figure 2.
Metabolic reprogramming of prematurely aged T cells in rheumatoid arthritis (RA). Glucose imported into the cell can either be broken down to generate ATP or be shunted into the pentose phosphate pathway (PPP) to generate reductive elements (NADPH). In RA T cells high activity of glucose-6-phosphate dehydrogenase (G6PD) and low activity of phosphofructokinase (PFK) result in excessive shunting of glucose, leaving the cell under reductive stress and impairing redox signaling. As a consequence, such cells insufficiently activate the cell cycle regulator ataxia telangiectasia mutated (ATM), which results in bypassing of the G2/M phase, hyperproliferation, and a bias toward proinflammatory effector functions. ROS = reactive oxygen species.
Notably, PFKFB3 is also involved in inducing autophagy, and PFK-deficient RA T cells fail to rely on autophagy as a means of energy generation (43, 44). The energy stress of aging T cells is aggravated by the disproportionate expression of the ATPase CD39 (45). Located on the cell membrane of CD4 T cells, CD39 activity critically controls the survival of effector T cells and their differentiation into long-lived memory T cells. In healthy older individuals, antigen-activated CD4 T cells overexpress this enzyme and become markedly apoptosis sensitive, resulting in poorer survival of effector T cells. This mechanism may be critically involved in curbing the induction of protective immune responses in such individuals, leaving them susceptible to infection and malignancy. Conversely, individuals with a genetic polymorphism in CD39 have improved antivaccine responses, emphasizing the role of the ATPase in controlling the pool of protective memory T cells.
Chronic energy stress in old T cells has been implicated in other age-dependent immunodeficiencies. Specifically, energy-deprived T cells up-regulate activation of the energy sensor 5′-AMP–activated protein kinase (AMPK) (46). A downstream target of inappropriately activated AMPK in aging T cells is the dual-specificity protein phosphatase 4 (DUSP4) (47), which negatively regulates members of the MAPK superfamily, in particular ERK1, ERK2, and JNK. ERK is also subject to increased negative regulation by another dual-specificity protein phosphatase, DUSP6 (48). ERK is a key regulator of the T-cell receptor signaling cascade, and its dephosphorylation by DUSP4 and DUSP6 functions as a suppressive mechanism, weakening the T-cell receptor–induced signal and dampening T-cell function. Old, DUSP4high T cells lose their ability to provide helper function for antibody-forming B cells, thus undermining protective immunity (47).
Conclusions
Aging of the immune system is associated with dramatic changes in the distribution and competence of immune cells (49). The overriding theme is the loss of adaptive immunity and the gain of nonspecific innate immunity, leaving older individuals susceptible to infection and cancer and unprotected from chronic tissue inflammation. The co-occurrence of weakened adaptive immunity with a bias toward nonspecific tissue inflammation is often captured in the term “inflammaging.” Mechanisms fostering inflammatory disease in the old include the loss of immunoinhibitory capacity, specifically the deterioration of regulatory T-cell (Treg) function (50–52). Immune aging is a risk factor for and amplifies many of the pathologies associated with the aging process. Therapeutic interventions holding or reversing immune aging would open opportunities to improve the management of aging-related morbidities and have a major impact on the health status of society (53).
Key molecular pathways involved in T-cell aging have been identified (Figures 1 and 2). The diversity of the T-cell repertoire declines markedly, subtracting from the immune system’s ability to specifically recognize antigen (6, 54–56). A major defect in older T cells lies in the inability to properly repair damaged DNA (10, 57), which possibly extends to telomeric ends and identifies telomeric loss as a manifestation of age-related genomic instability. Key enzymes related to defective DNA repair in aging T cells include ATM and DNA-PKcs.
A common denominator of T-cell aging is chronic energy stress (8, 58). Deficiency in the glycolytic enzyme PFKFB3 results in low ATP levels. Dysregulated expression of the ATPase CD39 aggravates the lack of ATP production, rendering T cells apoptosis sensitive and the host deprived of sufficient T-cell expansion. Favoring of phosphatases over kinases in aging T cells emerges as a mechanism elevating activation thresholds and resulting in low responsiveness (58). Antiaging therapy should aim at prolonging T-cell survival while weakening inflammation-prone innate immunity (7, 59).
Footnotes
Supported by the National Institutes of Health (R01 AR042547, R01 AI044142, HL 117913, R01 AI108906, and P01 HL058000 to C.M.W. and R01 AI108891, R01 AG045779, and I01 BX001669 to J.J.G.), and the Govenar Discovery Fund. The authors declare no competing financial interests.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201:S1–S7. doi: 10.1016/S0167-5273(15)31026-3. [DOI] [PubMed] [Google Scholar]
- 2.Hurme M, Korkki S, Lehtimäki T, Karhunen PJ, Jylhä M, Hervonen A, Pertovaara M. Autoimmunity and longevity: presence of antinuclear antibodies is not associated with the rate of inflammation or mortality in nonagenarians. Mech Ageing Dev. 2007;128:407–408. doi: 10.1016/j.mad.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Kooman JP, Shiels PG, Stenvinkel P. Premature aging in chronic kidney disease and chronic obstructive pulmonary disease: similarities and differences. Curr Opin Clin Nutr Metab Care. 2015;18:528–534. doi: 10.1097/MCO.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 4.Murray MA, Chotirmall SH. The impact of immunosenescence on pulmonary disease. Mediators Inflamm. 2015;2015:692546. doi: 10.1155/2015/692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone J, Parsons R, Botelho F, Millar J, McNeil S, Fulop T, McElhaney J, Andrew MK, Walter SD, Devereaux PJ, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One. 2014;9:e108481. doi: 10.1371/journal.pone.0108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goronzy JJ, Li G, Yang Z, Weyand CM. The Janus head of T cell aging—autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. doi: 10.3389/fimmu.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7– CD28– T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28– T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+CD28– T cells in immune disorders. Trends Mol Med. 2012;18:446–453. doi: 10.1016/j.molmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schönland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, Weyand CM. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci USA. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 21.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 22.Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzo G, Vallejo AN, Kopecky SL, Frye RL, Holmes DR, Goronzy JJ, Weyand CM. Molecular fingerprint of interferon-γ signaling in unstable angina. Circulation. 2001;103:1509–1514. doi: 10.1161/01.cir.103.11.1509. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell–mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima T, Goek O, Zhang X, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. De novo expression of killer immunoglobulin-like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ Res. 2003;93:106–113. doi: 10.1161/01.RES.0000082333.58263.58. [DOI] [PubMed] [Google Scholar]
- 27.Dehbi AZ, Radstake TR, Broen JC. Accelerated telomere shortening in rheumatic diseases: cause or consequence? Expert Rev Clin Immunol. 2013;9:1193–1204. doi: 10.1586/1744666X.2013.850031. [DOI] [PubMed] [Google Scholar]
- 28.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset–specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Žugich D, Kaye J, Nikolich-Žugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- 31.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 32.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci USA. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuerhahn S, Chen LY, Luke B, Porro A. No DDRama at chromosome ends: TRF2 takes centre stage. Trends Biochem Sci. 2015;40:275–285. doi: 10.1016/j.tibs.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas K, Westfall M, Pietenpol J, Olsen NJ, Aune T. Reduced p53 in peripheral blood mononuclear cells from patients with rheumatoid arthritis is associated with loss of radiation-induced apoptosis. Arthritis Rheum. 2005;52:1047–1057. doi: 10.1002/art.20931. [DOI] [PubMed] [Google Scholar]
- 36.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espejel S, Martín M, Klatt P, Martín-Caballero J, Flores JM, Blasco MA. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 2004;5:503–509. doi: 10.1038/sj.embor.7400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 39.Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015;6:1. doi: 10.3389/fimmu.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17:29. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, Weyand CM. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8:331ra38. doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins RH, Lack L, Killenberg PG. Rat hepatic bile acid sulfotransferase: enzyme response to androgens and estrogens. Am J Physiol. 1987;252:G276–G280. doi: 10.1152/ajpgi.1987.252.2.G276. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Goronzy JJ, Weyand CM. Autophagy in autoimmune disease. J Mol Med (Berl) 2015;93:707–717. doi: 10.1007/s00109-015-1297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, Ye Z, Le Saux S, Sultan W, Turgano E, Dekker CL, et al. Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Reports. 2016;14:1218–1231. doi: 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, Goronzy JJ. Signal inhibition by the dual-specific phosphatase 4 impairs T cell–dependent B-cell responses with age. Proc Natl Acad Sci USA. 2012;109:E879–E888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiting CC, Siebert J, Newman AM, Du HW, Alizadeh AA, Goronzy J, Weyand CM, Krishnan E, Fathman CG, Maecker HT. Large-scale and comprehensive immune profiling and functional analysis of normal human aging. PLoS One. 2015;10:e0133627. doi: 10.1371/journal.pone.0133627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology. 2014;60:130–137. doi: 10.1159/000355303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. 2012;189:2118–2130. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ, Weyand CM. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126:1953–1967. doi: 10.1172/JCI84181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olshansky SJ. Articulating the case for the longevity dividend. Cold Spring Harb Perspect Med. 2016;6:a025940. doi: 10.1101/cshperspect.a025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015;7:117. doi: 10.1186/s13073-015-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, Goronzy JJ. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. 2014;111:13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naive T cell repertoire in the elderly: thymic involution or peripheral homeostatic proliferation? Exp Gerontol. 2014;54:71–74. doi: 10.1016/j.exger.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells: a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hohensinner PJ, Goronzy JJ, Weyand CM. Targets of immune regeneration in rheumatoid arthritis. Mayo Clin Proc. 2014;89:563–575. doi: 10.1016/j.mayocp.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]