Abstract
Cellular senescence is a cell fate decision and stress response that entails a permanent arrest of cell proliferation coupled to a complex secretory phenotype. Senescent cells increase in number with age in most, if not all, mammalian tissues, including the airways and lungs. They also increase at greater than expected numbers, compared with age-matched controls, at sites of age-related pathologies such as chronic obstructive pulmonary disorder and emphysema. The senescence response is a double-edged sword. The proliferative arrest suppresses the development of cancer by preventing the propagation of stressed or damaged cells that are at risk for neoplastic transformation. However, this arrest can also curtail the proliferation of stem or progenitor cells and thus hamper tissue repair and regeneration. Similarly, the secretory phenotype can promote wound healing by transiently providing growth factors and the initial inflammatory stimulus that is required for tissue repair. However, when chronically present, the secretory phenotype of senescent cells can drive pathological inflammation, which contributes to a host of age-related pathologies, including cancer. There are now transgenes and prototype small molecules that can clear senescent cells, at least in mouse models, and thus improve health span and median life span. The next challenge will be to develop interventions and supplements that can abrogate the deleterious effects of senescent cells while preserving their beneficial effects.
Keywords: cancer, inflammation, regeneration, tumor suppressor genes, wound healing
Aging causes a decline in the structure and function of most, if not all, the major tissues in mammalian organisms, including the airways and lungs. Because these decrements affect multiple tissues, and in some cases can be delayed by single genetic changes (1), it is now thought that aging is caused by a few basic processes that have pleiotropic effects. As such, basic aging processes should then be amenable to targeted interventions that can broadly increase mammalian health span (years of healthy life) (2), although it remains an open question whether maximum life span can be substantially extended (3).
What are these basic aging processes, and are they really susceptible to health span–extending interventions? Undoubtedly, there is not a single basic aging process; rather, it is likely there are multiple processes that drive mammalian aging (4), which may explain why health span or median life span extension is often more robust than maximum life span extension. In recent years, one basic aging process or hallmark of aging has emerged as a promising target: the cell fate decision termed cellular senescence.
Cellular Senescence
Cellular senescence is arguably best viewed as a complex stress response that occurs in cells that have the potential to undergo cell division. The cellular senescence response can be induced by many stimuli, including genomic or epigenomic damage, mutational activation of oncogenes, mitochondrial dysfunction, metabolic imbalances, and other stressors (5–8). In each case, the response entails two important phenotypic changes. The first change is an essentially permanent arrest of cell proliferation (used here interchangeably with growth). The second change is a multicomponent senescence-associated secretory phenotype (SASP). Both of these phenotypic changes are proverbial double-edged swords; that is, they have potentially beneficial effects as well as potentially deleterious effects.
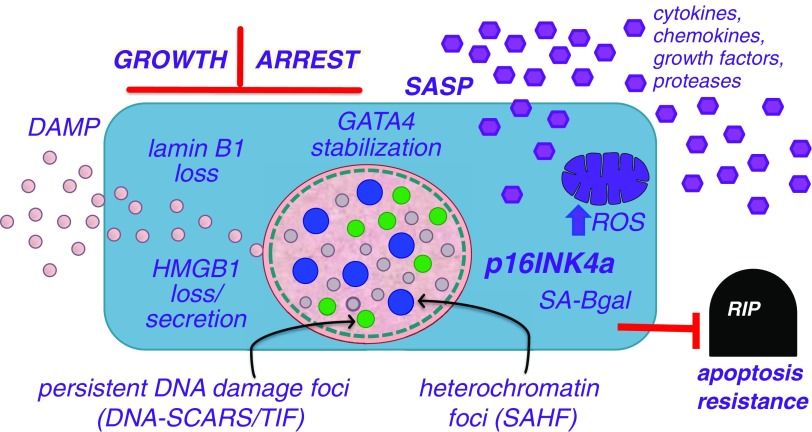
Senescent cells acquire a number of attributes or biomarkers that have enabled their identification in vertebrate tissues, including human tissues (see Figure 1) (9–15). To date, there are no biomarkers that are absolutely specific for senescent cells. Nonetheless, as discussed below, the identified biomarkers have been used in various combinations to determine when and where senescent cells occur in vivo.
Figure 1.
Senescence-associated markers and phenotypes. Cellular senescence causes cells to adopt a phenotype that includes an essentially irreversible arrest of cell proliferation (growth arrest) coupled to a secretory phenotype that includes numerous cytokines, chemokines, growth factors, and proteases (the senescence-associated secretory phenotype [SASP]) (39), as well as the alarmin HMGB1 (42). Senescent cells also upregulate expression of the cell-cycle inhibitor p16INK4a (5) and a neutral β-galactosidase (senescence-associated β-galactosidase [SA-Bgal]) (9), show increases in levels of reactive oxygen species (ROS) and the GATA4 transcription factor (15), and harbor nuclear structures termed senescence-associated heterochromatic foci (SAHF) (11) and DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS) (13). Finally, senescent cells acquire resistance to apoptosis, making them susceptible to drugs that inactivate proapoptotic proteins (23, 24). DAMP = damage-associated molecular pattern; HMGB1 = high-mobility group B1 protein; TIF = telomere dysfunction-induced foci.
One prominent senescence-associated biomarker is the induced expression of p16INK4a, a tumor suppressor protein and cyclin-dependent kinase inhibitor that is important for imposing the permanent growth arrest (6, 16). As discussed below, p16INK4a induction has been exploited to develop mouse models that allow the tracking, and in some cases the inducible killing, of senescent cells in living animals. Another important attribute of senescent cells is the fact that they remain alive and metabolically active for long periods of time, certainly in culture and probably in vivo as well. In culture, senescent cells do not, in general, undergo apoptosis or other forms of cell death, including cell death due to too much or too little autophagy (17, 18). Nonetheless, some cultures of senescent cells can undergo slow cell loss by poorly understood mechanisms. In vivo, it is not known whether senescent cells undergo apoptosis or other forms of cell death, but they can be recognized and eliminated by immune cells, primarily natural killer (NK) cells, which kill them by classic granzyme-mediated NK cell killing (19–22). Despite this elimination mechanism, senescent cells accumulate in vivo, most likely because the balance of pro- and antiapoptotic proteins is tipped somewhat in favor of antiapoptosis (23, 24), and also because the immune system is not totally efficient in eliminating them. These attributes may explain why senescent cells increase in number with age and age-related pathologies in many, if not most, vertebrate tissues, where they appear to be chronically present (5–8).
Senescent cells are also are present, albeit transiently, in young organisms, including developing embryos and during wound healing (7, 25). In general, it appears that the deleterious effects of senescent cells arise from their age-related chronic presence, as opposed to their transient presence during embryogenesis and wound healing, which appears to be beneficial (26).
In this regard, the senescence response is likely an example of evolutionary antagonistic pleiotropy (27)—that is, a trait that is beneficial early in life but deleterious later in life. An important point to note for understanding how the senescence growth arrest can promote cancer-free survival for only about half the maximum life span is that, throughout most of the evolutionary history of the majority of organisms, few individuals lived beyond what we now recognize as middle age. Prior to reaching middle age, most individuals died owing to predation, infection, starvation, and other extrinsic hazards. Thus, there has been very little selective pressure to optimize processes that have early life benefits but late life decrements. By what mechanisms do the main senescence-associated phenotypes—the growth arrest and SASP—have both beneficial and deleterious effects?
Senescence Growth Arrest
Many lines of evidence—from both cell culture experiments and in vivo manipulations and observations in model mammalian organisms and humans—strongly support the idea that the senescence growth arrest is a potent mechanism for suppressing the development of cancer (28, 29). Stressed cells often experience genomic, epigenomic, or metabolic disturbances that put them at risk for neoplastic transformation. The senescence growth arrest, by virtue of its permanency, prevents such cells from propagating. Thus, cellular senescence curtails an early stage of malignant tumorigenesis and helps ensure an early life period that is cancer-free. For most mammals, including humans, this period is about half of the maximum (species-specific) life span—that is, approximately 15–18 months for mice and 50–60 years for humans (30). Reasons why this mechanism might fail later in the latter half of the life span are discussed below in the section on the SASP.
Mitigating the early life benefits of the senescence growth arrest as an anticancer mechanism is the fact that many tissues rely on stem or progenitor cell proliferation for tissue repair, regeneration, and/or remodeling (31–33). These cells can, of course, undergo senescence in response to increasing age-related stress, in which case they lose the ability to proliferate and thus participate in tissue regeneration and repair. Furthermore, the senescence response can limit the ability of somatic cells to undergo induced pluripotency (34, 35). In addition, as discussed below, cells that comprise the stem cell niche can also undergo senescence, whereupon the SASP can create a niche environment that suppresses the ability of stem or progenitor cells to undergo proliferation and/or differentiation. Whether senescent stem/progenitor cells themselves are suppressed in their ability to proliferate, or whether the senescence of niche cells suppresses stem/progenitor cell proliferation, may be tissue-specific. For example, in skeletal muscle, senescent cells in the niche may be largely responsible for the loss of regenerative capacity (36). By contrast, in the skin, stem cell senescence and consequent loss of stem cell proliferative capacity may be the more important contributor to skin aging (37).
The Senescence-Associated Secretory Phenotype
A second important feature of senescent cells—the SASP—had been studied sporadically for some time (38), but only recently has it been comprehensively characterized (39–41). SASP components include many proinflammatory cytokines, chemokines, growth factors, and proteases—molecules that are known to have profound effects on neighboring or even distal cells. It also includes at least one alarmin or damage-associated molecular pattern, the high-mobility group B1 protein, which can initiate an inflammatory response by the immune system (42). Thus, the SASP has the potential to alter both the local tissue microenvironment and the overall systemic milieu. Similar to the senescence-associated growth arrest, the SASP can be beneficial or deleterious, depending on the biological context.
As noted above, senescent cells appear transiently during embryogenesis (43, 44) and also during wound healing or tissue repair (21, 45, 46). In both these cases, they appear to be beneficial by virtue of their SASP. In the liver, senescence is induced in the stellate cells in response to chemical injury; the senescent stellate cells, in turn, secrete proteases that degrade extracellular matrix proteins, which likely accounts for the ability of the senescent stellate cells to limit the extent of fibrosis (21). In the skin, fibroblasts and endothelial cells are induced to undergo senescence in response to wounding (45). In this tissue, the senescent cells also secrete a number of proteases that, likewise, are probably important for limiting fibrosis. In addition, they secrete growth factors, in particular platelet-derived growth factor A, which was shown to be critical for timely wound closure (45). A notable feature of the senescent cells that appear during tissue repair is that their presence is transient. The mechanisms by which senescent cells are cleared in these settings is incompletely understood, but, at least in the liver, they appear to be eliminated by NK cells (21).
Despite these beneficial effects of the SASP, there is mounting evidence that the SASP can also be deleterious. In some cases, the deleterious effects can emanate from the same SASP factors that can be beneficial, with the difference depending on whether the SASP is transiently or chronically present. For example, the chronic presence of matrix metalloproteinases that are secreted by senescent cells was shown to disrupt mammary gland tissue structure (alveolar formation and side branching) and function (milk production) (47). In the skin, these proteases can disrupt epidermal function by inducing an epithelial-to-mesenchymal transition in keratinocytes (48).
Perhaps one of the more serious consequences of a chronic SASP is the ability of SASP factors to promote cancer progression—a stark irony, given the role of the senescence growth arrest to suppress cancer. Thus, in both cell culture models and mouse xenografts, the presence of senescent cells can promote the growth of frankly malignant tumor cells, as well as the conversion of preneoplastic cells to malignant cells (49). Moreover, in some cases, this stimulation of tumor progression can occur through the same SASP factors that limit fibrosis during tissue repair, namely the proteases that are secreted by senescent cells (50). In other cases, specific nonprotease SASP factors such as osteopontin can promote neoplastic growth (51).
Senescent Cells and Age-related Phenotypes and Pathologies
As noted above, there is mounting evidence that the age-dependent accumulation of senescent cells, and particularly their SASP, can cause or significantly contribute to a variety of phenotypes and pathologies that are characteristic of aging. These age-related characteristics extend to many cell and tissue types, including those of the major airways and lung. For example, senescent fibroblasts, smooth muscle cells, and/or alveolar epithelial cells have been implicated in the etiology or progression of chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and emphysema (52–55). The stimuli that drive these cells into senescence are incompletely understood, but can include telomere dysfunction (which, in essence, is a particular type of DNA damage), oxidative stress, and inflammation due to infection and toxic environmental compounds, among others.
In recent years, several transgenic mouse models have been developed that permit critical assessments of when and where senescent cells arise in vivo, their roles in driving aging phenotypes and age-related diseases, and their ability to promote tissue repair. These models generally take advantage of the fact that senescent cells frequently express the genes encoding either the p21CIP1 (56) or the p16INK4a (45, 57, 58) cyclin-dependent kinase inhibitors. They therefore use the promoters of these genes to drive the expression of reporter and/or killer genes by senescent cells. They share the ability to visualize senescent cells in living animals by virtue of either a luminescent or a fluorescent reporter. Moreover, two of these models also offer the ability to selectively kill senescent cells (45, 58).
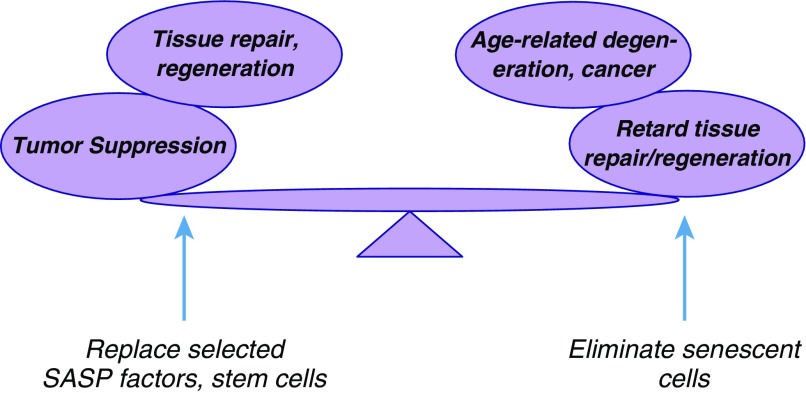
The senescence-reporter/killer transgenic mouse models have been used to confirm the findings from biomarker studies indicating that senescent cells increase in number during aging (45, 57, 58) and in response to environmental stresses (57, 59). These models were also used to confirm that senescent cells accumulate at sites of age-related pathology and in response to tissue damage or injury. Importantly, the transgenic models that permit the selective elimination of senescent cells confirm that senescent cells have both deleterious effects, in that they are important drivers of aging phenotypes (58), and beneficial effects, in that they are important contributors to tissue repair (45). Thus, manipulating senescent cells to delay, reverse, or treat age-related pathologies will require a balancing act: maintaining the benefits these cells subserve in maintaining tissue homeostasis while eliminating their deleterious effects, whether local or systemic (Figure 2).
Figure 2.
The double-edged sword of cellular senescence. Cellular senescence has both beneficial and deleterious effects. The growth arrest is a potent anticancer mechanism, and some senescence-associated secretory phenotype (SASP) components can promote tissue repair. However, the chronic presence of senescent cells, and especially inflammatory SASP components, can drive aging phenotypes and pathologies. Future efforts will entail developing interventions that eliminate senescent cells while replacing the factors they produce that optimize tissue repair.
Challenges for the Future
The transgenic mouse models, particularly those that permit the inducible elimination of senescent cells, provided the first proof of principle that cellular senescence is indeed a double-edged sword. A strategy for translation to humans, then, would be to develop pharmacological rather than transgenic interventions to eliminate senescent cells while replacing those factors produced by senescent cells that are beneficial in tissue repair and regeneration (Figure 2). But how feasible is it to substitute pharmacological approaches for transgenesis? Encouragingly, prototype small molecules have already been identified that appear to kill senescent cells, both in culture and in mouse models (23, 24). These compounds appear to act by specifically inactivating proapoptotic proteins that are important for preventing senescent cells from undergoing apoptosis. Although these early-stage compounds have some side effects and therefore are not yet ready for administration to human patients, their identification provides hope that improved versions are on the horizon.
Footnotes
Supported by National Institutes of Health grants AG009909, AG017242, and AG041122.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 6.Chandler H, Peters G. Stressing the cell cycle in senescence and aging. Curr Opin Cell Biol. 2013;25:765–771. doi: 10.1016/j.ceb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 8.Wiley CD, Campisi J. From ancient pathways to aging cells—connecting metabolism and cellular senescence. Cell Metab. 2016;23:1013–1021. doi: 10.1016/j.cmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 12.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [Published erratum appears in Nat Cell Biol 2009;11:1272.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ. The DNA damage response activates inflammation and senescence by protecting GATA4 from selective autophagy. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- 17.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavaré S, Arakawa S, Shimizu S, Watt FM, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 20.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 2016;8:328–344. doi: 10.18632/aging.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves J, Demaria M, Campisi J, Jasper H. Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging. Dev Cell. 2015;32:9–18. doi: 10.1016/j.devcel.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 28.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 29.Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol. 2008;20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- 31.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 32.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology. 2013;14:573–590. doi: 10.1007/s10522-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- 34.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velarde MC, Demaria M, Melov S, Campisi J. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA. 2015;112:10407–10412. doi: 10.1073/pnas.1505675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaquin N, Vercamer C, Bouali F, Martien S, Deruy E, Wernert N, Chwastyniak M, Pinet F, Abbadie C, Pourtier A. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One. 2013;8:e63607. doi: 10.1371/journal.pone.0063607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 51.Pazolli E, Luo X, Brehm S, Carbery K, Chung JJ, Prior JL, Doherty J, Demehri S, Salavaggione L, Piwnica-Worms D, et al. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Res. 2009;69:1230–1239. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amsellem V, Gary-Bobo G, Marcos E, Maitre B, Chaar V, Validire P, Stern JB, Noureddine H, Sapin E, Rideau D, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:1358–1366. doi: 10.1164/rccm.201105-0802OC. [DOI] [PubMed] [Google Scholar]
- 53.Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res. 2013;162:156–173. doi: 10.1016/j.trsl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Karrasch S, Holz O, Jörres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102:1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A, Chouaid C, et al. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011;109:543–553. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohtani N, Imamura Y, Yamakoshi K, Hirota F, Nakayama R, Kubo Y, Ishimaru N, Takahashi A, Hirao A, Shimizu T, et al. Visualizing the dynamics of p21Waf1/Cip1 cyclin-dependent kinase inhibitor expression in living animals. Proc Natl Acad Sci USA. 2007;104:15034–15039. doi: 10.1073/pnas.0706949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16INK4a-luciferase model. Cell. 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorrentino JA, Krishnamurthy J, Tilley S, Alb JG, Jr, Burd CE, Sharpless NE. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest. 2014;124:169–173. doi: 10.1172/JCI70960. [DOI] [PMC free article] [PubMed] [Google Scholar]