Abstract
Bartonella henselae is a gram-negative pathogen that causes angiogenesis. Here, I establish in vitro models to study Bartonella-induced blood vessel formation. I found that B. henselae induces long-term endothelial survival and tubular differentiation within type I collagen matrix.
Bartonella henselae is an emerging bacterial pathogen that causes febrile lymphadenopathy (cat scratch disease), endocarditis, and angioproliferative lesions in AIDS patients (bacillary angiomatosis) (12, 14, 17). Angioproliferative lesions are the most unique and defining feature of Bartonella infection. Composed of proliferating endothelial cells, these aggregates of immature vascular channels represent a distinctly abnormal form of angiogenesis. They differ from the less exuberant angiogenesis that occurs after stimulation by vascular endothelial growth factor (VEGF), a critical angiogenic cytokine, whether produced by tumors or introduced experimentally (18).
Relevant to its ability to cause angiogenesis, Bartonella induces proliferation of human umbilical endothelial cells (HUVEC) in vitro (reviewed in reference 6). Previously, we determined that this proliferation results from both mitotic induction and inhibition of apoptosis (10). VEGF displays similar properties, suggesting that Bartonella and VEGF act analogously. Interestingly, two studies found that B. henselae induces VEGF secretion from carcinoma (8) and monocytic (15) cell lines, respectively. Therefore, the organism may also promote angiogenesis indirectly by stimulating VEGF release from nonendothelial cell types. Thus, potential connections between Bartonella and VEGF clearly warrant further study.
Although Bartonella induces endothelial proliferation, this process is only one part of new vessel formation. During angiogenesis, endothelial cells must also migrate, survive in new types of extracellular matrix, differentiate, and organize into vessels. Unfortunately, in vivo models have not yet been established to investigate Bartonella-induced angiogenesis. Therefore, the goal of the present work is to characterize the effects of B. henselae in two well-established in vitro angiogenesis models in which the effects of VEGF have been well studied.
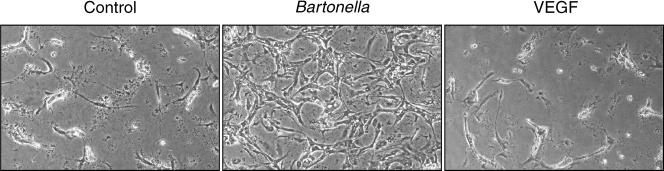
I first tested the effect of Bartonella in an in vitro cord formation assay (4, 19). This assay measures the ability of endothelial cells to form a web-like network of interconnected cells (cords), which are believed to model a late step in new vessel formation. To perform this assay, confluent endothelial monolayers, neonatal dermal microvascular endothelial cells (Clonetics) (Fig. 1) or HUVEC (data not shown), were overlaid with 0.5 mg of type I collagen gel (BD Biosciences) per ml (9). In control studies, endothelial cells reorganized into cords after 6 h (19, 20). As observed previously (20), cord formation was followed by massive endothelial cell death within 1 to 2 days, even in the presence of VEGF (Fig. 1). This was in marked contrast to experimental studies in which endothelial cells were infected with B. henselae ATCC 49882, as previously described (10), 24 h prior to collagen overlay. This bacterial strain was obtained from the American Type Culture Collection, displayed a smooth colonial phenotype, and was used at passage 3 (1, 11). Here, infected endothelial cells maintained long-term viability as cords (>5 days) (Fig. 1). Therefore, Bartonella protected vessel precursors from cell death under conditions where VEGF (VEGF 165; R&D Systems) did not (Fig. 1). Presumably in vivo, additional signals must normally maintain viability of vessel precursors during their invasion and differentiation within type I collagen-rich extracellular matrix, as Bartonella did in this assay.
FIG. 1.
B. henselae promotes survival of endothelial cords. Dermal microvascular endothelial cells were infected with B. henselae and/or treated with VEGF (10 ng/ml) and subsequently overlaid with type I collagen gel. The images shown (phase contrast; magnification, ×40) are from day 3 after collagen overlay and are representative of results from three different experiments.
I next tested the effect of Bartonella in a three-dimensional (3-D) collagen tube formation assay. This assay measures the ability of endothelial cells to invade, migrate, organize, and differentiate into capillary-like tubular structures within a 3-D matrix (3, 7). To perform this assay, HUVEC were trypsinized and resuspended in 2 mg of type I collagen gel per ml as described previously (7, 9). Ilan et al. demonstrated that within this matrix, phorbol myristate acetate (PMA), a potent tumor promoter and stimulator of protein kinase C, but neither VEGF nor basic fibroblastic growth factor induced HUVEC to form tubules (7). Therefore, the 3-D collagen assay appears to be a more stringent test for angiogenic potential than those assays using complex extracellular matrix preparations such as Matrigel and in vivo angiogenesis assays, where multiple cytokines and chemical stimuli have been found to be capable of inducing tubule formation or angiogenesis, respectively (2, 13, 16).
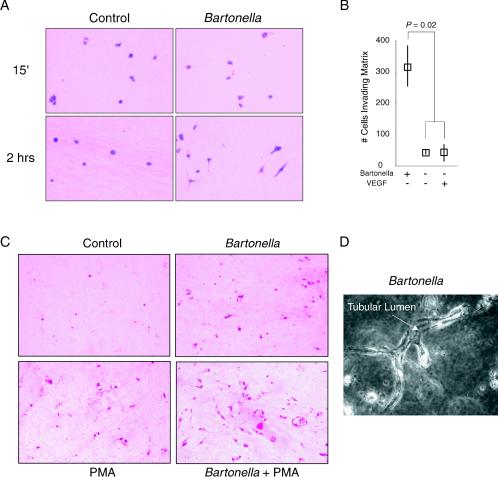
Interestingly, in this assay, Bartonella infection led to phenotypes previously observed only with PMA treatment. First, Bartonella induced matrix invasion. As observed in Fig. 2, both uninfected and infected endothelial cells were trapped in a roughly spherical conformation during gel formation. However, 2 h later, Bartonella-infected HUVEC had extended long projections into the matrix (Fig. 2A), with most cells demonstrating an extended morphology by 24 h (data not shown). In contrast, only a small fraction of control and VEGF-treated cells demonstrated cellular projections (Fig. 2A and B and data not shown), and, when present, these projections were fewer in number and shorter. At 2 h, approximately 10-fold more Bartonella-infected endothelial cells were found invading the extracellular matrix compared to untreated controls or controls treated with 10 ng of VEGF per ml (Fig. 2B) (P = 0.02 by the Mann-Whitney U test [StatView 4.51; Abacus Concepts]). Second, Bartonella promoted long-term survival of endothelial cells embedded in a 3-D collagen gel. By contrast, uninfected (Fig. 2C) and VEGF-treated (data not shown) controls lost viability within 3 days, as observed previously (7). Finally, Bartonella induced endothelial tubular differentiation (3, 4). As observed in two-dimensional hematoxylin and eosin (H&E) sections, Bartonella-infected endothelial cells developed central luminal spaces (Fig. 2C). In phase-contrast images, these intracellular spaces were observed to form luminal compartments within multicellular tubular structures (Fig. 2D) (3, 5). These findings were qualitatively similar to the tubular differentiation observed after treatment with 16 nM PMA (Fig. 2C) (7); however, structures formed in response to infection were less abundant. In contrast, tubular structures were not observed in either uninfected or VEGF-treated controls (data not shown) (7). Interestingly, B. henselae acted synergistically with PMA, increasing both the number and complexity of luminal structures (Fig. 2C).
FIG. 2.
Bartonella promotes invasion, survival, and differentiation in 3-D collagen gels. Infected HUVEC were embedded in type I collagen gel. Where indicated, VEGF (10 ng/ml) or PMA (16 nM) was maintained throughout the assay. (A) Endothelial morphology at 15 min and 2 h after collagen gel formation (H&E staining; magnification, ×400). Note, the elongated shape of infected HUVEC at 2 h. Some HUVEC are cut in cross section, and their elongated shape therefore cannot be appreciated. (B) Numbers of invading (elongated) cells at 2 h (medians and ranges from four separate assays, with one field [magnification, ×100] counted from each). (C) Endothelial morphology at day 4 (H&E staining; magnification, ×100). (D) Tubule from Bartonella infection assay at day 4 (phase contrast; magnification, ×400). The arrow points to an interior lumen running the length of a branching tubule. Parts of the tubule are outside the plane of focus, demonstrating its three-dimensional nature.
Taken together, these studies suggest that Bartonella orchestrates a series of events in addition to endothelial proliferation. These activities include matrix invasion, survival in type I collagen, and endothelial tubular differentiation. It should be noted that both Bartonella and PMA may either directly promote these activities or, alternatively, allow survival in a matrix that itself induces endothelial cells to undergo tubular differentiation. Importantly, my results indicate that Bartonella's activities exceeded those of VEGF in these in vitro angiogenesis assays. Cumulatively, I predict that Bartonella's diverse effects on endothelial cells, both those shared and those not shared with VEGF, help account for the abnormal and florid vascular proliferation observed during bacillary angiomatosis. Here, I have for the first time established in vitro models of Bartonella-induced angiogenesis. These systems will allow study of specific pathways and factors involved in the angioproliferative activities of this emerging infectious agent.
Acknowledgments
I thank Jeannie T. Lee, Lucius Chiaraviglio, and Shirley Stiver for critical reading of the manuscript.
This work was supported by an award from the American Heart Association and by the Beth Israel Hospital Pathology Foundation.
Editor: J. B. Bliska
REFERENCES
- 1.Batterman, H. J., J. A. Peek, J. S. Loutit, S. Falkow, and L. S. Tompkins. 1995. Bartonella henselae and Bartonella quintana adherence to and entry in cultured human epithelial cells. Infect. Immun. 63:4553-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benelli, R., and A. Albini. 1999. In vitro models of angiogenesis: the use of Matrigel. Int. J. Biol. Markers 14:243-246. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff, J. 1995. Approaches to studying cell adhesion molecules in angiogenesis. Trends Cell Biol. 5:69-73. [DOI] [PubMed] [Google Scholar]
- 4.Connolly, J. O., N. Simpson, L. Hewlett, and A. Hall. 2002. Rac regulates endothelial morphogenesis and capillary assembly. Mol. Biol. Cell 13:2474-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, G. E., and C. W. Camarillo. 1996. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224:39-51. [DOI] [PubMed] [Google Scholar]
- 6.Dehio, C. 2003. Recent progress in understanding Bartonella-induced vascular proliferation. Curr. Opin. Microbiol. 6:61-65. [DOI] [PubMed] [Google Scholar]
- 7.Ilan, N., S. Mahooti, and J. A. Madri. 1998. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 111:3621-3631. [DOI] [PubMed] [Google Scholar]
- 8.Kempf, V. A., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Riess, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 9.Kirby, J. E. 2004. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect. Immun. 72:430-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyme, P., B. Dillon, and J. Iredell. 2003. Phase variation in Bartonella henselae. Microbiology 149:621-629. [DOI] [PubMed] [Google Scholar]
- 12.Maguina, C. G. E. 2000. Bartonellosis: new and old. Infect. Dis. Clin. N. Am. 14:1-22. [DOI] [PubMed] [Google Scholar]
- 13.Malinda, K. M. 2001. In vivo matrigel migration and angiogenesis assays. Methods Mol. Med. 46:47-52. [DOI] [PubMed] [Google Scholar]
- 14.Maurin, M., R. Birtles, and D. Raoult. 1997. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 16:487-506. [DOI] [PubMed] [Google Scholar]
- 15.Resto-Ruiz, S. I., M. Schmiederer, D. Sweger, C. Newton, T. W. Klein, H. Friedman, and B. E. Anderson. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect. Immun. 70:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribatti, D., B. Nico, A. Vacca, L. Roncali, P. H. Burri, and V. Djonov. 2001. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 264:317-324. [DOI] [PubMed] [Google Scholar]
- 17.Spach, D. H., and J. E. Koehler. 1998. Bartonella-associated infections. Infect. Dis. Clin. N. Am. 12:137-155. [DOI] [PubMed] [Google Scholar]
- 18.Stiver, S. I., and H. F. Dvorak. 2000. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF). J. Clin. Ligand Assay 23:1-13. [Google Scholar]
- 19.Whelan, M. C., and D. R. Senger. 2003. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J. Biol. Chem. 278:327-334. [DOI] [PubMed] [Google Scholar]
- 20.Wright, T. J., L. Leach, P. E. Shaw, and P. Jones. 2002. Dynamics of vascular endothelial-cadherin and beta-catenin localization by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells. Exp. Cell Res. 280:159-168. [DOI] [PubMed] [Google Scholar]