Abstract
Infection of C57BL/6 mice with Mycobacterium avium leads to the activation of both CD4+ and CD8+ gamma interferon (IFN-γ)-producing T cells, although the CD8+ cells play no role in protection against infection. Using transfer of different lines of transgenic T cells with T-cell receptors (TCRs) which recognize irrelevant antigens, we show here that transferred CD8+ T cells from two of the three lines were activated to the same degree as the host cells, suggesting that the majority of the IFN-γ-producing CD8+ T cells of the host represented bystander activation. The third line, specific for the male HY antigen, showed no activation. Activation required the participation of the CD28 coreceptor on T cells and was unaffected by the removal of CD44hi (memory phenotype) T cells. The transferred CD8+ T cells proliferated in vivo, although this was not essential for IFN-γ production. Taken together, these data are highly reminiscent of homeostatic proliferation of TCR transgenic T cells upon transfer to lymphopenic hosts, and suggest low-affinity stimulation through the TCR, possibly by self peptides. The findings are discussed in relation to homeostatic proliferation and their significance in the possible induction of autoimmune disease.
While specific recognition of antigens is the basis of acquired immunity to infection, the simultaneous activation of irrelevant T lymphocytes has long been hypothesized to contribute to the induction of autoimmunity (27). The notion of bystander activation was supported by the detection of cross-reactive T cells after infection (3, 31) and by the appearance in infected lesions of transgenic T cells with receptors of irrelevant specificity (11). The extensive proliferation of T cells during viral infection also suggested bystander activation, since it greatly exceeded the numbers of specific cells estimated in limiting dilution assays (46). However, the more recent use of tetramers to estimate specific cells led to the conclusion with lymphocytic choriomeningitis virus (LCMV) infection (6, 30) and listeriosis (5) that between 50 and 70% of activated cells can be accounted for by known epitopes from the infecting virus or bacterium. Furthermore, transgenic T cells with receptors of irrelevant specificity showed no expansion when transferred to LCMV- or vaccinia virus-infected hosts (6, 50). In their work transferring LCMV-specific transgenic T cells to mice infected with vaccinia virus or Listeria monocytogenes, Ehl at al. (13) concluded that bystander activation only accounted for about 1/200 of the specific cytotoxic T lymphocytes and that it was mediated by cytokines.
It is indeed possible to show, both in vivo and in vitro, that cytokines typically induced by infection can lead to nonspecific activation, particularly of memory phenotype CD8+ T cells (46). The cytokines involved are notably alpha/beta interferons (IFN-α/β) (45) as well as IFN-γ, interleukin-12 (IL-12), IL-15, and IL-18 (46). Such cytokine-mediated activation of CD8+ T cells has been suggested to provide an early defense against infection (23, 48).
As in viral infection, Mycobacterium avium infection in mice results in a surprisingly high proportion of T cells with an activated phenotype (26). M. avium organisms occur ubiquitously in the environment and possibly induce subclinical infections which are readily controlled by most normal individuals. It is the commonest bacterial infection in untreated AIDS patients (33), and a high incidence of M. avium infection in certain families led to the recognition of immunodeficiencies in IL-12 and IFN-γ activity in humans (36). Mycobacteria are facultative intracellular organisms that survive within macrophages, and immunity requires augmentation of macrophage bactericidal activity by IFN-γ, produced classically by CD4+, but possibly also by CD8+ T cells (38). In a mouse model of M. avium infection, it has previously been shown that both CD4+ and CD8+ T cells proliferate in infected tissues (25) and can be activated in vitro to produce IFN-γ (16). Judged by intracellular cytokine (ICC) staining, approximately 10% of both CD4+ and CD8+ T cells produce IFN-γ at the height of the response. However, while depletion of CD4+ T cells exacerbates infection (34), depletion of CD8+ T cells does not (2, 39). This is in contrast with experimental Mycobacterium tuberculosis infection, where depletion of either CD4+ T cells or CD8+ T cells exacerbates infection (15, 29).
Thus, there is a question mark over the function of CD8+ T cells in M. avium infection. In addition, because these organisms represent an exogenous infection, antigen is not presented by the cytosolic pathway to CD8+ T cells in vitro. Hence, stimulation of the T-cell receptor (TCR) with anti-CD3 was used to demonstrate recall of IFN-γ production in vitro by CD8+ T cells. Anti-CD3 shows strong preference for stimulating cells already activated in vivo (7), but its use, rather than antigen recall, means that the specificity of the CD8+ T cells is unknown. The possibility that the activated CD8+ T cells are bystander cells rather than being specific for the infecting organism was therefore considered.
To investigate this possibility, T cells from transgenic mice expressing TCRs which recognized an irrelevant antigen were transfused into infected mice. Host and transferred cells were distinguished by the markers Ly5.1 and Ly5.2, respectively. We report here that the percentage of CD8+ T cells activated was similar for host and transgenic cells, indicating bystander activation. This is in marked contrast with similar experiments with virus-infected mice (6, 13, 50). The mechanism appeared in some respects to be analogous with homeostatic proliferation, the process that maintains T cells numbers outside the thymus at constant levels and involves low-affinity stimulation of the TCRs by self peptides (17). Homeostatic proliferation is particularly evident when T cells are transferred to T cell-depleted recipients, but in this case proliferation was clearly driven by infection and not depletion.
MATERIALS AND METHODS
Mice.
C57BL/6 (wild-type [WT] B6), ovalbumin (OVA)-specific OT-I (10), and herpes simplex virus (HSV)-specific gBTI.1 (28) mice were all pedigree bred and maintained under specific-pathogen-free conditions in the animal house of the Department of Microbiology and Immunology, University of Melbourne (Victoria, Australia). OT-I mice on a Rag−/− background were kindly provided by W. Heath (Walter and Elisa Hall Institute, Parkville, Victoria, Australia). B6 HY 7171 mice (43) whose transgenic TCR is specific for the male antigen HY in the context of H-2Db were obtained from the WEHI Animal Resource Facility (Kew, Victoria, Australia). CD28−/− mice on a B6 background, originally obtained from Jackson Laboratories (40) were supplied by Centenary Institute, Newtown, New South Wales, Australia. All these strains bear the Ly5.2 marker. Where necessary to distinguish two populations of cells, congenic B6.SJLPtprca (abbreviated B6 Ly5.1) mice were used as recipients in adoptive transfer experiments. All experiments were approved by the Animal Experimentation Ethics Committee of the University of Melbourne.
Infection of mice.
M. avium serovar 8, isolated from an AIDS patient (39), was of the smooth transparent colony type. It was grown in Middlebrook 7H9 broth (Difco, Detroit, Mich.) for 7 to 10 days. CFU were determined by plating serial dilutions on Middlebrook 7H11 agar. The bacteria were washed and stored at −70°C. Under a biosafety hood, lightly anesthetized C57BL/6 or B6 Ly5.1 mice were infected intranasally with 50 μl of the M. avium suspension, adjusted by turbidity to deliver 105 CFU. A retrospective dose check was made by culturing diluted samples onto Middlebrook agar.
Preparation of lymphocytes for adoptive transfer.
Lymph nodes (LN) (inguinal, axillary, brachial, superficial cervical, deep cervical, mesenteric, mediastinal, and lumbar) were aseptically removed from uninfected WT C57BL/6 and transgenic mice. Single-cell suspensions were obtained by gently teasing each LN apart with a fine forceps and passing it through 80-gauge and 80-mesh stainless steel sieves. Cells were washed thoroughly, and 5 × 107 cells per mouse, approximately equivalent to 1 donor per recipient, in 200 μl of phosphate-buffered saline were injected intravenously into Ly5.1 recipients. When Rag−/− OT-I cells were transferred, 7 × 106 lymphocytes were injected per mouse because of the reduced cell numbers recovered from these mice. In some experiments, CD44hi (memory phenotype) cells were removed from OT-I LN preparations prior to transfer by being stained with 5 μg of rat anti-mouse CD44 monoclonal antibody (MAb) (IM7; PharMingen)/ml, followed by fluorescein isothiocyanate (FITC)-conjugated anti-rat immunoglobulin (Silenus), then by being washed and excess conjugate absorbed with 1% normal rat serum. The cells were then stained with phycoerythrin (PE)-conjugated rat anti-mouse CD8 MAb and sorted on a MoFlo modular flow cytometer (Cytomation, Inc., Fort Collins, Colo.).
Recovery of transferred cells for analysis.
Unless otherwise indicated, WT B6 or transgenic cells (both Ly5.2) were injected into 6-week-infected and uninfected B6 Ly5.1 recipients and recovered 2 to 3 weeks later. All LN and spleens were then aseptically removed from each recipient, and single-cell suspensions were obtained by gentle passage through an 80-gauge and 80-mesh stainless steel sieve. Cells from three mice in each group were pooled and resuspended in Tris-NH4Cl to remove red blood cells (4). Cells were washed thoroughly and passed twice through nylon wool columns to deplete B cells (20).
ICC staining.
ICC staining was used to determine the frequency of IFN-γ-producing CD8+ T cells among the Ly5.1 (host) or Ly5.2 (transferred) populations, according to previous methods (21). Briefly, T cells were stimulated with protein G-purified immobilized anti-CD3 MAb (145-2C11; 3 μg/ml) (8) in the presence of 2 μM monensin (PharMingen, San Diego, Calif.) for 6 h at 37°C. Cells were stained with 2 μg of CyChrome-conjugated anti-mouse CD8 MAb (53-6.7)/ml, in conjunction with 5 μg of FITC-conjugated anti-mouse CD45.2 (104) MAb/ml specific for Ly5.2. The labeled cells were fixed, permeabilized, and then stained with 2 μg of PE-conjugated anti-mouse IFN-γ MAb (XMG1.2)/ml. For HY experiments, cells were also stained with a biotinylated anti-mouse TCR clonotype MAb (T3.70) followed by streptavidin-CyChrome to detect HY-specific cells. In these experiments, CD8+ cells were detected with 2 μg of antigen-presenting cell-conjugated anti-mouse CD8 MAb (53-6.7)/ml. Cells were analyzed by flow cytometry with FACSort and CellQuest software (Becton Dickinson, San Jose, Calif.). Routinely, 5,000 CD8+ CD45.2+ events were collected, and analysis gates were set on lymphocytes according to forward and side scatter properties. In all experiments, unstained cells and cells stained separately with each fluorochrome were included to optimize compensation settings. Specificity was determined with cells stained with the appropriate isotype control or with streptavidin-CyChrome complex alone. Results are expressed as the percentage of IFN-γ-producing cells in the host (CD45.2−) or transferred (CD45.2+) CD8+ lymphocyte population.
Proliferation of transgenic cells.
In some experiments, LN cells from OT-I mice were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, Oreg.) and washed thoroughly before adoptive transfer of 5 × 107 cells into B6 recipients. Two weeks later, spleen and LN cells were harvested and purified as above; the intensity of CFSE staining, an indicator of proliferation (24), was analyzed by fluorescence-activated cell sorting (FACS).
Quantitative analysis of IFN-γ transcripts.
Spleen cells pooled from two 6- to 8-week-M. avium-infected or uninfected mice were stained with FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 for sorting on a modular flow cytometer. Total RNA was extracted from 2 × 106 CD4+ and CD8+ cells with an RNeasy mini kit (QIAGEN, Valencia, Calif.). Reverse transcription and cDNA amplification was performed with a one-step reverse transcription-PCR kit (ABI Prism Systems, Foster City, Calif.) with predeveloped primer-probe reagents specific for 18S RNA or IFN-γ. The real-time PCR conditions consisted of 48°C for 30 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min; they were run on an AB17700 real-time PCR machine (Applied Biosystems, Foster City, Calif.). Results were detected using Sequence Detector (Applied Biosystems) software, and relative differences were determined using the Δct method according to the instructions of Applied Biosystems, in which 18S RNA is used as the standard against which other measurements on each sample are made.
RESULTS
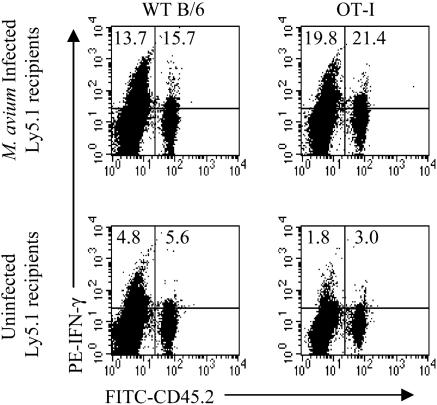
Nonspecific activation of CD8+ TCR transgenic cells. LN cells from B6 or OT-I transgenic T cells (both Ly5.2) were transferred to uninfected or 6- to 8-week-infected B6Ly5.1 mice at an approximate ratio of 1 donor per recipient. The donor OT-I cells were 49% CD8+; of these, 98% expressed the transgenic Vα2 chain. In contrast, B6 cells were 28% CD8+, and only 10% of these were Vα2+. Spleen and LN cells were harvested 3 weeks later, and cells from three mice were pooled to obtain enough cells to work with. The cells were stimulated in vitro with anti-CD3 before being stained for CD8, Ly5.2, and intracellular IFN-γ. The cells were gated for CD8 expression and analyzed for IFN-γ production by host and donor T cells (Fig. 1). Both B6 and OT-I cells became activated to produce IFN-γ upon transfer to infected hosts, with 15% or more of CD8+ T cells producing the cytokine. In normal mice, less than a third of this number was activated. While the necessary pooling of cells within groups precluded any statistical analysis, the results were absolutely reproducible in three experiments and consistent throughout the study.
FIG. 1.
Nonspecific activation of CD8+ OT-I cells. LN cells (5 × 107) from WT B6 (left) or OT-I (right) mice (both Ly5.2+) were transferred to uninfected or 6-week-infected B6Ly5.1 mice. Three weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations. The experiment was performed twice with similar results.
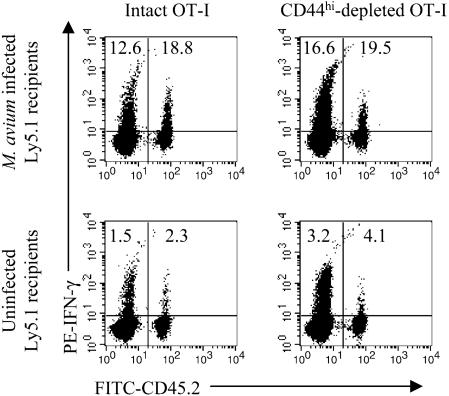
Even without deliberate antigen stimulation, T cells of the memory phenotype exist within the lymphocyte population. To test whether naïve or memory phenotype cells were predominantly involved, CD44hi cells were removed from LN cells pooled from OT-I mice prior to adoptive transfer. After sorting, CD44hi cells represented less than 0.3% of total CD8+ cells compared with 14.2% of unsorted cells. The CD44hi-depleted cells or undepleted cells were transferred to uninfected or 8-week-infected B6Ly5.1 mice, and production of IFN-γ was measured 2 weeks later. Depletion of the CD44hi cells had no effect on the percentage of IFN-γ-producing CD8+ cells among those transferred (Fig. 2).
FIG. 2.
Role of memory T cells in bystander activation. LN cells from OT-I mice (Ly5.2+) were FACS sorted to select CD8+ cells and to remove memory phenotype (CD44hi) cells. A total of 5 × 106 depleted (<0.3% CD44hi) (right) or 2 × 107 undepleted (14.2% CD44hi) (left) cells transferred to uninfected (bottom panels) or 6-week-infected (top panels) B6Ly5.1 mice. Two weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations.
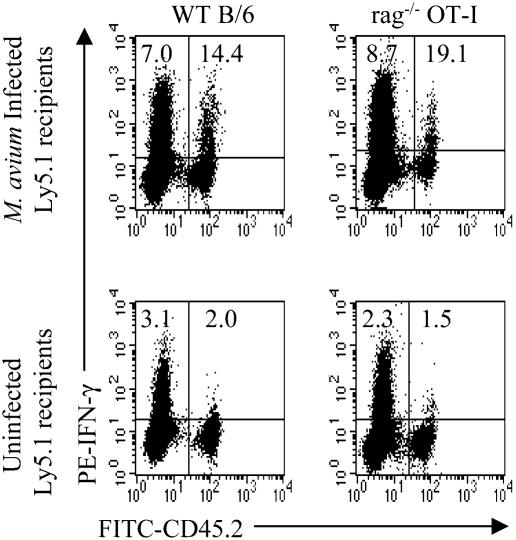
It is conceivable that activation of TCR transgenic T cells could be due to recognition of M. avium peptides by a second receptor on T cells (49). To test this possibility, OT-I Rag−/− mice in which a second receptor cannot be rearranged were used as donors for transfer to normal or 8-week-infected mice. LN cells from OT-I Rag−/− mice were 82% CD8+, and 98% expressed the Vα2+ TCRs. Again, there was substantial bystander activation upon transfer into infected mice (Fig. 3).
FIG. 3.
Bystander activation of CD8+ Rag−/− OT-I cells. LN cells from WT B6 (2 × 107) (left) or Rag−/− OT-I (7 × 106) (right) mice (both Ly5.2+) were transferred to uninfected or 8-week-infected B6Ly5.1 mice. Two weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations.
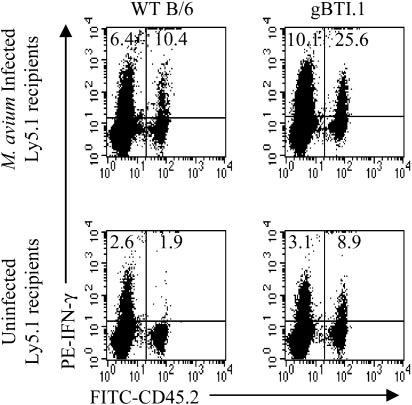
Similar experiments using cells from gBTI.1 mice, whose T cells are specific for the immunodominant antigen of HSV, showed that these cells were also activated to produce IFN-γ upon transfer to an infected host (Fig. 4), indicating that the apparent bystander activation was not due to a fortuitous cross-reaction between OVA and an M. avium antigen.
FIG. 4.
Bystander activation of CD8+ gBTI.1 cells. LN cells from WT B6 (left) or gBTI.1 (right) mice (number of cells, 5 × 107) were transferred to uninfected or 8-week-infected B6Ly5.1 mice. Three weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations. The experiment was performed twice with similar results.
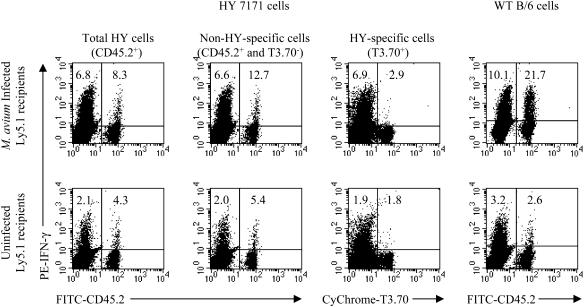
A third receptor specificity tested was that of HY transgenic mice (43). While all the T cells in HY mice express a single, transgenic Vβ8.2-containing TCR chain, only one-third to one-half of the CD8 T cells also express the transgenic Vα3 chain (43). Dual expression of these two transgenic TCRs imparts upon the T cell specificity for the male-specific HY antigen in the context of H-2Db and reactivity with the MAb T3.70, which detects a clonotypic determinant on the transgenic α chain (43). Cells from B6 or HY mice were transferred to 6-week-infected or uninfected B6Ly5.1 mice, and 3 weeks later spleen and LN cells were harvested for analysis of IFN-γ production. In this case, 8.3% of CD8+ cells from HY mice produced IFN-γ after transfer to infected mice, compared with 4.3% of cells in normal hosts (Fig. 5). However, the clonotype-negative CD8+ T cells were responsible for this production, as gating out the HY-specific T3.70+ cells increased the percentage of IFN-γ producers among CD8+ CD45.2+ cells to 12.7% (5.4% in uninfected animals). When HY-specific T3.70+ CD8+ cells were gated, very few IFN-γ-producing cells could be detected (Fig. 5). Unlike most other TCR transgenic T cells, HY T cells also failed to undergo homeostatic proliferation upon transfer into T cell-depleted female recipients (43), a failure which is interpreted as reflecting a deficiency in the recognition of low-affinity cross-reactive peptides in the periphery (17).
FIG. 5.
Lack of bystander activation of CD8+ HY-specific cells. LN cells (5 × 107) from WT B6 or HY 7171 mice (both Ly5.2+) were transferred to uninfected or 6-week-infected B6Ly5.1 mice. Three weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, T3.70, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations. The experiment was performed twice with similar results.
Requirement for costimulation.
LN cells from WT B6 or CD28−/− B6 mice were transferred to uninfected or 8-week-infected B6Ly5.1 mice. The CD28−/− mice lack the CD28 molecule, which acts as a costimulator for the TCR. Two weeks later, spleen and LN cells pooled from three mice were enriched for T cells. They were then stimulated in vitro with anti-CD3 before being stained for CD8, Ly5.2, and intracellular IFN-γ. The cells were gated for expression of CD8 and analyzed for IFN-γ production by host and donor T cells (Fig. 6). B6 cells transferred to infected mice showed levels of IFN-γ production equivalent to those of the host cells. In contrast, CD28−/− B6 cells were not activated, with only 2.1% producing IFN-γ despite 12.9% of host CD8+ cells becoming activated in the same animals. In uninfected hosts, only 1 to 2% of the cells produced IFN-γ. Therefore, there appeared to be a requirement for costimulation through the TCR via CD28 for CD8+ T cells to become activated.
FIG. 6.
Role of CD28 costimulation. LN cells (5 × 107) from WT B6 or CD28−/− B6 mice (both Ly5.2+) were transferred to uninfected or 8-week-infected B6Ly5.1 mice. Two weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations.
Proliferation of transferred cells and production of IFN-γ.
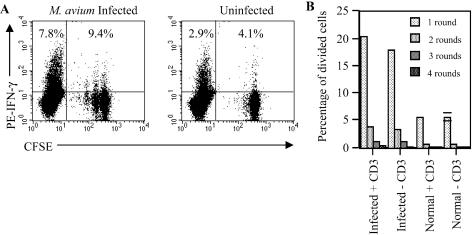
To investigate the relationship between proliferation and IFN-γ production, OT-I cells were CFSE labeled and transferred to 7-week-infected and uninfected B6 mice. The intensity of CFSE labeling is halved each time a cell divides (24). Two weeks later, cells were recovered from spleens and LN and enriched for T cells. Half of the cells were subjected to in vitro stimulation with an anti-CD3 MAb, while the other half were cultured without anti-CD3. The cells were then stained for CD8 and intracellular IFN-γ. Cells were gated for CD8 expression before analysis. Figure 7A shows the production of IFN-γ in relation to proliferation, as reflected in the serial halving of CFSE intensity. Without anti-CD3 stimulation in vitro, no IFN-γ was detected (results not shown). Anti-CD3 stimulation did not affect the CFSE profile (Fig. 7B). When cells from infected mice were analyzed without anti-CD3 activation, 23% of OT-I cells showed serial halving of CFSE over 2 weeks. This percentage increased only marginally to 26% when analyzed after anti-CD3 stimulation, once again showing that 6-h exposure to anti-CD3 did not induce significant further proliferation. In uninfected hosts, only 6% of the cells had divided during the 6-week period. When IFN-γ production was assessed following stimulation with anti-CD3, it was found that 9% of OT-I cells produced IFN-γ when transferred to infected mice, equivalent to the response of infected host CD8+ cells (8%) and more than double the percentage of OT-I cells in uninfected animals. Interestingly, while divided cells were more likely to produce IFN-γ, division was not required. Proliferating cells showed twice the rate of IFN-γ production compared with that of nonproliferating (15% versus 7%, respectively). However, 58% of the IFN-γ producers had never divided.
FIG. 7.
Proliferation and IFN-γ production by OT-I cells in M. avium-infected hosts. LN cells from OT-I mice (5 × 107 cells) were CFSE labeled and transferred to 7-week-infected (left) or uninfected (right) B6 mice. Spleen and LN cells pooled from three mice were recovered 2 weeks later and enriched for T cells. Cells were cultured with or without anti-CD3 MAb and stained for CD8 and IFN-γ. CD8+ cells were gated for analysis. (A) FACS analysis of CFSE dilution and IFN-γ production in infected (left) or uninfected (right) hosts following in vitro stimulation with anti-CD3 MAb. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host (CFSE−) or transferred (CFSE+) populations. The serial dilution of CFSE intensity was used to ascertain the cell division pattern of CD8+ OT-I cells. (B) Proportion of proliferating cells with or without stimulation by anti-CD3 MAb.
Role of T-cell depletion in proliferation.
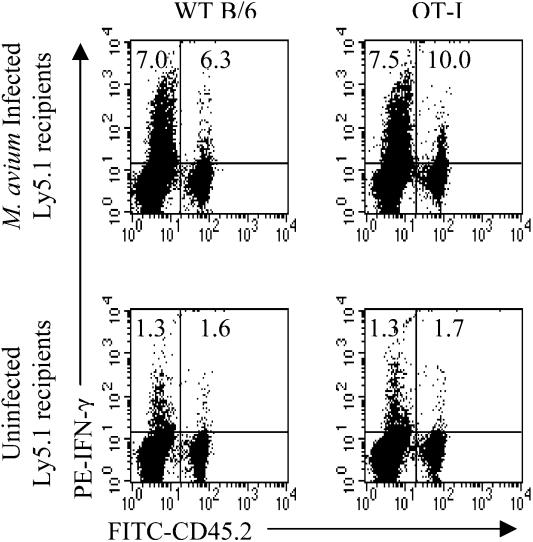
It is known that transfer of T cells into mice which have been depleted of T cells results in homeostatic proliferation and activation to produce IFN-γ upon in vitro stimulation (18). Since the behavior of T cells transferred into infected mice was reminiscent of homeostatic proliferation, the question arose as to whether the observations were an artifact of a depletion of T cells during infection. We considered this an unlikely explanation, since T cell numbers are constant up to 10 weeks postinfection with M. avium (16, 25). Nevertheless, to rule out this possibility, B6 or OT-I cells were transferred to 6-week-old B6Ly5.1 mice, half of which were infected 1 day later. At 6 weeks postinfection, the cells were nylon-wool purified and stimulated in vitro with anti-CD3 MAb for analysis of IFN-γ production. At this time, transferred OT-I cells represented 3% of all CD8+ T cells, while transferred B6 cells comprised 4% of the population, quite adequate to analyze IFN-γ production (Fig. 8). Of host CD8+ T cells, 7 to 7.5% produced IFN-γ, while 6.3% of transferred B6 cells and 10% of OT-I cells produced IFN-γ. In uninfected mice, 1.6% of transferred B6 cells and 1.7% of transferred OT-I cells produced IFN-γ. Since the host cells and the transferred cells shared the same history for the entire time of infection, it is reasonable to assume that the host population was also showing bystander activation.
FIG. 8.
Putative role of homeostatic proliferation. LN cells (5 × 107) from WT B6 or OT-I mice (both Ly5.2+) were transferred to uninfected B6Ly5.1 mice. The following day, half the mice were infected with M. avium. Six weeks later, spleen and LN cells pooled from three mice were enriched for T cells. Cells were stimulated with anti-CD3 MAb and stained for CD8, Ly5.2, and IFN-γ. CD8+ cells were gated for analysis. Numbers indicate the percentages of CD8+ cells producing IFN-γ in either the host or transferred populations. The experiment was performed twice with similar results.
IFN-γ production in vivo by CD8+ T cells.
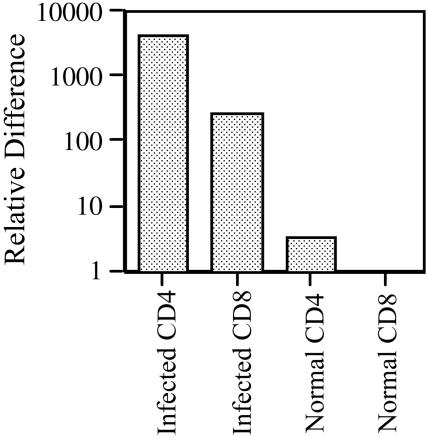
All the above observations on IFN-γ production required the in vitro restimulation of recovered T cells. It was therefore possible that only partial activation occurred in vivo, and this was completed to the point of IFN-γ production only following further stimulation with anti-CD3 MAb. Therefore, to study the in vivo relevance of this phenomenon, spleen cells were recovered from 6- to 8-week-infected mice or uninfected mice. The cells were immediately FACS sorted into CD4+ and CD8+ populations of more than 99.5% purity. Real-time PCR was used to determine the relative expression of mRNA specific for IFN-γ (Fig. 9). Normal CD8+ cells, being the lowest producers, were used to set the baseline. Normal CD4+ T cells produced marginally more IFN-γ-specific mRNA, while CD8+ T cells from infected mice produced approximately 250-fold more than background levels. This represented only 1/16th of the amount produced by CD4+ cells from the same mice. This ratio varied from 1/12 to 1/22 in three experiments, an order of magnitude higher than the 0.05% (1/200) contamination of CD4+ T cells among the sorted CD8+. It was therefore concluded that CD8+ T cells were activated in vivo, at least to the extent of proliferating and expressing IFN-γ-specific mRNA.
FIG. 9.
In vivo production of IFN-γ by CD8+ cells. Spleen cells pooled from two uninfected or 6- to 8-week-M. avium-infected mice were sorted on a MoFlo cytometer, and total RNA was extracted from 2 × 106 CD4+ and CD8+ cells. Real-time PCR was used to detect IFN-γ and 18S RNA. Results are expressed in relative terms compared with the lowest-producing group (normal CD8+ cells) given a value of 1. Results are representative of five independent experiments.
DISCUSSION
Exogenous antigens derived from bacterial infection are expected to activate CD4+ but not CD8+ T cells. Indeed, CD4+ IFN-γ-producing T cells are central to protection against experimental infection of mice with M. avium, while CD8+ T cells have no obvious role (2, 39). Nevertheless, 10 to 12% of CD8+ T cells are activated to produce IFN-γ upon in vitro restimulation with an anti-CD3 MAb (16). The present experiments strongly suggest that activation of these CD8+ T cells is nonspecific. Importantly, CD8+ T cells show an activation phenotype, proliferate in vivo in M. avium-infected mice (26), and contain significant amounts of mRNA specific for IFN-γ directly ex vivo, establishing the in vivo relevance of this model.
The basic experimental system used to demonstrate bystander activation was the transfer of TCR transgenic T cells to B6 mice harboring an established infection. Intranasal infection of B6 mice does not activate their T cells until about 5 to 6 weeks later (16, 39), presumably because it takes some time for this slow-growing organism to release enough antigen and trigger cytokines. Therefore, the transgenic cells were generally transferred at this time point. Both CD8+ OT-I cells specific for the OVA257-264 peptide and gBTI-1 cells specific for the immunodominant epitope of herpes simplex virus, gB498-505,were activated to produce IFN-γ upon transfer to infected but not uninfected mice, making a fortuitous cross-reaction between M. avium antigens and OVA an unlikely explanation for their activation. It has been suggested that TCR transgenic mice may sometimes express a second receptor (49), which could presumably recognize M. avium antigens specifically. This possibility was ruled out by testing OT-I Rag−/− cells that cannot rearrange another receptor. They also showed bystander activation. Interestingly, HY-specific cells, which recognize the male HY antigen on H-2Db, did not become activated upon transfer to infected female recipients. Unlike OT-I and gBTI-1 cells, HY cells do not undergo homeostatic proliferation in T-cell-depleted female mice (14). Homeostatic proliferation is the process where T-cell numbers outside the thymus are maintained at a constant level. It also leads to expression of IFN-γ (9).
Because this suggested that bystander activation and homeostatic proliferation may have a similar mechanism, the ability of OT-1 cells to proliferate upon transfer was tested with dilution of CFSE label as a measure (24). The OT-I cells proliferated upon transfer to infected hosts. The rate of proliferation was moderate, with 23% having divided at least once after 2 weeks. Such moderate proliferation again speaks against a fortuitous cross-reaction between OVA and M. avium epitopes, since encounter with the OVA epitope leads to rapid proliferation of virtually all the transferred OT-I cells (22). Proliferation favored but was not essential for IFN-γ production, as the majority of cells that produced IFN-γ had not divided.
Since homeostatic proliferation can lead to production of IFN-γ (9), we considered whether bystander activation in M. avium infection was actually a homeostatic response to depletion of T cells by infection. However, when OT-I cells were transferred prior to infection and were thus exposed to exactly the same sequence of stimuli as the natural host cells over the following 6 weeks, they still became activated. Furthermore, T-cell numbers do not decline in the infected mice until more than 10 weeks postinfection (16, 25), while the degree of proliferation we observed was similar to that seen in mice totally depleted of T cells (37).
Homeostatic proliferation, at least of naïve T cells, is believed to require low-affinity recognition by the TCRs of major histocompatibility complex (MHC) peptide complexes to drive that proliferation. It is the lack of this stimulus that is believed to account for the failure of HY T cells to respond upon transfer to T-cell-depleted female mice (14). The inference that TCR recognition of self peptides is required for the bystander activation during M. avium infection is reinforced by the fact that cells from CD28−/− mice did not respond on transfer to infected hosts. CD28 reacts with B7 molecules on the antigen-presenting cell to provide costimulation of the TCR. Interestingly, it is believed not to be involved in at least some types of homeostatic proliferation (37), although our own unpublished data indicate a role for CD28 in recruiting CD4+ T cells into division in lymphopaenic hosts.
Two hypotheses have been suggested to explain the mechanism of bystander activation during infection. The first suggests that bystander activation is due to nonspecific effects of cytokines without involvement of TCR stimulation. IFN-α/β, which is induced by both viruses and bacteria (47), is a favored candidate (45). The failure of HY cells and CD28−/− cells to respond in our system would seem to rule out this mechanism, which should act on all cells regardless of specificity. In addition, cytokines act preferentially on CD44hi T cells (46), whereas depletion of CD44hi had no effect on the activation we observed.
Alternatively, it has been suggested that bystander activation may result from low-affinity stimulation of the TCR (13, 50). While most writers favor a low-affinity cross-reactivity between the transgenic TCR and pathogen, we would suggest the possibility that self recognition is involved, based on the failure of HY cells to respond. This would be analogous with homeostatic proliferation, which is dependent on IL-7 and/or IL-15 but also requires low-affinity recognition of self peptide-MHC complexes (42). Our data indicate that naïve phenotype cells do respond, but we have no information on the response of memory cells. The major driver of homeostatic proliferation by naïve CD8+ T cells is IL-7, while memory CD8+ T cells use either IL-7 or IL-15 (19, 42). These cytokines also enhance survival of T cells upon activation by antigen presentation to the TCR (1, 32). It is known that the infection of macrophages and antigen-presenting dendritic cells by M. tuberculosis induces the release of both IL-7 and IL-15 (12, 41). The availability of IL-7 and IL-15 is believed to limit the homeostatic proliferation of T cells and to determine the numbers of cells at equilibrium (14), so logically the availability of an excess during infection could lead to proliferation above the normal limits. We therefore hypothesize a major role for induction of IL-7 and/or IL-15 in promoting the bystander proliferation during M. avium infection by a mechanism that is analogous to that of homeostatic proliferation but is not driven by lymphopenia. It is likely that other cytokines induced by infection, including IFN-α/β and IL-12 (46), are also needed. In addition, infection is known to increase expression in vitro of costimulatory molecules such as B7 (44), although in vivo regulation of costimulatory molecule expression is probably considerably more complex. Whatever the mechanism, bystander activation leads to 10% of CD8+ T cells being able to recall IFN-γ production in vitro, the same level of activation as seen among CD4+ T cells (16). The fact that their in vivo production of IFN-γ is lower may simply reflect a relatively low level of stimulation by low-affinity cross-reactive peptides.
If indeed low-affinity recognition of self peptide-MHC complexes is involved in bystander activation, it would serve as a potential trigger for autoimmunity, which is often associated with exposure to mycobacteria (35). Even if self recognition is not a requirement, activation of such a broad range of CD8+ T cells would be very likely to include self-reactive cells. It has already been suggested that bystander activation during infection may play a role in initiation and maintenance of autoimmune disease by expanding autoreactive T cells to break tolerance (27). The potential for ubiquitous exposure to infection with M. avium from the environment, resulting in a high incidence of frank infection among susceptible individuals and the probability of subclinical infections in others, suggests the need for further investigation.
Acknowledgments
The work was supported by grant number 145753 from the National Health and Medical Research Council of Australia.
We thank Claerwen Jones, Bill Heath, and Frank Carbone for technical advice and helpful discussion.
Editor: J. D. Clements
REFERENCES
- 1.Bennett, F., D. Luxenberg, V. Ling, I. M. Wang, K. Marquette, D. Lowe, N. Khan, G. Veldman, K. A. Jacobs, V. E. Valge-Archer, M. Collins, and B. M. Carreno. 2003. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 170:711-718. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., and M. Petrofsky. 1999. Host defense against Mycobacterium avium does not have an absolute requirement for major histocompatibility complex class I-restricted T cells. Infect. Immun. 67:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beverley, P. C. 1990. Is T-cell memory maintained by crossreactive stimulation? Immunol. Today 11:203-205. [DOI] [PubMed] [Google Scholar]
- 4.Boyle, W. 1968. An extension of the 51Cr release assay for the estimation of mouse cytotoxins. Transplantation 6:761-764. [DOI] [PubMed] [Google Scholar]
- 5.Busch, D. H., and E. G. Pamer. 1999. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol. Lett. 65:93-98. [DOI] [PubMed] [Google Scholar]
- 6.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne, J. A., J. L. Butler, and M. D. Cooper. 1988. Differential activation requirements for virgin and memory T cells. J. Immunol. 141:3249-3257. [PubMed] [Google Scholar]
- 8.Cherwinski, H. M., G. T. Semenuk, and J. T. Ransom. 1992. Stimulation of a T helper cell class 2 clone with immobilized anti-T cell receptor antibody activates a Ca2+ and protein kinase C- independent lethal signaling pathway. J. Immunol. 148:2996-3003. [PubMed] [Google Scholar]
- 9.Cho, B. K., V. P. Rao, Q. Ge, H. N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, S. R., M. Barnden, C. Kurts, F. R. Carbone, J. F. Miller, and W. R. Heath. 2000. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78:110-117. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande, S., M. Zheng, S. Lee, K. Banerjee, S. Gangappa, U. Kumaraguru, and B. T. Rouse. 2001. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J. Immunol. 167:2902-2910. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735-741. [PubMed] [Google Scholar]
- 13.Ehl, S., J. Hombach, P. Aichele, H. Hengartner, and R. M. Zinkernagel. 1997. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J. Exp. Med. 185:1241-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst, B., D. S. Lee, J. M. Chang, J. Sprent, and C. D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11:173-181. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I restricted T cells are required for immunity to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbertson, B., J. Zhong, and C. Cheers. 1999. Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with mycobacterium avium. J. Immunol. 163:2073-2080. [PubMed] [Google Scholar]
- 17.Goldrath, A. W., and M. J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldrath, A. W., L. Y. Bogatzki, and M. J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldrath, A. W., P. V. Sivakumar, M. Glaccum, M. K. Kennedy, M. J. Bevan, C. Benoist, D. Mathis, and E. A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julius, M. H., E. Simpson, and L. A. Herzenberg. 1973. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Immunol. 3:645-649. [DOI] [PubMed] [Google Scholar]
- 21.Jung, T., U. Schauer, C. Heusser, C. Neumann, and C. Rieger. 1993. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 159:197-207. [DOI] [PubMed] [Google Scholar]
- 22.Kurts, C., F. R. Carbone, M. Barnden, E. Blanas, J. Allison, W. R. Heath, and J. F. Miller. 1997. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186:2057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 24.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 25.Mannering, S. I., and C. Cheers. 2002. Interleukin-2 and loss of immunity in experimental Mycobacterium avium infection. Infect. Immun. 70:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannering, S. I., J. Zhong, and C. Cheers. 2002. T-cell activation, proliferation and apoptosis in primary Listeria monocytogenes infection. Immunology 106:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller, D. L., and M. K. Jenkins. 1997. Autoimmunity: when self-tolerance breaks down. Curr. Biol. 7:R255-R257. [DOI] [PubMed] [Google Scholar]
- 28.Mueller, S. N., W. Heath, J. D. McLain, F. R. Carbone, and C. M. Jones. 2002. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 80:156-163. [DOI] [PubMed] [Google Scholar]
- 29.Muller, I., S. B. Cobbold, H. Waldmann, and S. H. E. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4 and Lyt-2+ T cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 31.Nahill, S. R., and R. M. Welsh. 1993. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J. Exp. Med. 177:317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niedbala, W., X. Wei, and F. Y. Liew. 2002. IL-15 induces type 1 and type 2 CD4+ and CD8+ T cells proliferation but is unable to drive cytokine production in the absence of TCR activation or IL-12/IL-4 stimulation in vitro. Eur. J. Immunol. 32:341-347. [DOI] [PubMed] [Google Scholar]
- 33.Nightingale, S. D., L. T. Byrd, P. M. Southern, J. D. Jockusch, S. X. Cal, and B. A. Wynne. 1992. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J. Infect. Dis. 165:1082-1085. [DOI] [PubMed] [Google Scholar]
- 34.Orme, I. M., S. K. Furney, and A. D. Roberts. 1992. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retroviruses. Infect. Immun. 60:4747-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottenhoff, T. H., and R. R. de Vries. 1990. Antigen reactivity and autoreactivity: two sides of the cellular immune response induced by mycobacteria. Curr. Topics Microbiol. Immunol. 155:111-121. [DOI] [PubMed] [Google Scholar]
- 36.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 37.Prlic, M., B. R. Blazar, A. Khoruts, T. Zell, and S. C. Jameson. 2001. Homeostatic expansion occurs independently of costimulatory signals. J. Immunol. 167:5664-5668. [DOI] [PubMed] [Google Scholar]
- 38.Raupach, B., and S. H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 39.Saunders, B. M., and C. Cheers. 1995. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T cell subsets and gamma interferon. Infect. Immun. 63:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 41.Sieling, P. A., L. Sakimura, K. Uyemura, M. Yamamura, J. Oliveros, B. J. Nickoloff, T. H. Rea, and R. L. Modlin. 1995. IL-7 in the cell-mediated immune response to a human pathogen. J. Immunol. 154:2775-2783. [PubMed] [Google Scholar]
- 42.Tan, J. T., B. Ernst, W. C. Kieper, E. LeRoy, J. Sprent, and C. D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teh, H. S., H. Kishi, B. Scott, and H. Von Boehmer. 1989. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J. Exp. Med. 169:795-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurnher, M., R. Ramoner, G. Gastl, C. Radmayr, G. Bock, M. Herold, H. Klocker, and G. Bartsch. 1997. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J. Cancer 70:128-134. [DOI] [PubMed] [Google Scholar]
- 45.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 46.Tough, D. F., S. Sun, X. Zhang, and J. Sprent. 1999. Stimulation of naive and memory T cells by cytokines. Immunol. Rev. 170:39-47. [DOI] [PubMed] [Google Scholar]
- 47.Wood, P. R., A. M. Young, J. L. McKimm-Breschkin, and C. Cheers. 1984. Effect of splenectomy on production of interferon and colony-stimulating factor in Listeria monocytogenes-infected mice. Infect. Immun. 46:860-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yajima, T., H. Nishimura, R. Ishimitsu, T. Watase, D. H. Busch, E. G. Pamer, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J. Immunol. 168:1198-1203. [DOI] [PubMed] [Google Scholar]
- 49.Zal, T., S. Weiss, A. Mellor, and B. Stockinger. 1996. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc. Natl. Acad. Sci. USA 93:9102-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarozinski, C. C., and R. M. Welsh. 1997. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J. Exp. Med. 185:1629-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]