Abstract
Rationale: There is limited evidence of the effect of exposure to heat on chronic obstructive pulmonary disease (COPD) morbidity, and the interactive effect between indoor heat and air pollution has not been established.
Objectives: To determine the effect of indoor and outdoor heat exposure on COPD morbidity and to determine whether air pollution concentrations modify the effect of temperature.
Methods: Sixty-nine participants with COPD were enrolled in a longitudinal cohort study, and data from the 601 participant days that occurred during the warm weather season were included in the analysis. Participants completed home environmental monitoring with measurement of temperature, relative humidity, and indoor air pollutants and simultaneous daily assessment of respiratory health with questionnaires and portable spirometry.
Measurements and Main Results: Participants had moderate to severe COPD and spent the majority of their time indoors. Increases in maximal indoor temperature were associated with worsening of daily Breathlessness, Cough, and Sputum Scale scores and increases in rescue inhaler use. The effect was detected on the same day and lags of 1 and 2 days. The detrimental effect of temperature on these outcomes increased with higher concentrations of indoor fine particulate matter and nitrogen dioxide (P < 0.05 for interaction terms). On days during which participants went outdoors, increases in maximal daily outdoor temperature were associated with increases in Breathlessness, Cough, and Sputum Scale scores after adjusting for outdoor pollution concentrations.
Conclusions: For patients with COPD who spend the majority of their time indoors, indoor heat exposure during the warmer months represents a modifiable environmental exposure that may contribute to respiratory morbidity. In the context of climate change, adaptive strategies that include optimization of indoor environmental conditions are needed to protect this high-risk group from the adverse health effects of heat.
Keywords: chronic obstructive pulmonary disease, particulate matter, nitrogen dioxide, climate change, heat
Understanding the health effects of climate change has been identified as a research priority by the American Thoracic Society and other leading health organizations (1–7). The anticipated increases in temperature represent one aspect of climate change that has been associated with adverse health consequences. Global average temperatures are projected to increase by 1.4–5.8% by the end of the century, and heat waves are projected to be more frequent, intense, and longer lasting (8). It is critical to understand the health implications of heat exposure to protect those at greatest risk (9).
Previous population-level studies have demonstrated that heat waves are associated with increases in mortality (10, 11) and that certain populations, including those with underlying respiratory and cardiac disease, are likely to be at increased risk (12, 13). Studies investigating the impact of heat on morbidity using hospitalization and emergency visit records have also identified high-risk subgroups (14–17). To date, disease-specific indicators of morbidity have not been assessed, and studies have rarely used individual-level exposure assessment (18). Few studies have investigated the effect of indoor temperature on respiratory health, and the interactive effect between indoor temperature and indoor air pollution is unknown. It is important to understand the effects of the indoor environment because individuals spend the majority of their time indoors and this is projected to increase in the context of climate change (8, 19). Furthermore, actions such as using air conditioning, cooling centers, and energy-efficient building designs can reduce indoor heat exposure, and these can be deployed immediately at the individual and local levels.
To develop strategies to protect individuals from the adverse consequences of heat exposure, it is necessary to improve our understanding of the specific health consequences among high-risk groups. In this study, we sought to understand the health effects of heat among individuals with chronic obstructive pulmonary disease (COPD), using disease-specific respiratory health outcomes. We hypothesized that (1) increases in indoor and outdoor temperature during the warmer months would be associated with increases in daily respiratory symptoms and rescue medication use and decreases in lung function among participants with COPD, and (2) increases in air pollution exposure would modify the effects of temperature, enhancing the detrimental effects of increases in temperature on these daily indicators of COPD morbidity.
Methods
Participant Recruitment and Study Design
Participants provided written informed consent, and the Johns Hopkins Medical Institutional Review Board approved the protocol. Participants and methods have been described previously (20). Briefly, participants were former smokers with COPD who were recruited from the Baltimore area and studied at baseline and at 3 and 6 months as part of the COPD and Domestic Endotoxin Study. To determine the health effects of heat, we restricted analysis to the warm weather season, defined as the time between the first and last day that the maximal outdoor temperature exceeded 90°F in Baltimore for each calendar year. Sixty-nine of 84 participants were monitored during this warm weather season. Fifty-four were monitored for 1 week, and 15 were monitored for 2 weeks. Participants completed health and demographic questionnaires, and spirometry was performed according to American Thoracic Society criteria (21, 22).
Environmental Monitoring of Heat and Air Quality
A home environmental assessment was completed; this included a home inspection and continuous environmental monitoring over a 1-week period to capture daily indoor temperature and humidity and weekly particulate matter (PM) and nitrogen dioxide (NO2). Participants completed a daily activity diary during the environmental monitoring period. Air sampling occurred in the main living area, identified as a room other than the bedroom where the participant reported spending the most time. Additional methods are provided in the online supplement and in a prior publication (20). Outdoor temperature, humidity, and pollution concentrations (PM, NO2, and ozone) were obtained from publicly available data sets provided by the National Oceanic and Atmospheric Administration and the Environmental Protection Agency (see Table E1 in the online supplement) (23, 24).
COPD Daily Respiratory Health Outcomes
Participants completed daily questionnaires and performed spirometry during home environmental monitoring. The validated Breathlessness, Cough, and Sputum Scale (BCSS) contains three questions, each of which assesses a symptom using a Likert-type scale ranging from 0 to 4. A change in total score of 0.3–0.4 is considered mild, whereas a change of 1.0 is considered substantial (25). Handheld spirometry was also performed daily (PiKo-1; nSpire Health, Inc., Longmont, CO). Frequency of rescue inhaler medication use was captured in a daily diary as zero, one, two, three, or four or more times daily.
Statistical Analysis
Descriptive statistics were analyzed using Spearman correlations, chi-square tests, and t tests. At each time point, daily maximal temperature was used as the primary exposure variable in generalized estimating equation models to account for repeated measures. Models for indoor and outdoor temperature were run separately and were adjusted for age, sex, education, visit (baseline or 3 or 6 mo), and baseline percent predicted FEV1. Pack-years of smoking were used to account for disease severity for models in which the primary outcome was lung function.
Models were constructed to account for pollutant concentrations; models of indoor temperature included indoor daily average humidity and weekly average indoor PM2.5 and NO2. Models of outdoor temperature included daily average outdoor humidity, PM2.5, NO2, and ozone. Lag terms were created to assess same-day and subsequent-day health effects. To assess effect modification, interaction terms were created between pollution and temperature variables. To illustrate the temperature effects at given pollution concentrations, models were used to calculate the outcomes of interest for pollutant concentrations at the 25th, 50th, and 75th percentiles, using average or mode values for other variables.
Interaction terms and stratified models were created to investigate whether time spent outdoors modified the effect of outdoor temperature on COPD. Sensitivity analyses were performed using the 95th percentile values of temperature, rather than the maximal values, and excluding extreme outliers. Analyses were performed with Stata SE statistical software, version 11.0 (Stata Corp, College Station, TX). Statistical significance was defined as a P value <0.05.
Results
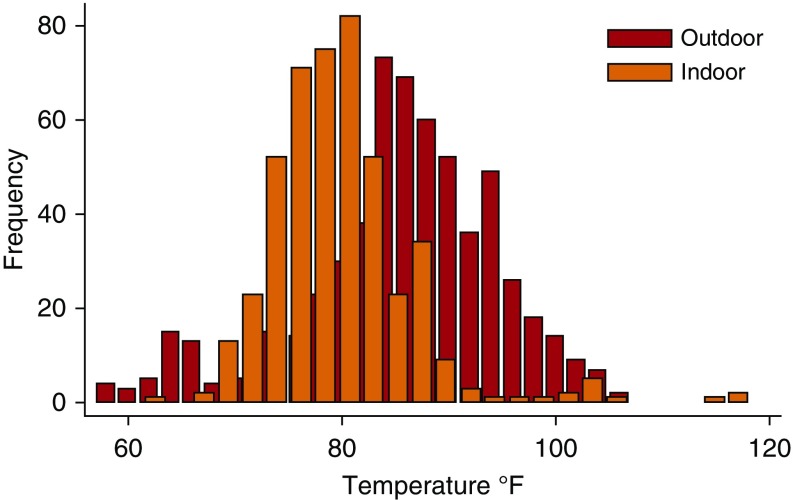
Study participants were older individuals with moderate or severe COPD and impaired pulmonary function (Table 1). Between 2009 and 2011, there were 601 participant study days in the warm weather season. Participants spent a substantial amount of time indoors and only went outdoors on 46% of these days. On the days during which participants went outdoors, the mean (SD) time outdoors was 2.0 (2.1) hours. The mean (SD) daily maximal indoor and outdoor temperatures were 80 (7)°F and 85(9)°F, respectively (Figure 1). There was only moderate correlation between daily indoor and outdoor maximal temperatures (Spearman’s ρ = 0.44, P < 0.01).
Table 1.
Participant characteristics (n = 69)
| Age, yr | 69 (8) |
| Sex, % male | 57 |
| White race, % | 90 |
| Smoking, pack-years | 56 (30) |
| Baseline post-bronchodilator FEV1 % predicted | 54 (16) |
| GOLD stage, % | |
| II | 48 |
| III | 40 |
| IV | 12 |
| Daily health assessment average values | |
| BCSS score | 2.7 (2.2) |
| Inhaler use, puffs/d | 0.88 (1.3) |
| Morning FEV1, L | 1.3 (0.6) |
| Evening FEV1, L | 1.3 (0.6) |
| Environmental characteristics (n = 601 study days) | |
| Daily maximal indoor temperature, °F | 80.1 (6.7) |
| Daily average indoor relative humidity, % | 40.8 (8.5) |
| Weekly average indoor PM2.5, μg/m3 | 13.1 (15.4) |
| Weekly average indoor NO2, ppb | 11.3 (11.7) |
| Days of reported central air conditioning use, % | 50 |
| Days of reported window-unit air conditioning use, % | 18 |
| Days reported with no air conditioning use, % | 37 |
| Days that participants went outdoors, % | 47 |
| Time outdoors on days that participants went outdoors, h | 1.95 (2.07) |
| Daily maximal outdoor temperature, °F | 84.9 (9.9) |
| Daily average outdoor PM2.5, μg/m3 | 12.8 (6.3) |
| Daily average outdoor NO2, ppb | 27.5 (11.1) |
| Daily average outdoor ozone, ppb | 35.2 (8.7) |
| Daily average outdoor relative humidity, % | 58.3 (13.1) |
Definition of abbreviations: BCSS = Breathlessness, Cough, and Sputum Scale; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PM = particulate matter.
Data are presented as mean (SD) unless otherwise indicated.
Figure 1.
Distribution of daily indoor and outdoor maximal temperatures during the warm weather season. Participants spent most of their time indoors; they went outside on only 47% of study days and spent about 2 hours outdoors on those days. Maximal daily indoor temperatures averaged 80°F, whereas maximal daily outdoor temperature averaged 85°F.
Eighty-five percent of participants had either central air conditioning or window units. Central air conditioning was reported to have been used on 50% of the study days, and window air conditioning unit use was reported to have been used on 18% of the study days. Participants reported that they did not use air conditioning at all on 37% of the study days that occurred during the warm season. The maximal indoor temperature was lower on days on which participants reported using central air conditioning compared with days on which they did not (mean [SD], 81.4 [6.4] vs. 79.0 [7.0]°F, respectively, P < 0.01)
Effect of Indoor Temperature on COPD Morbidity
Increases in maximal daily indoor temperature were associated with increases in symptoms as measured using the BCSS, and with increases in the frequency of rescue inhaler use. These associations persisted even after accounting for indoor pollutant concentrations, including indoor NO2 and indoor PM2.5. For example, a 10°F increase in indoor temperature was associated with a 0.38 increase in BCSS score (95% CI, 0.01–0.67; P = 0.01), even after adjustment for indoor relative humidity, PM2.5, and NO2. Inclusion of lag terms in these models suggested that increases in indoor temperatures had an immediate (same-day) effect, as well as an effect that was detectable 1–2 days after exposure.
We found no detectable effect of daily changes in indoor temperature on daily measurements of lung function, using either morning or evening FEV1 (evening values are shown in Table 2). Sensitivity analyses conducted excluding extreme temperature outliers (>110°F) and using the 95th percentile value of daily indoor temperature yielded similar results (Table E2). An evaluation of interaction terms and stratified models suggested an enhanced effect of indoor temperature on the use of rescue inhalers among participants who had more advanced COPD (coefficient 0.27, P = 0.06 for FEV1 ≤50% predicted; coefficient 0.01, P = 0.816 among those with FEV1 ≥50% predicted; P for interaction = 0.048) but did not suggest that disease severity influenced the effect of indoor heat on symptoms. Stratified models also suggested an enhanced effect of indoor temperature on symptoms and rescue inhaler use on days during which participants did not go outdoors (Table E3).
Table 2.
Association between indoor temperature and COPD symptoms, rescue medication use, and lung function
| Outcome | Coefficient* | 95% Confidence Interval | P Value |
|---|---|---|---|
| Breathlessness, Cough, and Sputum Scale | |||
| Daily temperature (limited model)† | 0.30 | 0.00–0.59 | 0.048 |
| Daily temperature (with humidity, NO2, PM2.5) | 0.38 | 0.01–0.67 | 0.013 |
| Lag 0 | 0.30 | 0.00–0.59 | 0.048 |
| Lag 1 | 0.36 | 0.01–0.70 | 0.042 |
| Lag 2 | 0.48 | 0.12–0.85 | 0.010 |
| Lag 3 | 0.10 | −0.27–0.47 | 0.602 |
| Rescue inhaler use | |||
| Daily temperature (limited model)† | 0.26 | 0.09–0.42 | 0.002 |
| Daily temperature (with humidity, NO2, PM2.5) | 0.23 | 0.06–0.41 | 0.008 |
| Lag 0 | 0.26 | 0.09–0.42 | 0.002 |
| Lag 1 | 0.17 | -0.02–0.36 | 0.077 |
| Lag 2 | 0.21 | -0.01–0.42 | 0.058 |
| Lag 3 | −0.02 | −0.24–0.20 | 0.845 |
| Lung function (evening FEV1) | |||
| Daily temperature (limited model)† | −0.02 | −0.05–0.02 | 0.419 |
| Daily temperature (with humidity, NO2, PM2.5) | −0.01 | −0.05–0.02 | 0.439 |
| Lag 0 | −0.02 | −0.05–0.02 | 0.419 |
| Lag 1 | −0.01 | −0.04–0.03 | 0.610 |
| Lag 2 | −0.01 | −0.05–0.02 | 0.418 |
| Lag 3 | −0.02 | −0.06–0.02 | 0.262 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PM = particulate matter.
Models include visit, age, sex, education, baseline FEV1. (Number of pack-years was used instead of baseline FEV1 in the lung function models).
Changes are expressed per 10°F increase in indoor temperature.
Interactive Effect of Indoor Pollution and Indoor Temperature
In models investigating the effect of indoor temperature on BCSS and on rescue inhaler use, significant positive interactions were detected between PM2.5 and temperature (interaction term P < 0.001 in both models). Similarly, significant positive interactions were detected between indoor NO2 and temperature (interaction term P < 0.05 in the BCSS model and <0.001 in the rescue inhaler model).
To illustrate the positive interactive effect, Table 3 provides estimates of the effect of a 10°F increase in temperature at given percentiles of indoor pollutant concentrations and demonstrates that the effect of temperature is greater with increasing indoor pollutant concentrations. For example, a participant residing in a home that had an indoor PM2.5 concentration at the 25th percentile of the study homes (5 μg/m3) would experience an increase of 0.4 in BCSS score (indicative of a mild increase in symptoms) for every 10°F increase in indoor temperature, whereas an individual in a home at the 75th percentile (16 μg/m3) would experience an increase of 1 in BCSS score (indicative of a severe increase in symptoms) (25). Models investigating lung function as the outcome did not demonstrate an interactive effect between indoor temperature and pollutants.
Table 3.
Illustration of the interactive effects between indoor temperature and pollutants
| Percentile of Indoor Air Pollutant Concentrations | Estimated Effect of 10°F Increase in Temperature at Increasing Indoor PM2.5 Concentrations |
Estimated Effect of 10°F Increase in Temperature at Increasing Indoor NO2 Concentrations |
||
|---|---|---|---|---|
| BCSS Score | Rescue Inhaler | BCSS Score | Rescue Inhaler | |
| 25 | 0.36 | 0.25 | 0.12 | 0.05 |
| PM2.5 4.99 μg/m3 | ||||
| NO2 4.37 ppb | ||||
| 50 | 0.54 | 0.42 | 0.20 | 0.13 |
| PM2.5 8.24 μg/m3 | ||||
| NO2 6.84 ppb | ||||
| 75 | 0.98 | 0.85 | 0.41 | 0.34 |
| PM2.5 16.20 μg/m3 | ||||
| NO2 13.00 ppb | ||||
| 95 | 2.21 | 2.02 | 1.12 | 1.08 |
| PM2.5 38.36 μg/m3 | ||||
| NO2 34.58 ppb | ||||
Definition of abbreviations: BCSS = Breathlessness, Cough, and Sputum Scale; PM = particulate matter.
The estimated effect of a 10°F increase in temperature becomes larger with increasing indoor pollutant concentrations (NO2 and PM2.5), demonstrated for given percentiles. Models used to predict estimated effect included age, sex, education, baseline FEV1, indoor humidity, and either indoor PM2.5 or indoor NO2.
Effect of Outdoor Temperature on COPD Morbidity
Daily maximal outdoor temperature was not significantly associated with respiratory symptoms, rescue medication use, or lung function in the overall cohort (Table 4). Because participants reported going outdoors on fewer than one-half of the study days in the warm weather season, we performed analyses stratified by days on which participants reported going outdoors and assessed for interaction by time outdoors. Stratified models suggested a significant association between increasing outdoor temperature and respiratory symptoms on days during which participants went outdoors. There were no statistically significant interactions between outdoor temperature and outdoor air pollutants, including PM2.5, NO2, and ozone (data not shown).
Table 4.
Daily maximal outdoor temperature and COPD health outcomes stratified by whether the participant went outdoors on a given day
| Outcomes | Overall (n = 485) |
Days Participants Stayed Indoors (n = 250) |
Days Participants Went Outdoors (n = 229) |
P Value for Interaction | |||
|---|---|---|---|---|---|---|---|
| Coefficient (95% Confidence Interval) | P Value | Coefficient (95% Confidence Interval) | P Value | Coefficient (95% Confidence Interval) | P Value | ||
| BCSS score | 0.13 (−0.06–0.32) | 0.18 | 0.00 (−0.29–0.29) | 0.985 | 0.38 (0.122–0.628) | 0.004 | 0.045 |
| Rescue inhaler | 0.01 (−0.09–0.10) | 0.912 | 0.01 (−0.14–0.16) | 0.899 | 0.03 (−0.10–0.17) | 0.619 | 0.554 |
| Evening FEV1* | 0.00 (−0.02–0.03) | 0.830 | 0.01 (−0.03–0.04) | 0.749 | −0.01 (−0.06–0.03) | 0.534 | 0.477 |
Definition of abbreviations: BCSS = Breathlessness, Cough, and Sputum Scale; COPD = chronic obstructive pulmonary disease; PM = particulate matter.
Multivariate analysis adjusted for age, sex, education, baseline lung function or smoking history, visit, outdoor PM2.5, outdoor NO2, outdoor ozone, and outdoor relative humidity.
n = 479 for overall, 187 for days indoors, and 211 for days participants went outdoors for evening FEV1.
Discussion
This study is among the first to describe the effect of heat exposure on disease-specific morbidity outcomes in those with COPD, a group that has been identified as at a high risk of the detrimental health effects of heat, and, to the best of our knowledge, it is the first to report an interactive effect between indoor temperature and indoor pollution.
In a cohort of participants with moderate to severe COPD, we found that increases in home indoor temperature during warmer weather were associated with increases in daily indicators of COPD morbidity, including respiratory symptoms and rescue inhaler medication use. There was a positive interaction between temperature and indoor air pollution, including PM2.5 and NO2, such that the effect of indoor heat was greater in the presence of higher indoor air pollutant concentrations.
In this study population, participants spent a great deal of time indoors and ventured outdoors on only one-half of the study days. Outdoor temperature was associated with increased respiratory symptoms on these days. In the context of the anticipated increase in temperatures related to climate change, these findings suggest that adaptive strategies targeting the indoor environment provide an opportunity to minimize the health risks for those with COPD.
Our results are consistent with and extend previous findings that have demonstrated adverse health consequences of heat exposure. Previous studies have largely used ambient data to assign exposure and have linked this to population health effects with compelling results. Such studies have identified elderly individuals and those with underlying cardiac and respiratory diseases, including COPD, as at an increased risk of adverse health effects of heat exposure (12, 15, 26–29).
For example, a time series study across 12 cities in the United States demonstrated increases in deaths attributable to COPD during hot weather, with differences between hot and cold cities. In cold cities, hot temperatures were associated with an increase in the risk of death attributable to COPD by as much as 25% (12) with immediate, same-day effects. In hot cities, the effect of hot temperatures was attenuated and delayed, with a 6% increase in COPD deaths at lags of 3 and 4 days.
A study in New York City examined COPD morbidity using hospitalization data and found that the same-day risk of COPD hospitalization increased by 7.6% for every one-degree Celsius increase above a threshold temperature of 29°C (17), and that there was a detectable but smaller association between temperature and respiratory hospitalization when applying a 1-day lag.
Other studies have used Medicare data to provide estimates of effect that are representative of a broader portion of the population of the United States (15, 30). In a study that included 12.5 million elderly individuals in 213 urban counties in the United States, there was a 4.7% increased risk of hospitalization for COPD for every 10°F increase in ambient temperature, and the findings were not attributable to air pollution health effects (15). Heat and respiratory hospitalizations were associated most strongly on the day of exposure in this study; the effect was still present and significant at a lag of 1 day and no longer detectable at 2 days. The ecologic design of such studies and the potential for bias caused by measurement error in exposure assignment have been limitations; these limitations are addressed in this study, which provides individual-level data.
Researchers who previously investigated the interactive effect of temperature and air pollution have reported mixed results, with little evidence for differences in COPD outcomes. For example, in a study conducted in Brisbane, Australia, PM10 modified the effect of temperature on respiratory hospital admissions, but there was no interactive effect for respiratory emergency visits (31). Basu and colleagues examined temperature and mortality in California and did not find that pollution modified the effect of temperature (32). In a study of nine European cities included in the EuroHEAT project, the effect of heat on overall mortality and cardiovascular mortality was increased on high-PM10 and high-ozone days, but the interactive effect of these pollutants and heat on respiratory mortality was less evident (33).
Ren and colleagues (31) analyzed data from 98 urban communities in the U.S. National Morbidity, Mortality, and Air Pollution Study and found that ozone modified the risk of cardiovascular mortality. However, their results did not include respiratory mortality as an outcome measure. Zanobetti and Schwartz studied nine cities in the United States during the warm season and did not find evidence that outdoor PM2.5 or ozone modified the relationship between increasing outdoor temperature and risk of death (34).
In the current study, we did not find an interaction between the effects of outdoor temperature and ozone or PM. However, there was a consistent signal demonstrating that increases in indoor pollution enhanced the adverse effect of increasing indoor temperature on COPD symptoms and rescue medication use. To our knowledge, this is the first study to report interactive effects of indoor temperature and air pollution on COPD morbidity.
Previous studies linking outdoor heat with hospitalization or death have demonstrated same-day health effects of heat exposure or lag times of 1 day (15). Our findings suggest the health effect of indoor heat exposure is immediate and may persist for 1 to 2 days. Although the mechanisms of health effects in COPD remain incompletely understood, proposed mechanisms include both thermoregulatory responses (35) and bronchoconstrictive effects of heat.
Patients with COPD may have an impaired ability to respond to heat stress. It has been proposed that heatstroke leads to increases in intravascular coagulation caused by the release of IL-1 or IL-6 into the systemic circulation with activation of microvascular thrombosis, which may trigger a respiratory distress syndrome (36, 37). Studies in asthma suggest that breathing hot humid air may result in bronchoconstriction and increased airways resistance that is mediated via cholinergic pathways (38, 39). We did not detect an association between indoor heat and FEV1. This suggests either that bronchoconstriction did not affect the airways resistance captured by the FEV1 outcome measurement or that bronchoconstriction was not the mechanism by which participants were affected by the heat.
Limitations
Because this study was limited to the Baltimore region, the results may not be generalizable to other areas of the country. We used education as an indicator of socioeconomic status because household income was not reflective of socioeconomic status in our largely retired population, and this approach may have resulted in some misclassification of socioeconomic status. Indoor air monitoring did not include ozone because previous studies in Baltimore city have documented that this exposure is typically low in homes (40).
Although each participant provided comprehensive and daily characterization of the indoor environment and COPD health outcomes, outdoor measurements of temperature and pollutant concentrations were obtained from central site monitors, which may have resulted in measurement error in assigning outdoor exposure to each individual. This may have contributed to the weaker associations between outdoor temperature and COPD morbidity, although the daily activity diary data would suggest that the short amount of time spent outdoors was also a key contributing factor.
Conclusions
The findings of this study suggest that additional indoor cooling may improve COPD respiratory health during the warmer months and that consideration should be given when traveling outdoors in warmer weather. Furthermore, our finding that increases in indoor air pollution exaggerated the adverse health effects of indoor heat exposure highlights the opportunity to improve COPD health through optimization of the indoor environment. The participants with COPD in this study spent the overwhelming majority of their time indoors during warm weather days.
Although 86% of the participants had some form of air conditioning available, air conditioning was not used on 37% of the study days. We did not find that education level was associated with the use of air conditioning; however, we were limited in our ability to understand the extent to which the use of air conditioning was influenced by financial hardship.
Addressing barriers to air conditioning use may have potential health benefits for those with COPD, and future studies may be needed to fully elucidate the impact of air conditioning use on respiratory health in COPD. However, although air conditioning may provide a short-term solution for this high-risk group (41), it is not without consequences. The use of air conditioning ultimately contributes to the cycle that perpetuates climate change and the steady rise in outdoor temperatures (42). Furthermore, the associated costs are problematic for disadvantaged populations in the United States and around the world.
Ultimately, in addition to adaptive strategies at the individual and population level, mitigation strategies are needed at a policy level to intercept the alarming rise in outdoor temperatures. To effectively address this and to provide the foundation for such policy will require the interdisciplinary work of urban planners, public health officials, engineers, economists, and climate scientists, in addition to medical practitioners.
Acknowledgments
Acknowledgment
The authors thank the study participants and the study staff for their contributions.
Footnotes
Supported by the National Institutes of Health (NIH)–National Institute of Environmental Health Science (NIEHS) (R21ES024021 [M.C.M.], R21ES015781 [N.N.H.], R21ES025840 [M.C.M.], R01 ES022607 [N.N.H.], R01ES019560 [R.D.P.], and R01 ES023500 [N.N.H.]), the NIH-National Institute of Allergy and Infectious Diseases (NIAID) (K24AI114769 [E.C.M.]), the NIEHS/Environmental Protection Agency (P50ES015903/RD83213901 [E.C.M. and G.B.D] and P01ES018176/RD83451001 [M.C.M., R.D.P., and N.N.H.]), the NIH-National Institute on Minority and Health Disparities (NIMHD) (P50 MD010431/RD83615201 [M.C.M., R.D.P., and N.N.H.]), and a Johns Hopkins Environment, Energy, Sustainability, and Health Institutes Faculty Award (M.C.M.).
The opinions expressed in this article are the authors’ own and do not reflect the views of the Centers for Disease Control, the Department of Health and Human Services, or the U.S. Government.
Author Contributions: M.C.M., G.B.D., P.N.B., E.C.M., and N.N.H. provided substantial contributions to the conception and design, acquisition of data, and analysis and interpretation of data; A.J.B., C.M.A., E.C.M., L.P., D.W., A.S., and R.D.P. contributed to the data analysis and interpretation of data; D.L.W. contributed to data acquisition; and all authors contributed to revising the manuscript critically for important intellectual content and provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Portier CJ, Thigpen Tart K, Carter SR, Dilworth CH, Grambsch AE, Gohlke J, Hess J, Howard SN, Luber G, Lutz JT, et al. A human health perspective on climate change: a report outlining the research needs on the human health effects of climate change. Journal of Current Issues in Globalization. 2013;6(4):621. [Google Scholar]
- 2.Huang C, Street R, Chu C. Adapting to climate change. JAMA. 2015;313:727. doi: 10.1001/jama.2014.18502. [DOI] [PubMed] [Google Scholar]
- 3.Pinkerton KE, Rom WN, Akpinar-Elci M, Balmes JR, Bayram H, Brandli O, Hollingsworth JW, Kinney PL, Margolis HG, Martin WJ, et al. American Thoracic Society Environmental Health Policy Committee. An official American Thoracic Society workshop report: climate change and human health. Proc Am Thorac Soc. 2012;9:3–8. doi: 10.1513/pats.201201-015ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Atlas of health and climate Geneva, Switzerland: World Health Organization; 2012[accessed 18 Jan 2016]. Available from: http://www.who.int/globalchange/publications/atlas/report/en/ [Google Scholar]
- 5.Bernstein AS, Rice MB. Lungs in a warming world: climate change and respiratory health. Chest. 2013;143:1455–1459. doi: 10.1378/chest.12-2384. [DOI] [PubMed] [Google Scholar]
- 6.Crowley RA Health, Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians. Ann Intern Med. 2016;164:608–610. doi: 10.7326/M15-2766. [DOI] [PubMed] [Google Scholar]
- 7.Patz JA, Frumkin H, Holloway T, Vimont DJ, Haines A. Climate change: challenges and opportunities for global health. JAMA. 2014;312:1565–1580. doi: 10.1001/jama.2014.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 9.Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health. 2008;29:41–55. doi: 10.1146/annurev.publhealth.29.020907.090843. [DOI] [PubMed] [Google Scholar]
- 10.Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, Michel JP, Herrmann FR. Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol. 2008;331:171–178. doi: 10.1016/j.crvi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Whitman S, Good G, Donoghue ER, Benbow N, Shou W, Mou S. Mortality in Chicago attributed to the July 1995 heat wave. Am J Public Health. 1997;87:1515–1518. doi: 10.2105/ajph.87.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002;110:859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner LR, Barnett AG, Connell D, Tong S. Ambient temperature and cardiorespiratory morbidity: a systematic review and meta-analysis. Epidemiology. 2012;23:594–606. doi: 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GB, Dominici F, Wang Y, McCormack MC, Bell ML, Peng RD. Heat-related emergency hospitalizations for respiratory diseases in the Medicare population. Am J Respir Crit Care Med. 2013;187:1098–1103. doi: 10.1164/rccm.201211-1969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, Biggeri A, Anderson HR, Katsouyanni K, Ballester F, et al. PHEWE Collaborative Group. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med. 2009;179:383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 17.Lin S, Luo M, Walker RJ, Liu X, Hwang SA, Chinery R. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology. 2009;20:738–746. doi: 10.1097/EDE.0b013e3181ad5522. [DOI] [PubMed] [Google Scholar]
- 18.Basu R, Samet JM. An exposure assessment study of ambient heat exposure in an elderly population in Baltimore, Maryland. Environ Health Perspect. 2002;110:1219–1224. doi: 10.1289/ehp.021101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. Climate change, the indoor environment, and health (2011) Washington, DC: The National Academies Press; 2015. [Google Scholar]
- 20.Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, Paulin L, Williams DL, Diette GB, Breysse PN. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.National Oceanic and Atmospheric Administration National Centers for Environmental Information2016 [accessed 18 Jan 2106]. Available from: http://www.nodc.noaa.gov/General/temperature.html
- 24.United States Environmental Protecation Agency Air Quality System database 2016[accessed 18 Jan 2016]. Available from: https://aqs.epa.gov/api
- 25.Leidy NK, Rennard SI, Schmier J, Jones MK, Goldman M. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest. 2003;124:2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 26.Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology. 2009;20:205–213. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz JD. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc Natl Acad Sci USA. 2012;109:6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro A, Carvalho V, Oliveira T, Sousa C. Excess mortality and morbidity during the July 2006 heat wave in Porto, Portugal. Int J Biometeorol. 2013;57:155–167. doi: 10.1007/s00484-012-0543-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Breitner S, Pan X, Franck U, Leitte AM, Wiedensohler A, von Klot S, Wichmann HE, Peters A, Schneider A. Associations between air temperature and cardio-respiratory mortality in the urban area of Beijing, China: a time-series analysis. Environ Health. 2011;10:51. doi: 10.1186/1476-069X-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O’Neill MS. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992-2006. Environ Health Perspect. 2014;122:1187–1192. doi: 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren C, Williams GM, Tong S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ Health Perspect. 2006;114:1690–1696. doi: 10.1289/ehp.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu R, Feng WY, Ostro BD. Characterizing temperature and mortality in nine California counties. Epidemiology. 2008;19:138–145. doi: 10.1097/EDE.0b013e31815c1da7. [DOI] [PubMed] [Google Scholar]
- 33.Analitis A, Michelozzi P, D’Ippoliti D, De’Donato F, Menne B, Matthies F, Atkinson RW, Iñiguez C, Basagaña X, Schneider A, et al. Effects of heat waves on mortality: effect modification and confounding by air pollutants. Epidemiology. 2014;25:15–22. doi: 10.1097/EDE.0b013e31828ac01b. [DOI] [PubMed] [Google Scholar]
- 34.Zanobetti A, Schwartz J. Temperature and mortality in nine US cities. Epidemiology. 2008;19:563–570. doi: 10.1097/EDE.0b013e31816d652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ. 2010;182:1053–1060. doi: 10.1503/cmaj.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.el-Kassimi FA, Al-Mashhadani S, Abdullah AK, Akhtar J. Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest. 1986;90:571–574. doi: 10.1378/chest.90.4.571. [DOI] [PubMed] [Google Scholar]
- 37.Malik AB, Johnson A, Tahamont MV, van der Zee H, Blumenstock FA. Role of blood components in mediating lung vascular injury after pulmonary vascular thrombosis. Chest. 1983;83:21S–24S. doi: 10.1378/chest.83.5_supplement.21s. [DOI] [PubMed] [Google Scholar]
- 38.Hayes D, Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med. 2012;185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aitken ML, Marini JJ. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. Am Rev Respir Dis. 1985;131:357–361. doi: 10.1164/arrd.1985.131.3.357. [DOI] [PubMed] [Google Scholar]
- 40.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, McCormack MC, Williams DL, Breysse PN. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115:1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostro B, Rauch S, Green R, Malig B, Basu R. The effects of temperature and use of air conditioning on hospitalizations. Am J Epidemiol. 2010;172:1053–1061. doi: 10.1093/aje/kwq231. [DOI] [PubMed] [Google Scholar]
- 42.Davis LW, Gertler PJ. Contribution of air conditioning adoption to future energy use under global warming. Proc Natl Acad Sci USA. 2015;112:5962–5967. doi: 10.1073/pnas.1423558112. [DOI] [PMC free article] [PubMed] [Google Scholar]