Abstract
Rationale: To date, EEG is the only quantifiable measure of the neural changes that define sleep. Although it is used widely for clinical testing, scalp-electrode EEG is costly and is poorly tolerated by sleeping patients.
Objectives: This was a pilot study to assess the agreement between EEG recordings obtained from a new ear-EEG sensor and those obtained simultaneously from standard scalp electrodes.
Methods: Participants were four healthy men, 25 to 36 years of age. During naps, EEG tracings were recorded simultaneously from the ear sensor and from standard scalp electrodes. A clinical expert, blinded to the data collection, analyzed 30-second epochs of recordings from both devices, using standardized criteria. The agreement between scalp- and ear-recordings was assessed.
Measurements and Main Results: We scored 360 epochs (scalp-EEG and ear-EEG), of which 254 (70.6%) were scored as non–REM sleep using scalp-EEG. The ear-EEG sensor had a sensitivity of 0.88 (95% confidence interval [CI], 0.82–0.92) and a specificity of 0.78 (95% CI, 0.70–0.84) in detecting N2/N3 sleep. The kappa coefficient between the scalp- and the ear-EEG was 0.65 (95% CI, 0.58–0.73). As a sleep monitor (all non–REM sleep stages vs. wake), the in-ear sensor had a sensitivity of 0.91 (95% CI, 0.87–0.94) and a specificity of 0.66 (95% CI, 0.56–0.75). The kappa coefficient was 0.60 (95% CI, 0.50–0.69).
Conclusions: Substantial agreement was observed between recordings derived from a new ear-EEG sensor and conventional scalp electrodes on four healthy volunteers during daytime naps.
Keywords: sleep, nocturnal EEG, obstructive sleep apnea, sleep-disordered breathing
Sleep is critical for health (1), with sleep disorders linked to an increased risk of systemic hypertension, cardiovascular disease, stroke (2, 3), cognitive dysfunction (4), and dementia (5). Nocturnal EEG, requiring the placement of up to 10 electrodes on the scalp in standardized positions (6), is the gold standard for the measurement of the neural changes that define sleep and is used routinely in the diagnosis of disorders. However, the approach is time consuming and can potentially disrupt sleep through the discomfort caused to the patient. It can also require an overnight stay in hospital, which is inconvenient and costly. A growing demand for ambulatory alternatives has led to the development of home sleep monitoring and a greater diagnostic role for primary care physicians (7). Indeed, it is now estimated that the combined market in the United States and Europe for clinical and ambulatory sleep devices is worth $96.5 million.
Portable sleep monitors that enable longer-term monitoring could facilitate the broader aim of establishing links between sleep disorders and daytime function. For example, sleep tests performed in the clinical environment cannot accurately quantify the safety and occupational risks of sleepiness (caused by lack of sleep) (8). There is, therefore, considerable need for comfortable, wearable monitors that can directly monitor long-term EEG in natural environments (9–11).
Existing EEG systems for the diagnosis of sleep-disordered breathing use scalp electrodes to define changes in the conscious state. This study tests, we believe for the first time, EEG recorded from within the ear canal during sleep. This was achieved by embedding electrodes on a viscoelastic earpiece to preserve the relevant signal components (11). The concept satisfies key wearable needs (it is comfortable, stable, and nonstigmatizing), thereby providing a convenient solution for long-term EEG recording in natural environments. The aim of this pilot study was to determine the agreement between the ear-EEG sensor and gold standard scalp-EEG electrodes in detecting sleep-related EEG changes.
Methods
Setting
This study was undertaken between May 2014 and March 2015 at Imperial College London, London, United Kingdom.
Participants
We recruited four healthy participants with no history of snoring, sleep disorders, or neurological disease. The participants were male, nonsmokers, and 25, 28, 32, and 36 years of age; and they had respective body mass indices of 25.7, 20.5, 28, and 24 kgm2. Written informed consent was obtained from all participants (Joint Research Office at Imperial College London, reference ICREC 12_1_1).
Ear-EEG Technology
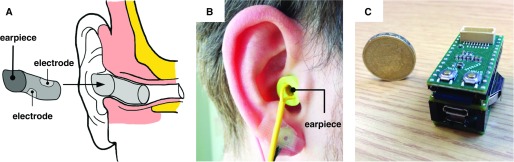
The recently developed ear-EEG sensor (11) comprises a memory-foam viscoelastic earpiece (diameter, approximately 12 mm; length, approximately 25 mm) with two electrodes at diametrically opposed locations, made from flexible conductive cloth (surface area, approximately 75 mm2), as shown in Figure 1. The viscoelastic nature of the earpiece, coupled with the flexibility of the electrodes, provided a key advantage compared with existing ear-EEG sensors, both personalized and generic (11).
Figure 1.
In-ear sleep sensor. (A) Right earpiece and its location inside the ear. (B) Prototype earpiece worn in ear. (C) Prototype electronics platform, comprising low-power microcontroller, analog front end for physiological signals, Secure Digital memory card, and 110 mAh battery.
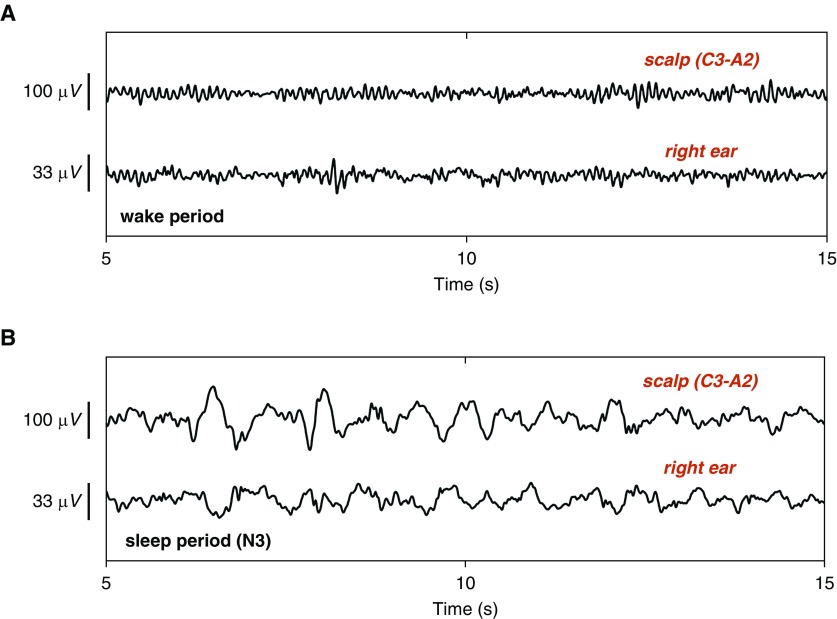
After insertion into the ear canal, the sensor expanded and redistributed pressure evenly along the entirety of its contact surface, thus providing a stable interface with the skin. The stability of the viscoelastic sensors was such that mechanical disturbances in the low-frequency range were greatly attenuated, facilitating uncompromised patterns of N2/N3 sleep, as illustrated in Figure 2.
Figure 2.
Time-domain EEG recorded from scalp and ear electrodes: 15 seconds of ear- and scalp-EEG obtained during (A) wake and (B) stage N3 sleep. Observe the presence of α activity (8–13 Hz) in ear-EEG in A, and the absence of α activity in B, as well as increased θ (4–7 Hz) and δ (1–3 Hz) activity. For reference, the simultaneously obtained trace using a conventional scalp electrode is also shown.
Protocol
Sleep studies were performed in the late afternoon. Participants were instructed to reduce their sleep to 4–5 hours on the night before the study and to refrain from napping or caffeine on the day of the study.
The setup time for each participant was between 60 and 90 minutes (ear-EEG sensors and scalp-EEG). Earwax was removed from the ear canals with cotton buds. Skin on the relevant outer parts of the ear (earlobe, helix) and scalp was abraded (Nuprep gel). EEG was recorded simultaneously from the in-ear and the standard on-scalp electrodes using the g-USBamp, a 24-bit biosignal amplifier (g.tec medical engineering GmbH, Schiedlberg, Austria) that enables up to four independent recording configurations.
Scalp-EEG was obtained from electrodes positioned according to the international 10–20 system: mastoid (A1, A2) and central (C3, C4) regions. The ground electrode was placed on the forehead. Standard reference configurations for sleep measurements (C3-A2 and C4-A1) were used.
Ear-EEG was obtained from the left (and right) ear from two electrodes placed at diametrically opposed locations along the ear canal wall, referenced to a gold-cup electrode placed behind the left (and right) helix. The ground electrode was placed on the left (and right) earlobe. All data were acquired with a sampling rate of 1.2 kS/s.
Once the electrodes were attached, the participants reclined in a chair in a dark and quiet room and were allowed to sleep. Recordings continued for at least 45 minutes, with bursts of 10 seconds of noise played through a loudspeaker at random intervals to increase the number of wake/sleep transitions.
Analysis
Preprocessing operations were applied to the EEG before scoring. In the case of the scalp-EEG, all data were band-pass filtered using a fourth-order Butterworth filter with cutoff frequencies at 1 and 20 Hz. To optimize the blinded nature of the scoring process, the ear-EEG amplitudes were scaled to match the range of the scalp-EEG. The low-pass cutoff was the same as that for the scalp-EEG (20 Hz), but the high-pass cutoff was adjusted depending on the level of low-frequency interference present in the ear-EEG recording; for participants 1 and 3, the cutoff was 1 Hz, and for participants 2 and 4, it was 2 Hz, because greater low-frequency interference was observed during data collection in participants 2 and 4.
The EEG recordings (four scalp, four in-ear) were randomized and blinded. The epochs were scored using American Academy of Sleep Medicine sleep-scoring criteria. Scoring was performed for the standard epoch size of 30 seconds, giving 90 epochs per recording. The clinical expert had 6 years of EEG scoring experience and was blinded to the data recording method.
Statistical Analysis
Analysis was performed to determine the level of agreement between the scores obtained with the ear- and scalp-EEG for two scenarios: (1) distinguishing between unambiguous N2/N3 sleep and wake plus light sleep (W/N1), and (2) distinguishing between non–REM (NREM) sleep (comprising stages N1, N2, and N3) and W.
The performance of ear-EEG for sleep detection was evaluated using true positive (TP), true negative (TN), false positive (FP), and false negative (FN) data by calculating the sensitivity (TP/[TP1FN]), specificity (TN/[TN + FP]) and accuracy ([TP + TN]/[TP + FP + TN + FN]). The kappa coefficient (12) was calculated for scenarios 1 and 2. A score of <0 indicates less than chance agreement; 0.01−0.20, slight agreement; 0.21−0.40, fair agreement; 0.41−0.60, moderate agreement; 0.61−0.80, substantial agreement; and 0.81−0.99, almost perfect agreement (13). The confidence interval (CI) of the coefficient was also calculated from its standard error.
Results
All participants were able to sleep and, as expected, no REM sleep was recorded. The number and percentage of W, N1, N2, and N3 epochs, scored using ear- and scalp-EEG, are shown in Table 1. The sensitivity, specificity, and accuracy of ear-EEG in detecting N2/N3 and all NREM sleep stages are given in Table 2.
Table 1.
Number and percentage of wake and N1, N2, and N3 sleep epochs
| EEG | Wake | N1 Sleep | N2 Sleep | N3 Sleep |
|---|---|---|---|---|
| Scalp | 106 (29.4) | 54 (15.0) | 153 (42.5) | 47 (13.1) |
| Ear | 92 (25.6) | 57 (15.8) | 181 (50.3) | 30 (8.3) |
Data are presented as No. (%). Total number of epochs = 360 (n = 4).
Table 2.
Performance of ear-EEG in correctly detecting N2/N3 sleep (stages N2 and N3) from wake and light sleep (W/N1) in scenario 1, and sleep (stages N1, N2, and N3) from wake in scenario 2, using scalp electrodes as the gold standard
| Scenario 1 | N2/N3 [scalp] | N2/N3 [ear] | TP | Sen | Spc | Acc |
|---|---|---|---|---|---|---|
| 1 | 17 (18.9%) | 9 (10.0%) | 9 | 0.53 | 1.00 | 0.91 |
| 2 | 66 (73.3%) | 59 (65.6%) | 55 | 0.83 | 0.83 | 0.83 |
| 3 | 81 (90.0%) | 78 (86.7%) | 78 | 0.96 | 1.00 | 0.97 |
| 4 | 36 (40.0%) | 65 (72.7%) | 33 | 0.92 | 0.41 | 0.61 |
| all | 200 (55.6%) | 211 (58.6%) | 175 | 0.88 | 0.78 | 0.83 |
| Scenario 2 | Sleep [scalp] | Sleep [ear] | TP | Sen | Spc | Acc |
|---|---|---|---|---|---|---|
| 1 | 30 (33.3%) | 14 (15.6%) | 14 | 0.47 | 1.00 | 0.82 |
| 2 | 76 (84.4%) | 75 (83.3%) | 70 | 0.92 | 0.64 | 0.88 |
| 3 | 82 (91.1%) | 89 (98.9%) | 82 | 1.00 | 0.13 | 0.92 |
| 4 | 66 (73.3%) | 90 (100%) | 66 | 1.00 | 0.00 | 0.73 |
| all | 254 (70.6%) | 268 (74.4%) | 232 | 0.91 | 0.66 | 0.84 |
Definition of abbreviations: Acc = accuracy; Sen = sensitivity; Spc = specificity; TP = true positive.
N2/N3 [scalp] denotes the number of N2 and N3 epochs defined by scalp-EEG scores in Scenario 1, N2/N3 [ear] number of N2 and N3 epochs defined by ear-EEG scores in Scenario 1, Sleep [scalp] denotes the number of sleep epochs defined by scalp-EEG scores in Scenario 2, Sleep [ear] number of sleep epochs defined by ear-EEG scores in Scenario 2.
In scenario 1, the sensitivity for the detection of N2/N3 using ear-EEG was 0.88 (95% CI 0.82–0.92) and the specificity was 0.78 (95% CI, 0.70–0.84). The level of agreement with scalp-EEG was substantial, as illustrated by the contingency table (Table 3) and a kappa coefficient of 0.65 (95% CI, 0.58–0.73). The higher sensitivity indicated a bias toward scoring wake epochs as sleep; this was likely caused by the presence of greater low-frequency activity in the ear-EEG during all epochs.
Table 3.
Contingency table for all participants
| Scenario 1 | Wake/N1 [scalp] | N2/N3 [scalp] |
|---|---|---|
| Wake/N1 [ear] | 124 (34.4%) | 25 (6.9%) |
| N2/N3 [ear] | 36 (10.0%) | 175 (48.6%) |
| Scenario 2 | Wake [scalp] | Sleep [scalp] |
|---|---|---|
| Wake [ear] | 70 (19.4%) | 22 (6.1%) |
| Sleep [ear] | 36 (10.0%) | 232 (64.4%) |
Kappa coefficient: scenario 1: 0.65 (95% CI, 0.58–0.73); scenario 2: 0.60 (95% CI, 0.50–0.69).
Scenario 1: wake and light sleep (Wake/N1) versus N2/N3 sleep (stages N2 and N3).
Scenario 2: wake versus sleep (N1, N2 and N3).
In scenario 2, the sensitivity, specificity, and accuracy of ear-EEG in detecting NREM sleep (all stages) are shown in Table 2. The corresponding contingency values are given in Table 3. The bias increased for the task of NREM sleep detection (all stages), and the specificity of ear-EEG decreased to 0.66 (95% CI, 0.56–0.75). This can be explained by the large number of N1 epochs (14.1%); the distinction between wake and N1 can be subtle and difficult to make accurately. The results for participant 4 were the weakest among all four participants. This was caused by the presence of low-frequency interference, such that wake epochs were scored incorrectly as sleep. The large CI of the group mean results was likely caused by the small number of participants in this proof-of-concept study. Overall, the ability of the ear-EEG to distinguish between wake and NREM sleep can be summarized by the kappa coefficient of 0.60 (95% CI, 0.50–0.69), a finding that indicates a moderate to substantial level of agreement with the scalp-EEG.
Discussion
The new ear-EEG sensor recorded sleep at a moderate to substantial level of agreement with the gold standard (scalp) EEG. The errors were caused, in part, by the similarity in the EEG patterns between wakefulness (W) and light (N1) sleep recorded by the ear-EEG sensor. Therefore, the agreement was closer between the ear- and scalp-EEG when distinguishing unambiguous N2/N3 sleep from wakefulness combined with light sleep (W/N1). In both cases, the specificity was lower than the sensitivity, which can be explained by the presence of low-frequency activity in the ear-EEG, resulting in some wake and N1 epochs being incorrectly labeled as N2/N3.
The ability to monitor EEG from a comfortable ear sensor could provide opportunities to improve the diagnosis of sleep disorders such as obstructive sleep apnea. Compared with other portable EEG systems, which rely on scalp electrodes with a cap and/or adhesives, the comfortable and stable nature of the in-ear sensor is more suitable for overnight recordings because it does not interfere with the patient’s sleep.
The viscoelastic in-ear sensor used in this study also offers several advantages compared with the original prototype that we have reported on previously (9, 10) and more recent ear-EEG developments (14, 15) that use silicone earpieces. First, the viscoelastic sensor is more cost effective because it fits any adult ear; the original prototype was customized to the ear like a hearing aid, requiring a costly and time-consuming manufacturing process. Second, unlike silicone earbuds, which provide suboptimal conformance to the shape of the ear canal, viscoelastic earbuds expand and redistribute pressure evenly along the contact surface. In this way, the sensor provides a more stable interface with the skin, which reduces mechanical artifacts caused by motion or cardiac pulsation (11). This feature is critical for sleep monitoring, where robust and stable recordings are paramount, particularly at low EEG frequencies. Future developments will use the interface to add additional physiological sensors such as heart rate and temperature.
Limitations
A limitation of the current study was the overly simple fashion in which the ear-EEG was adjusted to match the scalp-EEG (scaling, different filtering parameters for participants 2 and 4). To date, ear-EEG has been validated primarily on averaged responses that do not account for more qualitative differences with standard EEG or for occasional transient and spurious activity. Ear-EEG can exhibit greater low-frequency activity, making it visually dissimilar to activity recorded at more conventional scalp electrode locations. This is exemplified by Figure 2A, which illustrates that ear-EEG can contain low-frequency signal dynamics even during wakefulness. This is likely the cause for the sensitivity bias reported in this article, because low-frequency EEG is associated with sleep.
Another limitation is the small number of participants (n = 4). However, our intention was to provide an initial proof-of-concept study to illustrate the feasibility of in-ear sensing in sleep detection, shown by testing multiple epochs in several sleep stages, specifically linking N2 and N3 sleep to compare lower EEG frequencies.
Conclusions
The ear-EEG sensor offers a discreet way to continuously monitor sleep and, if verified on a broad spectrum of subjects, the potential clinical implications are many and varied. Monitoring excessive daytime sleepiness is currently difficult and is performed using subjective tests such as the Epworth Sleepiness Scale questionnaire (16) or objective tests such as the Maintenance of Wakefulness Test or the Multiple Sleep Latency Test. However, subjective tests are not always accurate (17), whereas access to objective tests is limited. The ear-EEG sensor may enable direct monitoring of sleep-related changes in EEG throughout both the day and night in real-world environments, and may ultimately offer a viable solution to the challenges of accurately but easily measuring sleep and sleepiness.
Acknowledgments
Acknowledgment
The authors thank Rachel Pickersgill for her technical assistance.
Footnotes
Supported by National Institute for Health Research Cardiovascular and Respiratory Biomedical Research Units Pump Priming Grants 2012; Engineering and Physical Sciences Research Council Ref:EP/K025643/1; Wellcome Trust Career Re-Entry Fellowship 103952/Z/14/Z (I.R.); and by the Rosetrees Trust.
Author Contributions: Conception and design: D.L., V.G., M.J.M., and D.P.M.; analysis and interpretation: D.L., V.G., M.J.M., and D.P.M.; review of project, data, and manuscript: D.L., V.G., I.R., M.J.M., and D.P.M.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, Malhotra A American Thoracic Society ad hoc Committee on Healthy Sleep. An official American Thoracic Society Statement. The importance of healthy sleep: recommendations and future priorities. Am J Respir Crit Care Med. 2015;191:1450–1458. doi: 10.1164/rccm.201504-0767ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, Soriano Y, Román-Sánchez P, Illa FB, Canal JM, Durán-Cantolla J. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twigg GL, Papaioannou I, Jackson M, Ghiassi R, Shaikh Z, Jaye J, Graham KS, Simonds AK, Morrell MJ. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. Am J Respir Crit Care Med. 2010;182:98–103. doi: 10.1164/rccm.200901-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3:404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 6.Campbell IG. EEG recording and analysis for sleep research. Curr Protoc Neurosci. 2009;10:2. doi: 10.1002/0471142301.ns1002s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai-Coetzer CL, Antic NA, Rowland LS, Reed RL, Esterman A, Catcheside PG, Eckermann S, Vowles N, Williams H, Dunn S, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309:997–1004. doi: 10.1001/jama.2013.1823. [DOI] [PubMed] [Google Scholar]
- 8.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, Hirshkowitz M, Daniel LL, Bailey D, Berry RB, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 9.Looney D, Kidmose P, Park C, Ungstrup M, Rank M, Rosenkranz K, Mandic D. The in-the-ear recording concept: user-centered and wearable brain monitoring. IEEE Pulse. 2012;3:32–42. doi: 10.1109/MPUL.2012.2216717. [DOI] [PubMed] [Google Scholar]
- 10.Kidmose P, Looney D, Ungstrup M, Rank ML, Mandic DP. A study of evoked potentials from ear-EEG. IEEE Trans Biomed Eng. 2013;60:2824–2830. doi: 10.1109/TBME.2013.2264956. [DOI] [PubMed] [Google Scholar]
- 11.Goverdovsky V, Looney D, Kidmose P, Mandic DP. In-ear EEG from viscoelastic generic earpieces: robust and unobtrusive 24/7 monitoring. IEEE Sens J. 2016;16:271–277. [Google Scholar]
- 12.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 13.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 14.Kidmose P, Looney D, Jochumsen L, Mandic DP. Ear-EEG from generic earpieces: a feasibility study. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:543–546. doi: 10.1109/EMBC.2013.6609557. [DOI] [PubMed] [Google Scholar]
- 15.Hoon Lee J, Min Lee S, Jin Byeon H, Sook Hong J, Suk Park K, Lee S-H. CNT/PDMS-based canal-typed ear electrodes for inconspicuous EEG recording. J Neural Eng. 2014;11:046014. doi: 10.1088/1741-2560/11/4/046014. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Damiani MF, Quaranta VN, Falcone VA, Gadaleta F, Maiellari M, Ranieri T, Fanfulla F, Carratù P, Resta O. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest. 2013;143:1569–1575. doi: 10.1378/chest.12-2174. [DOI] [PubMed] [Google Scholar]