Abstract
Rationale: In the intensive care unit (ICU), complex decision making by clinicians and families requires good communication to ensure that care is consistent with the patients’ values and goals.
Objectives: To assess the economic feasibility of staffing ICUs with a communication facilitator.
Methods: Data were from a randomized trial of an “ICU communication facilitator” linked to hospital financial records; eligible patients (n = 135) were admitted to the ICU at a single hospital with predicted mortality ≥30% and a surrogate decision maker. Adjusted regression analyses assessed differences in ICU total and direct variable costs between intervention and control patients. A bootstrap-based simulation assessed the cost efficiency of a facilitator while varying the full-time equivalent of the facilitator and the ICU mortality risk.
Measurements and Main Results: Total ICU costs (mean 22.8k; 95% CI, −42.0k to −3.6k; P = 0.02) and average daily ICU costs (mean, −0.38k; 95% CI, −0.65k to −0.11k; P = 0.006)] were reduced significantly with the intervention. Despite more contacts, families of survivors spent less time per encounter with facilitators than did families of decedents (mean, 25 [SD, 11] min vs. 36 [SD, 14] min). Simulation demonstrated maximal weekly savings with a 1.0 full-time equivalent facilitator and a predicted ICU mortality of 15% (total weekly ICU cost savings, $58.4k [95% CI, $57.7k–59.2k]; weekly direct variable savings, $5.7k [95% CI, $5.5k–5.8k]) after incorporating facilitator costs.
Conclusions: Adding a full-time trained communication facilitator in the ICU may improve the quality of care while simultaneously reducing short-term (direct variable) and long-term (total) health care costs. This intervention is likely to be more cost effective in a lower-mortality population.
Keywords: palliative care, critical care, communication, intensive care unit costs
The intensive care unit (ICU) is an important setting for improving communication between health care teams and family members of critically ill patients. Complex decision making on the part of both providers and families requires good communication to ensure that health care is consistent with patient values and preferences. Improving communication between clinicians and families is therefore important for improving the quality of care in the ICU (1).
A variety of interventions to improve communication in this setting, including providing printed information to families (2, 3), using primary and specialty palliative care interventions to enhance communication (4, 5), and implementing ethics consultation (6, 7), has been studied. Evidence suggests that these types of communication interventions can reduce treatment intensity and improve family emotional outcomes (8). However, to our knowledge, no study to date has evaluated the cost savings associated with the implementation of these interventions after factoring in the cost of the intervention.
Curtis and colleagues recently conducted a randomized trial of a nurse or social worker “facilitator” working with the ICU team and families to enhance communication between ICU clinicians and family, reduce family distress, and reduce intensity of care at the end of life (9). In this study, facilitators were trained in three techniques: (1) evidence-based approaches to improving ICU clinician-family communication; (2) attachment theory, allowing clinicians to adapt communication to individual family member’s needs; and (3) mediation to address conflict including clinician-family, clinician-clinician, and intrafamily conflict. Results suggested that the addition of a communication facilitator may result in reduced symptoms of depression for family members 6 months after the ICU.
Although other measures of families’ experiences of emotional distress (i.e., anxiety, post-traumatic stress at 3 and 6 mo, and depression at 3 mo), did not vary between groups, ICU length of stay and costs among decedents were reduced significantly for patients in the intervention arm. Furthermore, no difference in ICU mortality was found between the groups. These findings suggest that the addition of a communication facilitator may reduce unwanted high-intensity care with no evidence of increased family distress. However, the economic feasibility of including an ICU communication facilitator is not clear. By economic feasibility, we mean the overall costs of implementing such an intervention, incorporating the costs of the intervention itself.
The objective of this study was to assess the economic feasibility of staffing ICUs with a communication facilitator, incorporating the costs of the facilitator, and to identify the target population and employment arrangements that would lead to the most cost savings. This report is novel because it examines the cost savings associated with the intervention even after accounting for the costs of the intervention itself, and also because it examines the costs savings considering total ICU costs as well as the direct variable costs that can be realized on a short time horizon. Direct-variable costs include supply and drug costs and are responsive to patient characteristics and volume and thus can change in the short term. These results can help inform hospital administrators about whether staffing the ICU with communication facilitators can increase the value of care by reducing costs while improving the quality of care.
Methods
Design Overview
Data were collected as part of a parallel-group randomized trial of a “communication facilitator” intervention implemented in the ICU. Patients were randomly assigned with equal probability either to “usual care” or to the intervention, which consisted of a communication facilitator whose purpose was to assist families of critically ill patients during the ICU stay by providing communication support (9). Communication facilitators recorded the number of minutes spent during each encounter and the number of encounters with each intervention patient’s family member. Data from the randomized trial were linked to hospital financial records to obtain detailed cost information on hospital and ICU expenditures. All procedures were approved by the institutional review board at the University of Washington (UW HSC No. 33584).
Setting and Participants
The original randomized trial took place in ICUs in two hospitals, but only one hospital has a system of financial accounting that allows identification of direct variable costs. Therefore, this report is based on data from a single hospital, a 413-bed Level 1 trauma hospital located in Seattle, Washington. During the course of the study, ICU beds grew from 70 to 94 and were distributed among five ICUs (medical/cardiac, trauma/surgical, neurology/neurosurgical, burn, and pediatric), three of which provided patients for this study. ICU patients meeting the following criteria were eligible for random assignment: (1) being in ICU for >24 hours; (2) being older than 18 years; (3) being mechanically ventilated at the time of enrollment; (4) having a Sequential Organ Failure Assessment (SOFA) score ≥6 or diagnostic criteria predicting a ≥30% risk of hospital mortality (10, 11); (5) having a legal surrogate decision maker to consent for patient participation; and (6) having a family member able to come to the hospital. These criteria successfully identified patients with approximately 30% hospital mortality (9).
Intensive Care Unit Costs
ICU costs were obtained from administrative financial databases. Costs were used instead of charges because charges bear little resemblance to economic cost, and because use of charges as a proxy for economic cost may lead to unwarranted conclusions about economic efficiency (12). These costs represent the total costs for all services provided on each hospital day spent in the ICU, including overhead costs, labor costs, and supply costs. In this study, we report costs as two categories: total ICU costs and total direct variable ICU costs, to capture which costs could change in the long term or short term, respectively. Direct variable costs include supply and drug costs and are the most responsive to patient volume and individual patient characteristics such as severity of illness and reason for admission. Total costs include the fixed costs that hospitals pay, irrespective of patient volume. This institution uses the McKesson Explorer platform for hospital accounting; physician fees are generated from a separate organization and were not available at the ICU day level to be included in the analyses. All costs were adjusted for inflation and compared at the 2013 U.S. dollar value.
Statistical Analysis
Demographic and clinical characteristics of patients were summarized using mean ± SD for continuous variables and proportions for categorical variables. We used a generalized linear model with a γ error distribution and identity link function to explore the adjusted net difference in ICU costs between intervention and control subjects. We adjusted for variables that resulted in a change of ≥10% in the coefficient for the random assignment condition, compared with the baseline model. Potential adjustment variables were patient age, sex, race/ethnicity (categorized as minority status yes/no) and SOFA score; however, age was the only variable that met the criteria for adjustment. We tested whether the efficacy of the intervention was modified by patient mortality by including a cross-product term in the regression. All hypothesis tests were two sided, and all confidence intervals are reported at the 95% level. Data were analyzed using Stata, version 14.0 statistical software (StataCorp., College Station, TX).
Simulation Model
We used a bootstrap-based simulation model to estimate the net ICU cost savings that could result on a weekly basis if a salaried ICU communication facilitator was routinely available to family members of critically ill patients. To calculate the cost of the intervention, we obtained the average yearly salary of an ICU registered nurse (RN) on the basis of 1.0 full-time equivalent (FTE) (36 hours per week); at this institution, this amount was $139,000 including a 39.4% benefit load. We chose to consider an FTE facilitator rather than an hourly employee because it is more representative of a “real-world” scenario of hiring a communication facilitator to regularly staff the ICU.
Our simulation first calculated the amount of time a nurse with a given amount of FTE could spend with patients in 1 week, assuming 80% productivity (13, 14). Using to denote the level of FTE, the number of weekly hours available to see patients is equal to. Thus, a 1.0 FTE employee could produce 28.8 hours of clinical time per week. The training time for facilitators in the original randomized trial involved two 8-hour days, and maintaining the fidelity of the intervention involved a 1-hour monthly meeting to review cases. We did not factor training or maintenance into the model, but it could be considered a portion of the calculation for 80% productivity.
Next, we proceeded by sampling a single patient from the treatment group and calculating the total weekly time that patient’s family spent with a communication facilitator. This weekly time was calculated by multiplying the family’s daily minutes spent with a facilitator (contacts per day times minutes per contact) by the patient’s length of stay if it was <5 days, or by 5 days if the length of stay was ≥5 days. The resulting number represents the minutes per week that the communication facilitator spent with the sampled patient’s family on average. Five days was selected on the basis of the assumption that the ICU would be staffed with a communication facilitator Monday to Friday. This sampling continued until adding one more patient would exceed the amount of facilitator time available in 1 week. An equal number of control patients were then sampled with replacement. The average daily total ICU cost and average daily direct variable ICU cost were calculated for the resampled treatment and control patients. The difference in mean costs between the treatment and control arms represents the weekly potential cost savings that could result if the ICU was staffed with a communication facilitator.
We explored how mortality affected cost savings by adjusting sampling weights in the bootstrap resampling procedure. For example, to simulate a sample with mortality twice the level of the observed sample, decedents in the treatment and control groups were resampled twice as frequently as survivors. We varied the underlying mortality from one half (15%) to two times (60%) the mortality of the original study population (30%). We also varied the FTE of the communication facilitator between 0.5 and 1 FTE. For each mortality/FTE combination, 10,000 bootstrap samples of the average number of patients seen per week were generated, and we provide 95% Monte Carlo confidence intervals.
Results
Sample Characteristics
One hundred thirty-five patients were included in this study, with 66 in the control group and 69 in the intervention group. The mean age in the control group was slightly higher than in the intervention group (56 years [SD, 19.9] vs. 50.3 [SD, 17.5]). The mean SOFA score was similar in both groups (control, 10.0 [SD, 3.0] vs. intervention, 9.8 [SD, 3.5]), as was ICU mortality (29% in the control arm vs. 26% in the intervention arm). Having a Do Not Resuscitate order in place at the time of ICU admission was also similar in both groups (38% in the control arm vs. 33% in the intervention arm). Additional characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of study participants
| Characteristic | Control Group | Intervention Group |
|---|---|---|
| Patient characteristics | ||
| No. | 66 | 69 |
| Female | 22 (33) | 20 (29) |
| Age, mean (SD), yr | 56 (19.9) | 50.3 (17.5) |
| Race | ||
| White | 56 (85) | 55 (80) |
| African American | 2 (3) | 7 (10) |
| Asian | 4 (6) | 3 (4) |
| Native American | 2 (3) | 1 (1.5) |
| Native Hawaiian | 1 (1.5) | 0 |
| Other or mixed race | 1 (1.5) | 2 (2.9) |
| Racial/ethnic minority | 12 (19)* | 17 (25)† |
| SOFA score | ||
| Mean (SD)* | 10.0 (3.0) | 9.8 (3.5)‡ |
| Median (IQR) | 10.0 (8.0–12.0) | 10.0 (7.0–11.5) |
| ICU days | ||
| Mean (SD) | 23.5 (28.2) | 17.8 (12.7) |
| Median (IQR) | 16.4 (9.8–28.3) | 14.8 (9.8–22.3) |
| ICU survivors | ||
| Mean (SD) | 20.9 (17.7) | 20.5 (13.2) |
| Median (IQR) | 15.1 (9.9–23.9) | 18.8 (12.0–24.6) |
| ICU decedents | ||
| Mean (SD) | 30 (44.8) | 10.0 (6.9) |
| Median (IQR) | 17.4 (7.0–34.8) | 10.7 (3.5–14.5) |
| Died during or immediately after ICU stay | 19 (29) | 18 (26) |
| Palliative care service note during ICU admission | 17 (25.4) | 14 (20.2) |
| Social work consultation during ICU stay | 61 (91.0) | 65 (94.2) |
| Spiritual care services during ICU stay | 45 (67.2) | 41 (59.4) |
| Hospital days | ||
| Mean (SD) | 33.8 (30.9) | 25.4 (16.9) |
| Median (IQR) | 26.0 (17.0–42.0) | 23.0 (13.0–35.0) |
| Hospital death | 22 (33) | 18 (26) |
| DNR in place at time of ICU admit | 25 (38) | 23 (33) |
| Characteristics of decedents | ||
| No. | 19 | 18 |
| CPR or chemical code§ | 2 (10.5) | 1 (5.6) |
| Ventilator§ | 16 (84.2) | 17 (94.4) |
| Tube feeding§ | 18 (94.7) | 13 (72.2) |
| TPN§ | 4 (21.1) | 0 |
| Dialysis§ | 4 (21.1) | 2 (11.1) |
Definition of abbreviations: CPR = cardiopulmonary rescusitation; DNR = Do Not Resuscitate; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment; TPN = total parenteral nutrition.
Data are presented as No. (%) unless indicated otherwise.
Valid n = 65.
Valid n = 67.
Valid n = 68.
Valid n represents patients who died in the ICU or within 24 h of transfer out of the ICU.
Facilitator Time
Communication facilitators, on average, spent a total of 283 minutes per patient (SD, 218 min) assisting families of patients by providing communication support during the ICU stay. This time was divided among an average of 10.2 (SD, 6.7) encounters, resulting in an average of 27 minutes per encounter (SD, 13 min). Facilitators spent, on average, 25 minutes (SD, 11 min) per encounter with families of patients who survived and 36 minutes (SD, 14 min) per encounter with families of patients who died (Table 2).
Table 2.
Average facilitator time per patient
| Facilitator Time | Total Minutes | Number of Encounters | Minutes per Encounter | Encounters per Day* |
|---|---|---|---|---|
| All patients (n = 69) | ||||
| Mean (SD) | 283 (218) | 10.2 (6.7) | 27 (13) | 0.62 (0.24) |
| Median (IQR) | 240 (145–390) | 9 (7–13) | 25 (17–34) | 0.62 (0.50–0.70) |
| Survivors (n = 51) | ||||
| Mean (SD) | 300 (230) | 11.6 (6.9) | 25 (11) | 0.63 (0.26) |
| Median (IQR) | 250 (150–430) | 11 (8–14) | 24 (17–31) | 0.63 (0.49–0.71) |
| Decedents (n = 18) | ||||
| Mean (SD) | 227 (172) | 6.0 (4.4) | 36 (14) | 0.62 (0.20) |
| Median (IQR) | 208 (68–337) | 7 (2–9) | 35 (25–48) | 0.59 (0.53–0.70) |
Definition of abbreviation: IQR = interquartile range.
Time and encounter data were collected by the facilitators during the randomized trial.
Calculated as total number of encounters/total length of stay in days = encounters/day.
Intensive Care Unit Costs
Total ICU costs were significantly lower in the intervention arm (54.1k vs. 79.3k, P = 0.02). This reduction in costs was driven primarily by decedents, rather than survivors, although the trend in survivors also pointed toward cost savings (age-adjusted mean difference in cost = −8.6k) (Table 3). Direct variable costs were also significantly lower in the intervention arm (5.4k vs. 8.8k, P = 0.02). Once again, this reduction was driven primarily by decedents, rather than survivors, although the trend in survivors was also in the direction of savings (age-adjusted mean difference in cost = −1.3k). Average daily costs were significantly reduced in the intervention arm (3.0k vs. 3.4k, P = 0.006). Unlike the findings for total and direct variable costs, no evidence of interaction by mortality for average daily costs was found.
Table 3.
ICU costs of study population
| Outcome | Valid (n) | Control* | Intervention* | Age-adjusted Mean Difference† | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Total ICU costs | 135 | 79.3k (94.7k) | 54.1k (43.0k) | −22.8 | −42.0 to −3.6 | 0.020 |
| ICU Survivors‡ | 98 | 70.8k (65.1k) | 61.8k (44.1k) | −8.6 | −28.7 to 11.4 | 0.399 |
| ICU Decedents‡ | 37 | 100.4k (144.6k) | 32.2k (31.6k) | −61.0 | −117.7 to −4.4 | 0.035 |
| Direct variable costs | 135 | 8.8k (12.7k) | 5.4k (4.9k) | −2.7 | −4.9 to −0.53 | 0.015 |
| ICU Survivors‡ | 98 | 7.7k (10.2k) | 6.1k (5.1k) | −1.3 | −3.7 to 1.1 | 0.283 |
| ICU Decedents‡ | 37 | 11.6k (17.5k) | 3.5k (3.7k) | −6.9 | −13.9 to −0.10 | 0.053 |
| Average daily ICU costs§ | 135 | 3.4k (0.9k) | 3.0k (0.75k) | −0.38 | −0.65 to −0.11 | 0.006 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; GLM = generalized linear model.
Unadjusted mean (SD).
β-coefficient from γ GLM model with identity link function and robust standard errors, adjusted for age.
Based on ICU mortality.
No evidence of effect modification between random assignment and ICU mortality (interaction term P = 0.79).
Simulation Results
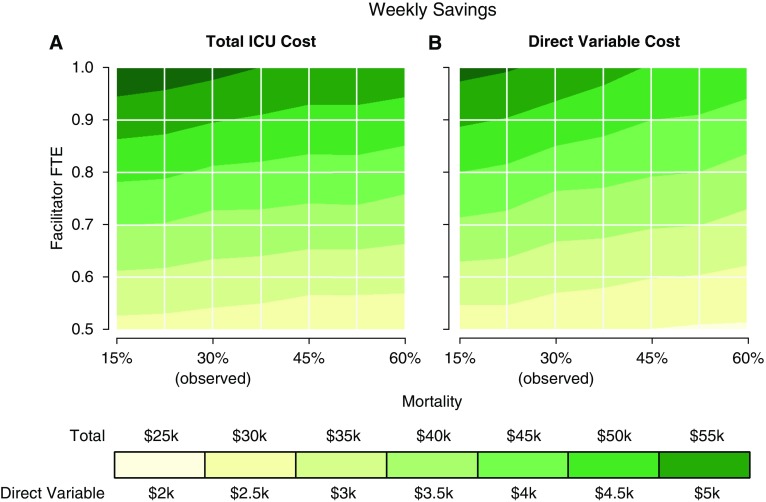
Results of the bootstrap-based simulation model are displayed in Table 4 and Figure 1. We found that the average number of patients seen per week by the facilitator ranged from 10.1 to 22.0, depending on the FTE and mortality of the ICU population. The average total ICU cost savings per week, after taking into account the cost of the facilitator, ranged from $26.3k to $58.4k, and the average direct variable savings per week ranged from $2.4k to $5.7k. In general, the simulation showed that increasing mortality led to fewer patients being seen, which, in turn, led to smaller cost savings. Conversely, increasing the FTE of the communication facilitator allowed more patients to be seen, resulting in greater cost savings. We have summarized the trends seen in the simulation results in Table 5. For both cost outcomes, the combination of targeting 0.5 × the mortality of the original study population (15% mortality risk) with a 1.0 FTE resulted in the most savings.
Table 4.
Simulated cost saving, by facilitator time and population mortality
| Mortality of ICU Population* | FTE of Communication Facilitator† | Total Cost Savings per Week ($) | Direct Variable Cost Savings per Week ($) | Patients Seen per Week | Cost Savings per Patient per Week ($) | Direct Variable Cost Savings per Patient per Week ($) |
|---|---|---|---|---|---|---|
| 0.5 | 0.5 | 28.4k (27.9k, 28.9k) | 2.7k (2.6k, 2.8k) | 10.8 (10.8k, 10.8k) | 2.7k (2.6k, 2.7k) | 0.25k (0.25k, 0.26k) |
| 1 | 0.5 | 27.8k (27.2k, 28.3k) | 2.6k (2.6k, 2.7k) | 10.5 (10.4k, 10.5k) | 2.7k (2.6k, 2.7k) | 0.25k (0.25k, 0.26k) |
| 2 | 0.5 | 26.3k (25.8k, 26.9k) | 2.4k (2.4k, 2.5k) | 10.1 (10.0k, 10.1k) | 2.7k (2.6k, 2.7k) | 0.24k (0.23k, 0.25k) |
| 0.5 | 0.75 | 43.0k (42.4k, 43.7k) | 4.2k (4.1k, 4.3k) | 16.4 (16.3k, 16.4k) | 2.6k (2.6k, 2.7k) | 0.26k (0.25k, 0.26k) |
| 1 | 0.75 | 41.2k (40.5k, 41.8k) | 3.9k (3.8k, 4.0k) | 15.9 (15.9k, 15.9k) | 2.6k (2.6k, 2.6k) | 0.25k (0.24k, 0.25k) |
| 2 | 0.75 | 39.5k (38.9k, 40.1k) | 3.6k (3.5k, 3.7k) | 15.3 (15.2k, 15.3k) | 2.6k (2.6k, 2.7k) | 0.23k (0.23k, 0.24k) |
| 0.5 | 1 | 58.4k (57.7k, 59.2k) | 5.7k (5.5k, 5.8k) | 22.0 (22.0k, 22.0k) | 2.7k (2.6k, 2.7k) | 0.26k (0.25k, 0.26k) |
| 1 | 1 | 56.5k (55.7k, 57.2k) | 5.4k (5.3k, 5.5k) | 21.3 (21.3k, 21.4k) | 2.7k (2.6k, 2.7k) | 0.25k (0.25k, 0.26k) |
| 2 | 1 | 53.0k (52.3k, 53.8k) | 4.8k (4.7k, 4.9k) | 20.5 (20.4k, 20.5k) | 2.6k (2.6k, 2.6k) | 0.23k (0.23k, 0.24k) |
Definition of abbreviations: FTE = full-time equivalent; ICU = intensive care unit.
Data are presented as mean (95% confidence interval).
Mortality = percent of baseline mortality; 1 represents mortality equal to the baseline population mortality of 30%.
Cost of FTE of communication facilitator on the basis of the average salary for 1.0 FTE (36 h) ICU registered nurse at Harborview Medical Center, which is 139k, including a 39.4% benefit load. This model also assumes productivity of 80%.
Figure 1.
Weekly cost savings as a result of varying the FTE of the communication facilitator (y-axis) and the mortality of the targeted ICU population (x-axis). For a given FTE and mortality, the intersection point estimates (A) average weekly total ICU cost savings and (B) average weekly direct variable cost savings. FTE = full-time equivalent; ICU = intensive care unit.
Table 5.
Summary of trends in simulation results
| Simulation Outcome | Increasing Mortality of ICU Population | Increasing FTE of Communication Facilitator |
|---|---|---|
| Average patients seen | ↓ | ↑ |
| Average total cost savings | ↓ | ↑ |
| Average direct cost savings | ↓ | ↑ |
Definition of abbreviations: FTE = full-time equivalent; ICU = intensive care unit.
Discussion
Our findings demonstrate that (1) staffing the ICU with a communication facilitator has the potential to result in net total ICU cost savings and direct variable cost savings even after accounting for the costs of the facilitator; (2) targeting these interventions toward a broader ICU population with a lower average mortality may achieve the most savings; and (3) staffing the ICU with a 1.0 FTE trained communication facilitator is an economically feasible model. These findings suggest that interventions aimed at improving communication between families of critically ill patients and the ICU team have the potential not only to improve the quality of care delivered in the ICU, but also to result in net ICU savings.
Previous studies have suggested that a proactive communication strategy for families of critically ill patients may improve family emotional outcomes (2, 9). Lautrette and colleagues found that a proactive family conference, resulting in longer family conferences and more time for family members to talk, as well as provision of a bereavement pamphlet, resulted in decreased levels of anxiety, depression, and post-traumatic stress disorder in family members 3 months after patient death (2). This recent randomized trial found that a trained communication facilitator was associated with some reduction in family distress for families of both survivors and decedents (9). Although the current literature has not established repeatedly or clearly an improvement in family outcomes, it seems reasonable to conclude that a communication facilitator certainly does not worsen family outcomes and can reduce costs.
Our findings in this current report suggest that staffing the ICU with a communication facilitator results in cost savings, even after factoring in the cost of the facilitator. Importantly, although an FTE facilitator is considered a fixed cost to the hospital, enough savings in direct variable costs offset the facilitator’s salary, suggesting both short- and long-term cost reductions.
Many studies have found that the primary mechanism for reducing ICU costs is by reducing length of stay for decedents (6, 7, 15–17), suggesting that targeting a higher-mortality population would result in more savings. Interestingly, the results of our simulation model suggest otherwise. We found that average daily costs were reduced in the intervention arm, indicating that a communication facilitator, in addition to being associated with a reduction in ICU length of stay for decedents (9), was also associated with a reduction in the average daily intensity of care for both survivors and decedents.
Not only did our simulation model demonstrate that savings could be achieved with patients at a lower risk of mortality, it also suggested that the maximal savings may occur in a patient population with an average 15% mortality, one-half that of the original study population. There are two main reasons for this: (1) average daily savings were seen for survivors, suggesting lower intensity of treatment; and (2) the amount of time per encounter that facilitators spent with families of survivors was lower, suggesting lower facilitator costs. Therefore, lowering the mortality of the target population enabled facilitators to see more patients and incur more savings overall.
Several studies have reported cost savings attributable to inpatient palliative care consultation (18–21), but the costs of these programs were not taken into consideration. To our knowledge, this is the first study evaluating the cost savings of an intervention to improve communication after factoring in the cost of the intervention, specifically a communication facilitator in the ICU. Additional studies are needed to evaluate the cost savings that factor in the cost of interventions aimed at improving communication between patients and families and health care teams. These types of studies will help inform hospital administrators about economically feasible interventions and programs.
Limitations
Our study has several limitations. First, because the sample size of the study population was small and cost data are skewed, our ability to provide precise estimates of savings is limited. However, our results indicate that the intervention pays for itself through reductions in ICU costs. Second, these data are from one institution, limiting the generalizability of our findings. Furthermore, this institution has been involved in previous quality improvement initiatives to improve palliative care in the ICU (22, 23), which could have improved care in the control group, resulting in more conservative estimates of savings.
Third, the costs of the intervention are based on the salary of an ICU RN at one institution. It is worthwhile noting that at this institution, salaries for ICU RNs are above the national average and include a 39.4% fringe benefit rate (the national average is 20%). This again results in more conservative estimates of net savings. Fourth, our results reflect savings seen in the ICU and do not factor in post-ICU costs. Finally, we were unable to obtain physician fees, which would have likely been lower in the intervention group given the shorter ICU length of stay, also suggesting that our estimates are conservative.
Conclusions
Our results suggest that staffing the ICU with a full-time trained communication facilitator has the potential to improve the quality of care delivered in the ICU while simultaneously resulting in cost savings. Importantly, the fixed cost incurred to the hospital by additional staff can be recouped in short-term, direct variable costs. Additional studies are needed to examine the benefits of a full-time facilitator and of targeting patients with a lower risk of mortality. Future economic analysis of other interventions to improve communication and larger-scale studies are needed to confirm and extend these findings.
Footnotes
Supported by the National Institute of Nursing Research (R01 NR05226), the National Institute of General Medical Sciences (5T32GM086270), and the Cambia Health Foundation.
Author Contributions: All authors made substantial contributions to the design of the work or the acquisition, analysis, or interpretation of the data; participated in revising it critically; provided final approval of the version to be published; and agree to be accountable for the work.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Curtis JR, Patrick DL, Shannon SE, Treece PD, Engelberg RA, Rubenfeld GD. The family conference as a focus to improve communication about end-of-life care in the intensive care unit: opportunities for improvement. Critical Care Med. 2001;29:N26–33. doi: 10.1097/00003246-200102001-00006. [DOI] [PubMed] [Google Scholar]
- 2.Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, Barnoud D, Bleichner G, Bruel C, Choukroun G, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay E, Pochard F, Chevret S, Jourdain M, Bornstain C, Wernet A, Cattaneo I, Annane D, Brun F, Bollaert PE, et al. Impact of a family information leaflet on effectiveness of information provided to family members of intensive care unit patients: a multicenter, prospective, randomized, controlled trial. Am J Respir Crit Care Med. 2002;165:438–442. doi: 10.1164/ajrccm.165.4.200108-006oc. [DOI] [PubMed] [Google Scholar]
- 4.Campbell ML, Guzman JA. A proactive approach to improve end-of-life care in a medical intensive care unit for patients with terminal dementia. Crit Care Med. 2004;32:1839–1843. doi: 10.1097/01.ccm.0000138560.56577.88. [DOI] [PubMed] [Google Scholar]
- 5.Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma. 2008;64:1587–1593. doi: 10.1097/TA.0b013e318174f112. [DOI] [PubMed] [Google Scholar]
- 6.Schneiderman LJ, Gilmer T, Teetzel HD. Impact of ethics consultations in the intensive care setting: a randomized, controlled trial. Crit Care Med. 2000;28:3920–3924. doi: 10.1097/00003246-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Schneiderman LJ, Gilmer T, Teetzel HD, Dugan DO, Blustein J, Cranford R, Briggs KB, Komatsu GI, Goodman-Crews P, Cohn F, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA. 2003;290:1166–1172. doi: 10.1001/jama.290.9.1166. [DOI] [PubMed] [Google Scholar]
- 8.Scheunemann LP, McDevitt M, Carson SS, Hanson LC. Randomized, controlled trials of interventions to improve communication in intensive care: a systematic review. Chest. 2011;139:543–554. doi: 10.1378/chest.10-0595. [DOI] [PubMed] [Google Scholar]
- 9.Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, Khandelwal N, Young JP, Engelberg RA. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193:154–162. doi: 10.1164/rccm.201505-0900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajdacsy-Balla Amaral AC, Andrade FM, Moreno R, Artigas A, Cantraine F, Vincent JL. Use of the sequential organ failure assessment score as a severity score. Intensive Care Med. 2005;31:243–249. doi: 10.1007/s00134-004-2528-6. [DOI] [PubMed] [Google Scholar]
- 11.Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The multiple organ dysfunction score (MODS) versus the sequential organ failure assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28:1619–1624. doi: 10.1007/s00134-002-1491-3. [DOI] [PubMed] [Google Scholar]
- 12.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96:102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 13.Westbrook JI, Duffield C, Li L, Creswick NJ. How much time do nurses have for patients? A longitudinal study quantifying hospital nurses’ patterns of task time distribution and interactions with health professionals. BMC Health Serv Res. 2011;11:319. doi: 10.1186/1472-6963-11-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farquharson B, Bell C, Johnston D, Jones M, Schofield P, Allan J, Ricketts I, Morrison K, Johnston M. Frequency of nursing tasks in medical and surgical wards. J Nurs Manag. 2013;21:860–866. doi: 10.1111/jonm.12110. [DOI] [PubMed] [Google Scholar]
- 15.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. 2015;43:1102–1111. doi: 10.1097/CCM.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, Cody S. An intensive communication intervention for the critically ill. Am J Med. 2000;109:469–475. doi: 10.1016/s0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 17.Lilly CM, Sonna LA, Haley KJ, Massaro AF. Intensive communication: four-year follow-up from a clinical practice study. Crit Care Med. 2003;31(5, Suppl):S394–S399. doi: 10.1097/01.CCM.0000065279.77449.B4. [DOI] [PubMed] [Google Scholar]
- 18.Morrison RS, Penrod JD, Cassel JB, Caust-Ellenbogen M, Litke A, Spragens L, Meier DE Palliative Care Leadership Centers’ Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 19.Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J, Meier DE. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood) 2011;30:454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 20.Penrod JD, Deb P, Dellenbaugh C, Burgess JF, Jr, Zhu CW, Christiansen CL, Luhrs CA, Cortez T, Livote E, Allen V, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13:973–979. doi: 10.1089/jpm.2010.0038. [DOI] [PubMed] [Google Scholar]
- 21.Penrod JD, Deb P, Luhrs C, Dellenbaugh C, Zhu CW, Hochman T, Maciejewski ML, Granieri E, Morrison RS. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9:855–860. doi: 10.1089/jpm.2006.9.855. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JR, Treece PD, Nielsen EL, Downey L, Shannon SE, Braungardt T, Owens D, Steinberg KP, Engelberg RA. Integrating palliative and critical care: evaluation of a quality-improvement intervention. Am J Respir Crit Care Med. 2008;178:269–275. doi: 10.1164/rccm.200802-272OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, Back AL, Rubenfeld GD, Engelberg RA. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]