Abstract
Organisms in the genus Anaplasma express an immunodominant major surface protein 2 (MSP2), composed of a central hypervariable region (HVR) flanked by highly conserved regions. Throughout Anaplasma marginale infection, recombination results in the sequential appearance of novel MSP2 variants and subsequent control of rickettsemia by the immune response, leading to persistent infection. To determine whether immune evasion and selection for variant organisms is associated with a predominant response against HVR epitopes, T-cell and linear B-cell epitopes were localized by measuring peripheral blood gamma interferon-secreting cells, proliferation, and antibody binding to 27 overlapping peptides spanning MSP2 in 16 cattle. Similar numbers of MSP2-specific CD4+ T-cell epitopes eliciting responses of similar magnitude were found in conserved and hypervariable regions. T-cell epitope clusters recognized by the majority of animals were identified in the HVR (amino acids [aa] 171 to 229) and conserved regions (aa 101 to 170 and 272 to 361). In contrast, linear B-cell epitopes were concentrated in the HVR, residing within hydrophilic sequences. The pattern of recognition of epitope clusters by T cells and of HVR epitopes by B cells is consistent with the influence of protein structure on epitope recognition.
Tick-borne pathogens in the family Anaplasmataceae infect and persist in reservoir mammalian hosts, with acute disease occurring after transmission to a naïve host. Acute infection is characterized by high-level rickettsemia resulting in clinical disease, whereas persistent infection is typified by recurrent low-level, subclinical rickettsemic peaks (10, 13, 17). Common to the pathogens in the genera Anaplasma and Ehrlichia are immunodominant outer membrane proteins (OMP) with defined conserved and variable domains (13, 15, 21, 23, 26, 30). In Anaplasma spp., gene conversion of msp2 pseudogenes into a single expression site provides an efficient mechanism to generate the large number of OMP variants seen during persistent infection (1, 2, 4, 12). Novel Anaplasma marginale MSP2 variants arise in sequential rickettsemic peaks, followed by clearance that is associated with generation of primary immunoglobulin G (IgG) antibody responses against variant-specific B-cell epitopes localized to the central hypervariable region (HVR) (12, 13). More recently, epitopes that elicited high levels of gamma interferon (IFN-γ) production by CD4+ T cells were identified in conserved and variable domains of A. marginale MSP2 (6, 7). The induction and recall of T-cell responses, including production of IFN-γ for macrophage activation and provision of help for antibody production, in particular IgG2, are proposed to be required for control of emergent variants (7, 25). The importance of CD4+ T cells and IFN-γ in controlling infection with other Ehrlichia spp. has also been reported (3, 14, 20).
Detailed knowledge of the epitope specificity of T- and B-lymphocyte responses to conserved and variable domains of A. marginale MSP2 is necessary to understand the selection for variants during infection. If the majority of IFN-γ-secreting CD4+ T lymphocytes were specific for HVR epitopes, control of emergent variants would require primary T-lymphocyte responses in addition to primary B-lymphocyte responses directed against variable region epitopes. Such predominant recognition of variable epitopes could facilitate immune evasion and persistent infection. Previous studies that identified T-cell epitopes in conserved and hypervariable regions of MSP2 were performed with proliferation assays using T-cell lines that were cultured for several weeks and obtained from only three animals (6, 7). Responses by in vitro propagated cell lines derived from few animals might be biased towards recognition of a few immunodominant epitopes. To more comprehensively test the hypothesis that the majority of T- and B-lymphocyte epitopes reside within the HVR, the present study used peripheral blood mononuclear cells (PBMC), rather than T-cell lines, from 16 outbred cattle immunized with MSP2. The animals expressed 10 different major histocompatibility complex (MHC) class II DRB3 alleles and 11 different combinations of DRB3 alleles. Furthermore, the present study measured the number of peptide-specific IFN-γ-secreting cells by enzyme-linked immunospot (ELISPOT) assay in addition to levels of proliferation and IFN-γ secretion. The strengths of this approach are that the ELISPOT assay specifically quantifies those IFN-γ-producing T lymphocytes that are important for the control of anaplasmosis and that the use of three T-cell assays may reveal a greater number of epitopes, as demonstrated by a study examining epitope recognition by PBMC from humans exposed to malaria (11). To determine B-cell epitopes on MSP2, immune sera from the MSP2-immunized animals were also used to identify peptides by enzyme-linked immunosorbent assay (ELISA).
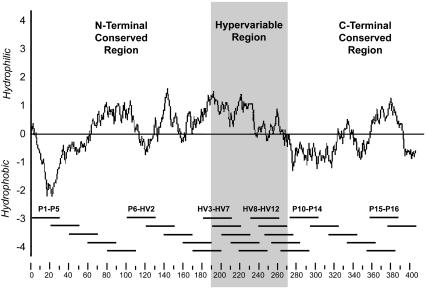
All cattle were previously immunized (29) with gel-purified MSP2 (22, 28). Twenty animals were allocated into five groups with four calves per group. Groups I to IV were immunized six times with 50 μg of MSP2 adsorbed in 2 mg of alum (29). The cattle received no additional adjuvant (group I) or the following: 1 mg of control non-CpG oligodeoxynucleotide (ODN) R2006 (group II), 1 mg of CpG ODN 2006 (group III), or 10 μg of recombinant human interleukin-12 (IL-12) (group IV). Calves in group V served as a control group and received alum plus CpG ODN 2006 but no MSP2. The Kyte and Doolittle method (18) was used to generate a hydropathic profile of MSP2 (Fig. 1). Imbricated 24- to 30-mer peptides that overlap by 10 to 20 amino acids and span the Florida strain MSP2 A variant sequence derived from msp2 clone 1-7 (GenBank accession no. AY138954) were synthesized (6, 7) and used for T- and B-cell epitope mapping. The MSP2 A variant was the most common variant transcript identified in the blood utilized for MSP2 immunization (6). The relative positions of the MSP2 peptides are shown in Fig. 1.
FIG. 1.
Hydropathic profile of full-length A. marginale MSP2. The central hypervariable region, aa 190 to 272, which represents the MSP2 A variant from the Florida strain (6), is predominantly hydrophilic and identified by shading. Amino acid position is indicated on the x axis, and the hydropathy index, representing the average value over a window of 19 amino acids, is indicated on the y axis. The overlapping peptides are represented under the hydropathic profile as black bars in groups of five to illustrate the relative position in the whole protein. The numbering of the amino acids corresponds to the msp2 11.2 genomic DNA clone (24).
Five to six months after immunization, 0.5 × 106 PBMC were assayed for IFN-γ production by ELISPOT assay (29). When PBMC from all animals were stimulated with a mixture of phytohemagglutinin (PHA; 1.0 μg per ml), IL-12 (0.01 ng per ml), and IL-18 (0.5 ng per ml), which served as a positive control for IFN-γ synthesis (27, 29), large numbers of IFN-γ-producing cells were detected, achieving or nearing saturation of the test wells. Antigens consisted of 10 and 1 μg of uninfected bovine erythrocyte membranes (URBC) per ml, A. marginale Florida strain homogenate, native MSP2, or MSP2-derived peptides diluted in complete RPMI 1640 medium (11) (Table 1). Significant responses to A. marginale antigen were seen in 73% of the MSP2-vaccinated animals (11 of 16), and 50% (8 of 16) of vaccinated animals had significant responses to MSP2 (Table 1). Four age-matched steers that were used as controls and given adjuvant without MSP2 responded significantly to PHA, IL-12, and IL-18 but not to A. marginale or MSP2 (data not shown).
TABLE 1.
MSP2 peptide-specific IFN-γ ELISPOT responses by PBMC from MSP2-vaccinated animals
| Antigen or peptide | No. of SFC per 106 PBMC from indicated calf in the following immunization groupa:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSP2 + alum
|
MSP2 + R2006 + alum
|
MSP2 + 2006 + alum
|
MSP2 + IL-12 + alum
|
|||||||||||||
| 70 (22/7)b | 80 (3/7) | 83 (24/8) | 85 (16/23) | 72 (8/23) | 77 (22/24) | 86 (16/24) | 88 (11/23) | 78 (8/11) | 79 (22/24) | 81 (8/23) | 87 (16/24) | 71 (8/14) | 75 (23/8) | 76 (22/21) | 82 (16/27) | |
| PHAc | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| A. marginale | 153 | 13 | 95 | 48 | 155 | 3 | 275 | 221 | 201 | 275 | 14 | 301 | 467 | 420 | 416 | 465 |
| MSP2 | 562 | 17 | 155 | 34 | 0 | 13 | 88 | 0 | 200 | 23 | 0 | 464 | 478 | 868 | 601 | 723 |
| P1 | 0 | 70 | 37 | 70 | 23 | 0 | 8 | 0 | 18 | 5 | 0 | 0 | 57 | 12 | 0 | 39 |
| P2 | 12 | 7 | 0 | 62 | 0 | 8 | 7 | 88 | 42 | 22 | 0 | 46 | 37 | 52 | 79 | 39 |
| P3 | 25 | 12 | 0 | 85 | 20 | 0 | 70 | 15 | 10 | 29 | 0 | 36 | 8 | 110 | 30 | 53 |
| P4 | 0 | 14 | 0 | 55 | 83 | 12 | 58 | 0 | 79 | 45 | 5 | 58 | 7 | 83 | 59 | 62 |
| P5 | 0 | 3 | 0 | 64 | 43 | 4 | 89 | 63 | 0 | 52 | 7 | 16 | 50 | 77 | 1 | 886 |
| P6 | 163 | 3 | 0 | 160 | 53 | 3 | 111 | 17 | 57 | 29 | 1 | 0 | 50 | 127 | 108 | 403 |
| P7 | 321 | 7 | 97 | 17 | 17 | 7 | 145 | 225 | 9 | 257 | 0 | 320 | 61 | 296 | 283 | 743 |
| P8 | 25 | 27 | 42 | 113 | 26 | 2 | 45 | 160 | 184 | 21 | 20 | 14 | 5 | 13 | 22 | 309 |
| P9 | 15 | 37 | 24 | 73 | 9 | 1 | 34 | 0 | 35 | 29 | 42 | 17 | 25 | 23 | 19 | 223 |
| HV2 | 137 | 8 | 77 | 95 | 271 | 0 | 0 | 84 | 0 | 239 | 28 | 54 | 5 | 296 | 485 | 522 |
| HV3 | 207 | 18 | 91 | 0 | 369 | 0 | 0 | 31 | 123 | 179 | 77 | 0 | 268 | 427 | 468 | 623 |
| HV4 | 255 | 24 | 377 | 0 | 376 | 0 | 15 | 0 | 233 | 167 | 144 | 21 | 592 | 636 | 391 | 161 |
| HV5 | 237 | 14 | 186 | 169 | 425 | 0 | 5 | 21 | 254 | 177 | 184 | 84 | 648 | 760 | 381 | 51 |
| HV6 | 61 | 22 | 118 | 14 | 48 | 0 | 15 | 7 | 139 | 89 | 24 | 7 | 280 | 436 | 64 | 57 |
| HV7 | 285 | 8 | 57 | 0 | 29 | 0 | 35 | 0 | 11 | 177 | 49 | 42 | 32 | 165 | 98 | 318 |
| HV8 | 579 | 6 | 69 | 0 | 0 | 0 | 0 | 8 | 39 | 93 | 15 | 92 | 71 | 83 | 15 | 101 |
| HV9 | 32 | 19 | 266 | 131 | 0 | 7 | 0 | 91 | 85 | 217 | 22 | 333 | 241 | 213 | 142 | 93 |
| HV10 | 19 | 9 | 240 | 96 | 0 | 5 | 0 | 0 | 3 | 108 | 7 | 57 | 58 | 21 | 60 | 4 |
| HV11 | 0 | 1 | 101 | 26 | 0 | 1 | 39 | 79 | 357 | 345 | 20 | 48 | 245 | 115 | 7 | 9 |
| HV12 | 3 | 4 | 106 | 3 | 0 | 0 | 116 | 113 | 390 | 443 | 115 | 62 | 412 | 563 | 17 | 0 |
| P10 | 13 | 65 | 130 | 139 | 33 | 10 | 92 | 198 | 106 | 137 | 93 | 298 | 299 | 329 | 15 | 604 |
| P11 | 5 | 25 | 100 | 62 | 18 | 0 | 162 | 184 | 67 | 56 | 63 | 94 | 253 | 233 | 285 | 68 |
| P12 | 169 | 34 | 31 | 43 | 116 | 11 | 53 | 0 | 71 | 209 | 36 | 94 | 105 | 261 | 575 | 15 |
| P13 | 61 | 50 | 50 | 59 | 75 | 8 | 343 | 143 | 359 | 68 | 147 | 453 | 63 | 87 | 533 | 831 |
| P14 | 25 | 17 | 18 | 83 | 29 | 9 | 111 | 76 | 151 | 73 | 80 | 247 | 29 | 13 | 237 | 411 |
| P15 | 13 | 30 | 32 | 15 | 0 | 3 | 66 | 13 | 46 | 57 | 59 | 0 | 47 | 45 | 166 | 58 |
| P16 | 9 | 31 | 26 | 8 | 10 | 0 | 64 | 0 | 164 | 29 | 49 | 116 | 280 | 219 | 292 | 673 |
| URBC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 74 |
PBMC obtained from calves 5 to 6 months after immunization with MSP2 were cultured for 40 h at a concentration of 5 × 105 cells per well. Results are presented as the mean number of SFC in triplicate wells of cells stimulated with 10 μg of A. marginale homogenate per ml, native MSP2, or overlapping peptides spanning MSP2 after subtracting the mean number of SFC in wells containing medium. Significant responses were determined by using one-way analysis of variance with Bonferroni's correction for comparison with control (medium alone) and are in bold. Data for PBMC stimulated with 1 μg of antigen or peptide per ml are not shown.
DRB3 haplotype.
For positive controls, PBMC were cultured with a mixture of 1 μg of PHA per ml, 0.01 ng of recombinant human IL-12 per ml, and 0.5 ng of recombinant human IL-18 per ml. All responses were considered to be positive, as most wells were at or near saturation and individual spots were not always discernable.
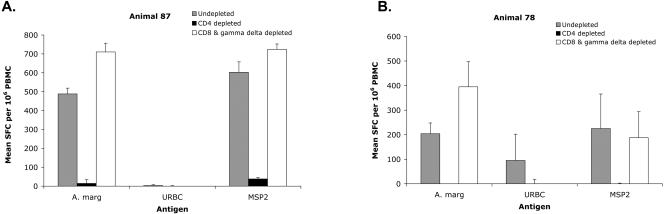
We next verified that the A. marginale-specific IFN-γ-secreting cells are predominantly CD4+ T lymphocytes by depleting specific cell subsets with monoclonal antibody (MAb) obtained from the Washington State University Monoclonal Antibody Center. Depletion of CD4+ T lymphocytes to <1% of total cells with CD4-specific MAb ILA-11A bound to magnetic beads (16) significantly reduced the number of lymphocytes responding to either A. marginale (P < 0.01) or MSP2 (P < 0.01) to numbers similar to background numbers from lymphocytes stimulated with medium alone or URBC (Fig. 2). In contrast, bead depletion of CD8+ T lymphocytes by using MAb 7C2B and of γδ T lymphocytes by using MAb GB21A to <1% of the total cells did not significantly reduce the response. This finding is consistent with our previous results from using CD4+ T clones to detect responses to MSP2-derived peptides, where peptide-specific T cells produced IFN-γ, which was measured by ELISA, and responses were blocked by anti-MHC class II-specific antibody (6-8).
FIG. 2.
Identification of T-lymphocyte subsets that secrete IFN-γ detected by ELISPOT assay. PBMC from MSP2-alum-CpG ODN-immunized animals no. 87 (panel A) and no. 78 (panel B), untreated and after depletion of either CD4+ cells or CD8+ cells and γδ T cells, were tested by an IFN-γ ELISPOT assay. The results are presented as the mean number of spot-forming cells (SFC) plus 1 standard deviation per 106 PBMC cultured with 10 μg of antigen per ml after subtracting the mean number of SFC in cells cultured with medium.
To test the hypothesis that the CD4+ T-cell response is predominantly directed to the HVR of MSP2, IFN-γ ELISPOT responses to conserved and hypervariable region peptides (Fig. 1) were evaluated and compared. The data in Table 1 are representative of independent experiments performed two or three times. When responses from all 16 vaccinated animals were considered together, 47% of the significant peptide-specific responses (52 of 110 responses) were directed at peptides from the conserved N- and C-terminal regions, and 53% (58 of 110 responses) were directed at HVR peptides. One-way analysis of variance with Bonferroni's correction revealed no significant differences in the magnitude of the responses to the conserved or hypervariable region peptides when the data for individuals or all 16 animals together were analyzed. Importantly, the equivalent distribution of T-cell epitopes in the conserved and hypervariable regions of MSP2 was observed regardless of the level of immunity achieved by administering a given adjuvant.
The ELISPOT analysis revealed that peptides that were recognized by the largest number of animals were from the HVR (Table 1). Peptides HV4 and HV5 were each recognized by PBMC from 10 of 16 vaccinated animals, and peptide HV3 was recognized by 7 of 16 vaccinated animals. In addition, peptides HV4 and HV5 stimulated one of the three strongest T-cell responses in 6 of the 10 responders. The HVR of MSP2 has been shown to undergo sequential, segmental gene conversion during A. marginale infection within specifically defined areas of change identified as blocks 1, 2, and 3 (5). These regions are proposed to be important for immune evasion and persistent infection, as changes in T-lymphocyte epitopes within blocks 1 and 2 resulted in significantly reduced T-lymphocyte recognition (6). Thus, peptides HV2 to HV5 spanning amino acids (aa) 171 to 229 constitute an epitope cluster in this important region of the protein shown to undergo sequential, segmental gene conversion in vivo.
During persistent infection, memory T-cell responses to conserved epitopes could activate macrophages and B cells to rapidly generate a protective response and thus provide a means to control the level of rickettsemia and ultimately clinical disease during persistence (7). We found that segments of both N- and C-terminal conserved regions of MSP2 also elicited strong IFN-γ-secreting CD4+ T-lymphocyte responses. Conserved region peptides P7 and P10, which most commonly elicited significant responses, were each recognized by PBMC from 8 of 16 vaccinated animals. Peptide P13 was recognized by PBMC from 7 of the 16 vaccinated animals. As for HVR peptides, commonly recognized peptides P7 and P13 stimulated one of the three strongest T-lymphocyte responses. One or more of peptides P6 to P8 were recognized by 10 of 16 animals, indicating that aa 101 to 170 constitute an epitope cluster in the N-terminal region. A second conserved epitope cluster in MSP2 was found in the C terminus at aa 272 to 361, as one or more of the peptides P10 to P13 were recognized by 14 of 16 vaccinated animals.
To verify the results obtained with the IFN-γ ELISPOT assay, proliferation assays and IFN-γ ELISAs were performed simultaneously using the same samples of PBMC and antigens. In addition, it was possible that certain peptide-specific T lymphocytes did not produce IFN-γ and these responses would be missed by the ELISPOT assay. Proliferation assays were carried out in replicate wells of round-bottomed 96-well plates for 6 days, essentially as described previously (7, 9). When proliferation was measured, 49.5% of the significant peptide-specific responses (57 of 115 responses) were directed at peptides from the conserved N- and C-terminal regions, and 50.4% of these (58 of 115 responses) were directed at peptides spanning the HVR (Table 2). The data in Table 2 are representative of results from independent experiments performed two or three times. IFN-γ ELISA also showed that IFN-γ responses directed at conserved and hypervariable region peptides were not significantly different (data not shown). Thus, these two assays confirmed the results from ELISPOT and also showed that the CD4+ T-cell response was evenly divided between hypervariable and conserved regions. The correlation of peptide-specific responses determined by ELISPOT assay with those determined by proliferation assay or ELISA for each individual and by adjuvant groups was determined by using multivariate linear regression and Pearson's correlation. Interestingly, in cattle with strong responses, such as those in the IL-12 group, there was a significant correlation in recognition of peptides with IFN-γ ELISPOT and proliferation assays (r2 ranged from 0.64 to 0.79 for individuals) or IFN-γ ELISPOT and IFN-γ ELISA (r2 ranged from 0.68 to 0.85 for individuals). However, in animals with weaker responses, the correlation between assays was less significant (data not shown) and similar to that reported for humans exposed to malaria (11).
TABLE 2.
MSP2 peptide-specific proliferative responses by PBMC from MSP2-vaccinated animals
| Antigen or peptide | Stimulation index (SI) by PBMC from indicated calf in the following groupa:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSP2 + alum
|
MSP2 + R2006 + alum
|
MSP2 + 2006 + alum
|
MSP2 + IL-12 + alum
|
|||||||||||||
| 70 (22/7)b | 80 (3/7) | 83 (24/8) | 85 (16/23) | 72 (8/23) | 77 (22/24) | 86 (16/24) | 88 (11/23) | 78 (8/11) | 79 (22/24) | 81 (8/23) | 87 (16/24) | 71 (8/24) | 75 (23/8) | 76 (22/21) | 82 (16/27) | |
| PHAc | 7 | 83 | 42 | 112 | NDd | NDd | NDd | NDd | 6 | 2 | 1 | 4 | 100 | 25 | 23 | 7 |
| TCGF | 2 | 29 | 16 | 87 | 101 | 66 | 2 | 18 | 6 | 5 | 4 | 15 | 1 | 1 | 23 | 0 |
| A. marginale | 2 | 4 | 11 | 6 | 72 | 25 | 3 | 28 | 34 | 11 | 9 | 17 | 150 | 32 | 62 | 28 |
| MSP2 | 5 | 3 | 28 | 26 | 3 | 2 | 1 | 5 | 2 | 2 | 1 | 0 | 125 | 100 | 126 | 39 |
| P1 | 1 | 1 | 2 | 17 | 1 | 2 | 0 | 1 | 2 | 2 | 6 | 1 | 3 | 2 | 10 | 1 |
| P2 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 1 |
| P3 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 5 | 0 |
| P4 | 5 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| P5 | 3 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 4 | 1 | 5 | 1 | 1 | 4 | 106 |
| P6 | 1 | 0 | 5 | 2 | 9 | 2 | 2 | 22 | 2 | 8 | 1 | 12 | 1 | 4 | 2 | 28 |
| P7 | 2 | 4 | 0 | 3 | 2 | 2 | 0 | 2 | 19 | 4 | 1 | 1 | 1 | 173 | 98 | 114 |
| P8 | 2 | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 3 | 0 | 2 | 1 | 2 | 2 | 22 |
| P9 | 4 | 1 | 1 | 1 | 1 | 9 | 1 | 1 | 16 | 5 | 2 | 5 | 1 | 1 | 2 | 31 |
| HV2 | 1 | 2 | 1 | 1 | 61 | 3 | 1 | 0 | 6 | 4 | 2 | 1 | 1 | 4 | 149 | 40 |
| HV3 | 2 | 0 | 12 | 1 | 68 | 2 | 0 | 1 | 21 | 2 | 1 | 1 | 27 | 147 | 147 | 41 |
| HV4 | 1 | 0 | 9 | 1 | 58 | 2 | 1 | 1 | 31 | 4 | 3 | 6 | 215 | 170 | 47 | 5 |
| HV5 | 1 | 3 | 15 | 8 | 7 | 4 | 1 | 1 | 2 | 2 | 1 | 0 | 303 | 148 | 9 | 1 |
| HV6 | 2 | 2 | 8 | 2 | 6 | 3 | 1 | 1 | 2 | 2 | 1 | 1 | 24 | 115 | 12 | 2 |
| HV7 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 3 | 26 | 1 | 12 | 12 |
| HV8 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 3 | 4 | 2 | 3 | 1 | 2 | 1 | 1 |
| HV9 | 2 | 0 | 5 | 9 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 34 | 21 | 2 | 4 |
| HV10 | 0 | 1 | 6 | 1 | 6 | 5 | 2 | 7 | 5 | 10 | 2 | 11 | 68 | 4 | 6 | 1 |
| HV11 | 1 | 0 | 10 | 1 | 6 | 5 | 2 | 8 | 5 | 15 | 1 | 10 | 187 | 4 | 3 | 1 |
| HV12 | 1 | 0 | 9 | 2 | 47 | 42 | 2 | 7 | 2 | 4 | 1 | 11 | 128 | 15 | 2 | 3 |
| P10 | 3 | 1 | 8 | 10 | 19 | 2 | 0 | 8 | 9 | 1 | 1 | 4 | 25 | 8 | 9 | 42 |
| P11 | 1 | 0 | 10 | 1 | 10 | 4 | 1 | 2 | 3 | 8 | 2 | 3 | 20 | 7 | 8 | 7 |
| P12 | 1 | 1 | 2 | 1 | 4 | 1 | 1 | 19 | 27 | 9 | 2 | 16 | 30 | 13 | 119 | 7 |
| P13 | 3 | 1 | 6 | 2 | 1 | 2 | 0 | 3 | 3 | 1 | 1 | 2 | 15 | 1 | 193 | 47 |
| P14 | 1 | 2 | 1 | 3 | 1 | 1 | 0 | 2 | 2 | 2 | 6 | 3 | 2 | 4 | 56 | 18 |
| P15 | 1 | 0 | 5 | 1 | 4 | 1 | 0 | 2 | 12 | 2 | 1 | 4 | 5 | 3 | 15 | 11 |
| P16 | 3 | 1 | 2 | 1 | 17 | 2 | 0 | 3 | 1 | 10 | 2 | 1 | 27 | 4 | 70 | 24 |
| URBC | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 0 | 3 | 3 |
PBMC obtained from calves 5 to 6 months after immunization with MSP2 were cultured for 6 days at a concentration of 2 × 105 cells per well. Results are presented as the mean SI of triplicate responses of cells stimulated with 10 μg of A. marginale per ml, MSP2, or overlapping peptides spanning MSP2. Responses were considered significant if the SI was ≥5 and counts per minute were >1,000; significant responses are in bold. Data for PBMC stimulated with 1.0 or 0.1 μg of antigen or peptide per ml are not shown. TCGF, T-cell growth factor.
DRB3 haplotype.
Positive controls consisted of either a mixture of 1 μg of PHA per ml, 0.01 ng of recombinant human IL-12 per ml, and 0.5 ng of recombinant human IL-18 per ml or T-cell growth factor used at a final dilution of 10%.
ND, not determined.
To map linear IgG epitopes on MSP2, sera were obtained 2 weeks following the last immunization. Immulon II 96-well ELISA plates were coated with 1 μg of antigen or overlapping peptides spanning MSP2 per well. Responses were considered positive if the mean optical density at 450 nm (OD450) of duplicate samples was ≥3 times the mean OD450 to peptide P2, which has repeatedly yielded results similar to those from medium alone in the IgG ELISA. Immune sera from all animals bound significantly to whole MSP2 (Table 3). As hypothesized, 72% of the peptides that were recognized significantly by sera from all animals are located in the HVR. The most commonly recognized peptides from the HVR include peptides HV3 (13 of 16 animals); HV2, HV6, and HV7 (12 of 16 animals); HV5 and HV11 (9 of 16 animals); and HV4 and HV10 (8 of 16 animals). The most commonly recognized peptides from the conserved region include peptides P9 (15 of 16 animals) and P4 and P8 (9 of 16 animals).
TABLE 3.
MSP2 peptide-specific IgG responses following immunization
| Antigen or peptide | OD450 value from the indicated calf in the following groupa:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSP2 + alum
|
MSP2 + R2006 + alum
|
MSP2 + 2006 + alum
|
MSP2 + IL-12 + alum
|
|||||||||||||
| 70 | 80 | 83 | 85 | 72 | 77 | 86 | 88 | 78 | 79 | 81 | 87 | 71 | 75 | 76 | 82 | |
| MSP2 | 1.26 | 0.79 | 0.86 | 1.08 | 1.15 | 0.76 | 1.33 | 1.09 | 1.26 | 1.22 | 1.30 | 1.23 | 1.20 | 1.26 | 1.44 | 1.29 |
| P1 | 0.15 | 0.17 | 0.20 | 0.11 | 0.23 | 0.13 | 0.24 | 0.13 | 0.14 | 0.12 | 0.14 | 0.11 | 0.15 | 0.21 | 0.09 | 0.12 |
| P2 | 0.20 | 0.13 | 0.07 | 0.08 | 0.18 | 0.11 | 0.14 | 0.18 | 0.10 | 0.17 | 0.08 | 0.24 | 0.09 | 0.20 | 0.11 | 0.16 |
| P3 | 0.18 | 0.18 | 0.07 | 0.14 | 0.19 | 0.10 | 0.19 | 0.18 | 0.11 | 0.40 | 0.09 | 0.14 | 0.14 | 0.26 | 0.11 | 0.13 |
| P4 | 1.14 | 0.14 | 0.19 | 0.29 | 0.39 | 0.11 | 1.19 | 0.26 | 0.93 | 1.27 | 0.48 | 0.71 | 0.74 | 0.56 | 0.48 | 0.63 |
| P5 | 0.35 | 0.15 | 0.16 | 0.12 | 0.44 | 0.11 | 1.09 | 0.21 | 0.82 | 1.14 | 0.68 | 0.35 | 0.82 | 0.24 | 1.00 | 0.47 |
| P6 | 0.12 | 0.08 | 0.07 | 0.06 | 0.07 | 0.12 | 0.07 | 0.08 | 0.07 | 0.17 | 0.08 | 0.08 | 0.07 | 0.12 | 0.36 | 0.08 |
| P7 | 0.17 | 0.24 | 0.07 | 0.12 | 0.21 | 0.13 | 0.18 | 0.15 | 0.24 | 0.34 | 0.19 | 0.13 | 0.17 | 0.25 | 0.12 | 0.17 |
| P8 | 1.35 | 0.22 | 0.09 | 0.24 | 0.43 | 0.12 | 1.09 | 0.19 | 0.90 | 1.21 | 0.69 | 0.47 | 0.62 | 0.56 | 1.18 | 0.51 |
| P9 | 0.86 | 0.21 | 0.46 | 0.37 | 0.93 | 0.51 | 1.16 | 0.54 | 0.94 | 1.22 | 0.85 | 0.86 | 0.66 | 0.99 | 1.33 | 1.18 |
| HV2 | 0.75 | 0.17 | 0.15 | 0.24 | 0.83 | 0.11 | 1.06 | 0.51 | 1.13 | 1.10 | 0.66 | 0.98 | 0.91 | 0.93 | 1.24 | 1.03 |
| HV3 | 1.20 | 0.34 | 0.25 | 0.55 | 0.89 | 0.18 | 1.28 | 0.86 | 1.13 | 1.26 | 0.83 | 1.04 | 1.08 | 1.03 | 1.26 | 1.09 |
| HV4 | 0.74 | 0.16 | 0.12 | 0.31 | 0.26 | 0.12 | 1.07 | 0.37 | 0.83 | 1.24 | 0.37 | 0.39 | 0.82 | 0.34 | 1.10 | 0.69 |
| HV5 | 0.85 | 0.22 | 0.20 | 0.24 | 0.48 | 0.15 | 1.08 | 0.39 | 0.89 | 1.27 | 0.41 | 0.64 | 0.98 | 0.41 | 1.22 | 0.89 |
| HV6 | 0.67 | 0.14 | 0.09 | 0.52 | 0.50 | 0.10 | 0.79 | 0.51 | 0.92 | 0.99 | 0.39 | 0.66 | 1.08 | 0.84 | 0.99 | 0.94 |
| HV7 | 0.77 | 0.13 | 0.17 | 0.47 | 0.62 | 0.10 | 1.08 | 0.35 | 1.00 | 1.19 | 0.38 | 0.52 | 1.06 | 0.81 | 1.08 | 1.00 |
| HV8 | 0.92 | 0.17 | 0.16 | 0.42 | 0.61 | 0.12 | 1.03 | 0.28 | 0.97 | 1.24 | 0.87 | 0.61 | 0.90 | 0.53 | 1.17 | 0.59 |
| HV9 | 0.34 | 0.24 | 0.11 | 0.25 | 0.29 | 0.11 | 0.61 | 0.18 | 0.60 | 0.77 | 0.28 | 0.21 | 0.42 | 0.33 | 0.23 | 0.42 |
| HV10 | 0.78 | 0.23 | 0.11 | 0.09 | 0.15 | 0.14 | 1.14 | 0.23 | 0.85 | 0.88 | 0.27 | 0.74 | 0.60 | 0.43 | 0.38 | 0.42 |
| HV11 | 0.33 | 0.83 | 0.18 | 0.26 | 0.43 | 0.15 | 0.97 | 0.25 | 0.83 | 1.04 | 0.49 | 0.78 | 0.53 | 0.40 | 0.70 | 0.58 |
| HV12 | 0.39 | 0.27 | 0.22 | 0.30 | 0.39 | 0.17 | 0.70 | 0.20 | 0.75 | 0.98 | 0.35 | 0.66 | 0.31 | 0.21 | 0.46 | 0.40 |
| P10 | 0.21 | 0.17 | 0.08 | 0.08 | 0.13 | 0.16 | 0.28 | 0.14 | 0.29 | 0.75 | 0.10 | 0.31 | 0.11 | 0.27 | 0.19 | 0.14 |
| P11 | 0.20 | 0.21 | 0.07 | 0.17 | 0.29 | 0.11 | 0.21 | 0.13 | 0.27 | 0.51 | 0.20 | 0.21 | 0.17 | 0.28 | 0.10 | 1.05 |
| P12 | 0.22 | 0.26 | 0.07 | 0.26 | 0.23 | 0.10 | 0.21 | 0.16 | 0.22 | 0.48 | 0.26 | 0.20 | 0.19 | 0.29 | 0.12 | 0.23 |
| P13 | 0.17 | 0.22 | 0.08 | 0.13 | 0.31 | 0.12 | 0.19 | 0.14 | 0.14 | 0.30 | 0.11 | 0.13 | 0.21 | 0.15 | 0.09 | 0.22 |
| P14 | 0.18 | 0.13 | 0.08 | 0.07 | 0.12 | 0.17 | 0.14 | 0.10 | 0.18 | 0.43 | 0.08 | 0.14 | 0.19 | 0.17 | 0.10 | 0.16 |
| P15 | 0.19 | 0.14 | 0.08 | 0.10 | 0.35 | 0.12 | 0.28 | 0.13 | 0.16 | 0.39 | 0.21 | 0.16 | 0.15 | 0.20 | 0.18 | 0.21 |
| P16 | 0.13 | 0.13 | 0.06 | 0.09 | 0.13 | 0.12 | 0.06 | 0.11 | 0.10 | 0.30 | 0.22 | 0.10 | 0.12 | 0.14 | 0.07 | 0.14 |
MSP2-specific IgG was measured by ELISA and determined by the OD values at 450 nm from serum of calves collected 2 weeks after the sixth immunization with MSP2 and respective adjuvant. Responses are considered significant if the mean OD450 of serum incubated with antigen is greater than three times the background OD. Values for significant responses are in bold. The background is the mean OD450 value of duplicate samples incubated with the known negative peptide P2, which has an OD450 similar to that of medium alone.
Evasion of immunity to A. marginale is believed to result, at least in part, from antigenic variation in the central HVR of MSP2 (6, 12, 23, 25). Because antigenic variation can lead to loss of both T- and B-lymphocyte recognition (6, 12), this study hypothesized that MSP2-specific T- and B-cell responses would be directed predominantly against epitopes within the HVR, which could foster organism persistence. To test the hypothesis, T-cell responses to MSP2 epitopes were determined by using PBMC, rather than cultured T-cell lines, from 16 animals expressing a broad repertoire of MHC class II molecules and by performing three separate assays to detect the largest number of potential epitopes. Our results showed that there was no significant difference in the number of T-cell stimulatory peptides in conserved and hypervariable regions of MSP2 or in the strength of the T-lymphocyte responses to peptides from conserved or hypervariable regions, and our first hypothesis is rejected. However, in accordance with our second hypothesis, the majority of peptides recognized by immune sera are located within the HVR. This serologic immunodominance of the MSP2 HVR supports the importance of antigenic variation in maintaining persistent infection. Furthermore, the preferential recognition of HVR B-cell epitopes is consistent with this hydrophilic region being surface exposed (24). Similarly, studies with related Ehrlichia chaffeensis showed that the amino-terminal hypervariable region of OMP-1 was also immunodominant for mice and humans and contained linear B-cell epitopes (19).
T cells from the majority of MSP2-immunized cattle recognized two T-lymphocyte epitope clusters within the conserved regions. Furthermore, one of these (aa 272 to 361, represented by peptides P10 to P13) is completely conserved in another antigenically variant surface protein, MSP3 (1, 4, 12). Thus, these immunodominant, conserved sequences could be important for providing accelerated memory T-cell responses during persistent infection with organisms that continually undergo variation in MSP2 and MSP3 (1, 4, 12). Although these results were obtained following immunization with MSP2 rather than infection with A. marginale, the recognition by T cells of defined epitope clusters in MSP2 and the preferential recognition by B cells of hydrophilic, variable region epitopes, regardless of the magnitude of the individual MSP2-specific responses, are consistent with protein structure influencing the pattern of epitope recognition.
Acknowledgments
We are grateful to Bev Hunter, Emma Karel, Kim Kegerreis, and Shelley Whidbee for excellent technical assistance.
This research was supported by NIH grants AI44005 and AI49276 and USDA NRICGP grant 02-35204-12352.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68:6133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-gamma by CD4 T cells is essential for resolving Ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 4.Brayton, K. A., P. F. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 71:6627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. C., K. A. Brayton, C. M. Styer, and G. H. Palmer. 2003. The hypervariable region of Anaplasma marginale major surface protein 2 (MSP2) contains multiple immunodominant CD4+ T lymphocyte epitopes that elicit variant-specific proliferative and IFN-gamma responses in MSP2 vaccinates. J. Immunol. 170:3790-3798. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., G. H. Palmer, K. A. Brayton, P. F. Meeus, A. F. Barbet, K. A. Kegerreis, and T. C. McGuire. 2004. CD4+ T lymphocytes from Anaplasma marginale major surface protein 2 (MSP2) vaccinees recognize naturally processed epitopes conserved in MSP3. Infect. Immun. 72:3688-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, W. C., D. Zhu, V. Shkap, T. C. McGuire, E. F. Blouin, K. M. Kocan, and G. H. Palmer. 1998. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect. Immun. 66:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriks, I. S., G. H. Palmer, T. C. McGuire, D. R. Allred, and A. F. Barbet. 1989. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J. Clin. Microbiol. 27:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan, K. L., E. A. Lee, M. B. Gravenor, W. H. Reece, B. C. Urban, T. Doherty, K. A. Bojang, M. Pinder, A. V. Hill, and M. Plebanski. 2001. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J. Immunol. 167:4729-4737. [DOI] [PubMed] [Google Scholar]
- 12.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganta, R. R., M. J. Wilkerson, C. Cheng, A. M. Rokey, and S. K. Chapes. 2002. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 70:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ijdo, J. W., W. Sun, Y. Zhang, L. A. Magnarelli, and E. Fikrig. 1998. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect. Immun. 66:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemshead, J. T., L. Heath, F. M. Gibson, F. Katz, F. Richmond, J. Treleaven, and J. Ugelstad. 1986. Magnetic microspheres and monoclonal antibodies for the depletion of neuroblastoma cells from bone marrow: experiences, improvements and observations. Br. J. Cancer 54:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58:1117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 19.Li, J. S., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 20.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy, C. I., J. R. Storey, J. Recchia, L. A. Doros-Richert, C. Gingrich-Baker, K. Munroe, J. S. Bakken, R. T. Coughlin, and G. A. Beltz. 1998. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect. Immun. 66:3711-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberle, S. M., G. H. Palmer, A. F. Barbet, and T. C. McGuire. 1988. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect. Immun. 56:1567-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer, G. H., J. R. Abbott, D. M. French, and T. F. McElwain. 1998. Persistence of Anaplasma ovis infection and conservation of the msp-2 and msp-3 multigene families within the genus Anaplasma. Infect. Immun. 66:6035-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer, G. H., G. Eid, A. F. Barbet, T. C. McGuire, and T. F. McElwain. 1994. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect. Immun. 62:3808-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer, G. H., F. R. Rurangirwa, K. M. Kocan, and W. C. Brown. 1999. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol. Today 15:281-286. [DOI] [PubMed] [Google Scholar]
- 26.Shkap, V., T. Molad, K. A. Brayton, W. C. Brown, and G. H. Palmer. 2002. Expression of major surface protein 2 variants with conserved T-cell epitopes in Anaplasma centrale vaccinates. Infect. Immun. 70:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoda, L. K., D. S. Zarlenga, A. Hirano, and W. C. Brown. 1999. Cloning of a cDNA encoding bovine interleukin-18 and analysis of IL-18 expression in macrophages and its IFN-gamma-inducing activity. J. Interferon Cytokine Res. 19:1169-1177. [DOI] [PubMed] [Google Scholar]
- 28.Tuo, W., G. H. Palmer, T. C. McGuire, D. Zhu, and W. C. Brown. 2000. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect. Immun. 68:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Y., G. H. Palmer, J. R. Abbott, C. J. Howard, J. C. Hope, and W. C. Brown. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307-3318. [DOI] [PubMed] [Google Scholar]
- 30.Zhi, N., N. Ohashi, Y. Rikihisa, H. W. Horowitz, G. P. Wormser, and K. Hechemy. 1998. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J. Clin. Microbiol. 36:1666-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]