Abstract
To investigate the factors associated with sexually transmitted infection and Human Immunodeficiency Virus (STI-HIV) co-infection among men who have sex with men (MSM). A total of 357 HIV-infected participants (84 STI-HIV co-infection and 273 HIV infections only) were recruited from Jiangsu, China. Logistic regression analyses were used to estimate the related factors associated with STI-HIV co-infection. Marginal structural models were adopted to estimate the effect of transmission drug resistance (TDR) on STI-HIV co-infection. For all participants, logistic regression analyses revealed that those who diagnosed with HIV-1 for longer duration (≥1.8 years) were significantly associated with reduced STI-HIV co-infection risk (OR = 0.55, 95%CI: 0.32–0.96, P = 0.036). In further stratification analysis by antiretroviral therapy (ART), individuals with longer duration showed consistent significant associations with STI-HIV co-infection risk (OR = 0.46, 95%CI: 0.26–0.83, P = 0.010) among MSM with ART-naïve status. In addition, significant reduced risk for STI-HIV co-infection (OR = 0.98, 95%CI: 0.96–0.99, P = 0.010) were observed in younger (under the average age of 31.03) MSM of the same group. Interestingly, we also found TDR was significantly associated with an increased risk of STI-HIV co-infection risk (OR = 3.84, 95%CI: 1.05–14.03, P = 0.042) in ART-naïve group. Our study highlights a pattern of STI-HIV co-infection among MSM in China and indicates that targeted interventions aimed at encouraging TDR monitoring in MSM with early HIV infection are warranted.
Introduction
Sexually Transmitted Infections and Human Immunodeficiency Virus (STI-HIV) co-infected individuals continue to expand at a rapid rate among men who have sex with men (MSM) in China. A previous review indicated that the syphilis prevalence in China among MSM increased from 6.8% (2003–2004) to 13.5% (2007–2008), whereas HIV prevalence rate increased from 1.3% (2003–2004) to 4.7% (2007–2008)[1]. STI also seems to contribute significantly to HIV burden inasmuch as it may facilitate HIV transmission by blocking protective mucosal barriers and recruiting susceptible immune cells (e.g, CD4+ T cells, macrophages) to the site of infection[2]. Similarly, HIV can accelerate progression of other STI and vice versa. When subjects are immune compromised, STI-HIV co-infection are more difficult to treat and disease periods may extend[3]. With the high incidence of syphilis among MSM (approximately 17 per 100 person years), and the heavy burden of STIs to HIV in China, it is important to figure out how these STI-HIV co-infection disease states impacted[4].
China was witnessing an increase in the proportion of MSM, especially they may play a bridge role in the spread of STI/HIV. In recent years, sexual exposure has become the primary route of HIV infection in China, and is the mode of transmission for STI infection[5]. Due to penile-anal intercourse and risk-related behavior, an approximately 45-fold higher risk of acquiring HIV was observed among MSM than other general male population in China[6]. STIs, associated with unsafe sex practices, intrinsic morbidity, have increased among MSM, while a recent research reported HIV-syphilis co-infection have increased from 1.4% during 2005–2006 to 2.7% during 2007–2008 among MSM in China[1]. Since STI and HIV share almost the same transmission routes and risk behaviors, strategies to control the epidemic of these infections should be combined together among MSM[7].
Currently, quite a few studies have aimed to monitor sexual behavior, STI-related knowledge, sexual network characteristics and health care consultation in HIV-infected MSM[8],[9],[10]. However, very few studies have explored molecular risk factors associated with STI-HIV co-infected MSM. Across the study, we analyzed the factors of molecular & socio-demographic risk factors among STI-HIV co-infected MSM, focusing on potential molecular risk factors. In addition, to overcome the time-varying covariates, we used marginal structural models to assessment the effect of risk factors on STI-HIV co-infected MSM. In studies, estimation of the causal effect of an exposure on an outcome may be biased because of confounding, i.e. covariates associated with treatment may also be associated with the potential response, so that the response differences cannot be attributed directly to the exposure. Proper estimation of causal effects must account for confounding. In studies where the exposure does not change, the traditional method of analysis is to model the probability of disease as a function of exposure. However, with a time-varying exposure, these traditional methods may be biased if time-varying covariates are simultaneously confounders and intermediates, if covariates are predictors of the outcome and also predict subsequent exposure, and past exposure history predicts resulting covariate level[11]. Such covariates are called time-dependent confounders, and they pose unique analytical challenges requiring specialized methods. To overcome the limitations of this standard approach, Robins and his colleagues[12]developed the marginal structural model. The advantage of MSM is that it can be adapted to unbiasedly estimate the causal effect of outcomes.
Materials and methods
Study population and blood sample
A total of 357 HIV-infected participants (84 STI-HIV co-infection and 273 HIV infection only controls) were recruited from 5 cities (Nantong, Suzhou, Taizhou, Wuxi and Yancheng) 2012 to 2015 in Jiangsu, China. HIV-infected individuals who had at least one of the STI were categorized into case group, while those without STI were categorized into control group. Demographic data including age, martial status, employment, and education status, HIV-1 subtype, CD4 at baseline and ART were directly collected from history cards of database of AIDS Direct Reporting Network System in the Center for Disease Control and Prevention (CDC) of 5 corresponding cities. Duration on HIV diagnosis was defined as the periods between the date of HIV diagnosis and the date of HIV-infected participants recruited, and categorized as two groups: ≤1.8 years and >1.8 years (the median of duration time). Marriage status was categorized as two groups: marital status and non-married status. CD4 count at baseline were categorized into three groups: < 500 cells/mm3, ≥ 500 cells/mm3 and unclear. The subtypes of HIV were categorized into three groups: CRF 01_AE, CRF 07_BC and others. The others were: CRF67_01B, CRF68_01B, CRF55_01B, CRF08_BC and subtype B. The information of STI were all confirmed by hospitals of county level and above after HIV diagnosis. Considering the influence of healthseeking behaviors, we further tested the association of the factors with STI-HIV co-infection risk. Two distinct subgroups were created: those taking ART and those without ART. Finally, blood samples of the 357 participants were obtained at HIV diagnosis, and plasma was isolated within 4 hours of sampling and stored at −80°C until HIV sequence genotyping.
Viral RNA extraction, PCR amplification and sequencing
HIV-1 genome RNA was extracted from 200 μl of stored plasma specimens using the QIAmp Viral RNA Mini kit (Qiagen, Valencia, CA, USA) as Manufacturer’s instructions. Reverse transcription and nested polymerase chain (nPCR) amplification for partial genes of pol and env were performed by a home brew PCR procedure as described in our previous reports[13,14]. A one-tube reverse transcriptase polymerase chain reaction kit (GoldScript one-step RT-PCR kit, Life Technologies, USA), and PCR kit (TaKaRa Ex Taq Kit, Takara Biotechnology Co, Ltd; Dalian, China) were used according to the manufacture’s recommendations for amplification of the HIV-1 pol gene (protease 1–99 amino acids and part of reverse transcriptase 1–254 amino acids). The PCR amplification was carried out in a thermal cycler (GeneAmp PCR System 9700, Applied Biosystems, USA).
Drug resistance
HIV-1 drug resistance mutations were determined according to the WHO 2009 Surveillance Drug Resistance Mutations (SDRM) list using the current Calibrated Population Resistance tool v5.0 of the Stanford University HIV Drug Resistance Database (http://cpr.stanford.edu/cpr.cgi)
Statistical analyses
The χ2 test or fisher exact test for categorical variables were used to analyze distribution differences of demographic characteristics and selected variables between cases and controls. Odd ratio (OR) and their 95% confidence intervals (CI) were calculated by using logistic regression analyses to evaluate the association between STI-HIV co-infection and its related factors with an adjustment for age, marriage status, duration time, education, occupation and DR. To make results more interpretable, the marginal structural models were used to estimate the impact of TDR on STI-HIV co-infection.
All the statistics were performed by SPSS 16 software (IBM Company, New York, USA). Marginal Structural Models analyses were conducted using SAS software Version 9.3.
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Review Board at the Human Medical Research Ethics Committee of the Center for Disease Control and Prevention in Nantong, Suzhou, Taizhou, Wuxi and Yancheng. All subjects were anonymous and provided written informed consent. The methods were carried out in accordance with the approved guidelines.
Results
Demographic characteristics of participants
A total of 357 participants were enrolled in the study, among which, 273 (76.47%) were infected with HIV only and 84 (23.53%) were STI-HIV co-infection. The distribution of selected characteristics between STI-HIV co-infection cases and controls among MSM were summarized in Table 1. Of these subjects, 92(25.77%) were on marital status, 265 (74.23%) were at non-married status. There were 102 (28.57%) participants had primary or junior school education, 116 (32.49%) were high school and 139 (38.94%) were post-graduate. HIV-infected individuals in our study were involved in different occupations in various proportions, in which students accounted for 5.60%. For the genotyping test, 214 (59.94%) infected with CRF01_AE, 80 (22.41%) infected with CRF07_BC, while other subtype (CRF67_01B, CRF68_01B, CRF55_01B, CRF08_BC, subtype B) were detected in 63 (17.65%) participants.
Table 1. Characteristics of 357 HIV infected MSM in Jiangsu.
| Variable | Total sample, (n = 357), n (%) | STI-HIV co-infection, (n = 84), n (%) | HIV infection without STI, (n = 273), n (%) | χ2 | p-value |
|---|---|---|---|---|---|
| Age at diagnosis, years; Mean (SD) | 31.14±10.05 | 31.38±9.34 | 31.06±10.27 | 0.811 | 0.368 |
| <30+ | 198 (55.46) | 43 (51.19) | 155 (56.78) | ||
| ≥30 | 159 (44.54) | 41 (48.81) | 118 (43.22) | ||
| Duration on diease++, years | 4.339 | 0.037 | |||
| ≤1.8 | 225 (63.03) | 61 (94.05) | 164 (60.07) | ||
| >1.8 | 132 (36.97) | 23 (5.95) | 109 (39.93) | ||
| Marriage Status | 0.149 | 0.700 | |||
| Non-married | 265 (74.23) | 61 (72.62) | 204 (74.73) | ||
| Married | 92 (25.77) | 23 (27.38) | 69 (25.27) | ||
| Education | 0.081 | 0.960 | |||
| Primary or junior school | 102 (28.57) | 23 (27.38) | 79 (28.94) | ||
| High school | 116 (32.49) | 27 (32.14) | 89 (32.60) | ||
| College or above | 139 (38.94) | 34 (40.48) | 105 (38.46) | ||
| Occupation | 1.906 | 0.753 | |||
| Students | 20 (5.60) | 4 (4.76) | 16 (5.86) | ||
| Workers and farmers | 123 (34.45) | 27 (32.14) | 96 (35.16) | ||
| Civil servants* | 27 (7.56) | 4 (4.76) | 23 (8.42) | ||
| Service industry** | 104 (29.13) | 26 (30.95) | 78 (28.57) | ||
| Others*** | 83 (23.25) | 23 (27.38) | 60 (21.98) | ||
| CD4 count, cells/mm3 | 1.513 | 0.469 | |||
| < 500 | 222 (62.18) | 48 (57.14) | 174 (63.74) | ||
| ≥ 500 | 123 (34.45) | 32 (38.10) | 91 (33.33) | ||
| unclear | 12 (3.37) | 4 (4.76) | 8 (2.93) | ||
| Region | 2.899 | 0.235 | |||
| Southern Jiangsu& | 250 (70.03) | 65 (77.38) | 185 (67.77) | ||
| Middle Jiangsu&& | 86 (24.09) | 14 (16.67) | 72 (26.37) | ||
| Northern Jiangsu&&& | 21 (5.88) | 5 (5.95) | 16 (5.86) | ||
| ART | 0.081 | 0.960 | |||
| Yes | 34 (9.52) | 9 (10.71) | 25 (9.16) | ||
| No | 323 (90.48) | 75 (89.29) | 248 (90.84) | ||
| Drug resistance | 3.025 | 0.105 | |||
| Yes | 14 (3.92) | 6 (7.14) | 8 (2.93) | ||
| No | 343 (96.08) | 78 (92.86) | 265 (97.07) | ||
| Subtype | 0.228 | 0.892 | |||
| 01_AE | 214 (59.94) | 50 (59.52) | 164 (60.07) | ||
| 07_BC | 80 (22.41) | 18 (21.43) | 62 (22.71) | ||
| Others# | 63 (17.65) | 16 (19.05) | 47 (17.22) | ||
+ The median of age;
++ Estimated as the period since the first seropositive HIV-1 antibody test date;
* Carders and staffs serving for governments, enterprises, school and hospital;
** People serving for commercial, catering and hair salon;
*** The unemployed, retirees and unclear;
& Suzhou, Wuxi;
&& Nantong, Taizhou;
&&& Yancheng;
# others refer to 67_01B, 68_01B, 55_01B, 08_BC, B
The mean age at diagnosis was 31.14 (±10.05) years old with a range of 18 to 67 years. DR was not significant with subtype of HIV-1 between two groups (P >0.05). Compared with controls, STI-HIV co-infected cases had a higher rate of short duration time (≤1.8 years) on diagnosis (94.05% vs 60.07%, P = 0.037). In addition, the education, occupation, CD count at baseline, region and ART between cases and controls had not statistic difference (P >0.05).
Factors associated with STI-HIV co-infection
All of the variables between STI-HIV co-infection cases and controls were included in the logistic regression model (Table 2). For all participants, logistic regression analyses revealed that those who diagnosed with HIV-1 for longer course of disease (≥1.8 years) were significantly associated with STI-HIV co-infection risk (OR = 0.55, 95%CI: 0.32–0.96, P = 0.036 with adjustment for age, occupation, marriage status, education and DR).
Table 2. Logistic regression analyses of related factors associated with STI-HIV co-infection among MSM in Jiangsu.
| Variable | STI-HIV co-infection rate, (N = 357), n (%) | STI-HIV co-infection rate on ART, (N = 34), n (%) | STI-HIV co-infection rate on ART-naïve (N = 323), n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | p-value | n/N (%) | OR (95% CI) | p-value | n/N (%) | OR (95% CI) | p-value | |
| Age at diagnosis, years; Mean (SD) | 31.14±10.05 | 0.99 (0.97–1.01) | 0.263 | 32.18±10.77 | 0.91 (0.78–1.06) | 0.231 | 31.03±9.98 | 0.98 (0.96–0.99) | 0.010 |
| <30+ | 43/198 (21.72) | ref | 5/16 (31.25) | ref | 38/182 (20.88) | ref | |||
| ≥30 | 41/159 (25.79) | 1.28 (0.71–2.31) | 0.412 | 4/18 (22.22) | 1.06 (0.13–8.71) | 0.956 | 37/141 (26.24) | 1.34 (0.71–2.50) | 0.365 |
| Duration on diease++, years | |||||||||
| <1.8 | 61/225 (27.11) | ref | 6/16 (37.50) | ref | 55/209 (26.32) | ref | |||
| ≥1.8 | 23/132 (17.42) | 0.55 (0.32–0.96) | 0.036 | 3/18 (16.67) | 0.28 (0.03–2.86) | 0.283 | 20/114 (17.54) | 0.46 (0.26–0.83) | 0.010 |
| Marriage Status | |||||||||
| Non-married | 61/265 (23.02) | ref | 7/24 (29.17) | ref | 54/241 (22.41) | ref | |||
| Married | 23/92 (25.00) | 1.20 (0.63–2.28) | 0.588 | 2/10 (20.00) | 1.25 (0.08–20.39) | 0.874 | 21/82 (25.61) | 1.40 (0.72–2.72) | 0.325 |
| Education | |||||||||
| Primary or junior school | 23/102 (22.55) | 0.84 (0.43–1.66) | 0.621 | 1/12 (8.33) | 0.10 (0.00–2.20) | 0.144 | 22/90 (24.44) | 0.99 (0.50–1.97) | 0.054 |
| High school | 27/116 (23.28) | 0.84 (0.45–1.56) | 0.583 | 3/10 (30.00) | 0.37 (0.03–4.62) | 0.441 | 24/106 (22.64) | 0.77 (0.41–1.44) | 0.631 |
| College or above | 34/139 (23.46) | ref | 5/12 (41.67) | ref | 29/127 (22.83) | ref | |||
| Occupation | |||||||||
| Workers and farmers | 27/123 (21.95) | ref | 3/12 (25.00) | ref | 24/111 (21.62) | ref | |||
| Students | 4/20 (20.00) | 0.70 (0.20–2.49) | 0.581 | 0/2 (0.00) | NA | NA | 4/18 (22.22) | 0.52 (0.16–1.71) | 0.281 |
| Civil servants* | 4/27 (14.81) | 0.56 (0.17–1.86) | 0.347 | 0/1 (0.00) | NA | NA | 4/26 (15.38) | 0.49 (0.15–1.59) | 0.238 |
| Service industry** | 26/104 (25.00) | 1.07 (0.61–1.89) | 0.817 | 6/17 (35.29) | 1.49 (0.13–16.66) | 0.747 | 20/87 (22.99) | 0.80 (0.46–1.37) | 0.413 |
| Others*** | 23/83 (27.71) | 1.04 (0.38–2.91) | 0.938 | 0/2 (0.00) | NA | NA | 23/81 (28.40) | 1.08 (0.38–3.09) | 0.879 |
| Drug resistance | |||||||||
| Yes | 6/14 (42.86) | 2.69 (0.86–8.40) | 0.089 | 1/3 (33.33) | 2.09 (0.09–47.03) | 0.644 | 5/11 (45.45) | 3.84 (1.05–14.03) | 0.042 |
| No | 78/343 (22.74) | ref | 8/31 (25.81) | ref | 70/312 (22.44) | ref | |||
+ The median of age;
++ Estimated as the period since the first seropositive HIV-1 antibody test date;
* Carders and staffs serving for governments, enterprises, school and hospital;
** People serving for commercial, catering and hair salon;
*** The unemployed, retirees and unclear
In further stratification analysis by ART, individuals with longer duration showed consistent significant associations with STI-HIV co-infection risk (OR = 0.46, 95%CI: 0.26–0.83, P = 0.010 with adjustment for age, occupation, marriage status, education and TDR) among MSM with ART-naïve status. In addition, significant reduced risk for STI-HIV co-infection (OR = 0.98, 95%CI: 0.96–0.99, P = 0.010 with adjustment for duration time on diagnosis, occupation, education, marriage status, and TDR) were observed in young (under the average age of 31.03) MSM of the same group. Interestingly, we also found TDR was significantly associated with an increased risk of STI-HIV co-infection risk (OR = 3.84, 95%CI: 1.05–14.03, P = 0.042 with adjustment for age, duration time on diagnosis, marriage status, education, occupation) of this group. However, no obvious variable was associated with STI-HIV co-infection among antiretroviral experienced MSM (Table 2).
Distribution of antiretroviral drug resistance
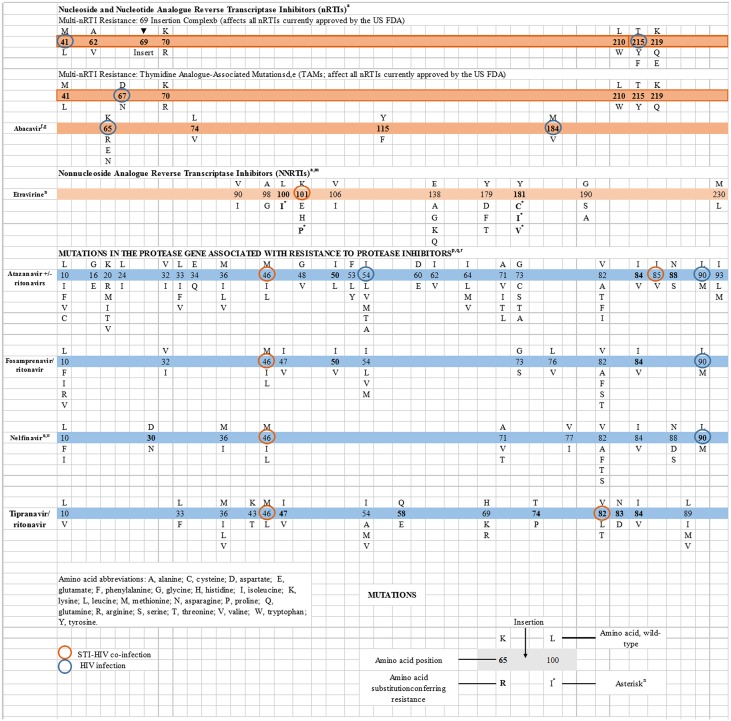
DR were detected in 14 of 357 subjects (3.9%). Among the 34 ART-receipt individuals, 3(8.8%) had evidence of resistance to at least one antiretroviral drug. For HIV-infected participants of ART-naïve status, 11(3.4%) with transmitted drug resistance were found. Resistance was observed most frequently for the PI (atazanavir +/- ritonavirs, fosamprenavir/ ritonavir, nelfinavir, tipranavir/ ritonavir). (Fig 1). We observed STI-HIV co-infection more frequently in participants with TDR (6.7%) and M46L was detected at higher frequency (40.0%) than any other mutation in STI-HIV co-infected participants.
Fig 1. Drug resistance mutation among HIV-infected MSM in Jiangsu.
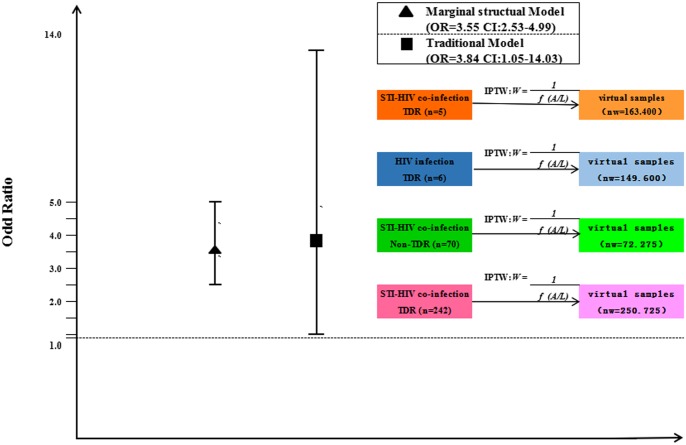
Estimation of Marginal Structural Models
Applying Marginal Structural Models, TDR was estimated to increase frequency in STI-HIV co-infection (OR = 3.55, 95%CI: 2.53–4.99, P<0.001). Though it was slightly lower than estimate from traditional models (OR = 3.84, 95%CI: 1.05–14.03, P = 0.042), the marginal structural models significantly increased the statistical power of this study (Fig 2).
Fig 2. Odd ratio from both traditional model and marginal structural model (MSM) for the risk of STI-HIV co-infection.
Discussion
In our research group, the prevalence rate of STI among HIV-infected MSM was 23.52% (84/357), this burden of STI is higher than Asia (median 17.4%) and Europe (median 14.7%), and greater than a meta-analysis (median 16.3%) in all HIV population in China[2]. The difference may result from different target population, since all the subjects in our study were MSM, who were regarded as high-risk behavior group of STI infection especially for anal intercourse (AI). One study showed that AI may give rise to STI (OR = 2.42). Additional, when compared with population of Asian, Europe and China, the sample size of our studied might not have been large enough to achieve a convincing conclusion about the prevalence of STI among HIV-infected MSM.
The logistic analysis found that subjects with longer duration had a significant decreased risk for STI-HIV co-infection risk with a total of 84 cases and 273 controls. After stratification by ART, two variables of age at diagnosis and duration time on diagnosis showed a significant association with STI-HIV co-infection, and TDR was associated with a significant increased STI-HIV co-infection risk in a case-control study of 75 STI-HIV co-infection cases and 248 HIV infection controls among MSM with ART-naïve status. In contrast, there is no significant association with STI-HIV co-infection risk among antiretroviral experienced MSM. To our knowledge, this is the first association study of TDR and STI-HIV co-infection risk among MSM.
In our research, we found a significant association of duration time with STI-HIV co-infection risk, which indicated MSM with early HIV infection may have potential threats of STI/HIV transmission to their partners, thus it is necessary that HIV care providers may be more pro-active in assessing ongoing risk behavior and suggesting STI testing among their clients. In addition, a recent research indicated that defective proviruses accumulate rapidly within the early period of infection to make up over 93% of all proviruses, hence promote harmful immune activation[15]. It is plausible that the weak individual immune system could further lead STI in HIV infected individuals, which may partly explain our result.
In addition, we also found young HIV infected MSM was associated with a significant decreased STI-HIV co-infection risk. There are several possible reasons for the results in our study. First, the young HIV-1 infected MSM were more acceptable to take protective measures once they had some information that suggested its potential as a useful intervention. Second, when compared with older population, the immune systems of younger population were stronger. The different level of immune system may be the potential reason for different ssusceptibility to STI among HIV-infected MSM.
Interestingly, our study showed STI-HIV co-infection occurred more frequently in individuals with TDR population. Previous studies showed a significant correlation between DR mutations and overlapping polymorphisms in the reverse transcriptase (RT) and protease (PR) viral genes[16], which can partly explain our result, high variation of HIV virus or rebuilt the HIV-specific CD4 or CD8 T-cell damage immune response for HIV infected individuals[17]. Key research priorities recently identified suppression of viral replication can reduce harmful immune activation, slowing pathogenesis, and stem the inhibition of CD4+T cells (‘treatment-as-prevention’) CD4 + T cells generated IL—2 and IFN–γ, enhancing the individual immune system, which further protect HIV infected individuals from STI. In particular, up to date, no direct experimental evidence has been reported for the hypothesis and future studies are warranted to prove the point[18],[19]
The prevalence of TDR among MSM in our study was 3.4% (11/323), which was lower than that of worldwide (10 ~ 20% of HIV-1 infection worldwide)[20]. According to the present investigation, the occurrence of drug resistance mutations both in viruses from individuals receiving ART and in transmitted viruses to ART-naïve individuals is increasing at a global level[21],[22] It is noteworthy that the prevalence of TDR is low in resource-limited settings, however it maybe show an increasing trend in recent years. That would result a heavy disease burden of STI to HIV infection as access to ART has been expanding[23]. Considering the dramatic health threats by sexually transmitted co-infections posing to people living with HIV/AIDS in recent years, the current study suggested surveillance drug resistance mutations (SDRMs) as a national surveillance system which was largely consistent with our suggestion[24]. Other studies further emphasize the association between TDR and HIV subtype among HIV infected MSM, which may be meaningful for STI control[25],[26],[27],[28].
It is possible that the results of our study could be affected by time-depending bias given that some of the participants were of a long duration time on disease and hence could not afford TDR or not which consequently switch to STI-HIV co-infection. Therefore, we used marginal structural models to estimate the causal effect of TDR on STI-HIV co-infection[29]. For MSM participants, TDR was estimated to increase the possibility to infect with STI in HIV by 3.552. This was slightly lower than the estimates from traditional association model. The results indicated that TDR status play an exceedingly promotive influence on STI-HIV co-infection after controlling time confounder.
Our study results should be interpreted in the context of some limitation. First, STIs can present in individuals without causing symptoms, so-called asymptomatic infections, and still potentially be transmitted to others, resulting in selection bias[30]. Second, the number of HIV infected MSM on ART status in our study was limited, leading to a difficult to further draw conclusion about acquired drug resistance (ADR) associated with STI-HIV co-infection. Studies with larger sample sizes are needed to evaluate our findings.
Despite the limitation above, our research also had several advantages. First, we shift the attention from sexual behaviors such as condom use and number of sex partners to molecular level of HIV for STI-HIV co-infection. Furthermore, the supplementary data analyse (marginal structural models) to control for confounding improved the robustness of the finding. When exposure level in a variable included in the research reaches a certain value, leading to all samples under a fixed exposure levels, Inverse Probability Of Treatment Weighting (IPTW) would produce deviation. It makes the strong assumption of no unmeasured confounders[31]. The marginal structural models can correctly adjust for measured time-varying confounders that were affected by exposure.
Conclusions
In summary, this research indicates the role of TDR in STI-HIV co-infection risk in HIV infected MSM as well as age and duration time with STI-HIV co-infection risk. Our study highlights a pattern of STI-HIV co-infection among MSM in Jiangsu and indicates that targeted interventions aimed at encouraging TDR monitoring in MSM with early HIV infection are urgently needed. Moreover, further researches should focus on TDR mechanisms on the etiology of STI-HIV co-infection development and illuminate TDR monitoring can be targeted for prevention strategies to mitigate the obviously increased prevalence of STI-HIV co-infection among MSM.
Supporting information
(XLS)
Acknowledgments
The authors would like to express their gratitude to staffs at Wuxi CDC, Nantong CDC, Taizhou CDC, Yancheng CDC, Suzhou CDC for their efforts for subject recruitment and blood sample collection. We are also particularly grateful to Ms. Yi Lin, Shanghai Municipal CDC, for her assistance for instruction in laboratory skills.
Abbreviations
- ART
antiretroviral therapy
- DR
Drug resistance
- IPTW
Inverse Probability Of Treatment Weighting
- MSM
Men who have sex with men
- PR
protease
- RT
reverse transcriptase
- SDRM
Surveillance drug resistance mutations
- STI
Sexually transmitted infection
- TDR
Transmission drug resistance
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Chow EP, Wilson DP, Zhang L. HIV and syphilis co-infection increasing among men who have sex with men in China: a systematic review and meta-analysis. PloS one. 2011; 6:e22768 10.1371/journal.pone.0022768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalichman SC, Pellowski J, Turner C. Prvalence of sexually transmitted co-infections in people living with HIV/AIDS: Systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011; 87:183–90. 10.1136/sti.2010.047514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White MK, Gorrill TS, Khalili K. Reciprocal transactivation between HIV-1 and other human viruses. Virology. 2006; 352:1–13. 10.1016/j.virol.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 4.Ruan Y, Jia Y, Zhang X, Liang H, Li Q, Yang Y, et al. Incidence of HIV-1, Syphilis, HepatitisB, and HepatitisC Virus Infections and Predictors Associated With Retention in a 12-Month Follow-Up Study Among Men Who Have Sex With Men in Beijing, China. Journal of Acquired Immune Deficiency Syndromes. 2009; 52:604–610. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Wu Y, Hong YA, Yang C, Cai W, Zhu Y, et al. Association between perceived social norm and condom use among people living with HIV/AIDS in Guangzhou, China. AIDS Care. 2016; 21:1–7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: A systematic review. Plos Medicine. 2007; 4:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Q, Wang Y, Lin P, Liu Y, Yang F, Fu X, et al. Potential bridges for HIV infection to men who have sex with men in Guangzhou, China. AIDS and behavior. 2006; 10 (4 Suppl):S17–23. 10.1007/s10461-006-9125-3 [DOI] [PubMed] [Google Scholar]
- 8.Gulov K, Coulter RW, Matthews DD, Uzzi M, Stall R. HIV and STIs Among MSM in Tajikistan: Laboratory-Confirmed Diagnoses and Self-Reported TestingBehaviors. AIDS Behav. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W, Best J, Zhang Y, Liu FY, Tso LS, Huang S, et al. Gay mobile apps and the evolving virtual risk environment: a cross-sectional online survey among men who have sex with men in China. Sexually Transmitted Infections. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnarrs PW, Rosenberger JG, Schick V, Delgado A, Briggs L, Dodge B, et al. Difference in Condom use Between Bear Concordant and Discordant Dyads During the Last Anal Sex Event. Journal of Homosexuality. 2016; 1–14. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Jensen A K, Ravn H, Sorup S, Andersen P. A marginal structural model for recurrent events in the presence of time-dependent confounding: non-specific effects of vaccines on child hospitalisations.[J]. Statistics in Medicine, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Hernan M, Brumback B, Robins J. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men.[J]. Epidemiology, 2000, 11(5):561–70. [DOI] [PubMed] [Google Scholar]
- 13.He X, Xing H, Ruan Y, Hong K, Cheng C, Hu Y, et al. A comprehensive mapping of HIV-1 genotypes in various risk groups and regions across China based on a nationwide molecular epidemiologic survey. Plos One. 2012; 7:e47289–e47289. 10.1371/journal.pone.0047289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong P, Pan Q, Ning Z, Xue Y, Gong J, Zhen X, et al. Genetic diversity and drug resistance of human immunodeficiency virus type 1 (HIV-1) strains circulating in Shanghai. Aids Research & Human Retroviruses. 2007; 23:847–56. [DOI] [PubMed] [Google Scholar]
- 15.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nature Medicine 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Huang Y, Ouyang Y, Xing H, Liao L, Jiang S, et al. Mutation covariation of HIV-1 CRF07_BC reverse transcriptase during antiretroviral therapy. Journal of Antimicrobial Chemotherapy. 2013; 68:2521–4. 10.1093/jac/dkt228 [DOI] [PubMed] [Google Scholar]
- 17.Schiffer JT, Swan DA, Magaret A, Schacker TW, Wald A, Corey L. Mathematical Modeling Predicts that Increased HSV-2 Shedding in HIV-1 Infected Persons Is Due to Poor Immunologic Control in Ganglia and Genital Mucosa. Plos One. 2016; 11:e0155124 10.1371/journal.pone.0155124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011; 365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermund SH, Hayes RJ. Combination Prevention: New Hope for Stopping the Epidemic. Current Hiv/aids Reports. 2013; 10:169–186. 10.1007/s11904-013-0155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frentz D, Boucher CA, van de Vijver DA. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS reviews. Jan-Mar 2012; 14:17–27. [PubMed] [Google Scholar]
- 21.Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, Renaud-Théry F, Shaffer N, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. Aids. 2013; 27:1403–1412. [DOI] [PubMed] [Google Scholar]
- 22.Manosuthi W, Thongyen S, Nilkamhang S, Manosuthi S, Sungkanuparph S. HIV-1 drug resistance-associated mutations among antiretroviral-naive thai patients with chronic HIV-1 infection. Journal of Medical Virology. 2013; 85:194–9. 10.1002/jmv.23452 [DOI] [PubMed] [Google Scholar]
- 23.Winand R, Theys K, Eusébio M, Aerts J, Camacho RJ, Gomes P, et al. Portuguese HIV-1 Resistance Study Group. Assessing transmissibility of HIV-1 drug resistance mutations from treated and from drug-naive individuals. Aids. 2015; 29:2045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendoza Y, Castillo Mewa J, Martínez AA, Zaldívar Y, Sosa N, Arteaga G, et al. HIV-1 Antiretroviral Drug Resistance Mutations in Treatment Naïve and Experienced Panamanian Subjects: Impact on National Use of EFV-Based Schemes. Plos One. 2016; 11:e0154317 10.1371/journal.pone.0154317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrowski M, Benko E, Yue FY, Kim CJ, Huibner S, Lee T, et al. Intensifying Antiretroviral Therapy With Raltegravir and Maraviroc During Early Human Immunodeficiency Virus (HIV) Infection Does Not Accelerate HIV Reservoir Reduction. Open Forum Infectious Diseases. 2015; 2:ofv138 10.1093/ofid/ofv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine. 2015; 373:795–807. 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Z, Lan G, Chen YQ, Zhu Q, Yang X, Shen Z, et al. HIV-1 Treatment-as-Prevention: A Cohort Study Analysis of Serodiscordant Couples in Rural Southwest China. Medicine. 2015; 94:e902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo JJ, Bower M, Brühlmann J, Novak U, Furrer H, Tanaka PY, et al. Prognostic factors for advanced-stage human immunodeficiency virus-associated classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine plus combined antiretroviral therapy: A multi-institutional retrospective study. Cancer. 2015; [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Petersen M, van der Laan MJ. Doubly Robust and Efficient Estimation of Marginal Structural Models for the Hazard Function. International Journal of Biostatistics. 2016; 12(1):233–252. 10.1515/ijb-2015-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva MC, Ximenes RA, Miranda Filho DB, Arraes LW, Mendes M, Melo AC, et al. Risk-factors for non-adherence to antiretroviral therapy. Revista Do Instituto De Medicina Tropical De So Paulo. 2009; 51:135–9. [DOI] [PubMed] [Google Scholar]
- 31.Robins J, Hernan M, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.