Abstract
Salmonella pathogenicity island 2 (SPI-2) is required for intramacrophage survival and systemic infection in mice. We have recently reported that Salmonella enterica causes activation of the protein kinase A (PKA) signaling pathway in a manner dependent on SPI-2, resulting in the upregulation of interleukin-10 expression in macrophages (K. Uchiya et al., Infect. Immun. 72:1964-1973, 2004). We show in the present study the involvement of SPI-2 in a signal transduction pathway that induces the expression of cyclooxygenase 2 (COX-2), an inducible enzyme involved in the synthesis of prostanoids. High levels of prostaglandin E2 (PGE2) and prostacyclin (PGI2), which are known to activate the PKA signaling pathway via their receptors, were induced in J774 macrophages infected with wild-type Salmonella compared to a strain carrying a mutation in the spiC gene, located within SPI-2. The increased production of both prostanoids was dependent on COX-2. COX-2 expression was dose dependently blocked by treatment with a specific inhibitor of the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway, and the phosphorylation level of ERK1/2 was higher in macrophages infected with wild-type Salmonella compared to the spiC mutant. Taken together, these results indicate that Salmonella causes an SPI-2-dependent ERK1/2 activation that leads to increased COX-2 expression, resulting in the upregulation of PGE2 and PGI2 production in macrophages. A COX-2 inhibitor inhibited not only Salmonella-induced activation of the PKA signaling pathway but also growth of wild-type Salmonella within macrophages, suggesting that Salmonella utilizes the COX-2 pathway to survive within macrophages and that the mechanism involves activation of the PKA signaling pathway.
Macrophages play a central role not only in host defense against infection by many pathogens but also in the regulation of immune responses and inflammation. The activation of macrophages to suppress bacterial growth in cells is an essential mechanism of defense against infection by intracellular pathogens. Several cytokines and eicosanoids, such as prostaglandins (PGs) and leukotrienes, are known to affect the function of macrophages (4, 34).
PGs produced in various types of cell are important mediators of inflammation or immune responses. The rate-limiting step in PG synthesis is catalyzed by cyclooxygenase (COX). COX converts arachidonic acid to PGH2, the common precursor to all PGs, prostacyclins, and thromboxanes (44). There are two isoforms of COX enzyme, encoded by distinct genes (35). Whereas COX-1 is constitutively expressed in most cell types and plays a role in gastrointestinal and reproductive function, COX-2 is normally expressed at very low levels but is strongly induced by various stimuli, including mitogens, cytokines, hormones, and oncogenes (27, 42). In addition to these stimulators, lipopolysaccharide (LPS) is known to induce COX-2 expression in monocytes/macrophages, and LPS-induced COX-2 expression is regulated by the mitogen-activated protein kinase (MAPK) signal transduction pathways (6, 11, 18, 28, 38).
PGE2, which is secreted in large quantities by macrophages, regulates a broad range of physiological functions (2, 37) and has been shown to have anti-inflammatory effects on macrophages through activation of the protein kinase A (PKA) signaling pathway. In fact, it has been demonstrated that PGE2 suppresses macrophage production of proinflammatory cytokines (24, 25) and nitric oxide (NO) radicals (29, 31) or enhances the synthesis of anti-inflammatory cytokines (45). In addition to PGE2, PGI2 is also known to activate the PKA signaling pathway by inducing an increase in intracellular cyclic AMP (cAMP) (16). These observations lead to the conclusion that PGE2 or PGI2 may participate in the inhibition of the host defense by deactivating macrophage responses against many types of infection.
Salmonella enterica is a facultative intracellular bacterium capable of surviving within macrophages. Specific virulence factors encoded within Salmonella pathogenicity island 2 (SPI-2), which is located at centisome 30.7 on the chromosome of S. enterica serovar Typhimurium, are required for growth within macrophages and for virulence in mice (8, 17, 20, 36, 41). Previous work showed that a mutant in the SPI-2 gene spiC was unable to survive within macrophages and unable to inhibit fusion of Salmonella-containing vacuoles (SCV) with lysosomal compartments (47). In addition, the SpiC protein is translocated into the cytosol of Salmonella-infected macrophages by the type III secretion system encoded within SPI-2 and interacts with host proteins such as TassC (26) and Hook3 (43), which are implicated in cellular trafficking. On the other hand, other researchers showed that SpiC is required for the translocation of SPI-2 effector proteins into target cells (13, 52). Thus, more research is needed to clarify the molecular function of SpiC. Recent studies have shown roles for SPI-2 in inhibiting fusion of SCV with vesicles of the endocytic pathway, such as inducible NO synthase-containing vesicles, and in inhibiting recruitment of NADPH oxidase components to SCV (5, 15, 49). These observations indicate that a role of SPI-2 is to interfere with intracellular vesicular trafficking to avoid exposure to toxic agents such as reactive oxygen intermediates (ROI) and reactive nitrogen intermediates in infected macrophages.
Our previous work has shown that SPI-2 is involved in Salmonella-induced activation of the PKA signaling pathway, resulting in upregulation of interleukin-10 expression in Salmonella-infected macrophages (48). However, the mechanism by which SPI-2 mediates activation of the PKA signaling pathway remains unknown. PGE2 and PGI2 are the main prostanoids reported to activate the PKA pathway through their interaction with plasma membrane G protein-coupled receptors (10, 21, 32). In the present study, we therefore focus on the COX pathway in Salmonella-infected macrophages and show the involvement of SPI-2 in Salmonella-induced expression of COX-2. Salmonella causes a SPI-2-dependent activation of extracellular signal-regulated kinase 1/2 (ERK1/2) that leads to COX-2 expression, resulting in upregulation of PGE2 and PGI2 production in macrophages. In addition, we discuss how COX-2 expression is involved in SPI-2 function in intramacrophage survival of Salmonella.
MATERIALS AND METHODS
Reagents.
Reagents for cell culture were purchased from Sigma-Aldrich (St. Louis, Mo.), and other reagents were purchased from the following sources: PD98052 and SB203580 were from Calbiochem (La Jolla, Calif.); SP600125 was from Biomol (Plymouth Meeting, Pa.); indomethacin, MDL 12,330A, and 2′,7′-dichlorodihydrofluorescein diacetate (HCFH-DA) were from Sigma-Aldrich; SC-58125 and AH6809 were from Cayman Chemicals (Ann Arbor, Mich.). ONO-AE3-208 (AE3-208) was kindly provided by Ono Pharmaceutical Co. (Osaka, Japan). PD98052, SB203580, SP600125, AH6809, SC-58125, and indomethacin were dissolved in dimethyl sulfoxide (DMSO). When these drugs were used, the final concentration of DMSO in the culture medium was 0.1%; this concentration of solvent did not affect the cell responses.
Bacterial strains and growth conditions.
Strains used in the present study are derived from the wild-type Salmonella enterica serovar Typhimurium strain 14028s. The spiC::kan derivative EG10128 and the purB::Tn10 strain EG9652 were described in Uchiya et al. (47). Bacteria were grown at 37°C in Luria broth. Kanamycin and tetracycline were used at 50 and 15 μg/ml, respectively.
Cell culture and macrophage survival assay.
J774 E clone, a mannose-receptor-positive murine macrophage cell line, was maintained in a 37°C incubator with 5% CO2 in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, Utah), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The day before infection, the macrophages were plated at a density of 0.4 × 106/well in 24-well tissue culture plates (Falcon; DB Biosciences, Franklin Lakes, N.J.) in medium without antibiotics. The macrophage survival assay was conducted as described previously (47). A multiplicity of infection of 25 bacteria per macrophage was used.
RNA extraction and semiquantitative RT-PCR.
Total RNA was prepared from macrophages as described previously (48). RNA (2 μg) was reverse-transcribed with SuperScript II reverse transcriptase (RT; Invitrogen, Carlsbad, Calif.) by using an oligo(dT) primer. PCR was conducted in 20-μl reactions consisting of reaction buffer (Perkin-Elmer, Forester City, Calif.), 0.5 μM concentrations of each deoxynucleoside triphosphate, 1 μM concentrations of each primer, 1 μl of cDNA, and 1 U of Taq DNA polymerase (Perkin-Elmer) in cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplification was carried out for 31 cycles for COX-1, 26 cycles for COX-2, 40 cycles for EP1, 37 cycles for EP2, 38 cycles for EP3, 28 cycles for EP4, 28 cycles for IP, and 16 cycles for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), followed by a 7-min final extension at 72°C. GAPDH was used as an internal standard for quantification of total RNA. In each case, the number of amplification cycles achieved exponential amplification, in which product formation was proportional to starting cDNA (data not shown). The PCR products were subjected to electrophoresis in a 1.5% agarose gel and visualized by ethidium bromide staining. Visual analysis and image analyzing software (Gel-Doc 2000 System; Bio-Rad, Hercules, Calif.) were used for the comparison of band intensities. The primer pairs were as follows: COX-1, 5′-ACT GGC TCT GGG AAT TTG TGA ATG-3′ and 5′-AGA GCC GCA GGT GAT ACT GTC GTT-3′ (451-bp fragment); COX-2, 5′-TTT GTT GAG TCA TTC ACC AGA CAG-3′ and 5′-CAG TAT TGA GGA GAA CAG ATG GGA-3′ (371-bp fragment); EP1, 5′-TGG CAC TAG CCG TGC TGG CTG CCA-3′ and 5′-CGA TGG CCA ACA CCA CCA ACA CCA-3′ (501-bp fragment); EP2, 5′-TTC ATA TTC AAG AAA CCA GAC CCT-3′ and 5′-AGG GAA GAG GTT TCA TCC ATG TAG-3′ (600-bp fragment); EP3, 5′-CCG GGC ACG TGG TGC TTC ATC AGC-3′ and 5′-TAG CAG CAG ATA AAC CCA GGG ATC-3′ (428-bp fragment); EP4, 5′-TTC CGC TCG TGG TGC GAG TGT TCA-3′ and 5′-GAG GTG GTG TCT GCT TGG GTC AGG-3′ (423-bp fragment); IP, 5′-GGC ACG AGA GGA TGA AGT TTA CC-3′ and 5′-GTC AGA GGC ACA GCA GTC AAT GG-3′ (407-bp fragment); and GAPDH, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (452-bp fragment).
PGE2 and 6-keto-PGF1α assays.
After macrophages in 24-well plates were infected with bacteria, levels of PGE2 and 6-keto PGF1α (the stable hydrolysis product of PGI2) in culture supernatants were measured by using enzyme immunoassay kit (Cayman Chemicals), according to the manufacturer's protocol. The detection limit of these assays was 10 pg/ml.
Western blot analysis.
Western blot analyses were performed essentially as described previously (48). Phosphorylation of ERK1/2 (p44/42) MAPK was determined with the PhosphoPlus p44/42 MAPK (Thr202/Tyr204) kit from New England Biolabs (Beverly, Mass.), and bands were analyzed by using a GS-800 Calibrated Densitometer (Bio-Rad).
Measurement of cAMP.
Macrophages were seeded at 5.0 × 104 per well in a 96-well plate. After infection with bacteria, cAMP in the cells was measured by using HitHunter EFC cAMP chemiluminescence assay kit (Applied Biosystems, Bedford, Mass.) according to the manufacturer's protocol. The detection limit of the assay was 0.04 pmol/well.
Measurement of ROI production.
Intracellular ROI production was determined by measuring oxidation of the fluorescent probe HCFH-DA as described previously (50). Macrophages (5.0 × 104) in a 96-well plate were infected with bacteria. After 5 h, medium was changed to 100 μl of Hanks balanced salt solution containing 40 μM DCFH-DA and further incubated for 1 h. The plate was washed with phosphate-buffered saline, and the fluorescence intensity was determined at 485-nm excitation and 530-nm emission wavelengths with a Wallac 1420 ARVOsx multi-label counter (Perkin-Elmer).
Statistical analysis.
Each experiment was performed at least three times. The results are expressed as means ± the standard deviations (SD). The data were analyzed by analysis of variance with Dunnett's test. A P value of <0.05 was considered statistically significant.
RESULTS
Upregulation of COX-2 expression is dependent on SPI-2.
We have previously reported that PKA activity is higher in macrophages infected with wild-type Salmonella than in macrophages infected with a strain carrying a nonpolar mutation in the spiC gene (48). In the present study, to investigate the involvement of the COX pathway in SPI-2-dependent activation of PKA, we examined the expression levels of COX-1 and -2 in macrophages infected with wild-type, spiC mutant, and purB mutant Salmonella strains. Both spiC and purB mutants survive poorly in macrophages (48). Thus, the purB mutant, which is defective in purine metabolism, serves as a control to test whether spiC defects are due simply to the reduction in intramacrophage survival. COX expression was analyzed by RT-PCR of total RNA extracted from macrophages at 5 h postinfection. Figure 1 shows that the level of COX-2 mRNA was higher in macrophages infected with wild-type Salmonella or with the purB mutant compared to those infected with the spiC mutant. This result indicates that the lower level of COX-2 expression induced by the spiC mutant Salmonella is due to defective SpiC function and not to reduced replication within macrophages. On the other hand, the expression of COX-1 showed no difference after infection with either wild-type or spiC mutant Salmonella, indicating that the spiC gene is involved in specific upregulation of COX-2 expression.
FIG. 1.
Expression of COX-1 and COX-2 mRNAs in macrophages infected with Salmonella. (A) At 5 h postinfection with wild-type (WT), spiC, or purB Salmonella strains, total RNA extracted from the pooled macrophages was reverse transcribed and amplified by PCR. The PCR products were run on 1.5% agarose gels. (B) Expression of COX-1 and COX-2, normalized to GAPDH expression. The data are the means and SD of three independent experiments. The level of COX-2 mRNA, but not COX-1 mRNA, is significantly lower in cells infected with the spiC mutant compared to wild-type or purB Salmonella. ∗, P < 0.001 (significantly different from macrophages infected with spiC Salmonella). UI, uninfected.
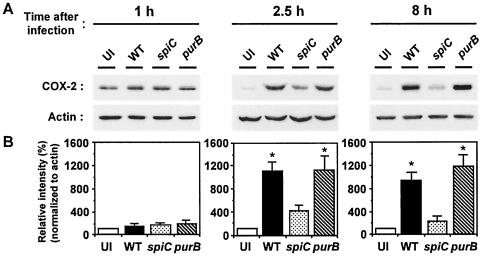
To confirm the results of the RT-PCR analysis, COX-2 expression in Salmonella-infected macrophages was quantified by Western blotting. As shown in Fig. 2, Salmonella infection did not cause a significant increase in COX-2 expression at 1 h postinfection, and there was no significant difference between wild-type Salmonella and each mutant. At 2.5 h postinfection, however, the level of COX-2 in wild-type Salmonella-infected macrophages was 2.5-fold greater than that in the spiC mutant-infected macrophages. At 8 h postinfection, the difference in COX-2 expression between the spiC mutant and the wild type was fourfold. In accord with the results of RT-PCR analysis, the purB mutant induced the same levels of COX-2 expression as did wild type.
FIG. 2.
Western blot analysis of COX-2 expression in macrophages infected with Salmonella. Cytosolic extracts from macrophages infected with wild-type (WT), spiC, or purB Salmonella strains were prepared at the indicated times postinfection and analyzed by using anti-COX-2 antibody. (A) Image of the original blots. (B) Levels of COX-2, normalized to actin levels. The graphs show percentages of the value in uninfected macrophages. The data are the means and SD of three independent experiments. ∗, P < 0.001 (significantly different from macrophages infected with spiC Salmonella). UI, uninfected.
In both RT-PCR and Western analyses (Fig. 1 and 2), COX-2 expression in the spiC mutant-infected macrophages was somewhat higher than that in uninfected cells. This modest induction of COX-2 expression may be caused by LPS on the bacterial surface. Indeed, LPS has been reported to induce COX-2 expression (6, 11, 18, 28, 38).
Involvement of ERK1/2 and p38 MAPK in Salmonella-induced expression of COX-2.
Subsequent studies focused on the signal transduction pathways that govern Salmonella-induced expression of COX-2. Many observations have shown that MAPK signal transduction pathways have a significant role in inducing COX-2 expression (7, 11, 18, 28, 38). Therefore, the effects of several MAPK inhibitors on Salmonella-induced COX-2 expression were examined. Figure 3 shows that COX-2 expression in wild-type Salmonella-infected macrophages was blocked by the ERK1/2 inhibitor PD98052, which inhibits the MAP/ERK kinase, and by the p38 MAPK inhibitor SB203580 in a concentration-dependent manner. In contrast, SP600125, an inhibitor of c-Jun amino-terminal kinase (JNK), did not have an inhibitory effect. The results indicate that both ERK1/2 and p38 MAPK signaling pathways could participate in Salmonella-induced expression of COX-2.
FIG. 3.
Effects of inhibitors of ERK1/2 (PD98052), p38 MAPK (SB203580), and JNK (SP600125) on Salmonella-induced expression of COX-2. Macrophages were infected with wild-type Salmonella, and inhibitors at the indicated concentrations or 0.1% DMSO solvent control were added simultaneously. At 5 h postinfection, cytosolic extracts were prepared and analyzed by using anti-COX-2 antibody. (A) Image of the original blots. (B) Levels of COX-2, normalized to actin levels. The graphs show percentages of the value in untreated macrophages. The data are the means and SD of three independent experiments. Both PD98052 and SB203580 caused a significant reduction in COX-2 expression. ∗, P < 0.05; ∗∗, P < 0.01; #, P < 0.001 (significantly different from macrophages treated with 0.1% DMSO). UI, uninfected.
Salmonella induces SPI-2-dependent ERK1/2 activation, resulting in the upregulation of COX-2 expression.
We previously measured the levels of p38 phosphorylation in Salmonella-infected macrophages (48). Wild-type Salmonella induced higher p38 phosphorylation in macrophages than did the spiC mutant, but there was no significant difference between the spiC mutant and the purB mutant, which, like the spiC mutant, is defective for intramacrophage survival. Although the results with the p38 MAPK inhibitor show that the p38 MAPK pathway is involved in Salmonella-induced expression of COX-2, these previous results imply that activation of the p38 MAPK pathway does not explain the spiC-dependent induction of COX-2 expression. To test whether ERK1/2 activation can account for spiC-dependent COX-2 induction, the level of ERK1/2 (p44/42) phosphorylation was measured by Western blotting. As shown in Fig. 4, increased ERK1/2 phosphorylation in wild-type Salmonella-infected macrophages was first observed at 2.5 h postinfection, and the elevation continued at 8 h. The purB mutant showed the same effect on ERK1/2 phosphorylation as wild type. The spiC mutant, however, induced ∼1.8-fold-lower ERK1/2 phosphorylation at 2.5 h postinfection. Taken together, these results demonstrate the involvement of the spiC gene in the activation of ERK1/2 but not p38 MAPK. This activation leads to the increase in COX-2 expression induced by wild-type or purB mutant Salmonella compared to the spiC mutant.
FIG. 4.
Western blot analysis of phospho-ERK1/2 (p44/42) in macrophages infected with Salmonella. Cytosolic extracts prepared at the indicated times after infection with wild-type (WT), spiC, or purB Salmonella strains were analyzed with phospho-ERK1/2 and ERK1/2 antibodies. (A) Image of the original blots. After analysis with an anti-phospho-ERK1/2 antibody (top), the membranes were stripped and reprobed with an antibody directed to ERK1/2 (bottom). (B) Densitometric analysis of the amounts of phospho-ERK1 (pP44) normalized to the amount of ERK1 in the same sample. The graphs show percentages of the value in uninfected macrophages. The data are the means and SD of three independent experiments. ∗, P < 0.01; ∗∗, P < 0.005 (significantly different from macrophages infected with spiC Salmonella). UI, uninfected.
Production of PGE2 and PGI2 by macrophages infected with Salmonella.
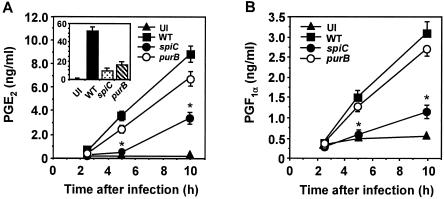
To characterize the relationship between Salmonella-mediated activation of PKA and COX pathways, we measured the production of PGE2 and PGI2, which are COX metabolites known to activate the PKA signaling pathway (10, 21, 32). As shown in Fig. 5, production of both prostanoids increased during the course of Salmonella infection. PGE2 production was lower for spiC mutant-infected macrophages than for wild-type-infected macrophages. The purB mutation did not significantly affect PGE2 levels up to 10 h postinfection, indicating that the lower level of PGE2 production induced by spiC mutant Salmonella depends on SpiC function. Similar results were obtained for PGI2 production (Fig. 5B). At 20 h postinfection, however, PGE2 production by wild-type Salmonella-infected macrophages was 3.2-fold higher than that of the purB mutant-infected macrophages (Fig. 5A, inset), presumably because the purB mutant had little or no capacity to survive within macrophages at 20 h postinfection. These results indicate that the production of PGE2 and PGI2 increased in a manner dependent on SPI-2 and suggest that these prostanoids are involved in Salmonella-mediated activation of PKA.
FIG. 5.
Time course of PGE2 and PGI2 production in macrophages infected with Salmonella. Macrophages were infected with wild-type (WT), spiC, or purB Salmonella strains. At the time points indicated, supernatants were harvested and tested for PGE2 (A) and PGF1α (B) content by enzyme-linked immunosorbent assay. The inset shows the results at 20 h postinfection. The data are the means and SD of three independent experiments. ∗, P < 0.001 (significantly different from macrophages infected with wild-type Salmonella). UI, uninfected.
Expression of prostanoid receptors in macrophages infected with Salmonella.
Four subtypes (EP1 to EP4) of PGE2 receptors have been identified thus far. EP2, EP4, and the PGI2 receptor IP are known to couple to and stimulate adenylate cyclase, and the resulting increase in cAMP leads to activation of the PKA signaling pathway (33). The expression levels of these receptors in Salmonella-infected macrophages are shown in Fig. 6. RT-PCR (28 cycles) demonstrated mRNAs encoding EP4 and IP receptors in wild-type Salmonella-infected macrophages (Fig. 6A). After 37 cycles, weak signals for EP2 and EP3 receptor mRNAs appeared, but a signal for EP1 mRNA could not be detected even with 40 cycles (data not shown). As shown in Fig. 6B and C, the EP3, EP4, and IP receptors were expressed constitutively in macrophages, and their expression levels showed no difference after infection with wild-type or spiC mutant Salmonella. In contrast, the level of EP2 expression was higher in macrophages infected with wild-type or purB mutant Salmonella at 5 h postinfection than in uninfected or spiC mutant-infected macrophages. The same pattern of expression was seen at 3 h postinfection, but expression returned to the basal level at 8 h postinfection (data not shown). These results indicate that the expression of EP2 induced by Salmonella is also dependent on SPI-2 and suggest that EP2, EP4, and IP receptors may be involved in Salmonella-mediated activation of the PKA signaling pathway.
FIG. 6.
Expression of prostanoid receptor mRNAs in macrophages infected with Salmonella. (A) At 5 h postinfection with wild-type Salmonella, total RNA extracted from the pooled macrophages was reverse transcribed and amplified by PCR with primer pairs specific to EP1 to EP4 and IP receptors. The PCR products were run on 1.5% agarose gels. (B) RT-PCR for EP2 to EP4 and IP receptors was performed with RNA isolated from macrophages infected with wild-type (WT), spiC, or purB Salmonella strains. (C) Expression of EP2 to EP4 and IP receptors, normalized to GAPDH expression. The data are the means and SD of three independent experiments. ∗, P < 0.001 (significantly different from uninfected macrophages). UI, uninfected.
We next examined the effects of MAPK inhibitors on Salmonella-induced expression of EP2. The expression of EP2, as well as COX-2, in wild-type Salmonella-infected macrophages was dose dependently blocked by the ERK1/2 inhibitor PD98052 and by the p38 MAPK inhibitor SB203580 (data not shown). Together with results with Fig. 4, these results show the involvement of ERK1/2 signaling pathways in SPI-2-dependent upregulation of EP2 expression in Salmonella-infected macrophages.
COX-2 pathway in Salmonella-mediated activation of PKA signaling.
We next tested the involvement of COX-2 in Salmonella-mediated activation of PKA by measuring intracellular cAMP levels in macrophages. For this purpose, indomethacin and SC-58125 were used. Indomethacin inhibits both COX-1 and COX-2, and SC-58125 is a selective COX-2 inhibitor. As shown in Fig. 7A, treatment with indomethacin (50 μM) or SC-58125 (10 μM) decreased PGE2 production by wild-type Salmonella-infected macrophages to the level produced by spiC mutant-infected macrophages. No difference was found in the levels of inhibition produced by the nonselective inhibitor and COX-2-selective inhibitor. These results establish that, of the two COX isoforms, COX-2 is mostly or entirely responsible for increased PGE2 production. This conclusion was also true for PGI2 production, since indomethacin and SC-58125 completely blocked the Salmonella-induced increase in PGI2 production (data not shown). In addition, both inhibitors prevented the increase in cAMP levels in wild-type Salmonella-infected macrophages (Fig. 7B), indicating that Salmonella-mediated PKA activation is dependent on COX-2 activity and suggesting the involvement of PGE2 and PGI2 in the PKA activation.
FIG. 7.
Involvement of the COX-2 pathway in PKA activation in Salmonella-infected macrophages. (A) Effects of COX inhibitors on PGE2 production. Macrophages were infected with the wild-type strain, and 50 μM indomethacin (IM), 10 μM SC-58125 (SC), or 0.1% DMSO solvent control was added simultaneously. At 5 h postinfection, supernatants were harvested and tested for PGE2 by ELISA. The data are the means and SD of three independent experiments. #, P < 0.001 (significantly different from wild-type-infected control macrophages). (B) Effects of COX inhibitors on cAMP content. Macrophages were infected with the wild-type strain, and 50 μM indomethacin (IM), 10 μM SC-58125 (SC), or 0.1% DMSO solvent control was added simultaneously. At 5 h postinfection, cell lysates were prepared and assayed for cAMP content. The data are the means and SD of three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01 (significantly different from wild-type strain-infected control macrophages). (C) Effects of AH6809 (an EP2 antagonist; EP2A) and AE3-208 (an EP4 antagonist; EP4A) on cAMP level. Macrophages (5.0 × 104) in a 96-well plate were infected with the wild-type strain, and 1 μM AH6809, 1 μM AE3-208, or 0.1% DMSO solvent control either alone or in the indicated combination was added simultaneously. At 5 h postinfection, cell lysates were prepared and assayed for cAMP content. The data are the means and SD of three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01 (significantly different from wild-type-infected control macrophages).
Next, the involvement of PGE2 in PKA activation was examined by using the EP2 receptor antagonist AH6809 and the EP4 receptor antagonist AE3-208. As shown in Fig. 7C, neither EP2 nor EP4 antagonist alone was sufficient for inhibition of the increase in cAMP induced by wild-type Salmonella infection. Combining the EP2 and EP4 antagonists produced a synergistic decrease, but the reduced level did not drop to the level seen in uninfected macrophages (uninfected, 0.20 ± 0.049 pmol/well; infected, 0.54 ± 0.042 pmol/well; infected plus both inhibitors, 0.33 ± 0.057 pmol/well; values are means ± the SD of triplicate cultures). Therefore, part of the Salmonella-mediated PKA activation is explained by PGE2 action via both EP2 and EP4 receptors in an autocrine manner. Because there is no antagonist available for the IP receptor, we estimated the contribution of IP receptor to the PKA activation by using PGI2 as an agonist. Stimulation with PGI2 or PGE2 increased cAMP levels in macrophages (data not shown), suggesting strongly the contribution of PGI2, in addition to PGE2, in the PKA activation.
Involvement of the PKA signaling pathway in intramacrophage survival of Salmonella.
The findings described above strongly showed the possibility that SPI-2-dependent COX-2 expression leads to PGE2 and PGI2 production, resulting in activation of the PKA signaling pathway through the stimulation of EP2, EP4, and IP receptors coupled to adenylate cyclase. Therefore, we were interested in examining the effects of the PKA signaling pathway on intramacrophage survival of Salmonella. As shown in Fig. 8A, treatment with the adenylate cyclase inhibitor MDL 12,330A reduced intracellular growth of wild-type Salmonella, whereas the survival level of the spiC mutant did not significantly change, indicating that the activation of the PKA pathway via these prostanoid receptors is important for intramacrophage survival of Salmonella. In the present study, since Salmonella-mediated activation of PKA was shown to contribute to SPI-2-dependent COX-2 expression, we examined whether the COX-2 is involved in intramacrophage survival of Salmonella. At 5 h postinfection, treatment with the COX-2 inhibitor SC-58125 resulted in significant inhibition of the intracellular growth of wild-type Salmonella (Fig. 8B). These results indicate that the COX-2 pathway plays a significant role in intramacrophage survival of Salmonella and suggest that the mechanism is dependent on activation of the PKA pathway through both PGE2 and PGI2 production.
FIG. 8.
Involvement of PKA activation in Salmonella survival within macrophages. (A and B) Effects of the adenylate cyclase inhibitor MDL 12,330A (A) and the COX-2 inhibitor SC-58125 (B) on Salmonella survival within macrophages. Macrophages were infected with the wild-type strain or spiC mutant, and MDL 12,330A or SC-58125 at the indicated concentrations or a 0.1% DMSO solvent control was added simultaneously. Samples were taken at 0 and 5 h and plated onto Luria agar to determine the number of viable bacteria. The percentage survival is relative to that of the wild-type strain within untreated macrophages. (C) Effect of MDL 12,330A on intracellular ROI production in macrophages infected with Salmonella. Macrophages were infected with the wild-type strain or spiC mutant, and MDL 12,330A (5 μM) was added simultaneously. ROI production was analyzed fluorometrically with 40 μM DCFH-DA as the fluorescent probe. The data are the means and SD of three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01 (significantly different from wild-type-infected control macrophages).
Finally, to investigate the mechanism by which PKA activation is involved in intramacrophage survival of Salmonella, the levels of intracellular ROI in macrophages were measured. ROI production was assessed by oxidation of HCFH-DA as the fluorescent probe (50). As shown in Fig. 8C, ROI production was significantly increased by Salmonella infection, but no difference was seen between wild-type Salmonella and the spiC mutant, suggesting that PKA activation could counter the ROI production induced by wild-type Salmonella infection. Treatment with MDL 12,330A significantly increased ROI production in wild-type-infected macrophages, whereas this effect was not observed in spiC mutant-infected macrophages. These results demonstrate that SPI-2-dependent PKA activation is involved in the inhibition of enhanced ROI production in wild-type Salmonella-infected macrophages, which leads to Salmonella survival within macrophages.
DISCUSSION
We show here the involvement of SPI-2 in a signal transduction pathway that induces COX-2 expression in Salmonella-infected macrophages. Salmonella causes SPI-2-dependent activation of the ERK1/2 signaling pathway that leads to COX-2 expression, resulting in the upregulation of PGE2 and PGI2 production. In addition, we found that COX-2 is involved in intramacrophage survival of Salmonella through the activation of the PKA signaling pathway.
Our data showed that high levels of PGE2 and PGI2 production were induced in macrophages infected with wild-type Salmonella compared to a strain carrying a mutation in spiC gene encoded within SPI-2, and the increased production of both prostanoids was dependent on activity of COX-2, but not COX-1, suggesting that the spiC gene influences the signal transduction pathway involved in COX-2 expression. There have been many reports that ERK1/2 or p38 MAPK is involved in LPS-induced COX-2 expression in monocytes/macrophages (6, 11, 18, 28, 38). Chen et al. (7) also reported that tumor necrosis factor alpha-induced COX-2 expression in epithelial cell lines is inhibited by treatment with an ERK1/2 inhibitor, indicating a significant role of the MAPK signal transduction pathway in COX-2 expression. Consistent with these reports, our results showed that inhibition of ERK1/2 or p38 MAPK blocked Salmonella-induced COX-2 expression, supporting the involvement of both ERK1/2 and p38 MAPK signaling pathways in this process. We previously showed that wild-type Salmonella induces phosphorylation of p38 MAPK in macrophages, but we did not find evidence for SpiC involvement in p38 MAPK phosphorylation (48). On the other hand, we demonstrate here that wild-type Salmonella induces ERK1/2 phosphorylation in a SpiC-dependent manner, indicating that upregulation of COX-2 expression could occur through SpiC-dependent activation of ERK1/2. However, more research is needed to clarify the mechanism by which the spiC gene affects the ERK1/2 signaling pathway.
PGE2 is known to have deactivating properties on macrophages. It has been demonstrated that PGE2 suppresses the production of NO radicals (29, 31) and proinflammatory cytokines (24, 25) or enhances the synthesis of anti-inflammatory cytokines (45) in macrophages. In addition, PGE2 was reported to inhibit the generation of superoxide anion by activated human neutrophils (51). This information suggests that PGE2 affects host defenses against infection with various pathogens. Indeed, Takano et al. (46) showed that PGE2 diminished bacterial exclusion in the peritoneal cavity, spleen, and liver after murine Escherichia coli infection. It has been reported Leishmania donovani, a facultative intracellular parasite, stimulates production of PGE2 in macrophages (30, 40), and the administration of indomethacin can significantly decrease metastatic lesions in Leishmania-infected mice (12). In the present study, we show that treatment of wild-type Salmonella-infected macrophages with the selective COX-2 inhibitor SC-58125 had significant inhibitory effects not only on PGE2 and PGI2 production but also on intramacrophage survival of Salmonella, indicating the importance of these prostanoids for the intracellular growth of Salmonella. In addition to in vitro experiments with macrophages, our data further show that COX-2 expression is increased in the livers and spleens of mice infected with wild-type Salmonella compared to those infected with the spiC mutant, and the survival rate of mice infected with wild-type Salmonella is significantly increased by treatment with indomethacin (data not shown), suggesting that the COX-2 pathway may play an important role in the establishment of systemic infection of Salmonella.
The effects of prostanoids, including PGE2 or PGI2, are exerted by specific receptors on the plasma membrane of target cells (9, 19). Four PGE2 receptor subtypes have been identified (EP1, EP2, EP3, and EP4) and have been shown to differ in their signal transduction pathways. They are coupled to Ca2+ mobilization (EP1 and EP3) or the stimulation (EP2 and EP4) or inhibition (EP3) of adenylate cyclase (10, 21). Like EP2 and EP4, the PGI2 receptor IP is also known to participate in activation of the PKA signaling pathway through the stimulation of adenylate cyclase (32). Several groups have shown that these receptors, except for EP1, are expressed in murine macrophage-like cell lines such as J774.1 and RAW264.7, and mainly EP2 expression is transiently upregulated by stimuli such as LPS (1, 22, 23). Consistent with these reports, our results showed that, in J774 macrophages, EP4 and IP were the most strongly expressed among these five receptors, and EP2 expression was increased by Salmonella infection in a manner dependent on SPI-2, indicating the possibility that EP2, EP4, and IP receptors are involved in Salmonella-mediated activation of the PKA signaling pathway. By measuring intracellular cAMP levels in Salmonella-infected macrophages treated with receptor antagonists or agonists, we found that all three of these receptors contribute to Salmonella-mediated PKA activation.
As described above, the present study showed that an SPI-2-dependent COX-2 expression leads to PGE2 and PGI2 production, resulting in activation of the PKA signaling pathway through the stimulation of EP2, EP4, and IP receptors coupled to adenylate cyclase. Therefore, it was of interest to examine the involvement of the PKA signaling pathway in intramacrophage survival of Salmonella. Our result showed that the adenylate cyclase inhibitor MDL 12,330A inhibits the intracellular growth of wild-type Salmonella but not that of the spiC mutant, indicating that the activation of the PKA signaling pathway via these prostanoid receptors is important for the intramacrophage survival of Salmonella and suggesting that PKA activation could be a critical mechanism that Salmonella uses to survive within macrophages. Indeed, PKA is reported to affect components of the NADPH oxidase complex and reduce its ability to produce superoxide radicals in phagocytes (3, 14, 39), suggesting that PKA plays a negative role, downregulating the superoxide-dependent mechanisms of killing ingested bacteria in phagocytes. Taken together with these observations, our results indicated that the mechanism by which PKA activation promotes intramacrophage survival of Salmonella could be the inhibition of the increase in ROI production induced by wild-type Salmonella infection. Previous studies have shown that one of the mechanisms by which SPI-2 promotes the survival within macrophages of Salmonella is interference with intracellular vesicular trafficking so as to avoid exposure to toxic agents such as ROI or reactive nitrogen intermediates (5, 15, 47, 49). The present study demonstrates that SPI-2 also mediates inhibition of ROI production through COX-2-dependent PKA activation.
In conclusion, we show that Salmonella causes the activation of the ERK1/2 signaling pathway in a manner dependent on SPI-2, which leads to COX-2 expression and results in the upregulation of PGE2 and PGI2 production in macrophages. Our results also demonstrate that COX-2 affects intramacrophage survival of Salmonella through the activation of the PKA signaling pathway. Thus, Salmonella appears to utilize the COX-2 pathway to survive within macrophages.
Acknowledgments
We thank Emi Okumura for technical assistance.
This study was supported by Grants-in-Aid for Specially Promoted Research of Meijo University Research Institute and by the Scientific Frontier Research Project of Meijo University from the Ministry of Education, Culture, Sports, Science, and Technology.
Editor: F. C. Fang
REFERENCES
- 1.Arakawa, T., O. Laneuville, C. A. Miller, K. M. Lakkides, B. A. Wingerd, D. L. DeWitt, and W. L. Smith. 1996. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells: structure and expression of the murine prostaglandin EP4 receptor gene. J. Biol. Chem. 271:29569-29575. [DOI] [PubMed] [Google Scholar]
- 2.Astiz, M., D. Saha, D. Lustbader, R. Lin, and E. Rackow. 1996. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J. Lab. Clin. Med. 128:594-600. [DOI] [PubMed] [Google Scholar]
- 3.Bengis-Garber, C., and N. Gruener. 1996. Protein kinase A downregulates the phosphorylation of p47phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell Signal. 8:291-296. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C., Y., and C. Nathan. 1993. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann. N. Y. Acad. Sci. 685:713-739. [DOI] [PubMed] [Google Scholar]
- 5.Chakravortty, D., I. H. Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, B. C., Y. H. Chen, and W. W. Lin. 1999. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 97:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. C., Y. T. Sun, J. J. Chen, and Y. J. Chang. 2001. Tumor necrosis factor-α-induced cyclooxygenase-2 expression via sequential activation of ceramide-dependent mitogen-activated protein kinases, and IκB kinase 1/2 in human alveolar epitherial cells. Mol. Pharmacol. 59:493-500. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, R. A., I. Kennedy, P. P. A. Humphrey, K. Bunce, and P. Lumley. 1990. Prostanoid and their receptors, p. 643-714. In C. Hansch, P. G. Sammes, J. B. Taylor, and J. C. Emmett (ed.), Comprehensive medical chemistry, 2nd ed. Pergamon, Oxford, England.
- 10.Coleman, R. A., W. L. Smith, and S. Narumiya. 1994. Classification of prostanid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 46:205-229. [PubMed] [Google Scholar]
- 11.Dean, J. L. E., M. Brook, A. R. Clark, and J. Saklatvala. 1999. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 274:264-269. [DOI] [PubMed] [Google Scholar]
- 12.Farrell, J. P., and C. E. Kirkpatrick. 1987. Experimental cutaneous leishmaniasis. II. A possible role for prostaglandins in exacerbation of disease in Leishmania major-infected BALB/c mice. J. Immunol. 138:902-907. [PubMed] [Google Scholar]
- 13.Freeman, J. A., C. Rapple, V. Kuhle, M. Hensel, and S. I. Miller. 2002. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon protein SseB and SseC. J. Bacteriol. 184:4971-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabig, T. G., C. D. Cren, P. L. Mantel, and R. Rosli. 1995. Function of wild-type or mutant Rac2 and Rap1a GTPases in differentiated HL60 cell NADPH oxidase activation. Blood 85:804-811. [PubMed] [Google Scholar]
- 15.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 16.Gorman, R. R., F. A. Fitzpatrick, and O. V. Miller. 1978. Reciprocal regulation of human platelet cAMP levels by thromboxane A2 and prostacyclin. Adv. Cyclic Nucleotide Res. 9:597-609. [PubMed] [Google Scholar]
- 17.Groisman, E. A., A.-B. Blanc-Portard, and K. Uchiya. 1999. Pathogenicity island and the evolution of Salmonella virulence, p. 127-150. In J. B. Kaper and J. Hacker (ed.), Pathogenicity island and other mobile virulence elements. ASM Press, Washington, D.C.
- 18.Guan, Z., S. Y. Buckman, A. P. Pentland, D. J. Templeton, and A. R. Morrison. 1998. Induction of cyclooxygenase-2 by the activated MEKK1→SEK/MKK4→p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 273:12901-12908. [DOI] [PubMed] [Google Scholar]
- 19.Halushka, P. V., Mais, D. E., P. R. Mayeux, and T. A. Morinelli. 1989. Thromboxane, prostaglandin and leukotriene receptors. Annu. Rev. Pharmacol. Toxicol. 29:213-239. [DOI] [PubMed] [Google Scholar]
- 20.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa, A., Y. Sugimoto, and A. Negishi. 1996. Molecular aspects of the structures and functions of the prostaglandin E receptors. J. Lipid Mediat. Cell Signal. 14:83-87. [DOI] [PubMed] [Google Scholar]
- 22.Ikegami, R., Y. Sugimoto, E. Segi, M. Katsuyama, H. Karahashi, F. Amano, T. Maruyama, H. Ymane, S. Tsuchiya, and A. Ichikawa. 2001. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipopolysaccharide in C3H/HeN peritoneal macrophages. J. Immunol. 166:4689-4696. [DOI] [PubMed] [Google Scholar]
- 23.Katsuyama, M., R. Ikegami, H. Karahashi, F. Amano, Y. Sugimoto, and A. Ichikawa. 1998. Characterization of the LPS-induced expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem. Biophys. Res. Commun. 251:727-731. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel, S. L., M. Spengler, M. A. May, R. Spengler, J. Larrick, and D. Remick. 1988. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 263:5380-5384. [PubMed] [Google Scholar]
- 25.Kunkel, S. L., R. C. Wiggins, S. W. Chensue, and J. Larrick. 1986. Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochem. Biophys. Res. Commun. 137:404-410. [DOI] [PubMed] [Google Scholar]
- 26.Lee, A. H., M. P. Zareei, and S. Daefler. 2002. Identification of a NIPSNAP homologue as host cell target for Salmonella virulence protein SpiC. Cell Microbiol. 4:739-750. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. H., E. Soyoola, P. Chanmugam, S. Hart, W. Sun, H. Zhong, S. Liou, D. Simmons, and D. Hwang. 1992. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 267:25934-25938. [PubMed] [Google Scholar]
- 28.Lo, C. J. 2003. MAPK regulation of prostaglandin E2 production by lipopolysaccharide-stimulated macrophages is not dependent on nuclear factor κB. J. Surg. Res. 113:189-194. [DOI] [PubMed] [Google Scholar]
- 29.Marotta, P., L. Sautebin, and M. Di Rosa. 1992. Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br. J. Pharmacol. 107:640-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matte, C., G. Maion, W. Mourad, and M. Olivier. 2001. Leishmania donovani-induced macrophages cyclooxygenase-2 and prostaglandin E2 synthesis. Parasite Immunol. 23:177-184. [DOI] [PubMed] [Google Scholar]
- 31.Milano, S., F. Arcoleo, M. Dieli, R. D'Agostino, P. D'Agostino, G. De Nucci, and E. Cillari. 1995. Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins 49:105-115. [DOI] [PubMed] [Google Scholar]
- 32.Namba, T., H. Oida, Y. Sugimoto, A. Kakizuka, M. Negishi, A. Ichikawa, and S. Narumiya. 1994. cDNA cloning of a mouse prostacyclin receptor- multiple signaling pathways and expression in thymic medulla. J. Biol. Chem. 269:9986-9992. [PubMed] [Google Scholar]
- 33.Narumiya, S., Y. Sugimoto, and F. Ushikubo. 1999. Prostanoid receptors: structure, properties and functions. Physiol. Rev. 79:1193-1226. [DOI] [PubMed] [Google Scholar]
- 34.Nathan, C. R., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Banion, M. K., V. D. Winn, and D. A. Young. 1992. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl. Acad. Sci. USA 89:4888-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phipps, R. P., S. H. Stein, and R. L. Roper. 1991. A new view of prostaglandin E regulation of the immune response. Immunol. Today 12:349-352. [DOI] [PubMed] [Google Scholar]
- 38.Pouliot, M., J. Baillargeon, J. C. Lee, L. G. Cleland, and M. J. James. 1997. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J. Immunol. 158:4930-4937. [PubMed] [Google Scholar]
- 39.Qilliam, L., H. Mueller, B. Bohl, V. Prossnitz, L. Sklar, C. Der, and G. Bokoch. 1991. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J. Immunol. 147:1628-1635. [PubMed] [Google Scholar]
- 40.Reiner, N. E., and C. J. Malemud. 1984. Arachidonic acid metabolism in murine leishmaniasis (donovani): ex-vivo evidence for increased cyclooxygenase and 5-lipoxygenase activity in spleen cells. Cell. Immunol. 88:501-510. [DOI] [PubMed] [Google Scholar]
- 41.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheng, H., C. S. Williams, J. Shao, P. Liang, R. N. DuBois, and R. D. Beauchamp. 1998. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J. Biol. Chem. 273:22120-22127. [DOI] [PubMed] [Google Scholar]
- 43.Shotland, Y., H. Krämer, and E. A. Groisman. 2003. The Salmonella SpiC protein targets the mammalian Hook3 protein function to alter cellular trafficking. Mol. Microbiol. 49:1565-1576. [DOI] [PubMed] [Google Scholar]
- 44.Smith, W. L., and L. J. Marnett. 1991. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim. Biophys. Acta 1083:1-17. [DOI] [PubMed] [Google Scholar]
- 45.Strassmann, G., V. Patil-koota, F. Finkelman, M. Fong, and T. Kambayashi. 1994. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 180:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takano, M., H. Nishimura, Y. Kimura, J. Washizu, Y. Mokuno Y. Nimura, and Y. Yoshikai. 1998. Prostaglandin E2 protects against liver injury after Escherichia coli infection but hampers the resolution of the infection in mice. J. Immunol. 161:3019-3025. [PubMed] [Google Scholar]
- 47.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchiya, K., E. A. Groisman, and T. Nikai. 2004. Involvement of Salmonella pathogenicity island 2 in the up-regulation of interleukin-10 expression in macrophages: role of protein kinase A signal pathway. Infect. Immun. 72:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 50.Wan, C. P., E. Myung, and B. H. S. Lau. 1993. An automated micro-fluorometric assay for monitoring oxidative burst activity of phagocytes. J. Immunol. Methods 159:131-138. [DOI] [PubMed] [Google Scholar]
- 51.Wheeldon, A., and C. J. Vardey. 1993. Characterization of the inhibitory prostanoid receptors on human neutrophils. Br. J. Pharmacol. 108:1051-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, X.-J., J. Ruiz-Albert, K. E. Unsworth, S. Garvis, M. Liu, and D. W. Holden. 2002. SpiC is required for secretion of Salmonella pathogenicity island 2 type III secretion system proteins. Cell Microbiol. 4:531-540. [DOI] [PubMed] [Google Scholar]