Abstract
Chlamydia trachomatis is an obligate intracellular gram-negative bacterium responsible for a wide spectrum of diseases in humans. Both genital and ocular C. trachomatis infections are associated with tissue inflammation and pathology. Dendritic cells (DC) play an important role in both innate and adaptive immune responses to microbial pathogens and are a source of inflammatory cytokines. To determine the potential contribution of DC to the inflammatory process, human DC were infected with C. trachomatis serovar E or L2. Both C. trachomatis serovars were found to infect and replicate in DC. Upon infection, DC up-regulated the expression of costimulatory (B7-1) and cell adhesion (ICAM-1) molecules. Furthermore, chlamydial infection induced the secretion of interleukin-1β (IL-1β), IL-6, IL-8, IL-12p70, IL-18, and tumor necrosis factor alpha (TNF-α). The mechanisms involved in Chlamydia-induced IL-1β and IL-18 secretion differed from those of the other cytokines. Chlamydia-induced IL-1β and IL-18 secretion required infection with viable bacteria and was associated with the Chlamydia-induced activation of caspase-1 in infected host cells. In contrast, TNF-α and IL-6 secretion did not require that the Chlamydia be viable, suggesting that there are at least two mechanisms involved in the Chlamydia-induced cytokine secretion in DC. Interestingly, an antibody to Toll-like receptor 4 inhibited Chlamydia-induced IL-1β, IL-6, and TNF-α secretion. The data herein demonstrate that DC can be infected by human C. trachomatis serovars and that chlamydial components regulate the secretion of various cytokines in DC. Collectively, these data suggest that DC play a role in the inflammatory processes caused by chlamydial infections.
Chlamydia trachomatis infections cause a wide spectrum of human diseases. Trachoma, the major cause of preventable blindness, with an estimated 146 million cases of active trachoma worldwide (39), is the long-term consequence of repeated chlamydial infections of the conjunctival epithelium. C. trachomatis infection of the genital tract is now recognized as the most common sexually transmitted disease in the United States (40). Without treatment, genital chlamydial infections in women can cause pelvic inflammatory disease (PID) and its sequelae of infertility or ectopic pregnancy. The pathological mechanisms by which C. trachomatis induces conjunctival scarring or PID are not well understood. In all cases, however, the pathology seems to be related to a chronic inflammation caused by a persistent chlamydial infection or by repeated infections with the bacterium.
C. trachomatis is an obligate intracellular gram-negative bacterium with a unique biphasic developmental cycle (14). After entering host cells, metabolically inert elementary bodies (EB) rapidly transform into metabolically active reticulate bodies (RB) that replicate by binary fission within a membrane-bound vesicle termed an inclusion. After logarithmic bacterial cell division, the RB reorganizes to the infective EB, which is adapted for survival in the extracellular environment of the host. The C. trachomatis strains causing disease in humans are classified into trachoma and lymphogranuloma venerium (LGV) biovars with significantly different clinical features. The trachoma biovar (serovars A to K) is associated with ocular (serovars A to C) and genital infections (serovars D to K) of mucosal surfaces, and the more invasive LGV biovar (serovars L1, L2, and L3) is associated with systemic disease following a genital infection. It has been recently suggested that the tissue tropisms of C. trachomatis serovars may be associated with the presence (genital serovars D to L3) or absence (ocular serovars A to C) of a partial functional tryptophan operon mediating gamma interferon (IFN-γ) resistance via an indol rescue mechanism (8).
After initial infection of the host with C. trachomatis, dendritic cells (DC) are likely the first professional antigen-presenting cells encountering the bacteria. DC are present in the epithelium of the vagina and the cervix (24), as well as in the conjunctiva (1). Not only are DC potent antigen-presenting cells crucial for initiation of cell-mediated immune responses to foreign antigens (32), they also contribute to the innate resistance against microbial pathogens (23). In some instances, however, DC may also be involved in chronic inflammation (32). DC are found throughout the body as immature precursors. After microbial encounter, DC lose their phagocytic and endocytic ability, migrate to the draining lymph nodes, and increase their capacity for antigen processing and presentation (5). Their ability to prime T cells, to modulate the type of T-cell responses (Th1/Th2), and to contribute to the inflammatory response largely depends on the up-regulation of costimulatory and adhesion molecules and on the secretion of inflammatory cytokines (5, 23, 28).
Protection against chlamydial infection has been shown to be primarily mediated by IFN-γ-producing T cells (26, 31), and it has been shown that DC can process and present chlamydial antigens to T cells (15, 21, 22, 35). Little is known, however, about the interaction between human DC and Chlamydia spp. The purpose of the present study was to characterize the effect of a C. trachomatis infection on cytokine production and on expression of surface molecules by human monocyte-derived DC. Since C. trachomatis infections with the trachoma biovar are restricted to the epithelial and mucosal surfaces of the ocular and urogenital tract, we compare herein the effects of C. trachomatis serovar E of the trachoma biovar, a strain associated with localized urogenital infections, with those of the more invasive serovar L2 of the LGV biovar, a Chlamydia serovar disseminating to the draining lymph nodes of the urogenital system by infecting macrophages in the urogenital submucosae. A better understanding of the interaction between DC and Chlamydia spp. may contribute to the understanding of the role DC play either in control of chlamydial infection or in Chlamydia-induced pathology.
MATERIALS AND METHODS
Reagents.
Cells were cultured in RPMI medium (Gibco BRL, Grandville, N.Y.) supplemented with fetal calf serum (FCS) (HyClone Laboratories, Logan, Utah), gentamicin (Gibco BRL), and l-glutamine (Gibco BRL). A human HeLa cervical epitheloid cell line (HeLa 229; ATCC CCL-2.1) was purchased from the American Type Culture Collection (ATCC; Manassas, Va.). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) was generously provided by Amgen Corporation, recombinant human interleukin-4 (IL-4) was produced in Escherichia coli and purified at Corixa Corporation, and recombinant human IFN-γ was obtained from Pharmingen (San Diego, Calif). Monoclonal antibodies specific for CD1a; CD3; CD11a; CD14; CD16; CD19; CD40; CD54; CD80; CD86; DC-SIGN; HLA A, B, and C; HLA DP, DQ, and DR; and mouse isotype controls (Pharmingen) were used as direct conjugates to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) for flow cytometric analysis. Lipopolysaccharide (LPS) from E. coli O127:B8 was purchased from Sigma Chemical Co. (St. Louis, Mo.). Cell wall skeleton (CWS) was prepared from Mycobacterium phlei as described previously (3). The recombinant human cytokines IL-1α, IL-1β, IL-6, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α) were purchased from Pharmingen, and IL-12 was purchased from Sigma Chemical Co. For cytokine-specific enzyme-linked immunosorbent assays (ELISAs), the following monoclonal antibody pairs (Pharmingen) were used: for IL-1α, #18931D (capture) and #18662D (detection); for IL-6, #18871D (capture) and #18882D (detection); for IL-8, #20781D (capture) and #20792D (detection); for IL-10, #18551D (capture) and #18562D (detection); for IL-12, 20512D (detection); and for TNF-α, #18631D (capture) and #18642D (detection). IL-12p70 antibody #24910.1 (capture) was purchased from R&D Systems Inc. (Minneapolis, Minn.). IL-1β #M-421B-E (capture) and #M-420B-B (detection) antibodies were purchased from Endogen (Woburn, Mass.). An IL-18 ELISA kit was purchased from Medical and Biological Laboratories (Nagoya, Japan), and an IFN-α ELISA kit was purchased from Biosource International (Camarillo, Calif.). Toll-like receptor 4 (TLR4) blocking antibody (#16-9917-82) and isotype control (#16-4724-82) were purchased from eBioscience (San Diego, Calif.).
Chlamydia preparations.
C. trachomatis serovar L2 (L2/434/Bu; ATCC 902B-VR) and serovar E (ATCC 348B-VR) were propagated in HeLa 229 (ATCC CCL-2.1) cell monolayers as described previously (18). Briefly, HeLa cell monolayers were infected with either C. trachomatis serovar L2 or serovar E by centrifugation. EB were purified by two-step Hypaque-70 discontinuous gradient ultracentrifugation (Nycomed Inc., Princeton, N.J.) and stored at −80°C in sucrose-phosphate-glutamate buffer. The infectivity of the Chlamydia preparations was defined by determination of inclusion-forming unit (IFU) levels per milliliter on HeLa cells. Chlamydial preparations were routinely negative for mycoplasma, as shown by a mycoplasma-specific PCR. Heat inactivation of chlamydial EB was performed by twice incubating EB at 56°C for 30 min followed by flash freezing in liquid nitrogen. The 100% inactivation of infectious EB by heat treatment was confirmed by titration on HeLa cells.
Cell preparation.
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque 1.077 (Sigma Chemical Co.) centrifugation of a leukapheresis product prior to aliquoting and freezing. Monocyte-derived DC were prepared following the method of Romani et al. (30) from adherent PBMC as previously described (27). Phenotypic analysis of the cells revealed that more than 95% of the cells were positive for DC-SIGN, CD4, CD11a, CD40, CD54, CD86, and major histocompatibility complex class I and class II. Expression levels for CD1a ranged between 85 and 95%. The DC were negative for CD3, CD16, and CD19. Expression levels for CD14 differed between experiments, with cells having either no or low-level expression of CD14.
Infection of DC with C. trachomatis.
DC were plated in 24-well plates (Costar, Cambridge, Mass.) at 2 × 105 cells per well in RPMI medium containing 10% FCS. Cells were incubated overnight before infection. The medium was replaced with 0.3 ml of RPMI medium-10% FCS containing the infection inoculum, and plates were centrifuged for 1 h at 1,400 × g. The medium was replaced after centrifugation with 1 ml of RPMI medium-10% FCS, and plates were placed under conditions of 37°C and 7% CO2. Multiplicities of infection (MOI) were calculated as the number of chlamydial IFU/number of human cells. Supernatants were removed at 24 h postinfection and frozen at −20°C until cytokine quantification by ELISA. In TLR blocking experiments, TLR4 blocking antibody and isotype control (eBioscience) (20 μg/ml) were added to DC cultures 2 h prior to infection. The blocking antibody remained in the DC cultures for the 24-h culture period.
Cytokine assays.
Quantification of IL-1α, IL-6, IL-8, IL-10, IL-12p70, and TNF-α production was done by cytokine-specific ELISA as previously described (27). The working sensitivity of all ELISAs was shown to be approximately 10 pg/ml. IFN-α and IL-18 levels were determined using ELISA kits according to the manufacturer's protocol. Cytokines were not detectable in the absence of stimulation.
Detection of IL-1β by Western blot analysis.
At 24 h after chlamydial infection, DC were harvested, washed, and lysed by addition of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample-loading buffer (50 mM Tris-Cl [PH 6.8], 3% beta-mercaptoethanol, 2% SDS, 0.1% bromophenol blue, 10% glycerol). Lysates derived from equivalent numbers of cells were run on SDS-polyacrylamide electrophoresis gels (Novex, San Diego, Calif.) and transferred to nitrocellulose membranes (Schleicher & Schuell Inc., Keene, N.H.). Membranes were blocked overnight at 4°C in phosphate-buffered saline (PBS)-Tween containing 5% nonfat dry milk. IL-1β was detected by incubating membranes for 2 h with a dilution of 1:100 in blocking buffer of a goat anti-human IL-1β antibody (SC-1250; Santa Cruz Biotechnology) followed by washing membranes in blocking buffer and then incubating for 30 min with peroxidase-conjugated anti-goat immunoglobulin G (SC-2056; Santa Cruz Biotechnology) (1:1,000). IL-1β was then visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, England). Recombinant IL-1β (Pharmingen) was used as a positive control for the mature form of IL-1β.
Flow cytometry.
Cell surface marker expression analysis was performed by flow cytometry following standard procedures with a FACSCalibur apparatus (Becton Dickinson).
Caspase-1 activity.
At 24 h after infection, DC were harvested, washed once, and stained with a Caspatag Caspase-1 (YVAD) Activity kit (Intergen) according to the manufacturer's protocol. Active caspase-1 was detected by flow cytometry.
Chlamydial infection rates.
Infection rates were determined for each of the cytokine experiments. After supernatant removal, cells were incubated for an additional 24 h at 37°C to allow for chlamydial growth and fixed in 100% methanol followed by 50% methanol-50% acetone for 5 min each. Inclusions were stained with an FITC-conjugated anti-Chlamydia monoclonal antibody (Chlamydia AccuCLONE; Biowhittaker, Walkersville, Md.), counterstained with Evans blue, and then counted by using a fluorescence microscope.
Fluorescence microscopy.
DC were plated at 2 × 105 cells per well onto a 24-well plate containing a 12-mm-diameter coverslip. Plates were incubated overnight and then infected with C. trachomatis serovar L2 or E (MOI, 10:1) in RPMI medium-10% FCS. After infected DC were grown in cultures for the indicated times, DC were fixed in 100% methanol for 10 min. After being washed in PBS, cells were blocked with 3% bovine serum albumin in PBS at 37°C for 30 min before labeling with monoclonal antibody (LV-22) specific for chlamydial major outer membrane protein (MOMP) for 30 min at 37°C. Coverslips were removed and inverted onto a microscope slide in a drop of mounting fluid. Fluorescence was visualized with a 63× objective of a Zeiss epifluorescence microscope, and photomicrographs were taken with a Nikon UFX-11A camera.
Transmission electron microscopy (TEM).
At various time points post-chlamydial infection, DC were harvested. Cells in suspension were washed twice in 0.1 M Sorenson's buffer (PH 7.2; 28 ml of 0.1 M KH2PO4 with 72 ml of 0.1 M Na2HPO4) and fixed in 2% formaldehyde-0.5% gluteraldehyde (Sigma Chemical Co.) prepared in 0.1 M Sorenson's buffer for 1 h on ice. The samples were washed three times (10 min each) in cold 0.1 M Sorenson's buffer and postfixed in 0.5% osmium tetroxide prepared in 0.1 M Sorenson's buffer. Samples were washed three times in cold 0.1 M Sorenson's buffer and dehydrated in graded (30, 50, 70, and 95% ethanol) steps followed by two steps of 100% ethanol. Cells were washed twice in a transitional solvent, propylene oxide (Ted Pella Inc., Redding, Calif.), and infiltrated at room temperature in increasing concentrations of Eponate 12 (Ted Pella) (Epon:propylene oxide ratios were 1:1 and 2:1) and 100% Eponate 12 overnight at 4°C and 100% Eponate 12 for 2 days at 60°C. Sections 90 nm thick were cut on a Dupont MT 6,000 ultramicrotome and collected on 200-mesh copper grids with a nitrocellulose support film. The sections were stained with 5% aqueous uranyl acetate and lead citrate for 10 min, rinsed with water, dried, and examined in a JEOL 100S transmission electron microscope operated at 60 kV.
RESULTS
C. trachomatis infects and replicates in human DC.
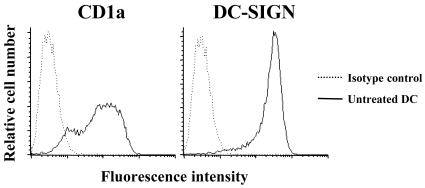
It has recently been shown that different C. trachomatis strains can infect and survive in murine macrophages (17). However, even though it has been shown that C. trachomatis can infect human DC, it is not clear that C. trachomatis can complete its developmental cycle in human DC (19, 22). Moreover, the ability of C. trachomatis serovar E (causing a localized sexually transmitted disease) to infect human DC and induce inflammatory cytokines has not yet been examined. In the present study, we determined whether C. trachomatis serovars L2 and E can multiply in human monocyte-derived DC. DC were generated following the method of Romani et al. (30) and expressed the DC markers DC-SIGN and CD1a (Fig. 1). Infection of DC with serovar L2 or E resulted in the formation of inclusions, as detected by staining with an anti-Chlamydia antibody followed by fluorescence microscopy (Fig. 2).
FIG. 1.
Human monocyte-derived dendritic cell (DC) CD1a and DC-SIGN surface marker expression. DC were harvested after a 7-day incubation of human monocytes in GM-CSF- and IL-4-containing medium, and expression of CD1a and DC-SIGN was determined by fluorescence-activated cell sorter (FACS) analysis. Results are representative of four different donors.
FIG. 2.
Fluorescence microscopy (A and B) and TEM (C to E) of human monocyte-derived DC infected with C. trachomatis serovar E or L2. After human DC were infected with serovar E or L2 at an MOI of 10:1, DC were cultured for the indicated time periods and then fixed for fluorescence microscopy or TEM. (A) DC infected with serovar L2 for 24 h and stained with Chlamydia MOMP-specific antibody (green) and Evans blue (red). (B) DC infected with serovar E for 48 h and stained with Chlamydia MOMP-specific antibody (green) and Evans blue (red). (C and D) TEM of DC infected with serovar L2 for 24 h (C) and 48 h (D). (E) TEM of DC infected with serovar E for 72 h. Elementary bodies (EB) and reticulate bodies (RB) can be distinguished within the chlamydial inclusion.
Infection rates ranged from 10 to 30% of cells for serovar E and from 30 to 100% of cells for serovar L2. By fluorescence microscopy, typical Chlamydia-containing inclusions were detected 24 h after infection with L2 (Fig. 2A) and 48 h after infection with E serovar (Fig. 2B). To morphologically compare the developmental cycles of serovars E and L2 in infected DC, inclusions were analyzed by TEM at 2, 24, 48, and 72 h postinfection. EB and RB were detected in inclusions of DC 24 h postinfection with serovar L2 (Fig. 2C). At 48 h postinfection with L2, chlamydial inclusions were slightly larger and showed more EB (Fig. 2D). In contrast, RB- and EB-containing inclusions were identified in serovar E-infected DC only 72 h after infection (Fig. 2E). Viability of DC by trypan blue exclusion was generally over 90% at 24 h postinfection (data not shown). EB from both serovars passaged on DC could be further propagated on HeLa 229 cells (data not shown).
Interaction of human monocyte-derived DC with C. trachomatis up-regulates costimulatory and adhesion molecules.
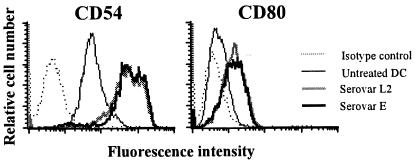
Exposure of DC to microbial products induces the up-regulation of adhesion and costimulatory molecules. Therefore, the surface expression of these molecules was analyzed in human DC following infection with C. trachomatis. The surface expression of CD11a, CD40, CD54, CD80, CD86, and HLA class I and class II was analyzed 24 h post-chlamydial infection. As shown in Fig. 3, DC infected with serovar E or serovar L2 up-regulated expression of CD54 (ICAM-1) and CD80 (B7-1). Expression of all other costimulatory and adhesion molecules measured remained unchanged after infection (data not shown). Both heat-inactivated and viable chlamydial EB induced expression of CD54 and CD80 in the same dose-dependent manner (data not shown).
FIG. 3.
Chlamydia infection up-regulates the expression of CD54 (ICAM-1) and CD80 (B7-1) on human monocyte-derived DC. DC were infected with C. trachomatis serovar L2 or E at a MOI of 10:1. Expression of cell surface markers CD54 and CD80 was determined by FACS analysis 24 h postinfection. Results are representative of four different donors.
C. trachomatis infection induces the production of inflammatory cytokines in human DC.
The results of cytokine induction in monocyte-derived DC by either infection with viable EB or exposure to heat-inactivated EB were compared. As a control, DC were stimulated with 1 μg of LPS derived from E. coli/ml. Infection with serovar L2 induced the production of IL-1β, IL-6, IL-8, and TNF-α in DC generated from the PBMC of five study subjects (Table 1). No IL-1α or IFN-α secretion was detected in supernatants from infected cells (data not shown). As shown in Table 1, heat-inactivated EB from serovar L2 as well as E. coli LPS induced the production of IL-6, IL-8, and TNF-α by human DC but did not induce the secretion of IL-1β. Treatment of human macrophages with viable or heat-killed EB from serovar L2 induced a cytokine profile similar to that shown for DC (data not shown). Bioactive IL-12p70 was only produced when DC were activated with IFN-γ at the time of infection or exposure to heat-inactivated EB (Table 1). The IL-12 data are consistent with previous reports demonstrating that the induction of IL-12p70 by E. coli LPS requires that macrophages and DC be activated with IFN-γ (27). Infection of human DC with serovar E yielded results similar to those obtained with serovar L2 (Table 2). DC produced IL-6, IL-8, and TNF-α after infection with viable or heat-inactivated serovar E EB, and viable EB induced the production of IL-1β. In contrast to the results of treatment with heat-inactivated serovar L2, however, heat-inactivated EB from serovar E consistently induced small amounts of IL-1β. Collectively, the data demonstrate that serovar E and the more invasive serovar L2 induce comparable inflammatory cytokine responses in DC. The presence of heat-inactivated EB is sufficient to induce the production of IL-6, IL-8, IL-12p70, and TNF-α, whereas it appears that a second mechanism requiring infection with viable C. trachomatis is involved in the secretion of IL-1β.
TABLE 1.
Viable and heat-inactivated EB from C. trachomatis serovar L2 induce the secretion of IL-6, IL-8, TNF-α, and IL-12p70 from human monocyte-derived DC, and only viable Chlamydia induces the secretion of IL-1β
| Treatment | Cytokine levela
|
||||
|---|---|---|---|---|---|
| IL-1β (pg/ml) | IL-6 (pg/ml) | IL-8 (ng/ml) | TNF-α (pg/ml) | IL-12p70b (pg/ml) | |
| Medium | <10 | <10 | 50 ± 10 | <10 | <10 |
| E. coli LPS | <10 | 2,100 ± 300 | 300 ± 50 | 14,000 ± 5,500 | 6,900 ± 4,800 |
| L2 | 2,000 ± 500 | 2,100 ± 300 | 300 ± 80 | 17,000 ± 5,200 | 3,200 ± 2,100 |
| HK L2 | <10 | 1,800 ± 400 | 300 ± 100 | 9,000 ± 6,000 | 1,700 ± 1,500 |
Monocyte-derived DC (2 × 105) were either infected as indicated with C. trachomatis serovar L2 (L2) treated with heat-inactivated L2 (HK L2) at an MOI of 10:1 or stimulated with 1 μg of LPS/ml. Cytokines were determined by ELISA in supernatants taken at 24 h posttreatment. Mean ± SD were calculated from the results obtained with five different donors.
IFN-γ was added to cultures at 10 ng/ml.
TABLE 2.
Comparison of cytokine induction results obtained with DC infected with C. trachomatis serovar E or serovar L2
| DC treatment | Cytokine level (pg/ml)a
|
||
|---|---|---|---|
| IL-1β | IL-6 | TNF-α | |
| Medium | <10 | <10 | <10 |
| L2 | 500 ± 100 | 1,400 ± 60 | 5,200 ± 200 |
| HK L2 | <10 | 1,100 ± 100 | 2,300 ± 700 |
| E | 300 ± 110 | 1,000 ± 20 | 7,300 ± 100 |
| HK E | 70 ± 15 | 1,200 ± 50 | 3,300 ± 1,000 |
Monocyte-derived DC (2 × 105) were infected as indicated with C. trachomatis serovar L2 (L2) and serovar E (E) or treated with heat-inactivated L2 (HK L2) and heat- inactivated E (HK E) at an MOI of 20:1. Cytokine levels were determined by ELISA in supernatants taken at 24 h posttreatment. Means ± SD were calculated from the results obtained with two different donors.
A neutralizing TLR4 antibody inhibits C. trachomatis-induced cytokine production.
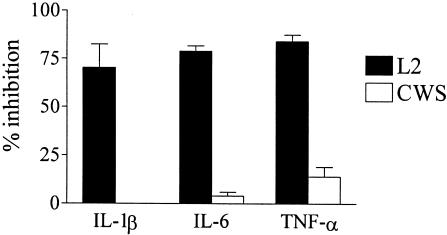
The recognition of microbial products by TLR4 induces the activation of human DC; therefore, the role of TLR4 in the C. trachomatis-induced cytokine production was examined. Monocyte-derived DC were incubated for 2 h with a TLR4-neutralizing antibody and then infected with serovar L2. As shown in Fig. 4, the TLR4 antibody blocked 70 to 80% of the Chlamydia-induced production of IL-1-β, IL-6, and TNF-α compared to the isotype control (IL-1-β, 1,100 pg/ml; IL-6, 2,700 pg/ml; TNF-α, 12 ng/ml). TLR4 antibody treatment had no effect on the IL-6 (6,700 pg/ml) and TNF-α (560 pg/ml) secretion in response to the presence of the CWS of M. phlei, a TLR2 agonist, indicating the TLR4 specificity of the neutralizing antibody. Similarly, the IL-6 and TNF-α secretion of DC stimulated with heat-inactivated EB was inhibited by 80 to 90% with the anti-TLR4 antibody (data not shown), suggesting that the activation of monocyte-derived DC by C. trachomatis EB is primarily dependent on a Chlamydia component signaling through TLR4.
FIG. 4.
TLR-dependent induction of IL-1β, IL-6, and TNF-α secretion by C. trachomatis in DC. DC (2 × 105) from two human donors were treated with a neutralizing antibody to TLR4 (20 μg/ml) or isotype control antibody 2 h before and during stimulation. DC were either infected with C. trachomatis L2 (MOI, 1:1) or were stimulated with CWS from M. phlei (5 μg/ml). Cytokine levels were determined by ELISA in supernatants taken 24 h postinfection. Data are presented as percent inhibition of antibody treatment compared to the isotype control antibody results. Results are shown as means ± standard deviations (SD) for the two donors tested. The TLR4 antibody treatment used in the study was efficient at the reduction of IL-6 production in DC in response to LPS (1 ng/ml) from 3,600 pg/ml to 400 pg/ml. The figure is representative of three separate experiments.
IL-1β secretion requires chlamydial protein synthesis.
To further characterize the mechanism involved in the induction of IL-1β secretion by Chlamydia-infected DC, DC infected with serovar L2 were treated with chloramphenicol. Chloramphenicol treatment selectively blocks chlamydial protein synthesis but has no effect on the protein synthesis of the host cell. As shown in Fig. 5, chloramphenicol treatment of L2-infected DC inhibited IL-1β secretion. Notably, the inhibitory effect on IL-1β secretion in chloramphenicol-treated DC was higher at the lower MOI (ninefold reduction at an MOI of 5:1 compared to the results seen with untreated, infected DC) than in DC infected with a higher dose of C. trachomatis (fourfold reduction at an MOI of 20:1). This effect was specific for the release of IL-1β, because similar treatment had no effect on the production of IL-6 and TNF-α by L2-infected DC. These data suggest that the release of IL-1β by Chlamydia-infected DC could be mediated by a chlamydial protein.
FIG. 5.
Chloramphenicol inhibits IL-1β secretion but not IL-6 or TNF-α secretion in Chlamydia-infected DC. DC (2 × 105) from two human donors were treated in the presence or absence of chloramphenicol (50 μg/ml) 2 h prior to and during infection with C. trachomatis serovar L2 (MOI, 10:1). Cytokine levels were determined by ELISA in supernatants taken 24 h postinfection. Data represent means ± SD of cytokine release from two human donors. The figure is representative of three separate experiments.
Both viable and inactivated chlamydial EB induce the production of pro-IL-1β.
The induction of IL-1β secretion by macrophages and DC requires two distinct processes. First, after a microbial stimulus the 31-kDa intracellular pro-form is synthesized. Then, in a second step, pro-IL-1β is posttranslationally cleaved by active caspase-1 into the secreted 17-kDa mature form that is released from the cells in the same process (29, 38). To determine whether heat-inactivated chlamydial EB can induce pro-IL-1β in DC, lysates of human monocyte-derived DC infected with viable or heat-inactivated EB were analyzed by Western blotting. As shown in Fig. 6, nontreated DC (lane 1) failed to produce pro-IL-1β. Treatment of DC with E. coli LPS (lane 4), inactivated EB (lane 3), or viable EB (lane 2) led to the accumulation of pro-IL-1β in the cytoplasm of DC. Interestingly, there was some intracellular mature IL-1β detectable in DC treated with LPS or inactivated EB. Secreted IL-1β, however, was detected only in the supernatants of DC infected with viable EB (3,200 pg of IL-1β/ml) but not in DC stimulated with E. coli LPS or inactivated EB, as determined by ELISA (data not shown). Thus, C. trachomatis EB do not need to be viable to induce the expression of pro-IL-1β in human DC. The Chlamydia-induced release of mature processed IL-1β by DC, however, requires infection with viable C. trachomatis EB.
FIG. 6.
Both viable and inactivated Chlamydia bacteria induce the production of pro-IL-1β by human monocyte-derived DC. DC (1.5 × 106) were infected with C. trachomatis serovar L2 (MOI, 10:1) (lane 2), pulsed with heat-inactivated serovar L2 (MOI, 10:1) (lane 3), or treated with 1 μg of LPS/ml (lane 4) as indicated. Untreated DC (lane 1) and mature recombinant IL-1β (lane 5) were used as controls. DC lysates were prepared 24 h after treatment, and the presence of IL-1β in DC lysates was analyzed by Western blotting. The results are representative of three independent experiments.
Only viable Chlamydia infection induces caspase-1 activation and secretion of IL-18.
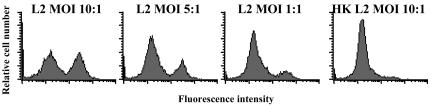
Since host caspase-1 is present in most cells as latent procaspase-1, one explanation for the data above is that chlamydial infection but not exposure to inactivated EB induces the activation of caspase-1 in DC. Alternatively, a chlamydial component of viable EB could directly process pro-IL-1β to mature IL-1β. To address these possibilities, we quantified the levels of active caspase-1 in C. trachomatis-infected DC by intracellular staining of active caspase-1 and analysis by flow cytometry. As shown in Fig. 7, active caspase-1 was readily detected in DC infected with viable chlamydial EB. Notably, the frequency of DC with detectable levels of active caspase-1 correlated with the rate of C. trachomatis infection in DC (approximately 50% infection at an MOI of 10:1 in this experiment). The number of DC with intracellular active caspase-1 was reduced in a dose-dependent manner with lower chlamydial MOIs. No active intracellular caspase-1 was detected in DC treated with heat-inactivated EB. Collectively, these data suggest that viable C. trachomatis infection of DC induces the secretion of IL-1β by activating caspase-1 by a mechanism requiring chlamydial protein synthesis.
FIG. 7.
Active caspase-1 is detected in human monocyte-derived DC after infection with viable but not heat-killed C. trachomatis. DC were either infected with C. trachomatis serovar L2 (L2) at MOIs of 10, 5, and 1:1 or pulsed with heat inactivated serovar L2 (HK L2) (MOI, 10:1). Intracellular levels of active caspase-1 were determined by FACS analysis in DC 24 h postinfection. Results of one representative experiment of five are shown.
Finally we were interested in the ability of C. trachomatis to regulate IL-18 production, because secretion of bioactive IL-18 also requires processing of procaspase-1 to active caspase-1 (13). As seen with IL-1β, IL-18 was also produced in DC infected with viable serovar E and L2 EB but not in DC treated with heat-inactivated EB (Fig. 8).
FIG. 8.
Infection of DC with viable C. trachomatis but not culture with heat-inactivated C. trachomatis induces the production of IL-18. Monocyte-derived DC (2 × 105) were either infected as indicated with C. trachomatis serovar L2 (L2) and serovar E (E), pulsed with heat-inactivated L2 (HK L2) and heat-inactivated E (HK E) at an MOI of 10:1, or left untreated (Medium). IL-1β and IL-18 levels were determined by ELISA in supernatants taken 24 h posttreatment. Means ± SD were calculated for two different donors. The results are representative of three independent experiments.
DISCUSSION
Dendritic cells are likely to be the first professional antigen-presenting cells that encounter C. trachomatis following genital and ocular chlamydial infections. DC are important for priming cellular mediated immune responses and innate inflammatory processes and therefore potentially play important roles in both host immunity and chronic inflammation, the hallmark of chlamydial disease. The goal of this study was to examine the interaction between human DC and C. trachomatis with respect to DC activation and induction of proinflammatory immune responses.
It has been demonstrated that chlamydial species are capable of infecting and surviving in murine macrophage cell lines (17). The fate of C. trachomatis in human DC is, however, less clear. Previously, two groups have reported on the ability of the L2 serovar to multiply in human monocyte-derived DC. Larsson et al. (19) showed that a small percentage of L2-infected human DC contained typical inclusions of fused vesicles, whereas Matyszak et al. (22) found that L2-containing vacuoles in GM-CSF-activated DC did not develop into characteristic inclusions. Since both studies used monocytes to generate DC, it appears that GM-CSF activation of DC, in similarity to the effect of IFN-γ, may alter the chlamydial developmental cycle. In the present study, large inclusions containing both developmental forms of C. trachomatis were detected by fluorescence microscopy and TEM after infection of DC with serovar L2 or E. Flow cytometry analysis of DC cultures, the morphology of the infected cells, and the infection rates indicate that both serovars infected and replicated in DC-SIGN-positive DC. As shown in Fig. 1, over 95% of the cells are DC-SIGN positive and 85% of the cells are CD1a positive. DC-SIGN is a specific marker for immature dendritic cells in peripheral blood, and since 10 to 30% of the cells were infected with serovar E and 30 to 100% were infected with serovar L2, we conclude that DC were the predominant cell type infected in these studies.
The protection induced by immunization of mice with viable C. trachomatis has been correlated to the presence of DC at the site of immunization (41). Protective immunity has also been achieved with adoptive transfer of DC pulsed with inactivated chlamydial organisms, and this protection correlates with the induction of a Th1 immune response (15, 21, 35, 37). The protective ability of the Chlamydia-pulsed DC correlates with their ability to secrete IL-12 (21, 35, 41) and induce a protective Th1 type of response when transferred in vivo. However, adoptive transfer of DC pulsed with recombinant chlamydial MOMP induced a nonprotective Th2 response (34). Thus, in vivo animal models indicate that DC need to be activated to induce a protective Th1 immune response. In the present study, both viable and heat-inactivated chlamydial EB induced the activation of DC, as characterized by the production of the inflammatory cytokines IL-6, IL-12 p70, and TNF-α and the up-regulation of cell surface markers ICAM-1 (CD54) and B7-1 (CD80) on DC. Furthermore, a neutralizing TLR4 antibody inhibited the secretion of IL-6, TNF-α, and IL-1β, suggesting that binding of a chlamydial component to TLR4 induces the production of these cytokines. This TLR4 binding component is most likely chlamydial LPS, since a heat-stable component of chlamydial EB induces IL-6, TNF-α, and pro-IL-1β, verifying the results of a study by Ingalis et al. in which chlamydial LPS induced TNF-α production in whole-blood cultures (16).
Although heat-inactivated chlamydial EB induced the production of IL-6, IL-12p70, and TNF-α, the secretion of IL-1β and IL-18 by DC was shown to be largely dependent upon infection with viable C. trachomatis. Interestingly, heat-inactivated EB from serovar E but not serovar L2 consistently induced small amounts of IL-1β. This IL-1β release could be due to limited cell lysis occurring during treatment with serovar E, since small amounts of mature IL-1β were detected in the cytoplasm of heat-inactivated EB. The secretion of mature IL-1β and IL-18 requires the processing of pro-IL-1β and pro-IL-18 by activated caspase-1. In fresh human monocytes, expression of active caspase-1 is constitutive; therefore, the presence of either E. coli LPS or heat-killed chlamydial EB is sufficient to induce the secretion of mature IL-1β (data not shown). In human DC and macrophages, however, only the latent procaspase-1 is present and LPS does not induce the secretion of IL-1β and IL-18. Infection with viable C. trachomatis induces the cleavage of procaspase-1 to form active caspase-1 homodimers. A recent report by Lu et al. (20) showed that Chlamydia-induced IL-18 secretion in epithelial cell lines was regulated at the posttranscriptional level and was dependent on caspase-1 activation and chlamydial protein synthesis. We extend these findings to human monocyte-derived DC and in addition demonstrate that two Chlamydia-dependent mechanisms, the induction of pro-IL-1β and the activation of caspase-1, are involved in the secretion of IL-1β. It has previously been shown that both Shigella flexneri and Salmonella enterica serovar Typhimurium are capable of activating caspase-1 and inducing the secretion of IL-1β from infected macrophages and DC by two homologous proteins of the type III secretion system, IpaB from S. flexneri and SipB from S. enterica serovar Typhimurium (7, 12, 25, 33). Sequencing of several Chlamydia genomes revealed the presence of a type III secretion system (36). Thus, it is possible that the chlamydial type III secretion system is involved in the activation of caspase-1.
The potential role of caspase-1 activation in chlamydial virulence or host immunity is unknown; interestingly, though, the production of IL-1β has been shown to potentiate the IFN-γ-mediated inhibition of chlamydial growth in epithelial cells (4) and human macrophages (9). IL-18 has been shown to be important not only for the induction of innate resistance but also (together with IL-12) in the differentiation of Th1 T cells (6). Furthermore, caspase-1 activation (2) and subsequent IL-1β (11) and IL-18 (10) production by DC are required for Langerhans cell migration into the local lymph node and, therefore, T-cell priming. Thus, it seems more likely that caspase-1 activation in DC is a protective host response to a chlamydial infection than a chlamydial virulence determinant. We speculate that following genital infection with C. trachomatis, cervical DC (Langerhans cells) may be involved in early inflammatory processes and may initiate a C. trachomatis-specific Th1 immune response by providing costimulatory molecules and producing the Th1-skewing cytokines IL-12 and IL-18 to initiate a protective immune response against a C. trachomatis infection.
Acknowledgments
We thank Ajay Bhatia for his expertise in Western blot analysis, Hang Fang for generating the Chlamydia preparations, Liz Caldwell, Bobbie Schneider, Franque Remington, and Judy Bousman from the electron microscope laboratory at the Fred Hutchinson Cancer Research Center and Daniel Luchtel and John Boykin from the Environmental Health, Toxicology Program at the University of Washington for their help and advice with the electron microscope, and Robert A. Henderson, Lee Ann Campbell, Sheila Lukehart, and Wesley Van Voorhis for fruitful discussions and suggestions.
This work was supported in part by grant T32AI07509 from the National Institutes of Health.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abu el-Asrar, A. M., K. Geboes, K. F. Tabbara, S. A. al Kharashi, L. Missotten, and V. Desmet. 1998. Immunopathogenesis of conjunctival scarring in trachoma. Eye 12(Pt. 3a):453-460. [DOI] [PubMed] [Google Scholar]
- 2.Antonopoulos, C., M. Cumberbatch, R. J. Dearman, R. J. Daniel, I. Kimber, and R. W. Groves. 2001. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J. Immunol. 166:3672-3677. [DOI] [PubMed] [Google Scholar]
- 3.Azuma, I., E. E. Ribi, T. J. Meyer, and B. Zbar. 1974. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J. Natl. Cancer Inst. 52:95-101. [DOI] [PubMed] [Google Scholar]
- 4.Babcock, T. A., and J. M. Carlin. 2000. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 12:588-594. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj, N. 2001. Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol. Med. 7:388-394. [DOI] [PubMed] [Google Scholar]
- 6.Biet, F., C. Locht, and L. Kremer. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80:147-162. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell, H. D., H. Wood, D. Crane, R. Bailey, R. B. Jones, D. Mabey, I. Maclean, Z. Mohammed, R. Peeling, C. Roshick, J. Schachter, A. W. Solomon, W. E. Stamm, R. J. Suchland, L. Taylor, S. K. West, T. C. Quinn, R. J. Belland, and G. McClarty. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J. Clin. Investig. 111:1757-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin, J. M., and J. B. Weller. 1995. Potentiation of interferon-mediated inhibition of Chlamydia infection by interleukin-1 in human macrophage cultures. Infect. Immun. 63:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumberbatch, M., R. J. Dearman, C. Antonopoulos, R. W. Groves, and I. Kimber. 2001. Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-alpha- and IL-1beta-dependent mechanism. Immunology 102:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1997. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 92:388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgeworth, J. D., J. Spencer, A. Phalipon, G. E. Griffin, and P. J. Sansonetti. 2002. Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur. J. Immunol. 32:1464-1471. [DOI] [PubMed] [Google Scholar]
- 13.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Hatch, T.P. 1999. Developmental biology, p. 29-67. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 15.Igietseme, J. U., G. A. Ananaba, J. Bolier, S. Bowers, T. Moore, T. Belay, F. O. Eko, D. Lyn, and C. M. Black. 2000. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J. Immunol. 164:4212-4219. [DOI] [PubMed] [Google Scholar]
- 16.Ingalls, R. R., P. A. Rice, N. Qureshi, K. Takayama, J. S. Lin, and D. T. Golenbock. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect. Immun. 63:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo, C. C., M. Puolakkainen, T. M. Lin, M. Witte, and L. A. Campbell. 2002. Mannose-receptor positive and negative mouse macrophages differ in their susceptibility to infection by Chlamydia species. Microb. Pathog. 32:43-48. [DOI] [PubMed] [Google Scholar]
- 18.Kuo, C.-C., S.-P. Wang, and J. T. Grayston. 1977. Growth of trachoma organisms in HeLa 229 cell culture, p. 328-336. In D. Hobson and K.K. Holmes (ed.), Nongonococcal uretritis and related infections. American Society for Microbiology, Washington, D.C.
- 19.Larsson, M., M. Majeed, J. D. Ernst, K. E. Magnusson, O. Stendahl, and U. Forsum. 1997. Role of annexins in endocytosis of antigens in immature human dendritic cells. Immunology 92:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, H., C. Shen, and R. C. Brunham. 2000. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J. Immunol. 165:1463-1469. [DOI] [PubMed] [Google Scholar]
- 21.Lu, H., and G. Zhong. 1999. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect. Immun. 67:1763-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matyszak, M. K., J. L. Young, and J. S. Gaston. 2002. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur. J. Immunol. 32:742-751. [DOI] [PubMed] [Google Scholar]
- 23.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 24.Miller, C. J., M. McChesney, and P. F. Moore. 1992. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab. Investig. 67:628-634. [PubMed] [Google Scholar]
- 25.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probst, P., Y. A. Skeiky, M. Steeves, A. Gervassi, K. H. Grabstein, and S. G. Reed. 1997. A Leishmania protein that modulates interleukin (IL)-12, IL-10 and tumor necrosis factor-alpha production and expression of B7-1 in human monocyte-derived antigen-presenting cells. Eur. J. Immunol. 27:2634-2642. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 29.Reed, J. C. 1999. Caspases and cytokines: roles in inflammation and autoimmunity. Adv. Immunol. 73:265-299. [DOI] [PubMed] [Google Scholar]
- 30.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rottenberg, M. E., A. Gigliotti-Rothfuchs, and H. Wigzell. 2002. The role of IFN-gamma in the outcome of chlamydial infection. Curr. Opin. Immunol. 14:444-451. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., and A. Lanzavecchia. 1999. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 189:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 34.Shaw, J., V. Grund, L. Durling, D. Crane, and H. D. Caldwell. 2002. Dendritic cells pulsed with a recombinant chlamydial major outer membrane protein antigen elicit a CD4+ type 2 rather than type 1 immune response that is not protective. Infect. Immun. 70:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw, J. H., V. R. Grund, L. Durling, and H. D. Caldwell. 2001. Expression of genes encoding Th1 cell-activating cytokines and lymphoid homing chemokines by Chlamydia-pulsed dendritic cells correlates with protective immunizing efficacy. Infect. Immun. 69:4667-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 37.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornberry, N. A., H. G. Bull, J. R. Calaycay, K. T. Chapman, A. D. Howard, M. J. Kostura, D. K. Miller, S. M. Molineaux, J. R. Weidner, and J. Aunins. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768-774. [DOI] [PubMed] [Google Scholar]
- 39.Thylefors, B., A. D. Negrel, R. Pararajasegaram, and K. Y. Dadzie. 1995. Global data on blindness. Bull. W. H. O. 73:115-121. [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. World Health Organization, Geneva, Switzerland.
- 41.Zhang, D., X. Yang, H. Lu, G. Zhong, and R. C. Brunham. 1999. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlates with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production and with dendritic cell-like maturation. Infect. Immun. 67:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]