Abstract
To preserve genome integrity, the S-phase checkpoint senses damaged DNA or nucleotide depletion and when necessary, arrests replication progression and delays cell division. Previous studies, based on two pol2 mutants have suggested the involvement of DNA polymerase epsilon (Pol ε) in sensing DNA replication accuracy in Saccharomyces cerevisiae. Here we have studied the involvement of Pol ε in sensing proper progression of DNA replication, using a mutant in DPB2, the gene coding for a non-catalytic subunit of Pol ε. Under genotoxic conditions, the dpb2-103 cells progress through S phase faster than wild-type cells. Moreover, the Nrm1-dependent branch of the checkpoint, which regulates the expression of many replication checkpoint genes, is impaired in dpb2-103 cells. Finally, deletion of DDC1 in the dpb2-103 mutant is lethal supporting a model of strand-specific activation of the replication checkpoint. This lethality is suppressed by NRM1 deletion. We postulate that improper activation of the Nrm1-branch may explain inefficient replication checkpoint activation in Pol ε mutants.
Author Summary
The viability of living organisms depends on the integrity of their genomes. Each cell has to constantly monitor DNA replication and coordinate it with cell division to avoid genomic instability. This is achieved through pathways known as cell cycle checkpoints. Therefore, upon replication perturbation, DNA synthesis slows down and cell division is delayed. For that, a specific signal is induced and propagated through a mechanism that have already been identified but still need investigations. We have isolated a mutated form of Dpb2, the essential subunit of DNA polymerase epsilon (Pol ε) holoenzyme. This mutated form of Pol ε impairs proper activation of the cellular response to replication stress. We show that yeast cells with mutations in the DPB2 gene fail to activate the Nrm1-regulated branch of the checkpoint, which controls numerous genes expressed in response to replication stress. Moreover, our results support the model of parallel activation of replication checkpoint from the leading and lagging DNA strands. This strongly suggests that Pol ε, the leading strand replicase, is involved in replication checkpoint activation from this strand. Our results contribute to the understanding of mechanisms of cellular response to replication stress, which are necessary to preserve genome stability.
Introduction
DNA integrity of living organisms is affected by perturbations that induce replication stress including nucleotide depletion or collision with lesions encountered in DNA exposed to alkylating agents [1]. Therefore, each cell must constantly monitor its genome integrity and coordinate DNA replication with cell division in order to avoid genetic instability [2]. Cell cycle checkpoints that monitor the accuracy of each phase of the cycle play crucial role in this control. The replication checkpoint monitors DNA duplication, and when activated, regulates transcription of specific genes, arrests replication progression, stabilizes replication forks, increases the dNTP pool, suppresses late-origin firing, delays cell division and finally restarts DNA synthesis after removal of replication stress [3–10]. It also prevents homologous recombination (HR) at double strand breaks (DSB) and stressed replication forks during S phase, presumably by blocking DNA ressection, to prevent genetic instability [11,12].
Checkpoint mechanisms encompass many proteins that act as sensors, mediators and effectors in a cascade of phosphorylation events [13]. In the first step, uncoupling of helicase and polymerase activities, unsynchronized leading and lagging strand replication or replication fork collapse result in accumulation of ssDNA [14,15]. After an activation threshold is reached [16], large stretches of RPA-coated ssDNA recruit the apical protein kinase Mec1 bound to Ddc2 [17]. Then, the Ddc1 subunit of the 9-1-1 sensor checkpoint clamp (Ddc1-Rad17-Mec3 in Saccharomyces cerevisiae) is recruited to the ds-ssDNA junctions and activates the signaling network [18]. The checkpoint response is completely dependent on the 9-1-1 complex in G1 phase while in G2 Dpb11 is also involved in this process [19]. In the S-phase, multiple factors are needed to trigger checkpoint activation including Dna2 in addition to Ddc1, Dpb11 [20–22] reviewed in [13,23]. It has been shown that a ddc1Δ dpb11-1 double mutant is partially defective in phosphorylation of the checkpoint effector kinase, Rad53 [20,24], indicating that there is an additional S-phase checkpoint activation pathway. Since Dna2 is probably involved in this additional activation mechanism, in the triple dpb11Δ ddc1Δ dna2Δ mutant only negligible phosphorylation of Rad53 was detected [21]. Finally, there is also evidence that DNA polymerase epsilon (Pol ε) is involved in the 9-1-1 independent activation pathway (Dpb11 recruitment to stalled replication forks) [25] suggesting separation of replication stress sensors on the leading and lagging DNA strands [20,26].
Upon checkpoint activation, the phosphorylated signaling kinase Mec1, transmits the signal to the downstream effector kinase Rad53 [27]. Its activation during replication stress is facilitated by checkpoint mediator protein Mrc1 [28,29] which promotes Mec1-Rad53 interactions [30]. Importantly, both Mec1 and Rad53 are essential genes in S. cerevisiae while not in Schizosaccharomyces pombe [31]. Rad53-dependent control of the replication stress response is divided into two branches: (i) the well-characterized Dun1-Crt1 pathway, also called DNA damage response (DDR) branch [32,33], which mainly up-regulates the dNTP pool, and (ii) the Nrm1-MBF pathway, also called the G1/S cell cycle (CC) branch [34,35], which up-regulates dozens of genes involved in many processes e.g., TOS4, TOS2, MCD1, CDC21 [36].
Pol ε is one of the major replicative polymerases that generally replicates the leading DNA strand while DNA polymerase delta (Pol δ) replicates the lagging strand [37–40]. Recently, an in vitro study of a reconstituted replisome has shown that Pol ε is targeted to the leading strand by the CMG complex (Cdc45, Mcm2-7 and GINS) while Pol δ is targeted to the lagging strand by PCNA (proliferating cell nuclear antigen) [41]. Moreover, a chromatin immunoprecipitation based method (eSPAN) was used to demonstrate the same strand bias patterns of Pol δ and Pol ε [42].
Pol ε is composed of the catalytic Pol2 subunit and three non-catalytic subunits Dpb2, Dpb3 and Dpb4 [43–45], for review see [46,47]. Dpb3 and Dpb4 subunits are involved in stabilization of Pol ε interaction with DNA, and their deletion affects replication fidelity [48]. Pol2 and Dpb2 subunits are essential in yeast, although deletion of the N-terminal polymerase catalytic domain of Pol2 gives viable cells [49,50]. In contrast, its C-terminal half is necessary and sufficient to support growth and is involved in both interaction with the Dpb2 subunit and S-phase checkpoint activation [50–52]. The interaction of Dpb2 subunit with Psf1, a subunit of the GINS complex, is important for the CMG complex assembly. Therefore, Dpb2 is involved in initiation of DNA replication but also links Pol ε to the CMG complex during elongation [53–57]. Finally, Pol ε –GINS interaction enables the preferential recruitment of Pol ε over Pol δ to the leading strand [41].
The dpb2 mutants isolated in our laboratory demonstrate temperature-sensitivity and an increased number of replication errors (MMR-dependent mutator phenotype) [58,59]. In these mutator strains, Pol ζ participates in DNA replication more often although the mutator phenotype of dpb2 mutants results not only from this error-prone TLS polymerase activity [60]. Moreover, these Dpb2 mutants are impaired in interaction with Pol2 and the GINS subunits Psf1 and Psf3 [56,58,61] which may result in increased participation of Pol δ on the leading strand and be partially responsible for the mutator phenotype [61].
In this work, we investigate the involvement of the Dpb2 subunit of Pol ε in triggering the response to replication stress. For this purpose, we use the dpb2-103 mutant carrying T342I S343F T345I P347S P348S substitutions, isolated in our laboratory [58]. We found that this mutant demonstrates phenotypes characteristic for replication checkpoint mutants. The dpb2-103 cells are sensitive to MMS (methyl methanesulfonate) and HU (hydroxyurea), and fail to delay cell cycle progression when treated with these agents. Although, dpb2-103 cells undergo checkpoint-induced Rad53 phosphorylation, they cannot properly activate the Nrm1/MBF branch of downstream response. Finally, we observed a lethal effect of dpb2-103 mutation combined with ddc1Δ. We propose that the observed synergy suggests independent roles in checkpoint activation and that 9-1-1 may recognize damage on the lagging strand while dpb2, as a subunit of Pol ε, acts on the leading strand.
Results
The dpb2-103 mutant demonstrates an S-phase checkpoint deficiency phenotype
Studies of the replication stress checkpoint have suggested the involvement of the catalytic subunit of DNA polymerase epsilon, Pol2, in checkpoint activation [62,63]. Later, it was suggested that Dpb2, the essential non-catalytic subunit of Pol ε interacts with Mrc1, the checkpoint mediator, and that thus Dpb2 may also be involved in activation of the S-phase checkpoint through modulation of Pol2-Mrc1 interactions [64]. Dpb2 variants that contribute to a spontaneous mutator phenotype have been analyzed in our laboratory for many years [58–61].
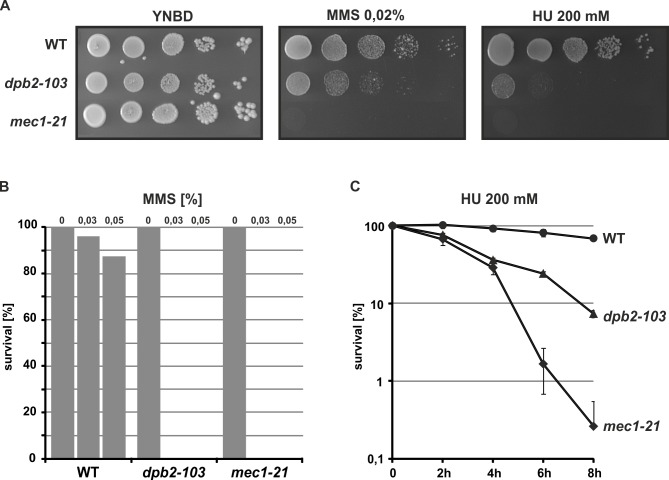
Replication stress can be generated either by nucleotide depletion using HU or by blocking replication due to fork collision with MMS-generated DNA lesions, which are detected only during replication [1,65]. To determine whether Dpb2 protein is involved in proper execution of replication checkpoint, first we analyzed the sensitivity of yeast cells with the dpb2-103 allele to the genotoxic agent methyl methanesulfonate (MMS) or to hydroxyurea (HU), the ribonucleotide reductase inhibitor (Fig 1). When compared to wild type cells, those with the dpb2-103 allele demonstrate increased sensitivity to both MMS and HU, although these cells were not as sensitive as the canonical S-phase checkpoint deficient mutant mec1-21 (Fig 1A, 1B and 1C).
Fig 1. dpb2-103 mutation affects yeast viability under genotoxic or replication stress.
(A) Cultures of indicated strains were grown exponentially, serially diluted and spotted on YNBD supplemented with MMS or HU. Plates were incubated at 23°C for 5 days. (B) Log-phase cultures of yeast strains were appropriately diluted and plated on YNBD medium without MMS or supplemented with 0,03% or 0,05% MMS. Plates were incubated at 23°C for 5 days. (C) Log-phase cultures of yeast strains were supplemented with HU to final concentration 200 mM. Samples were collected at 2 hours intervals, plated on YNBD medium, and incubated at 23°C for 5 days.
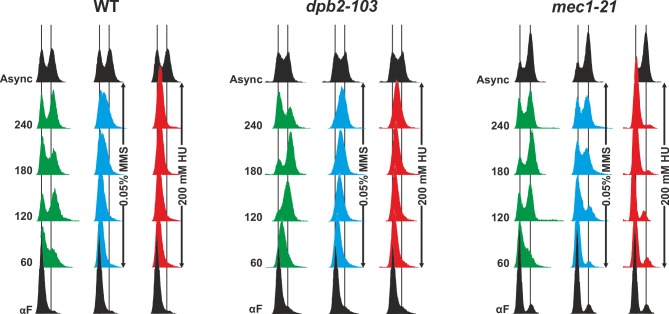
Yeast cells challenged with genotoxic or replication stress activate the checkpoint and delay their cell cycle progression. Slowing down the progression through S-phase gives more time to complete perturbed DNA replication and may result from inhibition of dormant or late origin firing [1,3,47] or inhibition of replication elongation [66]. This delay can be observed by flow cytometry analysis of DNA synthesis progression in the population of yeast synchronized in G1 and released under specific conditions [9,67,68]. We synchronized dpb2-103 mutant yeast cells with α-factor and released them from G1 in the absence or presence of MMS or HU. Then, we performed a flow cytometry analysis of DNA content to monitor G1-S-G2 transitions. Under MMS treatment in minimal media at 23°C, dpb2-103 cells reached the 2C DNA content after 240 min while wild-type cells remained at the G1-S transition after the same time in the same conditions. (Fig 2). Under HU treatment dpb2-103 cells entered S-phase very slowly, whereas wild-type cells remained blocked in G1 phase. These results demonstrate that, similarly to the mec1-21 checkpoint mutant, dpb2-103 cells are defective in delaying cell cycle progression and DNA synthesis when challenged with replication stress.
Fig 2. Functional DPB2 is required to prevent cell cycle progression under genotoxic or replication stress.
Cultures of indicated strains were synchronized in G1 and released from α-factor in YNBD (green), YNBD with 0,05% MMS (blue) or YNBD with 200 mM HU (red). Samples were taken every 60 minutes. For each strain, the DNA content was monitored by flow cytometry.
The checkpoint-induced delay of cell cycle progression in cells exposed to HU enables replication fork stabilization and DNA synthesis restart after release from replication stress. Therefore, we synchronized dpb2-103 cells in G1, and released them in the presence of HU. After 90 minutes, we washed out HU and shifted cells into fresh medium. Unexpectedly, unlike the control strain mec1-21 cells, the dpb2-103 cells were able to restart DNA synthesis after release from HU (S1 Fig). This result shows that the dpb2-103 mutant retains partial S-phase checkpoint activity, perhaps due to 9-1-1 checkpoint clamp sensing from the lagging strand.
Deletion of DDC1 in dpb2-103 is lethal
Previous work has suggested the involvement of the leading strand DNA polymerase ε in replication checkpoint activation [62,69]. At the same time, the 9-1-1 (Ddc1-Rad17-Mec3) complex has been proposed to be involved in sensing lagging strand replicative stress [20]. If the 9-1-1 checkpoint clamp and Pol ε act in parallel, strand-specific pathways to induce the response to replication stress, one can expect reduced ability to induce the checkpoint in the double mutant. To test whether Dpb2 is the Pol ε subunit involved in inducing the leading strand pathway of the replication stress checkpoint, we decided to introduce a DDC1 deletion in the dpb2-103 cells. Interestingly, the attempts to substitute DDC1 with nourseothricin resistance cassette (NAT1) were unsuccessful. Similarly, the dissection of tetrads obtained from a DPB2/dpb2-103 DDC1/ddc1Δ strain (Fig 3A) failed to generate dpb2-103 ddc1Δ cells, suggesting that the double mutant dpb2-103 ddc1Δ is inviable. In order to verify this, we attempted to introduce a DDC1 deletion into dpb2-103 cells carrying the pMJDPB2 plasmid [58] that provides Dpb2 protein. Transformants obtained in this experiment were cultured and serial dilutions were plated on YNBD medium and YNBD supplemented with 5-FOA to obtain plasmid-free clones. As expected, in contrast to the wild-type, dpb2-103 and ddc1Δ strains the dpb2-103 ddc1Δ cells became inviable after plasmid loss (Fig 3B) supporting the conclusion that the double mutant phenotype is lethal.
Fig 3. Synthetic lethality dpb2-103 ddc1Δ cells.
(A) Tetrad analysis of a heterozygous dpb2-103/DPB2, ddc1Δ/DDC1 strain. (B) Indicated strains with plasmid pMJDPB2 (source of DPB2 allele) were grown exponentially, serially diluted and spotted on YNBD or YNBD supplemented with 5-FOA.
The S-phase checkpoint induction deficiency of canonical mec1 or rad53 mutants is rescued by increasing dNTP formation by deletion of the ribonucleotide reductase inhibitor gene SML1 [4,70]. Therefore, we attempted to obtain dpb2-103 ddc1Δ sml1Δ cells through tetrad dissection of an appropriate heterozygous strain. However, after prolonged incubation, we obtained only small colonies of inviable double or triple mutants (S2 Fig). These results demonstrate that the sml1Δ (increased dNTP level) does not rescue lethality of dpb2-103 ddc1Δ cells. Together, these results demonstrate synthetic lethality of the dpb2-103 mutation combined with deletion of the DDC1 gene.
The Dun1 / Crt1 pathway is properly activated in dpb2-103 cells
Checkpoint activation in the S-phase induces a cascade of phosphorylation events. To test the stage at which checkpoint activation fails in dpb2-103 cells, first we analyzed activation of the checkpoint kinase Rad53. [27,71]. We compared the phosphorylation of Rad53 in dpb2-103 cells after MMS or HU treatment to the Rad53 status in checkpoint defective mec1-21 and pol2-12 cells. Migration of the phosphorylated form of Rad53 in polyacrylamide gels is retarded compared to unmodified Rad53. In dpb2-103 cells, after MMS or HU treatment during 180 minutes, phosphorylated Rad53 was detected (S3 Fig). As expected, in the checkpoint defective control strain mec1-21, after either MMS or HU treatment no Rad53 phosphorylated form was observed. In pol2-12 cells Rad53 was phosphorylated after HU treatment and residual phosphorylation was observed under MMS-induced genotoxic stress (S3 Fig). These observations suggest that although the dpb2-103 mutant seems to be impaired in S-phase checkpoint activation, the checkpoint kinase Rad53 is phosphorylated. However, it is not clear whether the protein is phosphorylated properly and the downstream signal amplified and propagated correctly.
Rad53-dependent phosphorylation of Dun1 (DNA-damage un-inducible) up-regulates the dNTP pool, primarily through two mechanisms. First, Dun1 phosphorylates and thus inhibits the Crt1 (constitutive RNR transcription) repressor which regulates a small part of the checkpoint-dependent transcriptional response i.e. the RNR2, RNR3 and RNR4 genes which encode subunits of the ribonucleotide reductase RNR [32,72]. Crt1 also represses the expression of gene HUG1 (hydroxyurea and UV and gammaradiation induced) whose product inhibits RNR through binding the Rnr2 subunit [73,74]. In parallel, Dun1 phosphorylates and promotes degradation of Sml1, the inhibitor of RNR [4,75] Moreover, Dun1-dependent phosphorylation of Dif1 (damage-regulated import facilitator 1), results in inactivation of the nuclear import of the Rnr2 and Rnr4 subunits of RNR, resulting in their cytoplasmic localization [76,77].
Therefore, to test whether in the dpb2-103 cells the checkpoint was interrupted downstream of Rad53, we analyzed the degradation of Sml1 and induction of RNR3 and HUG1 genes. The amount of the Sml1 protein was analyzed immunologically in extracts from cells treated with MMS for 60 or 120 minutes and compared with untreated cells. In wild-type cells, under genotoxic stress Sml1 is degraded after 60 minutes. Similar results were observed for dpb2-103 cells treated with MMS (Fig 4A). Next, using quantitative RT-PCR we analyzed the expression of the ribonucleotide reductase gene RNR3 and gene HUG1 encoding the RNR inhibitor [73,74]. Expression of these two genes is upregulated by the Dun1-Crt1 branch of replication stress response. Wild-type or dpb2-103 cells were synchronized in G1 and released into S-phase in the presence of 200 mM HU. The amount of RNR3 or HUG1 transcripts was normalized to wild-type untreated cells. After 120 and 240 minutes of HU treatment, the RNR3 and HUG1 expression levels in dpb2-103 were similar to those observed in wild-type cells under the same treatment (Fig 4B). It is noteworthy, that the RNR3 mRNA levels at the time of release from G1 and after 120 or 240 from release into S-phase were about 2-fold higher than in wild-type cells. In the control experiment, in mec1-21 cells, induction of RNR3 or HUG1 was not observed. These results demonstrate that the dpb2-103 cells activate the Dun1-Crt1 branch in response to HU or MMS induced replication stress.
Fig 4. The Crt1/Dun1 pathway of the replication stress checkpoint is activated in dpb2-103 cells under MMS or HU treatment.
(A) Western-Blot detection of Sml1 degradation. Extracts from WT or dpb2-103 yeast cells treated with 0,05% MMS were analyzed. (B) Quantitative RT-PCR analysis of RNR3 and HUG1 transcripts in yeast cells released from G1-arrest in YNBD (green) or YNBD with 200 mM HU (red). Transcript levels were analyzed after 120 and 240 minutes of growth and normnalized to wild-type G1-synchronized cells.
The Nrm1 / MBF pathway is not activated properly in dpb2-103 cells
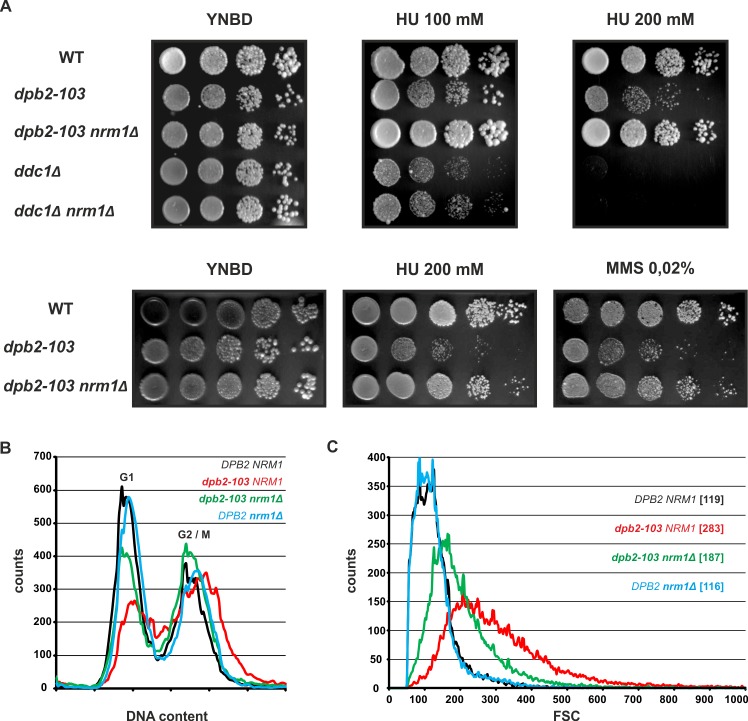
The replication checkpoint pathway downstream of Rad53 also encompasses a second branch, parallel to the Dun1/Crt1, i.e. the Nrm1/MBF branch. The Nrm1 co-repressor (negative regulator of MBF targets 1) together with the MBF (MluI-binding factor) repressor complex recognize the MCB (MluI cell-cycle box) DNA sequence in promoter regions of dozens of genes to repress transcription upon exit from G1 phase. Under replication stress, Rad53-mediated phosphorylation of the Nrm1 repressor prevents its binding to MBF promoters and allows upregulation of a set of MCB G1/S transition genes [34,35,78–83]. As a consequence, deletion of NRM1 and expression of MCB genes increases cell survival of checkpoint-deficient rad53Δ or mec1Δ yeast cells challenged with replication stress [78,79]. Therefore, we hypothesized that upregulation of G1/S transition genes would also rescue dpb2-103 sensitivity to replication stress. We saw that indeed nrm1Δ bypasses the sensitivity of dpb2-103 cells in the presence of HU or MMS (Fig 5A). Interestingly, this was not the case for ddc1Δ cells–deletion of NRM1 does not rescue HU sensitivity resulting from DDC1 deletion (Fig 5A).
Fig 5. The MBF pathway of the replication stress checkpoint is not activated (derepressed) properly in dpb2-103 cells under MMS or HU treatment.
(A) Deletion of NRM1 (coding for the MBF repressor) rescues dpb2-103 but not ddc1Δ-mediated MMS or HU sensitivity. Cultures of indicated strains were grown exponentially, serially diluted and spotted on YNBD supplemented with MMS or HU. (B) The abnormal progression of replication and (C) the large-cell phenotype of dpb2-103 cells are partialy rescued by NRM1 deletion. Asynchronous cells were analysed by flow cytometry to evaluate DNA content (FL2) and cell size (FSC, forward scatter). FSC median values are given in brackets.
Flow cytometry shows that asynchronous dpb2-103 cells have perturbed cell cycle, i. e. lower 1C DNA content and slow progression through S-phase (Figs 2 and 5B). Moreover, light scattering measurements indicate that dpb2-103 cells are larger than wild-type cells (Fig 5C). This observation is confirmed by microscopic observations of dpb2-103 cells (S4 Fig). Deletion of NRM1 in dpb2-103 cells partially suppressed these effects: the DNA content in dpb2-103 nrm1Δ cells shows higher 1C DNA content and lower proportion of S-phase cells (Fig 5B). Moreover, forward scatter (FSC) measurement indicates a decrease in cell size of dpb2-103 mutants after NRM1 deletion (Fig 5C). Together these results demonstrate that upregulation of MBF G1/S transition genes rescues several dpb2-103 phenotypes.
To support the hypothesis that activation of G1/S transition genes (the Nrm1/MBF branch) is impaired in dpb2-103 cells under replication stress, we tested the induction of TOS2, TOS4, MCD1 and CDC21 MCB genes repressed by Nrm1 and upregulated during S phase to promote cellular tolerance to replication stress [34]. Tos4 contains an FHA (ForkHead-associated) domain which interacts with components of the HADAC (histone deacetylase) complex involved in the response to various environmental stresses including replication stress [84]. Mcd1 is a subunit of cohesion complex involved in sister chromatide cohesion and chromosome condensation [85], Tos2 is involved in morphogenesis [86], Cdc21 is a thymidylate synthase [87]. Wild type and dpb2-103 cells were synchronized in G1 and released into S-phase in the presence of 200 mM HU. The amount of TOS2, TOS4, MCD1 and CDC21 RNA was normalized to wild-type untreated cells synchronized in G1 (time “0”) (Fig 6A and S1 Table). In parallel, cell cycle progression of these cells was monitored by flow cytometry analysis of DNA content (Fig 6B) demonstrating that both wild-type and dpb2-103 cells reached the S-phase 60–90 minutes after release from G1. Interestingly, a difference between wild-type and dpb2-103 cells, in transcription of these genes can be observed even in normal growth conditions. In wild-type cells, expression of G1/S transition genes is upregulated after release from G1 block, reaches the maximum level after 60 minutes, and decreases after 90–120 minutes. In contrast, in dpb2-103 cells, G1/S transition transcripts are most abundant after 30 minutes of growth and reach the minimum after 60–90 minutes. More important, HU-generated replication stress induced elevated transcription of TOS2, TOS4, MCD1 and CDC21 genes in wild-type cells but not in dpb2-103 cells as observed at 90 and 120 minutes time points. (Fig 6A). We conclude there is a defect in the Nrm1 branch of the checkpoint pathway.
Fig 6. The expression of G1/S transition genes (repressed by Nrm1) from the MBF pathway is not activated in dpb2-103 cells under replication stress.
(A) quantitative RT-PCR analysis of TOS2, TOS4, MCD1, CDC21 transcripts in wild-type (solid line) and dpb2-103 (dashed line) cells under unperturbed growth conditions (green) or under replication stress generated by 200 mM HU (red). Transcript levels were normalized to G1-synchronized wild-type cells. Yeast cultures were synchronized in G1 and released from α-factor in YNBD or YNBD 200 mM HU. Samples were taken at time 0 (G1 arrest), 30, 60, 90, 120 and 180 minutes after release from G1 block. Standard deviations were omitted for clarity and are presented in S1 Table. (B) Flow cytometry analysis of DNA content (cell cycle progression) at 0, 30, 60, 90, 120 and 180 minutes time points in wild-type (solid line) and dpb2-103 (dashed line) cells under unperturbed growth conditions (green) or under replication stress generated by 200 mM HU (red). (C and D) Deletion of NRM1 gives viable dpb2-103 ddc1Δ nrm1Δ cells. (C) Deletion of DDC1 in dpb2-103 cells carrying plasmid pMJDPB2 (source of DPB2). Transformants were were grown exponentially and dilutions were spotted on YNBD or YNBD with 5-FOA. (D) Tetrad analysis of a dpb2-103/DPB2, ddc1Δ/DDC1 nrm1Δ/ nrm1Δ strain.
Derepression of Nrm1-regulated genes rescues dpb2-103 ddc1Δ lethality
Positive effects of nrm1Δ on dpb2-103 survival, DNA content and cell size suggest that nrm1Δ may restore viability of dpb2-103 ddc1Δ cells. Therefore, we introduced nrm1Δ into dpb2-103 ddc1Δ pMJDPB2 cells and attempted to obtain plasmid-free cells on 5-FOA. Indeed, in contrast to dpb2-103 ddc1Δ, we were able to obtain viable dpb2-103 ddc1Δ nrm1Δ cells without the plasmid carrying the gene encoding WT Dpb2 (Fig 6C). Similar results were obtained after tetrad dissection from a dpb2-103/DPB2 ddc1Δ/DDC1 nrm1Δ/ nrm1Δ diploid strain (Fig 6D), demonstrating that the lethal effect of the dpb2-103 ddc1Δ is suppressed by derepression of genes that are up regulated in checkpoint proficient cells challenged with replication stress. This strengthens our conclusion that the Nrm1 pathway is affected in dpb2-103 and that Dpb2 and Ddc1 are involved in two separate branches of the checkpoint activation pathway.
Discussion
Early studies of Pol ε suggested that its catalytic subunit, Pol2, is involved in replication checkpoint activation. This function has been mapped to the essential C-terminal part of the protein as shown in temperature sensitive pol2-11 and pol2-12 mutants, which encode subunits lacking 31 or 27 C-terminal amino acids, respectively [62,88]. Besides replication perturbations, these mutants demonstrate a subset of checkpoint deficiency phenotypes including impaired DUN1 activation after MMS or HU treatment, low viability and elongated spindle formation after release from G1 synchronization into HU [62]. Therefore, such pol2 mutations allow entry into mitosis despite uncompleted DNA replication. The C-terminus of Pol2 is also involved in the interaction with Dpb2, the second essential subunit of Pol ε [58,59,63]. This interaction is facilitated by cell-cycle dependent phosphorylation of Dpb2 by CDK in late G1 phase. Inactivation of phosphorylation sites of Dpb2 in the pol2-11 strain dramatically reduces its viability, demonstrating that the Dpb2-Pol2 interaction is essential [89]. Pol2 also interacts with Mrc1 the fork-associated protein that mediates the Mec1-dependent activation of Rad53 [64]. Moreover, mrc1Δ pol2-11 cells are inviable and overexpression of MRC1 rescues pol2-11 temperature sensitivity [64].
The Dpb2 subunit of Pol ε plays an essential role in maintaining the proper architecture of the replisome as it links the Pol ε with GINS and therefore the CMG helicase complex (Cdc45, Mcm2-7 and GINS) through the interaction of the Dpb2 with the Psf1 and Psf3 GINS subunits [53,55–57,90]. Therefore, it is not surprising that increased amounts of Dpb2 [22], or the four subunits of GINS [53] suppress the pol2-11 mutation. Interestingly, both pol2-11 and dpb2-103 mutations impair interaction between Pol2 and Dpb2 [58,59,63]. Consequently, mutations in DPB2 affecting proper interactions of Dpb2 with either Pol2 or GINS may disrupt the replisome integrity and influence correct activation of the DNA replication checkpoint on the leading strand. Therefore, the dpb2-103 mutant encoding Dpb2-103 which has impaired interaction not only with the catalytic subunit Pol2 but also with Psf1 and Psf3 subunits of the GINS complex (S2 Table), was a good candidate for studies of DNA replication checkpoint-defective phenotypes. Using flow cytometry, we found that dpb2-103 mutant cells, similarly to mec1-21 mutant cells, failed to delay DNA replication after release from G1 block into MMS. (Fig 2). Checkpoint deficient mutant cells (mec1 or rad53) have been shown previously to be unable to delay replication progression [68,91]. However, when compared to mec1-21, dpb2-103 cells under MMS treatment progress through the S phase slowly and hardly reach the G2 phase, suggesting possible residual checkpoint activation (Fig 2). Another agent that induces replication stress, HU, slows DNA replication progression due to nucleotide depletion [8] resulting in elongating the time of origin firing [92]. Our flow cytometry experiments with G1 synchronized cells released into HU confirm that wild-type cells remain in early S even after 240 minutes. In contrast, the dpb2-103 mutant released from G1 synchronization into HU progressed through the S phase, albeit slowly, likely due to insufficient nucleotide precursors (Fig 2). One can therefore speculate that S phase progression of this mutant under HU treatment results from inefficient delay of DNA replication combined with alleviation of nucleotide depletion by the slight induction of RNR3 expression (Fig 4B). This hypothesis is reinforced by the observation that when compared to the wild type strain, dpb2-103 cells progress very slowly through unperturbed S phase (Fig 2).
Checkpoint-deficient cells such as mec1 mutants are also unable to resume DNA synthesis after transient HU treatment (S1 Fig) [20,31]. However, in our experiments, dpb2-103 cells resume DNA replication after the HU-generated block is removed in both permissive and restrictive temperature (S1 Fig). This observation explains why dpb2-103 cells are less sensitive to these drugs when compared to the mec1 mutant (Fig 1).
We also observed that similarly to pol2-11 and pol2-12 mutants [88] untreated dpb2-103 cells are larger, (Fig 5C), and that DNA content in asynchronous cells indicates that the relative proportion of S phase dpb2-103 cells is higher when compared to DPB2 cells (Fig 5B) demonstrating cell cycle control perturbations. However, the relative amount of cells in S phase may also be due to replication perturbations resulting from impaired interactions both within Pol ε and between Pol ε and the CMG helicase complex [59,61]. Nonetheless, these observations reinforce the conclusion that the cell cycle control is perturbed in the dpb2-103 mutant most probably due to inefficient replication checkpoint activation.
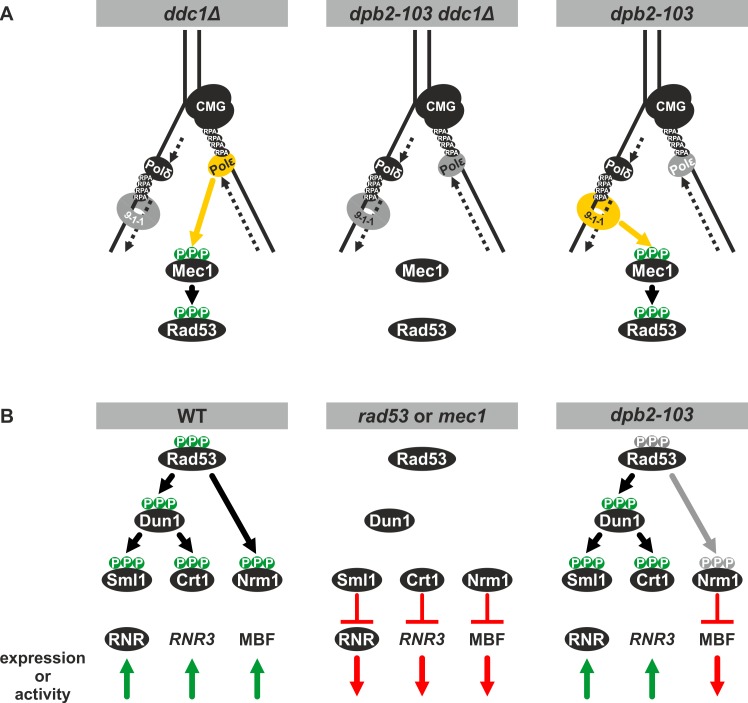
Because Dpb2 is a subunit of Pol ε, the leading strand polymerase [38,41], it has been suggested that its role in sensing replication perturbations is oriented mainly toward the leading strand [20]. Then, the signal from the lagging strand would come from the 9-1–1 (Ddc1-Rad17-Mec3) checkpoint clamp which is loaded specifically at the 5’ junctions of RPA-coated ssDNA and duplex DNA [93]. The existence of two parallel leading and lagging strand-specific checkpoint activation pathways would explain partial checkpoint activation in the pol2 mutants, the dpb2-103 or the ddc1Δ cells. Flow cytometry analysis of DNA content in ddc1Δ cells treated with MMS demonstrated progression through S phase similar to that observed in our study for dpb2-103 cells [18], although none of these mutants abolishes Rad53 phosphorylation in response to MMS or HU treatment [20,71] (S3 Fig). Consequently, it was suggested that in S. cerevisiae ddc1Δ cells the partial checkpoint activation is mediated by Dpb11 recruited to the replication fork by Pol ε in a 9-1-1 independent manner [20,25]. Therefore, it is not surprising that our experiments showed that the double dpb2-103 ddc1Δ mutant is lethal (Fig 3), supporting a model of separate sensing of replication stress on the two DNA strands and points out the involvement of Dpb2 in this process. The interpretation that the synthetic lethality of dpb2-103 and ddc1Δ results from the fact that replication defects in dpb2-103 cells can only be bypassed by a proficient replication checkpoint is also possible. However, given that the ddc1Δ mutant is only partially impaired in checkpoint activation and even combined with the dpb11-1 mutation retains low Rad53 activity that prevent replication fork breakdown this would not explain the synthetic lethality of dpb2-103 and ddc1Δ. Therefore, we favor the hypothesis of separation of replication problems sensing on leading and lagging strands (Fig 7A).
Fig 7. Model for replication checkpint activation and response in dpb2-103 cells.
(A) In ddc1Δ and dpb2-103 cells replication checkpoint activation is partially impared whereas in ddc1Δ dpb2-103 cells is abolished. (B) The two branches i. e. DDR (Dun1/Crt1) and CC (Nrm1/MBF) are activated in wild type cells and inactive in rad53 or mec1 checkpoint mutants. In dpb2-103 cells the Nrm1/MBF branch is inactive.
SML1 deletion can rescue mec1Δ or rad53Δ lethality, although it cannot restore checkpoint activation. However, after tetrad dissection of a heterozygous triple mutant we obtained colonies of very sick dpb2-103 ddc1Δ sml1Δ strains (S2 Fig), what is in accordance with our observation that the Dun1/Crt1 pathway, which regulates Sml1 degradation is properly activated in dpb2-103 cells. It also demonstrates that the lethal effect of mec1Δ or rad53Δ mutations (rescued by sml1Δ) results from replication checkpoint activation/execution defects other than those occurring in dpb2-103 ddc1Δ cells.
The involvement of Pol ε and Pol δ in the majority of replication of the leading and lagging DNA strands, respectively, is very well documented. However, it has been proposed recently, that Pol δ is the major replicase of both DNA strands [94]. However, mutation rate data obtained using Pol2 and Pol3 mutants that incorporate unique strand specific substitutions and studies of ribonucleotide incorporation into DNA by Pol ε and Pol δ as well as DNA in vitro studies [37,40,95,96] strongly support the model in which Pol ε acts as the major leading strand DNA polymerase. Moreover, the model in which Pol δ is the major replicase of both strands still locates Pol ε in association with the CMG complex on the leading strand with a role in correcting replication errors and proofreading rNMPs [94].
The replication checkpoint activation results in phosphorylation of the Rad53 effector kinase and subsequent cellular response to DNA synthesis problems. The best analyzed branch of this response is the Rad53-Dun1-dependent upregulation of dNTP pool [33,75] which normally limits DNA replication, but is upregulated 6- to 8-fold under replication stress to promote fork progression [8,97]. Interestingly, although the dpb2-103 mutant is impaired in correct response to replication stress, we detected Rad53 phosphorylation (S3 Fig), degradation of the RNR inhibitors Sml1 and induction of expression of the RNR3 and HUG1 genes (Fig 4). We can therefore conclude that dpb2-103 cells activate the Dun1-Crt1 branch of replication stress response correctly (Fig 7B). This suggests that Pol ε checkpoint mutants are partially proficient in activation of first steps of replication stress response although with modifications in the pattern of Rad53 phosphorylation. Such incomplete phosphorylation may be undetectable in the gel retardation assay of Rad53 which has been shown to contain multiple phosphorylation sites [98–100]. Alternatively, the Pol ε signal may act downstream of or independently of Rad53 in checkpoint activation. Importantly, these observations rule out the possibility that inefficiency of replication checkpoint activation in dpb2-103 cells results from the fact that the number of affected origins is not high enough to reach an activation threshold as demonstrated for the orc2-1 mutant defective in initiation of DNA replication and Rad53 phosphorylation under MMS treatment [16].
The results of our analysis of the second branch of Rad53-dependent response to replication stress, the Nrm1/MBF pathway, clarify the checkpoint-deficiency phenotypes of the dpb2-103 mutant. Rad53-dependent phosphorylation of the Nrm1 corepressor of MBF genes prevents its binding to MBF promoters in response to the S phase checkpoint [80] to activate expression of many genes involved in the replication stress response [36]. We found that NRM1 deletion suppresses the MMS and HU sensitivity of dpb2-103 cells (Fig 5A) and that dpb2-103 nrm1Δ cells partially rescue their cell size as well as their DNA content, demonstrating proficient progression through S phase (Fig 5B and 5C). We also tested nrm1Δ dependent checkpoint-deficiency phenotypes rescue in pol2-12 cells. Our flow cytometry analysis shows, that the pol2-12 mutant demonstrates a defect in S-phase progression (although less severe when compared to dpb2-103 cells), and that deletion of NRM1 restores proper DNA content (S5 Fig). Moreover, NRM1 deletion alleviates pol2-12 HU and temperature sensitivity (S5 Fig). These results suggest that the dpb2-103 and pol2-12 mutations in Pol ε may similarly affect the replication stress response.
The observed nrm1Δ dependent rescue of dpb2-103 phenotypes during unperturbed growth can be explained by upregulation of Nrm1-regulated genes at the G1/S transition, which are prematurely downregulated in dpb2-103 cells, when compared to the wild-type cells (Fig 6). Moreover, we observed that expression of Nrm1-repressed genes TOS2, TOS4, MCD1, CDC21, which is upregulated in wild-type cells even after 90–120 minutes in response to replication stress, remain uninduced in dpb2-103 cells (Fig 6). Finally, the lack of Nrm1 repressor (nrm1Δ) partially rescued the synthetic lethality of dpb2-103 ddc1Δ cells; a similar lethality bypass by nrm1Δ has been observed for rad53Δ and mec1Δ mutants [78,79]. This demonstrates that checkpoint deficiencies in dpb2-103 cells are due mainly to impaired derepression of Nrm1-regulated genes (Fig 7B). However, uncovering of the mechanism of Dpb2-dependent derepression of the Nrm1 branch of the replication stress response needs further investigation.
The question remains whether the failure of dpb2-103 mutant to fully activate the replication checkpoint results from direct involvement of the Dpb2 in replication stress sensing / activation, protein stability changes, impaired phosphorylation or from defects in Pol ε association within the replisome. Indeed, mutations in dpb2-103 partially impair interaction of Dpb2 with the catalytic subunit Pol2 of Pol ε and strongly impair its interaction with the Psf1 subunit of GINS. However, it would be expected that the destabilization of Pol ε in the replication fork and possibly dissociation would induce replication checkpoint activation rather than abolish it. Therefore, we favor the hypothesis of direct involvement of Dpb2 in the replication stress response, which still needs further investigation.
Materials and Methods
Yeast strains, media and growth conditions
S. cerevisiae strains listed in Table 1. were grown in standard media [101] [102]. When nutrition selection was not required yeast complete medium YPD (1% bacto-yeast extract, 2% bacto-peptone, 2% glucose liquid or solidified with 2% bacto-agar) was used. Yeast transformants were selected on YPD supplemented with appropriate antibiotics (Hygromycin B 300 μ-ml or Nourseothricin 100 μg-ml). When necessary yeasts were selected for prototrophy on YNBD minimal medium (0.67% yeast nitrogen base without amino acids, 2% glucose, liquid or solidified with 2% bacto-agar) supplemented with appropriate amino acids and nucleotides. For selection of URA3-plasmid-free cells, YNBD medium supplemented with 1 mg/ml 5-fluoroorotic acid (5-FOA) was used [103]. Escherichia coli DH5α (F-, gyrA96, recA1, relA1, endA1, thi1, hsdR17, supE44, deoR, Δ(lacZYA-argF)U169, [φ80Δ (lacZ)M15]) cells were grown routinely at 37°C in L broth–liquid or solidified with 1.5% agar and supplemented when needed with ampicillin (100 μg-ml).
Table 1. Yeast strains used in this work.
| Strain | Genotype | Source |
|---|---|---|
| SC228 | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 DPB2 | [58] |
| SC232 | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-103 | [58] |
| SC765 | MATa CAN1 his7-2 leu2-Δ::hisG ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 | [56] |
| Y306 | MATa can1-100 ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 mec1-21 | [9,108] |
| Y445MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 DPB2 ddc1-Δ::HPH | This work |
| Y446MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-10 3 ddc1-Δ::HPH pMJDPB2 | This work |
| Y447MD | MATα CAN1 his7-2 leu2-Δ::hisG ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 | This work |
| Y448MD | MATα CAN1 his7-2 leu2-Δ::hisG ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 ddc1-Δ::HPH | This work |
| Y449MD | MATa /MATα CAN1/CAN1 his7-2/his7-2 leu2-Δ::kanMX4/leu2-Δ::hisG ura3-Δ/ura3-Δ trp1-289/trp1-289 ade2-1/ade2-1 lys2-ΔGG2899-2900/lys2-ΔGG2899-2900 DPB2/ dpb2-103 DDC1/ddc1-Δ::HPH | This work |
| Y450MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-103 sml1-Δ::NAT1 | This work |
| Y451MD | MATa /MATα CAN1/CAN1 his7-2/his7-2 leu2-Δ::kanMX4/leu2-Δ::hisG ura3-Δ/ura3-Δ trp1-289/trp1-289 ade2-1/ade2-1 lys2-ΔGG2899-2900/lys2-ΔGG2899-2900 DPB2/ dpb2-103 DDC1/ddc1-Δ::HPH SML1/sml1-Δ::NAT1 | This work |
| Y452MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 DPB2 nrm1-Δ::NAT1 | This work |
| Y453MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-103 nrm1-Δ::NAT1 | This work |
| Y454MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-10 3 ddc1-Δ::HPH nrm1-Δ::NAT1 pMJDPB2 | This work |
| Y455MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 dpb2-10 3 ddc1-Δ::HPH nrm1-Δ::NAT1 | This work |
| Y458MD | MATa /MATα CAN1/CAN1 his7-2/his7-2 leu2-Δ::kanMX4/leu2-Δ::hisG ura3-Δ/ura3-Δ trp1-289/trp1-289 ade2-1/ade2-1 lys2-ΔGG2899-2900/lys2-ΔGG2899-2900 DPB2/ dpb2-103 DDC1/ddc1-Δ::HPH nrm11-Δ::NAT1/nrm11-Δ::NAT1 | This work |
| Y459MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 DPB2 pol2-12 | This work |
| Y460MD | MATa CAN1 his7-2 leu2-Δ::kanMX4 ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900 DPB2 nrm1-Δ::NAT1 pol2-12 | This work |
DNA manipulations
Yeast strains were transformed using the LiAc/ssDNA/PEG method [104]. Total yeast DNA was isolated using the Genomic Mini AX Yeast Spin kit (A&A Biotechnology, Gdansk, POLAND). E. coli cells were transformed as previously described [105] and bacterial plasmids were isolated using the Plasmid mini kit (A&A Biotechnology, Gdansk, POLAND).
Construction of yeast strains
Deletions of DDC1, SML1 or NRM1 were performed by replacement of their coding regions by PCR-amplified HPH or NAT1 cassettes obtained with primers listed in Table 2. Gene replacement was verified using PCR with primers listed in Table 2 and sequencing. The pol2-12 mutation was introduced as previously described [106] and verified using primers POL2DO35 and POL2DR20 (Table 2).
Table 2. Primers used in this work.
| Primer | Sequence 5’-3’ |
|---|---|
| ddc1HphU | GCTTAGACATATATGTCATTTAAGGCAACTATCACCGAGTCGGGGAGATCTGTTTAGCTTGCC |
| ddc1HphD | TATACCCCTTGGCTTTTCTACTTGTGTTAGACCCAGCCCATCTTCTTCGAGCTCGTTTTCGACAC |
| nrm1-P_U | AGGCTGCGGAGAGGGCCAATTGCAGCAGCGACGTACGCATAGAGGAAAAACAACCGTTCATGAGATCTGTTTAGCTTGCC |
| nrm1-T_D | TATTATTATATACACAATGAAGGAGAATGAAAATGAAAGATAGAAATATTGGGGGTGGGATTCGAGCTCGTTTTCGACAC |
| 3-sml1 | TGGCGCTAGCGATATCTAGC |
| 5-sml1 | CCAAACGGGCTCCACTACC |
| UFddc1 | GCCGCCAAAGGTAATCATAGAC |
| UFddc1_2 | GGTGCACTCAATTTGCCGAAAG |
| DRddc1 | GCACGCTCACCAAATTGAGAC |
| DRddc1_2 | TAGCGTTCCGGAGTATGTAGG |
| DDC1UO | TCAGCAGCCGTTAACTGATTCC |
| DDC1DO | ACACTCTGTGGCTGGAACTC |
| NRM1-UF | CCGACATTGACTCACTATCC |
| NRM1-DR | ACTTTCAGTGCTCGTGTCTC |
| NRM1-UO | GCCACTGCTCCTCATTAGGG |
| NRM1-DO | ACATCTTTCCGCGGTGTCAG |
| 5T-sml1 | CCGTGTCAACAAGAGTGTCAAGACC |
| sml1UO | GTCACGGTACGCAATGTGGAAG |
| hph UO | ACAGACGTCGCGGTGAGTTCAG |
| hph DO | TCGCCGATAGTGGAAACCGACG |
| nat1UO | ACCGGTAAGCCGTGTCGTCAAG |
| nat1DO | GCTTCGTGGTCGTCTCGTACTC |
| POL2DO35 | GGTTCCCATCTGAATGTG |
| POL2DR20 | GGTAAAGAGGCCATTGAACC |
| ACT1RTup | ACCGCTGCTCAATCTTCTTC [109] |
| ACT1RTlo | GTAGTTTGGTCAATACCGGC [109] |
| RNR3F682 | CAAAGAGCCCGTTCAATTCG |
| RNR3R539 | TCCAGCTCAACGGTAGTAAC |
| HUG1F801 | TGACGATGTCGTCCTACAAG |
| HUG1R935 | AGACCGCCGCGACGTTCGAC |
| CDC21F022 | GAAGGAGAAGGATCCGGTAAG |
| CDC21R873 | GAACTCACCTGGCTCCATGTC |
| MCD1F215 | TCGGTAGACGATTCAGTCCAGATG |
| MCD1R362 | TCGTGCGTATCAAAGTTGCGTGAG |
| TOS2F961 | AGTGATTCCCGAATCGTCTC |
| TOS2R836 | ACGGGAGCCTTTGCAGAGTG |
| TOS4F942 | AGCCTAATTGTCCCTCATCC |
| TOS4R802 | TATTCGACCTAACGGGAGAC |
Sensitivity tests
For MMS sensitivity tests, yeast strains were grown in YPD medium until OD600 reached 0,6, harvested and resuspended in 0,9% NaCl. Appropriate dilutions were plated on YNBD medium supplemented with MMS (0%, 0,01%, 0,02% or 0,03%). Colonies were counted after 5 days incubation at 23°C. For HU sensitivity tests, yeast strains were grown in YPD medium until OD600 reached 0,6 before adding HU to 200 mM final concentration. Samples were collected at indicated time points, washed with distilled water and plated on YNBD medium. Colonies were counted after 5 days incubation at 23°C.
α-factor synchronization and cell cycle progression analysis
Yeasts were precultured overnight in YNBD medium at 23°C and appropriately diluted in YNBD medium to grow at 23°C until OD600 reached 0,4. Cells were harvested, resuspended in fresh YNBD medium with the α-factor mating pheromone (4 mg/ml) and grown for 2–3 hours at 23°C. Then to release them from α-factor, cells were harvested and washed three times with water. Next they were released from G1-arrest into fresh YNBD medium and incubated at 23°C. When necessary, cells were released from G1-arrest in YNBD medium containing 0,05% MMS or 200 mM HU. Samples were taken at indicated time points and fixed in 70% ethanol.
Flow cytometry analysis
Ethanol-fixed cells were harvested, washed and resuspended in 1 ml of sodium citrate (50 mM, pH 7,0). After brief sonication they were treated with RNaseA (0,25 mg-ml) at 50°C for 1 hour and with proteinase K (1 mg/ml) for another hour at 50°C. Then, samples were diluted in sodium citrate containing propidium iodide (16 μg-ml) and incubated overnight at 4°C. The DNA content was identified by measuring the propidium iodide fluorescence signal (FL2) using Becton Dickinson FACSCalibur and the CellQuest software (BD Bioscience). To evaluate the size of yeast cells, the forward scatter (FSC) was analyzed.
Western-blot analysis
Yeast cells were grown until OD600 reached 0,4–0,6. Then, 0,05% MMS or 200 mM HU was added and cells were grown for 2 h. Cells were collected and prepared for SDS-PAGE as described previously [107]. For immunodetection, goat polyclonal anti-Rad53 antibody (sc-6749) from Santa Cruz Biotechnology), rabbit polyclonal anti-Sml1 antibody (AS10 847) from Agrisera and mouse monoclonal anti-actin antibody (MAB1501) from Millipore were used.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using the Syngen Tissue RNA Mini Kit (Syngen Biotech, POLAND) as indicated in the manufacturer’s instruction. Reverse transcription was performed using the RevertAid™ First Strand cDNA Synthesis Kit (ThermoFisher Scientific) and Real-Time PCR was done using Real-Time 2xHS-PCR Master Mix SYBR (A&A Biotechnology) and LightCycler 480 (Roche). Transcript levels were normalized to actin mRNA (ACT1).
Supporting Information
Yeast cultures of indicated strains were synchronized in G1 and released from α-factor in YNBD medium supplemented with 200 mM HU (red). After 90 minutes, cells were washed and released in YNBD medium in 23°C (permissive temperature for dpb2-103 mutant) or 37°C (restrictive temperature for dpb2-103 mutant). Samples were collected after 30 and 60 minutes.
(TIF)
(TIF)
Extracts from WT, dpb2-103, mec1-21 or pol2-12 yeast cells treated with 200 mM HU or 0,05% MMS were resolved by SDS-PAGE and Anti-Rad53 antibodies were used for Western-blot analyzis. Unspecific bands detected using the same antibodies were used as loading control.
(TIF)
Yeasts were grown in YNBD medium to log phase.
(TIF)
(A) The abnormal progression of replication in pol2-12 cells is rescued by NRM1 deletion. Asynchronous cells were analysed by flow cytometry to evaluate DNA content. (B) Deletion of NRM1 (coding for the MBF repressor) rescues pol2-12 HU and temperature sensitivity. Cultures of indicated strains were grown exponentially, serially diluted and spotted on YNBD supplemented with HU and incubated at 23 or 37°C
(TIF)
(PDF)
(PDF)
Acknowledgments
We thank Dr. Karolina Makiela-Dzbenska for assistance with strain construction.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grant no. 5714/B/P01/2010/39 from the Ministry of Science and Higher Education, Poland (www.ncn.gov.pl) to PJ and grant no. TEAM/2011-8/1 from the Foundation for Polish Science (www.fnp.org.pl), co financed from European Union – Regional Development Fund “New players involved in the maintenance of genomic stability” to IJF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tercero J a, Longhese MP, Diffley JFX. A central role for DNA replication forks in checkpoint activation and response. Mol Cell. 2003;11: 1323–1336. [DOI] [PubMed] [Google Scholar]
- 2.Skoneczna A, Kaniak A, Skoneczny M. Genetic instability in budding and fission yeast—sources and mechanisms [Internet]. FEMS Microbiology Reviews. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395: 615–8. 10.1038/27001 [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Chabes a, Domkin V, Thelander L, Rothstein R. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 2001;20: 3544–3553. 10.1093/emboj/20.13.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachant J, Jessen SR, Kavanaugh SE, Fielding CS. The yeast S phase checkpoint enables replicating chromosomes to bi-orient and restrain spindle extension during S phase distress. J Cell Biol. 2005;168: 999–1012. 10.1083/jcb.200412076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourrière H, Pasero P. Maintenance of fork integrity at damaged DNA and natural pause sites. DNA Repair (Amst). 2007;6: 900–13. [DOI] [PubMed] [Google Scholar]
- 7.Szyjka SJ, Aparicio JG, Viggiani CJ, Knott S, Xu W, Tavaré S, et al. Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev. 2008;22: 1906–20. 10.1101/gad.1660408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poli J, Tsaponina O, Crabbé L, Keszthelyi A, Pantesco V, Chabes A, et al. dNTP pools determine fork progression and origin usage under replication stress. EMBO J. 2012;31: 883–94. 10.1038/emboj.2011.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8: 2401–2415. [DOI] [PubMed] [Google Scholar]
- 10.Aboussekhra A, Vialard JE, Morrison DE, de la Torre-Ruiz MA, Cernáková L, Fabre F, et al. A novel role for the budding yeast RAD9 checkpoint gene in DNA damage-dependent transcription. EMBO J. 1996;15: 3912–22. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=452098&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 11.Alabert C, Bianco JN, Pasero P. Differential regulation of homologous recombination at DNA breaks and replication forks by the Mrc1 branch of the S-phase checkpoint. EMBO J. Nature Publishing Group; 2009;28: 1131–1141. 10.1038/emboj.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prado F. Genetic instability is prevented by Mrc1-dependent spatio-temporal separation of replicative and repair activities of homologous recombination: Homologous recombination tolerates replicative stress by Mrc1-regulated replication and repair activities opera. BioEssays. 2014; 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hustedt N, Gasser SM, Shimada K. Replication checkpoint: tuning and coordination of replication forks in s phase. Genes (Basel). 2013;4: 388–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branzei D, Foiani M. The checkpoint response to replication stress. DNA Repair (Amst). 2009;8: 1038–46. [DOI] [PubMed] [Google Scholar]
- 15.Branzei D, Foiani M. The DNA damage response during DNA replication. Curr Opin Cell Biol. 2005;17: 568–75. 10.1016/j.ceb.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Shimada K, Pasero P, Gasser SM. ORC and the intra-S-phase checkpoint: a threshold regulates Rad53p activation in S phase. 2002; 3236–3252. [DOI] [PMC free article] [PubMed]
- 17.Ball HL, Ehrhardt MR, Mordes D a, Glick GG, Chazin WJ, Cortez D. Function of a conserved checkpoint recruitment domain in ATRIP proteins. Mol Cell Biol. 2007;27: 3367–3377. 10.1128/MCB.02238-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longhese MP, Paciotti V, Fraschini R, Zaccarini R, Plevani P, Lucchini G. The novel DNA damage checkpoint protein ddc1p is phosphorylated periodically during the cell cycle and in response to DNA damage in budding yeast. EMBO J. 1997;16: 5216–26. 10.1093/emboj/16.17.5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Elledge SJ. Genetic and physical interactions between DPB11 and DDC1 in the yeast DNA damage response pathway. Genetics. 2002;160: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puddu F, Piergiovanni G, Plevani P, Muzi-Falconi M. Sensing of replication stress and Mec1 activation act through two independent pathways involving the 9-1-1 complex and DNA polymerase ε. PLoS Genet. 2011;7: e1002022 10.1371/journal.pgen.1002022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Burgers PM. Lagging strand maturation factor Dna2 is a component of the replication checkpoint initiation machinery. Genes Dev. 2013;27: 313–21. 10.1101/gad.204750.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki H, Leem SH, Phongdara A, Sugino A. Dpb11, which interacts with DNA polymerase II(ε) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc Natl Acad Sci U S A. 1995;92: 11791–5. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=40488&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanrooij PH, Burgers PM. Yet another job for Dna2: Checkpoint activation. DNA Repair (Amst). 2015;32: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navadgi-Patil VM, Burgers PM. The unstructured C-terminal tail of the 9-1-1 clamp subunit Ddc1 activates Mec1/ATR via two distinct mechanisms. Mol Cell. Elsevier Ltd; 2009;36: 743–53. 10.1016/j.molcel.2009.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navadgi-Patil VM, Burgers PM. A tale of two tails: activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair (Amst). 2009;8: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Rodríguez LJ, De Piccoli G, Marchesi V, Jones RC, Edmondson RD, Labib K. A conserved Polϵ binding module in Ctf18-RFC is required for S-phase checkpoint activation downstream of Mec1. Nucleic Acids Res. 2015; gkv799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoch NC, Chen ES-W, Buckland R, Wang S-C, Fazio A, Hammet A, et al. Molecular basis of the essential s phase function of the Rad53 checkpoint kinase. Mol Cell Biol. 2013;33: 3202–13. 10.1128/MCB.00474-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborn AJ, Elledge SJ. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. 2003; 1755–1767. [DOI] [PMC free article] [PubMed]
- 29.Katou Y, Kanoh Y, Bando M, Noguchi H, Tanaka H, Ashikari T, et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424: 1078–83. 10.1038/nature01900 [DOI] [PubMed] [Google Scholar]
- 30.Chen S-H, Zhou H. Reconstitution of Rad53 activation by Mec1 through adaptor protein Mrc1. J Biol Chem. 2009;284: 18593–604. 10.1074/jbc.M109.018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desany BA, Alcasabas AA, Bachant JB, Elledge SJ. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12: 2956–70. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=317167&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang M, Zhou Z, Elledge SJ. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell. 1998;94: 595–605. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Smolka MB, Zhou H. Mechanism of Dun1 activation by Rad53 phosphorylation in Saccharomyces cerevisiae. J Biol Chem. 2007;282: 986–95. 10.1074/jbc.M609322200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastos de Oliveira FM, Harris MR, Brazauskas P, de Bruin RAM, Smolka MB. Linking DNA replication checkpoint to MBF cell-cycle transcription reveals a distinct class of G1/S genes. EMBO J. Nature Publishing Group; 2012;31: 1798–1810. 10.1038/emboj.2012.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer VR, Horak CE, Scafe CS, Botstein D, Snyder M, Brown PO. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature. 2001;409: 533–538. 10.1038/35054095 [DOI] [PubMed] [Google Scholar]
- 36.Smolka MB, Bastos De Oliveira FM, Harris MR, De Bruin RAM. The checkpoint transcriptional response: Make sure to turn it off once you are satisfied. Cell Cycle. 2012;11: 3166–3174. 10.4161/cc.21197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PMJ, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30: 137–44. 10.1016/j.molcel.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18: 521–7. 10.1016/j.tcb.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyabe I, Kunkel T a, Carr AM. The major roles of DNA polymerases epsilon and delta at the eukaryotic replication fork are evolutionarily conserved. PLoS Genet. 2011;7: e1002407 10.1371/journal.pgen.1002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pursell ZF, Isoz I, Lundström E-B, Johansson E, Kunkel T a. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science. 2007;317: 127–30. 10.1126/science.1144067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgescu RE, Schauer GD, Yao NY, Langston LD, Yurieva O, Zhang D, et al. Reconstitution of a eukaryotic replisome reveals suppression mechanisms that define leading/lagging strand operation. Elife. 2015;4: e04988 10.7554/eLife.04988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu C, Gan H, Han J, Zhou Z, Jia S, Chabes A, et al. Strand-specific analysis shows protein binding at replication forks and PCNA unloading from lagging strands when forks stall. Mol Cell. 2014;56: 551–63. 10.1016/j.molcel.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamatake RK, Hasegawa H, Clark AB, Bebenek K, Kunkel TA, A S. Purification and characterization of DNA polymerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;265: 4072–4088. [PubMed] [Google Scholar]
- 44.Chilkova O, Jonsson B-H, Johansson E. The quaternary structure of DNA polymerase ε from Saccharomyces cerevisiae. J Biol Chem. 2003;278: 14082–6. 10.1074/jbc.M211818200 [DOI] [PubMed] [Google Scholar]
- 45.Pospiech H, Syväoja JE. DNA polymerase ε—more than a polymerase. ScientificWorldJournal. 2003;3: 87–104. 10.1100/tsw.2003.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogg M, Johansson E. The Eukaryotic Replisome: a Guide to Protein Structure and Function. 2012;62: 237–257. [Google Scholar]
- 47.Zegerman P, Diffley JFX. DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst). 2009;8: 1077–88. [DOI] [PubMed] [Google Scholar]
- 48.Aksenova A, Volkov K, Maceluch J, Pursell ZF, Rogozin IB, Kunkel T a, et al. Mismatch repair-independent increase in spontaneous mutagenesis in yeast lacking non-essential subunits of DNA polymerase ε. PLoS Genet. 2010;6: e1001209 10.1371/journal.pgen.1001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesti T, Flick K, Keränen S, Syväoja JE, Wittenberg C. DNA Polymerase ε Catalytic Domains Are Dispensable for DNA Replication, DNA Repair, and Cell Viability. Mol Cell. 1999;3: 679–685. [DOI] [PubMed] [Google Scholar]
- 50.Dua R, Levy DL, Campbell JL. Analysis of the essential functions of the C-terminal protein/protein interaction domain of Saccharomyces cerevisiae pol ε and its unexpected ability to support growth in the absence of the DNA polymerase domain. J Biol Chem. 1999;274: 22283–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/10428796 [DOI] [PubMed] [Google Scholar]
- 51.Feng W, D’Urso G. Schizosaccharomyces pombe Cells Lacking the Amino-Terminal Catalytic Domains of DNA Polymerase Epsilon Are Viable but Require the DNA Damage Checkpoint Control. Mol Cell Biol. 2001;21: 4495–4504. 10.1128/MCB.21.14.4495-4504.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isoz I, Persson U, Volkov K, Johansson E. The C-terminus of Dpb2 is required for interaction with Pol2 and for cell viability. Nucleic Acids Res. 2012; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muramatsu S, Hirai K, Tak YS, Kamimura Y, Araki H. CDK-dependent complex formation between replication proteins Dpb11, Sld2, Pol ε, and GINS in budding yeast. Genes Dev. 2010;24: 602–612. 10.1101/gad.1883410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka S, Araki H. Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb Perspect Biol. 2013;5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 2003;17: 1153–65. 10.1101/gad.1065903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grabowska E, Wronska U, Denkiewicz M, Jaszczur M, Respondek A, Alabrudzinska M, et al. Proper functioning of the GINS complex is important for the fidelity of DNA replication in yeast. Mol Microbiol. 2014;7. [DOI] [PubMed] [Google Scholar]
- 57.Sengupta S, Van Deursen F, De Piccoli G, Labib K. Dpb2 Integrates the Leading-Strand DNA Polymerase into the Eukaryotic Replisome. Curr Biol. Elsevier Ltd; 2013;23: 543–552. 10.1016/j.cub.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 58.Jaszczur M, Flis K, Rudzka J, Kraszewska J, Budd ME, Polaczek P, et al. Dpb2p, a noncatalytic subunit of DNA polymerase ε, contributes to the fidelity of DNA replication in Saccharomyces cerevisiae. Genetics. 2008;178: 633–47. 10.1534/genetics.107.082818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaszczur M, Rudzka J, Kraszewska J, Flis K, Polaczek P, Campbell JL, et al. Defective interaction between Pol2p and Dpb2p, subunits of DNA polymerase epsilon, contributes to a mutator phenotype in Saccharomyces cerevisiae. Mutat Res. 2009;669: 27–35. 10.1016/j.mrfmmm.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraszewska J, Garbacz M, Jonczyk P, Fijalkowska IJ, Jaszczur M. Defect of Dpb2p, a noncatalytic subunit of DNA polymerase ɛ, promotes error prone replication of undamaged chromosomal DNA in Saccharomyces cerevisiae. Mutat Res. Elsevier B.V.; 2012;737: 34–42. 10.1016/j.mrfmmm.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 61.Garbacz M, Araki H, Flis K, Bebenek A, Zawada AE, Jonczyk P, et al. Fidelity consequences of the impaired interaction between DNA polymerase epsilon and the GINS complex. DNA Repair (Amst). 2015;29: 23–35. [DOI] [PubMed] [Google Scholar]
- 62.Navas TA, Zhou Z, Elledge SJ. DNA polymerase ε links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80: 29–39. [DOI] [PubMed] [Google Scholar]
- 63.Dua R, Levy DL, Campbell JL. Role of the putative zinc finger domain of Saccharomyces cerevisiae DNA polymerase epsilon in DNA replication and the S/M checkpoint pathway. J Biol Chem. 1998;273: 30046–55. Available: http://www.ncbi.nlm.nih.gov/pubmed/9792727 [DOI] [PubMed] [Google Scholar]
- 64.Lou H, Komata M, Katou Y, Guan Z, Reis CC, Budd M, et al. Mrc1 and DNA polymerase ε function together in linking DNA replication and the S phase checkpoint. Mol Cell. 2008;32: 106–17. 10.1016/j.molcel.2008.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ensminger M, Iloff L, Ebel C, Nikolova T, Kaina B, Lobrich M. DNA breaks and chromosomal aberrations arise when replication meets base excision repair. J Cell Biol. 2014;206: 29–43. 10.1083/jcb.201312078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groth P, Ausländer S, Majumder MM, Schultz N, Johansson F, Petermann E, et al. Methylated DNA Causes a Physical Block to Replication Forks Independently of Damage Signalling, O6-Methylguanine or DNA Single-Strand Breaks and Results in DNA Damage. J Mol Biol. Elsevier Ltd; 2010;402: 70–82. 10.1016/j.jmb.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 67.Pabla R, Pawar V, Zhang H, Siede W. Characterization of checkpoint responses to DNA damage in Saccharomyces cerevisiae: basic protocols. Methods Enzymol. 2006;409: 101–17. 10.1016/S0076-6879(05)09006-3 [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature. Nature Publishing Group; 2010;467: 479–83. 10.1038/nature09377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navas T a, Sanchez Y, Elledge SJ. RAD9 and DNA polymerase ε form parallel sensory branches for transducing the DNA damage checkpoint signal in Saccharomyces cerevisiae. Genes Dev. 1996;10: 2632–2643. [DOI] [PubMed] [Google Scholar]
- 70.Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2: 329–40. [DOI] [PubMed] [Google Scholar]
- 71.Pellicioli A, Lucca C, Liberi G, Marini F, Lopes M, Plevani P, et al. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 1999;18: 6561–6572. 10.1093/emboj/18.22.6561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Z, Elledge SJ. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 73.Basrai MA, Velculescu VE, Kinzler KW, Hieter P. NORF5/HUG1 is a component of the MEC1-mediated checkpoint response to DNA damage and replication arrest in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19: 7041–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meurisse J, Bacquin a., Richet N, Charbonnier J-B, Ochsenbein F, Peyroche a. Hug1 is an intrinsically disordered protein that inhibits ribonucleotide reductase activity by directly binding Rnr2 subunit. Nucleic Acids Res. 2014;42: 13174–13185. 10.1093/nar/gku1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao X, Rothstein R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc Natl Acad Sci U S A. 2002;99: 3746–51. 10.1073/pnas.062502299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee YD, Wang J, Stubbe J, Elledge SJ. Dif1 Is a DNA-Damage-Regulated Facilitator of Nuclear Import for Ribonucleotide Reductase. Mol Cell. 2008;32: 70–80. 10.1016/j.molcel.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu X, Huang M. Dif1 controls subcellular localization of ribonucleotide reductase by mediating nuclear import of the R2 subunit. Mol Cell Biol. 2008;28: 7156–7167. 10.1128/MCB.01388-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Bruin RAM, Kalashnikova TI, Chahwan C, McDonald WH, Wohlschlegel J, Yates J, et al. Constraining G1-Specific Transcription to Late G1 Phase: The MBF-Associated Corepressor Nrm1 Acts via Negative Feedback. Mol Cell. 2006;23: 483–496. 10.1016/j.molcel.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 79.de Bruin RAM, Kalashnikova TI, Aslanian A, Wohlschlegel J, Chahwan C, Yates JR, et al. DNA replication checkpoint promotes G1-S transcription by inactivating the MBF repressor Nrm1. Proc Natl Acad Sci U S A. 2008;105: 11230–11235. 10.1073/pnas.0801106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Travesa A, Kuo D, de Bruin R a M, Kalashnikova TI, Guaderrama M, Thai K, et al. DNA replication stress differentially regulates G1/S genes via Rad53-dependent inactivation of Nrm1. EMBO J. Nature Publishing Group; 2012;31: 1811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caetano C, Limbo O, Farmer S, Klier S, Dovey C, Russell P, et al. Tolerance of Deregulated G1/S Transcription Depends on Critical G1/S Regulon Genes to Prevent Catastrophic Genome Instability. Cell Rep. The Authors; 2014;9: 2279–2289. 10.1016/j.celrep.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 82.Travesa A, Kalashnikova TI, de Bruin R a M, Cass SR, Chahwan C, Lee DE, et al. Repression of G1/S transcription is mediated via interaction of the GTB motifs of Nrm1 and Whi5 with Swi6. Mol Cell Biol. 2013;33: 1476–86. 10.1128/MCB.01333-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harris MR, Lee D, Farmer S, Lowndes NF, de Bruin R a M. Binding Specificity of the G1/S Transcriptional Regulators in Budding Yeast. PLoS One. 2013;8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma VM, Tomar RS, Dempsey AE, Reese JC. Histone deacetylases RPD3 and HOS2 regulate the transcriptional activation of DNA damage-inducible genes. Mol Cell Biol. 2007;27: 3199–3210. 10.1128/MCB.02311-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guacci V, Koshland D, Strunnikov A. A Direct Link between Sister Chromatid Cohesion and Chromosome Condensation Revealed through the Analysis of MCD1 in S. cerevisiae. Cell. 1997;91: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howell AS, Lew DJ. Morphogenesis and the cell cycle. Genetics. 2012;190: 51–77. 10.1534/genetics.111.128314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vernis L, Piskur J, Diffley JFX. Reconstitution of an efficient thymidine salvage pathway in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31: 120e–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Budd ME, Campbell JL. DNA polymerases δ and ε are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kesti T, McDonald WH, Yates JR, Wittenberg C. Cell cycle-dependent phosphorylation of the DNA polymerase epsilon subunit, Dpb2, by the Cdc28 cyclin-dependent protein kinase. J Biol Chem. 2004;279: 14245–55. 10.1074/jbc.M313289200 [DOI] [PubMed] [Google Scholar]
- 90.Langston LD, Zhang D, Yurieva O, Georgescu RE, Finkelstein J, Yao NY, et al. CMG helicase and DNA polymerase ε form a functional 15-subunit holoenzyme for eukaryotic leading-strand DNA replication. Proc Natl Acad Sci U S A. 2014;111: 15390–5. 10.1073/pnas.1418334111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulovich a G, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82: 841–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/7671311 [DOI] [PubMed] [Google Scholar]
- 92.Alvino GM, Collingwood D, Murphy JM, Delrow J, Brewer BJ, Raghuraman MK. Replication in hydroxyurea: it’s a matter of time. [Internet]. Molecular and cellular biology. 2007. pp. 6396–406. 10.1128/MCB.00719-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Majka J, Binz SK, Wold MS, Burgers PMJ. Replication protein A directs loading of the DNA damage checkpoint clamp to 5’-DNA junctions. J Biol Chem. 2006;281: 27855–61. 10.1074/jbc.M605176200 [DOI] [PubMed] [Google Scholar]
- 94.Johnson RE, Klassen R, Prakash L, Prakash S. A Major Role of DNA Polymerase δ in Replication of Both the Leading and Lagging DNA Strands. Mol Cell. Elsevier Inc.; 2015;59: 163–175. 10.1016/j.molcel.2015.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Georgescu RE, Schauer GD, Yao NY, Langston LD, Yurieva O, Zhang D, et al. Reconstitution of a eukaryotic replisome reveals suppression mechanisms that define leading/lagging strand operation. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lujan S a, Clausen AR, Clark AB, MacAlpine HK, MacAlpine DM, Malc EP, et al. Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition. Genome Res. 2014;24: 1751–64. 10.1101/gr.178335.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell. 2003;112: 391–401. [DOI] [PubMed] [Google Scholar]
- 98.Sweeney FD, Yang F, Chi A, Shabanowitz J, Hunt DF, Durocher D. Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr Biol. 2005;15: 1364–1375. 10.1016/j.cub.2005.06.063 [DOI] [PubMed] [Google Scholar]
- 99.Smolka MB, Albuquerque CP, Chen S, Schmidt KH, Wei XX, Kolodner RD, et al. Dynamic changes in protein-protein interaction and protein phosphorylation probed with amine-reactive isotope tag. Mol Cell Proteomics. 2005;4: 1358–69. 10.1074/mcp.M500115-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pellicioli A, Foiani M. Signal transduction: how rad53 kinase is activated. Curr Biol. 2005;15: R769–71. 10.1016/j.cub.2005.08.057 [DOI] [PubMed] [Google Scholar]
- 101.Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 102.Amberg DC, Burke DJ, Strathern JN. Methods in Yeast Genetics. A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- 103.Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking 5’ phosphate decarboxylase activity in yeast: 5 fluoro-orotic acid resistance. Mol Gen Genet. 1984;197: 345–346. [DOI] [PubMed] [Google Scholar]
- 104.Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. John Wiley & Sons, Ltd.; 1995;11: 355–360. 10.1002/yea.320110408 [DOI] [PubMed] [Google Scholar]
- 105.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press, 2001. [Google Scholar]
- 106.Edwards S, Li CM, Levy DL, Brown J, Snow PM, Campbell JL. Saccharomyces cerevisiae DNA polymerase ε and polymerase σ interact physically and functionally, suggesting a role for polymerase ε in sister chromatid cohesion. Mol Cell Biol. 2003;23: 2733–2748. 10.1128/MCB.23.8.2733-2748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kushnirov V V. Rapid and reliable protein extraction from yeast. Yeast. 2000;16: 857–860. [DOI] [PubMed] [Google Scholar]
- 108.Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-Like Kinases MEC1 and TEL1 in Yeast Cell Cycle Checkpoint Pathways. Science (80-). 1996;271: 357–360. [DOI] [PubMed] [Google Scholar]
- 109.Malc E, Dzierzbicki P, Kaniak A, Skoneczna A, Ciesla Z. Inactivation of the 20S proteasome maturase, Ump1p, leads to the instability of mtDNA in Saccharomyces cerevisiae. Mutat Res—Fundam Mol Mech Mutagen. 2009;669: 95–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Yeast cultures of indicated strains were synchronized in G1 and released from α-factor in YNBD medium supplemented with 200 mM HU (red). After 90 minutes, cells were washed and released in YNBD medium in 23°C (permissive temperature for dpb2-103 mutant) or 37°C (restrictive temperature for dpb2-103 mutant). Samples were collected after 30 and 60 minutes.
(TIF)
(TIF)
Extracts from WT, dpb2-103, mec1-21 or pol2-12 yeast cells treated with 200 mM HU or 0,05% MMS were resolved by SDS-PAGE and Anti-Rad53 antibodies were used for Western-blot analyzis. Unspecific bands detected using the same antibodies were used as loading control.
(TIF)
Yeasts were grown in YNBD medium to log phase.
(TIF)
(A) The abnormal progression of replication in pol2-12 cells is rescued by NRM1 deletion. Asynchronous cells were analysed by flow cytometry to evaluate DNA content. (B) Deletion of NRM1 (coding for the MBF repressor) rescues pol2-12 HU and temperature sensitivity. Cultures of indicated strains were grown exponentially, serially diluted and spotted on YNBD supplemented with HU and incubated at 23 or 37°C
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.