Abstract
Mannheimia haemolytica serotype 1 (S1) is the most common bacterial isolate found in shipping fever pneumonia in beef cattle. Currently used vaccines against M. haemolytica do not provide complete protection against the disease. Research with M. haemolytica outer membrane proteins (OMPs) has shown that antibodies to one particular OMP from S1, PlpE, may be important in immunity. In a recently published work, members of our laboratory showed that recombinant PlpE (rPlpE) is highly immunogenic when injected subcutaneously into cattle and that the acquired immunity markedly enhanced resistance to experimental challenge (A. W. Confer, S. Ayalew, R. J. Panciera, M. Montelongo, L. C. Whitworth, and J. D. Hammer, Vaccine 21:2821-2829, 2003). The objective of this work was to identify epitopes of PlpE that are responsible for inducing the immune response. Western blot analysis of a series of rPlpE with nested deletions on both termini with bovine anti-PlpE hyperimmune sera showed that the immunodominant region is located close to the N terminus of PlpE. Fine epitope mapping, in which an array of overlapping 13-mer synthetic peptides attached to a derivatized cellulose membrane was probed with various affinity-purified anti-PlpE antibodies, identified eight highly reactive regions, of which region 2 (R2) was identified as the specific epitope. The R2 region is comprised of eight imperfect repeats of a hexapeptide (QAQNAP) and is located between residues 26 and 76. Complement-mediated bactericidal activity of affinity-purified anti-PlpE bovine antibodies confirmed that antibodies directed against the R2 region are effective in killing M. haemolytica.
Bovine respiratory disease arises from the interaction of numerous contributing factors, including physical stresses associated with weaning, shipment, inclement weather, and overcrowding coupled with viral and bacterial infections (8, 31, 32, 59, 63). The result in severe cases is colonization of the lungs with pathogenic bacteria resulting in severe pneumonia. Pasteurella multocida, Haemophilus somnus, and Mannheimia (formerly Pasteurella) haemolytica are associated with bovine pneumonia. However, M. haemolytica serotype 1 (S1) is by far the most important bacterial pathogen in the development of the often-fatal fibrinous pleuropneumonia in beef cattle known as pneumonic pasteurellosis or shipping fever (31, 32).
Immunity against M. haemolytica is thought to be primarily through production of serum antibodies that neutralize the secreted leukotoxin (LKT) and antibodies against surface antigens (45). The mechanism of activity of antisurface antibodies and the specific surface antigens involved in anti-M. haemolytica immunity are not known; however, complement-mediated bacterial lysis and bacterial phagocytosis and killing are thought to be important in defense against M. haemolytica infection (45). Complement-mediated bactericidal activity against M. haemolytica and phagocytosis of M. haemolytica by bovine neutrophils has been demonstrated with bovine immune serum (12, 17, 40, 46).
Little is known about the specific surface antigens that are important in stimulating host immunity to M. haemolytica. However, several studies point toward the importance of outer membrane proteins (OMPs). Pandher et al. (45) identified 21 surface-exposed immunogenic OMPs in M. haemolytica S1 by protease treatment and Western blotting. High antibody responses to several specific OMPs correlated with resistance to challenge with virulent M. haemolytica S1 (18, 43). Vaccination of cattle with OMP-enriched cellular fractions from M. haemolytica S1 also significantly enhanced the resistance of cattle to experimental challenge (42) even in the absence of antibodies to LKT.
A major 45-kDa OMP was one of the M. haemolytica OMPs to which high antibody responses correlated with resistance to experimental challenge (43). In 1999, Pandher et al. (46) reported the cloning, sequencing, and characterization of the gene encoding the 45-kDa M. haemolytica S1 OMP (designated PlpE), which was found to be genetically similar to an immunogenic lipoprotein (OmlA) of Actinobacillus pleuropneumoniae serotypes 1 and 5 (34). Affinity-purified anti-PlpE antibodies recognized an OMP of similar size in all serotypes of M. haemolytica except serotype 11 (46), which was later classified as Mannheimia glucosida. In addition, PlpE is surface exposed and immunogenic in cattle, and in complement-mediated killing assays, a significant reduction in killing of M. haemolytica occurred when bovine immune serum was depleted of anti-PlpE antibodies (43). Our laboratory recently cloned and expressed the gene for M. haemolytica OMP PlpE, and the recombinant PlpE (rPlpE) was purified and used in immunological and vaccination studies (15). In that study, rPlpE with an adjuvant was shown to be highly immunogenic in cattle and vaccination of cattle with 100 μg of rPlpE markedly enhanced resistance to experimental challenge with virulent M. haemolytica (15). Finally, the addition of rPlpE to a commercial M. haemolytica vaccine significantly enhanced (P < 0.05) the protection afforded by the vaccine against experimental challenge (15). All of these results indicate that antibodies against PlpE may significantly contribute to host defense against the bacterium.
Since extended portions of the molecule are predicted to be buried in the outer membrane, most of the OMP molecule would play no significant role in inducing protective immune responses. Only short, surface-exposed epitopes of these proteins represent the major immunogenic regions of the protein. Identification of such surface-exposed epitopes as protective antigens in animal models has been the goal of peptide vaccine design strategies for various pathogenic bacteria including nontypeable Haemophilus influenzae (3, 4, 44), Pseudomonas aeruginosa (62), Neisseria meningitidis (61), and Streptococcus mutans (48). Since the M. haemolytica PlpE is an important immunogen, this study was undertaken to characterize surface-exposed and immunologically important epitopes of this OMP.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. haemolytica 89010807N (45), which is S1, was used as a source of the plpE gene (43) and in complement-mediated bactericidal assays. The organism was routinely cultured in brain heart infusion (BHI) broth or on BHI blood agar plates (Hardy Diagnostics, Mesa, Ariz.) supplemented with 5% sheep blood. Escherichia coli DH5α (Invitrogen, Carlsbad, Calif.) was used for subcloning and propagation of recombinant plasmids. Recombinant proteins were overexpressed in and purified from E. coli BL21(DE3) or BL21(DE3)(pLysS) (Novagen, Madison, Wis.). E. coli strains were grown on Luria-Bertani (LB) medium supplemented with the appropriate antibiotic when needed. All plates were incubated at 37°C with 5% CO2.
Construction of recombinant plasmids containing deletions in plpE.
Truncated forms of the plpE gene carrying various deletions were generated from M. haemolytica 89010807N genomic DNA by PCR with the primers listed in Table 1. PCR products were cut with BamHI plus EcoRI or BamHI plus HindIII and ligated to pRSETA (Invitrogen) and/or pET28 (Novagen) that were cut with the same pair of enzymes. Chemically competent E. coli DH5α (Invitrogen) cells were transformed with 1 to 5 μl of the ligation mixture and plated on LB agar plates supplemented with either 50 μg of carbenicillin/ml or 30 μg of kanamycin/ml. Transformants were screened by restriction enzyme analysis, and appropriate subclones were identified. Plasmid DNA isolated from such subclone was submitted to the Oklahoma State University Core Facility where the nucleotide sequence was determined with the ABI model 3700 (Applied Biosystems, Foster City, Calif.) automated DNA sequencing system. Nucleotide sequences of representative subclones were compared to that deposited in GenBank (accession no. AF059036). The final eight recombinant plasmids constructed in this study and the truncated recombinant proteins they encode are listed in Table 2.
TABLE 1.
PCR primers used in this study
| Name | Sequence (5′-3′)a | Orientationb | Target nucleotidesc |
|---|---|---|---|
| plpEBH | GTCAggatccTGCGGAGGAAGCGGTAGC | F | 198-215 |
| plpEER | GACTgaattcTTATTTTTTCTCGCTAACCATTA | R | 1208-189 |
| plpBM-1 | CTTggatccCAAGCACAAAATGTT | F | 282-296 |
| plpBM-2 | CCTggatccCAAGCAGAGGTTACT | F | 426-440 |
| plpBM-3 | ATTggatccAATGCTGAACAACTC | F | 648-662 |
| HNplp-1 | GATaagcttTTACCGTGCGGCAAATTC | R | 890-876 |
| HNplp-2 | AAAaagcttTTATTTAATTTCTACATC | R | 920-906 |
| HNplp-3 | TTTaagcttTTATATACTTCCTTGAGC | R | 950-936 |
Lowercase portions of sequences are restriction sites.
F, forward; R, reverse.
Numbering is according to GenBank accession no. AF059036.
TABLE 2.
Plasmid constructs used in this study, characteristics of recombinant proteins, and binding properties of anti-PlpE antibodies, determined densitometrically
| Plasmid | Recombinant protein | Deleted region (amino acids) | Binding capacity of anti-PlpE antibodies
|
|
|---|---|---|---|---|
| Vola (INT/mm2) | % Binding | |||
| pSAC3 | rPlpE (pRSETA) | 0b | 2,472 | 100 |
| pSAC30 | rPlpEΔC106 | 232-337 | 2,333 | 94.3 |
| pSAC31 | rPlpEΔC96 | 242-337 | 2,450 | 99.1 |
| pSAC32 | rPlpEΔC86 | 252-337 | 2,635 | 106 |
| pSAC63 | rPlpEΔN28 | 1-28 | 2,528 | 102 |
| pSAC64 | rPlpEΔN76 | 1-76 | 699 | 28.0 |
| pSAC65 | rPlpEΔN150 | 1-150 | 310 | 12.5 |
| pSAC67 | rPlpE (pET28) | 0b | 2,472 | 100 |
Sum of the intensities of the pixels inside the volume boundary × area of a single pixel (in millimeters squared).
Mature rPlpe with 337 amino acids.
Expression and purification of truncated forms of rPlpE.
Each recombinant plasmid listed in Table 2 was introduced into BL21(DE3)(pLysS) cells (Novagen) to express and purify recombinant forms of PlpE with His tags on their N termini according to the manufacturer's protocols. Briefly, single colonies of BL21(DE3)(pLysS) harboring the truncated plpE in pRSETA or pET28 were inoculated into 250 to 500 ml of LB broth supplemented with 50 μg of carbenicillin/ml and 34 μg of chloramphenicol/ml (pRSETA-based constructs) or 30 μg of kanamycin/ml and 34 μg of chloramphenicol/ml (pET28-based constructs). The cultures were incubated at 37°C until reaching an absorbance (A600) of 0.5, at which time expression was induced by adding isopropyl-β-d-galactopyranoside (IPTG) to a final concentration of 1 mM. Induction was continued for at least 3 h. Cells were then harvested by centrifugation at 10,000 × g at 4°C, resuspended in binding buffer (6 M urea, 500 mM NaCl, 20 mM Tris-HCl, 5 mM imidazole [pH 7.9]), and lysed by applying 20,000 lb/in2 in an Aminco French pressure cell (SLM Instruments, Inc., Rochester, N.Y.). Cellular debris was removed by centrifugation at 14,000 × g for 30 min at 4°C, and the supernatant containing the recombinant protein was passed through a 0.45-μm-pore-size filter (Nalge Nunc, Rochester, N.Y.). The clarified supernatant was loaded onto a 10-mg-binding-capacity His•Bind column (Novagen) prepacked with Ni2+-charged His•Bind resin that was preequilibrated with 10 ml of binding buffer. Unbound proteins were removed by applying 10 ml of binding buffer followed by 10 ml of wash buffer (6 M urea, 500 mM NaCl, 20 mM imidazole, and 20 mM Tris-HCl [pH 7.9]). The recombinant protein was then eluted in small fractions with an elution buffer (6 M urea, 1 M imidazole, 250 mM NaCl, 10 mM Tris-HCl [pH 7.9]). Fractions containing the recombinant protein were pooled and dialyzed against phosphate-buffered saline (PBS) containing decreasing concentrations of urea to remove the denaturant and, at the same time, aid in the refolding of the recombinant protein. The identity, purity, and integrity of purified proteins were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie staining and Western blotting with murine anti-PlpE polyclonal ascites.
Production of polyclonal mouse ascites.
Anti-PlpE polyclonal mouse ascites was produced by the Hybridoma Center of Oklahoma State University. Briefly, three female Swiss Webster (CFW) mice (Charles River Laboratories, Wilmington, Mass.) were immunized three times with 50 μg of truncated rPlpE diluted in RIBI (Corixa Corp., Seattle, Wash.) adjuvant. The first immunization was given subcutaneously. The second immunization was given intraperitoneally after 14 days and repeated 10 days later. A test bleed was performed, and the sera were screened for antibodies to rPlpE by enzyme-linked immunosorbent assay (ELISA). Because the antibody titer was less than 1:3,200, two additional intraperitoneal immunizations were given at 10-day intervals. The mice were then injected with approximately 2 × 106 sarcoma cells (catalog no. TIB-66; American Type Culture Collection, Manassas, Va.). Between 7 and 10 days after sarcoma injection, ascites was removed from each mouse three times. A total of 4 to 18 ml of ascitic fluid was removed from each mouse before euthanization.
Bovine antisera.
PlpE-specific sera were obtained from 6- to 8-month-old calves that were used for immunogenicity studies of rPlpE (15). Sera from these calves were screened for anti-M. haemolytica antibodies, as measured by ELISA, to formalin-killed M. haemolytica whole cells (16, 18). Calves that had anti-M. haemolytica antibody concentrations of less than 0.50 ng of immunoglobulin G binding to the antigen at a 1:400 serum dilution (normal background) were used in this study. Each of three calves was vaccinated once with 10, 50, or 100 μg of rPlpE in a commercial proprietary adjuvant (Pfizer, Inc., Lincoln, Neb.) and, on day 23, had endpoint antibody titers of 1:12,800, 1:25,600, and 1:25,600, respectively, as measured by an ELISA for rPlpE (15). A nonvaccinated calf had an endpoint titer of only 1:400. In that study, rPlpE-vaccinated calves were subsequently challenged with 5.0 × 109 CFU of virulent M. haemolytica and had between 71 and 81% smaller lung lesions than did the M. haemolytica-challenged nonvaccinated calf (15).
Anti-M. haemolytica serum was obtained from a weanling beef calf that spontaneously developed high anti-M. haemolytica antibodies with an endpoint titer of 1:25,600 after natural exposure. Serum was also available from a calf that had been subcutaneously vaccinated twice, 14 days apart, with 2 ml of 109 CFU of live M. haemolytica each time. The serum was obtained 14 days after the last vaccination and had an endpoint titer to PlpE of 1:25,600.
Preparation of affinity columns and purification of anti-PlpE antibodies.
Purified rPlpE was coupled to N-hydroxylsuccinimide (NHS)-activated Sepharose 4 Fast Flow (Amersham Biosciences, Uppsala, Sweden) according to the manufacturer's recommendations. Briefly, defined bed volumes of NHS-activated Sepharose 4 Fast Flow in an Econo column (Bio-Rad, Hercules, Calif.) was washed with 10 to 15 volumes of cold 1 mM HCl and equilibrated with PBS. Purified rPlpE (3 to 7 mg) in PBS was mixed with a 2-ml bed volume in a ratio of 0.5:1 (coupling solution and medium), and the column was incubated at 4°C on a rocking platform overnight. The nonreacted groups were blocked by 0.1 M Tris (pH 8.0) and washed with alternating high- and low-pH buffers (Tris-HCl [pH 8.0] and acetate buffer [pH 4.0], respectively). An affinity column was prepared for each truncated rPlpE as well as for whole rPlpE.
Anti-rPlpE antibodies against specific regions of PlpE were purified by using the affinity columns described above. A column with NHS-activated Sepharose coupled to an rPlpE of interest was fitted with a flow adaptor according to the recommendation of the manufacturer (Bio-Rad) and equilibrated with Dulbecco's PBS (DPBS) at a flow rate of 1 ml/min. Hyperimmune serum produced by immunizing calves with full-length rPlpE was diluted 1:10 with DPBS and passed through Nalgene 0.45-μm-pore-size PES filters (Nalge). The filtered serum was then applied to the equilibrated column via a peristaltic pump (pump P-1; Pharmacia LKB) at a flow rate of 1 ml/min. The flowthrough was reapplied to the column several times to reextract the serum by connecting the flowthrough to the reservoir of the initial serum. The column was then washed with DPBS until complete removal of nonspecific proteins was achieved, as determined by a UV monitor (optical unit UV-1; Pharmacia LKB) attached to a chart recorder. The specifically bound antibody was eluted with glycine buffer (100 mM glycine, 140 mM NaCl [pH 3.0]) by collecting fractions into microcentrifuge tubes containing a 1/10 volume of 1 M Tris-HCl (pH 8.0). The absorbance of fractions was determined at 280 nm, and those having a reading of at least two to three times that of the background were pooled and dialyzed overnight against DPBS at 4°C in a Slide-A-Lyzer dialysis cassette (Pierce, Rockford, Ill.). The concentration of affinity-purified antibodies was determined with a BCA protein assay kit (Pierce). Specific antibodies against rPlpE with 28-, 76-, and 150-amino-acid deletions on their N termini, rPlpEΔN28 (pSAC63), rPlpEΔN76 (pSAC64), and rPlpEΔN150 (pSAC65), respectively, were purified as described previously.
Antibodies against regions of PlpE that are exposed on the surfaces of M. haemolytica cells were purified as previously described (45, 60). Briefly, intact M. haemolytica cells from 500 ml in late log phase were harvested by centrifugation and washed with PBS. The washed cells were resuspended in 10 ml of a 1:10 dilution of hyperimmune bovine anti-rPlpE serum or anti-PlpE mouse ascites diluted in PBS on ice for 2 to 4 h with gentle agitation. The cells were pelleted and washed several times with PBS. The antibodies bound to the surface were eluted by resuspending and agitating the cells in 2 ml of 0.1 M glycine and 140 mM NaCl (pH 3.0) for at least 30 min. The cells were centrifuged at 14,000 × g, and the eluted antibodies were collected in the supernatant, which was neutralized immediately by adding a 1/10 volume of 1 M Tris (pH 8.0).
Epitope mapping of PlpE by peptide array.
A peptide array comprising a total of 109 overlapping 13-mer peptides with a 3-amino-acid offset (Table 3) was custom made by Sigma-Genosys LP (The Woodlands, Tex.). Briefly, the synthesis of peptides was performed on cellulose membranes in which hydroxyl functions of a commercially available filter paper were derivatized with 9-fluorenylmethoxy carbonyl-B-alanine (Fmoc-B-Ala) with subsequent removal of the Fmoc group. The synthesis areas were defined by spotting an Fmoc-B-alanine-pentafluorophenyl ester solution to distinct areas on the membrane. Blocking the remaining amino functions between spots provided discrete reaction sites on the membrane for further standard solid-phase peptide synthesis with amino acid pentafluorophenyl esters. Peptides remained covalently attached to the cellulose membrane by the C terminus and had a free N terminus.
TABLE 3.
Overlapping peptides spanning PlpEa
| No. | Mol wt | Peptide sequence | No. | Mol wt | Peptide sequence | |
|---|---|---|---|---|---|---|
| 1 | 1,022.4 | CGGSGSGGSSSTP | ||||
| 2 | 1,153.4 | SGSGGSSSTPNHP | ||||
| 3 | 1,246.5 | GGSSSTPNHPKPV | ||||
| 4 | 1,354.6 | SSTPNHPKPVLVP | ||||
| 5 | 1,436.6 | PNHPKPVLVPKTQ | ||||
| 6 | 1,429.6 | PKPVLVPKTQNNL | ||||
| 7 | 1,434.3 | VLVPKTQNNLQAQ | ||||
| 8 | 1,433.2 | PKTQNNLQAQNVP | ||||
| 9 | 1,433.9 | QNNLQAQNVPQAQ | ||||
| 10 | 1,350.0 | LQAQNVPQAQNAS | ||||
| 11 | 1,364.8 | QNVPQAQNASQAQ | ||||
| 12 | 1,305.9 | PQAQNASQAQNAP | ||||
| 13 | 1,336.8 | QNASQAQNAPQAQ | ||||
| 14 | 1,305.9 | SQAQNAPQAQNAP | ||||
| 15 | 1,346.8 | QNAPQAQNAPQAQ | ||||
| 16 | 1,315.9 | PQAQNAPQAQNAP | ||||
| 17 | 1,375.9 | QNAPQAQNAPQVE | ||||
| 18 | 1,345.0 | PQAQNAPQVENAP | ||||
| 19 | 1,375.9 | QNAPQVENAPQAQ | ||||
| 20 | 1,345.0 | PQVENAPQAQNAP | ||||
| 21 | 1,377.0 | ENAPQAQNAPQVE | ||||
| 22 | 1,345.0 | PQAQNAPQVENAP | ||||
| 23 | 1,377.0 | QNAPQVENAPQAE | ||||
| 24 | 1,361.1 | PQVENAPQAEVTP | ||||
| 25 | 1,330.2 | ENAPQAEVTPPVP | ||||
| 26 | 1,369.0 | PQAEVTPPVPQPQ | ||||
| 27 | 1,416.1 | EVTPPVPQPQSQK | ||||
| 28 | 1,372.2 | PPVPQPQSQKIDG | ||||
| 29 | 1,428.3 | PQPQSQKIDGSFD | ||||
| 30 | 1,404.6 | QSQKIDGSFDKIG | ||||
| 31 | 1,375.9 | KIDGSFDKIGSVK | ||||
| 32 | 1,374.9 | GSFDKIGSVKLNK | ||||
| 33 | 1,411.7 | DKIGSVKLNKEAQ | ||||
| 34 | 1,398.6 | GSVKLNKEAQTLE | ||||
| 35 | 1,511.8 | KLNKEAQTLELSR | ||||
| 36 | 1,517.8 | KEAQTLELSRFTL | ||||
| 37 | 1,531.8 | QTLELSRFTLVDK | ||||
| 38 | 1,460.9 | ELSRFTLVDKLGT | ||||
| 39 | 1,453.9 | RFTLVDKLGTPPK | ||||
| 40 | 1,439.9 | LVDKLGTPPKFDK | ||||
| 41 | 1,355.8 | KLGTPPKFDKVSG | ||||
| 42 | 1,426.9 | TPPKFDKVSGKKI | ||||
| 43 | 1,503.0 | KFDKVSGKKIIEE | ||||
| 44 | 1503.0 | KVSGKKIIEEKDF | ||||
| 45 | 1,514.1 | GKKIIEEKDFLVL | ||||
| 46 | 1,515.0 | IIEEKDFLVLNLS | ||||
| 47 | 1,501.9 | EKDFLVLNLSDIN | ||||
| 48 | 1,457.7 | FLVLNLSDINAEQ | ||||
| 49 | 1,355.6 | LNLSDINAEQLSG | ||||
| 50 | 1,390.6 | SDINAEQLSGDFL | ||||
| 51 | 1,500.8 | NAEQLSGDFLIRR | ||||
| 52 | 1,503.8 | QLSGDFLIRRSDD | ||||
| 53 | 1,599.1 | GDFLIRRSDDLFY | ||||
| 54 | 1,663.2 | LIRRSDDLFYGYY | ||||
| 55 | 1,634.0 | RSDDLFYGYYHDT | ||||
| 56 | 1,575.0 | DLFYGYYHDTNGK | ||||
| 57 | 1,525.9 | YGYYHDTNGKNLV | ||||
| 58 | 1,399.7 | YHDTNGKNLVDAA | ||||
| 59 | 1,374.7 | TNGKNLVDAADKF | ||||
| 60 | 1,480.7 | KNLVDAADKFSQY | ||||
| 61 | 1,470.6 | VDAADKFSQYFVV | ||||
| 62 | 1,592.7 | ADKFSQYFVVYDE | ||||
| 63 | 1,661.8 | FSQYFVVYDEKRV | ||||
| 64 | 1,642.8 | YFVVYDEKRVNDN | ||||
| 65 | 1,548.7 | VYDEKRVNDNISD | ||||
| 66 | 1,513.8 | EKRVNDNISDKLT | ||||
| 67 | 1,435.7 | VNDNISDKLTATY | ||||
| 68 | 1,520.0 | NISDKLTATYRKK | ||||
| 69 | 1,539.0 | DKLTATYRKKEGF | ||||
| 70 | 1,501.9 | TATYRKKEGFVYG | ||||
| 71 | 1,526.9 | YRKKEGFVYGSNP | ||||
| 72 | 1,445.8 | KEGFVYGSNPHTK | ||||
| 73 | 1,478.8 | FVYGSNPHTKEFA | ||||
| 74 | 1,409.8 | GSNPHTKEFAARI | ||||
| 75 | 1,480.0 | PHTKEFAARISKL | ||||
| 76 | 1,415.9 | KEFAARISKLGDV | ||||
| 77 | 1,381.9 | AARISKLGDVEIK | ||||
| 78 | 1,473.9 | ISKLGDVEIKFEN | ||||
| 79 | 1,401.6 | LGDVEIKFENGQA | ||||
| 80 | 1,388.4 | VEIKFENGQAQGS | ||||
| 81 | 1,403.5 | KFENGQAQGSIKD | ||||
| 82 | 1,371.4 | NGQAQGSIKDEKD | ||||
| 83 | 1,314.5 | AQGSIKDEKDGNA | ||||
| 84 | 1,447.8 | SIKDEKDGNAEIF | ||||
| 85 | 1,461.8 | DEKDGNAEIFTIK | ||||
| 86 | 1,362.7 | DGNAEIFTIKGDT | ||||
| 87 | 1,445.8 | AEIFTIKGDTKQL | ||||
| 88 | 1,475.8 | FTIKGDTKQLETT | ||||
| 89 | 1,441.6 | KGDTKQLETTPTE | ||||
| 90 | 1,498.6 | TKQLEITPTESNR | ||||
| 91 | 1,480.9 | LEITPTESNRIII | ||||
| 92 | 1,422.9 | TPTESNRIIIAIL | ||||
| 93 | 1,480.8 | ESNRIIIAILDQN | ||||
| 94 | 1,493.8 | RIIIAILDQNQKS | ||||
| 95 | 1,472.6 | IAILDQNQKSYTP | ||||
| 96 | 1,492.5 | LDQNQKSYTPGME | ||||
| 97 | 1,448.7 | NQKSYTPGMEKAI | ||||
| 98 | 1,439.8 | SYTPGMEKAIMET | ||||
| 99 | 1,477.0 | PGMEKAIMETKFI | ||||
| 100 | 1,522.0 | EKAIMETKFIDSK | ||||
| 101 | 1,435.9 | IMETKFIDSKAGN | ||||
| 102 | 1,392.6 | TKFIDSKAGNSDQ | ||||
| 103 | 1,420.7 | IDSKAGNSDQKYL | ||||
| 104 | 1,404.7 | KAGNSDQKYLIGE | ||||
| 105 | 1,434.7 | NSDQKYLIGEAKS | ||||
| 106 | 1,533.8 | QKYLIGEAKSDNW | ||||
| 107 | 1,426.7 | LIGEAKSDNWQAI | ||||
| 108 | 1,460.6 | EAKSDNWQAIMVS | ||||
| 109 | 1,517.7 | SDNWQAIMVSEKK |
Peptides comprising R2 are shown in boldface type.
Typically, prior to blotting, the membrane was blocked with SuperBlock dry blend (Pierce) blocking buffer in Tris-buffered saline (TBS) (pH 7.4). All incubations were done at room temperature. The membrane was then incubated in blocking buffer containing primary antibody at a dilution of 1:1,000 to 1:5,000 for an hour. Following several washes with TBS (pH 7.4) supplemented with 0.05% Tween 20 and 0.2% Triton X-100 (TBSTT), the membrane was incubated in SuperBlock containing a goat anti-bovine or anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) at dilutions of 1:100,000 to 1:200,000 for 1 h. The membrane was washed several times with TBSTT, incubated with SuperSignal West Pico chemiluminescent substrate (Pierce) working solution (0.125 ml/cm2) for 5 min, placed in a plastic membrane protector, and exposed to a CL-X Posure X-ray film (Pierce) for various durations of time. The X-ray film was then developed in a Konica (Wayne, N.J.) SRX-101A medical film processor. The developed X-ray film was scanned on an Arcus 1200 Agfa scanner (Taipei, Taiwan), and scanned images were analyzed with the Gene Pix Pro, version 4.0 (Axon Instruments, Union City, Calif.). Signal intensities were defined as the median pixel intensity following the subtraction of the local median background signal. The peptide array was stripped with Restore Western blot stripping buffer (Pierce) according to the procedure recommended by the manufacturer. Complete stripping was confirmed by autoradiography. The blot procedure was repeated several times with anti-PlpE antibodies obtained from different sources or purified in a variety of ways.
The peptide array was sequentially probed according to the manufacturer's recommendations. Nonspecifically binding spots were initially identified by probing the peptide array with HRP-conjugated goat anti-bovine and rabbit anti-bovine secondary antibodies. The membrane was then probed with a naive serum obtained from a colostrum-deprived newborn calf to further identify spots that nonspecifically bound bovine antibodies. Finally, the peptide array was probed with antibodies produced against PlpE by either vaccination with rPlpE or live M. haemolytica or by natural exposure.
Complement-mediated bactericidal assay.
Serum from a newborn colostrum-deprived Holstein calf was used as a source of complement. Sources of antibodies used in the complement-mediated bactericidal assay were hyperimmune sera from calves vaccinated with the full-length rPlpE and affinity-purified antibodies with the affinity columns described above. Each of these antibodies, with the exception of the complement source, was incubated at 56°C for 30 min to inactivate resident complement prior to use.
The complement-mediated killing assay was performed as previously described (45), except that a decapsulation procedure (12) was added. Decapsulation was done to maximize the exposure of surface protein epitopes to anti-PlpE antibodies (12). Briefly, well-isolated colonies of M. haemolytica 89010807N from overnight growth on BHI blood agar plates were transferred into tubes with BHI broth and grown in a shaker-incubator (200 rpm) at 37°C overnight. The overnight starter culture was transferred into a 125-ml conical flask containing 50 ml of BHI and incubated at 37°C in a shaker-incubator for 2 to 3 h. The cells were pelleted, washed once with PBS, resuspended in 40 ml of PBS, and decapsulated by incubation at 41°C with 100-rpm shaking for 1 h to remove the polysaccharide capsule (12). The decapsulated cells were resuspended in PBS to an A600 of 0.50. A 1:1,000 dilution of the latter was used in a killing assay. The assay, plating, and incubation were done as previously described (45). In each assay, heat-inactivated antibodies without any complement source and complement source without antibodies were used as negative controls. The percent killing was calculated with the following formula: % killing = [(CFUt0 − CFUt30)/CFUt0](100%).
Western blotting.
Whole-cell lysates, outer membrane preparations of M. haemolytica or rPlpE, were resolved on SDS-12.5% PAGE gels by electrophoresis and transferred onto 0.2-μm nitrocellulose membranes in a Mini Trans-Blot cell (Bio-Rad). Membranes were blocked with TBS containing 1% casein for 1 h at room temperature. Each membrane was transferred into blocking buffer containing an appropriately diluted anti-PlpE antibody, where it was incubated for 1 h at room temperature. Following extensive washing with TBSTT, bound antibodies were detected by either alkaline phosphatase-conjugated goat anti-bovine or anti-mouse antibodies with 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)-nitroblue tetrazolium as the substrate (Kirkegaard & Perry Laboratories).
ELISA.
ELISA was performed as described previously (14). Briefly, high-binding, 96-well, flat-bottom polystyrene Costar 9018 (Corning Inc., Corning, N.Y.) plates were coated with 0.5 μg of recombinant protein/ml in coating solution (12.8 mM Na2CO3, 34.8 mM NaHCO3 [pH 9.6]) at 4°C overnight or at 37°C for 2 h on a rocking platform. The plates were washed four times with 1× PBS supplemented with 0.05% Tween (PBST) (Sigma, St. Louis, Mo.) and blocked with PBST-1% bovine serum albumin for 1 h at room temperature. Primary bovine or murine antibody diluted in PBS-1% bovine serum albumin was added to the wells and incubated at 37°C for 1.5 h. In most instances, an initial 1:400 dilution followed by twofold serial dilutions of the primary antibody were used. After washing with PBST, a 1:400 dilution of goat anti-mouse or goat anti-bovine HRP conjugate (Kirkegaard & Perry Laboratories) was added and the plates were incubated at 37°C for 1 h and 30 min. Following washing with PBST, o-phenylenediamine tablets (Amresco, Solon, Ohio) were reconstituted and used as the substrate according to the manufacturer's recommendations. The absorbance at 490 nm was determined for each well by using a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, Calif.).
RESULTS
Immunogenicity of rPlpE.
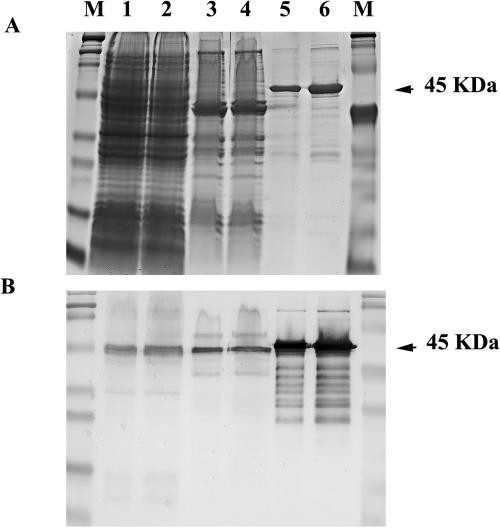
The purity of the rPlpE preparation (Fig. 1A) and the specificity of the immune response of an rPlpE-vaccinated calf were evident (Fig. 1B), in that PlpE in the OMP and whole-cell lysate was demonstrated. Evidence of mild proteolysis in the Western blots and several trace contaminants were seen on the stained gel.
FIG. 1.
Twenty and 40 μl of whole-cell lysate (lanes 1 and 2), the same volumes of outer membrane proteins (lanes 3 and 4), and 0.5 and 1.0 μg of rPlpE (lanes 5 and 6) were separated by SDS-12.5% PAGE. M, molecular mass markers. One gel was stained with Coomassie brilliant blue (A), and the second was transferred onto nitrocellulose for a Western blot with hyperimmune calf serum immunized with rPlpE (B).
Epitope mapping with truncated rPlpE.
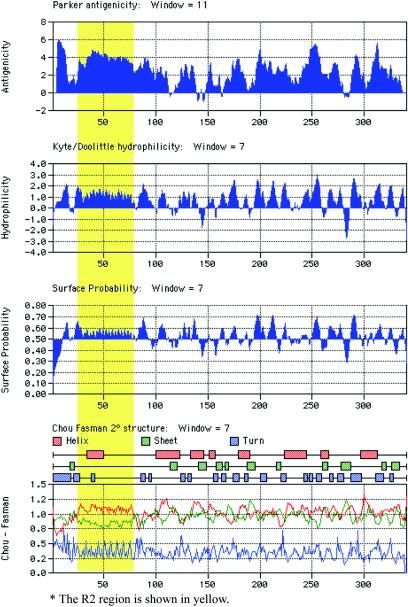
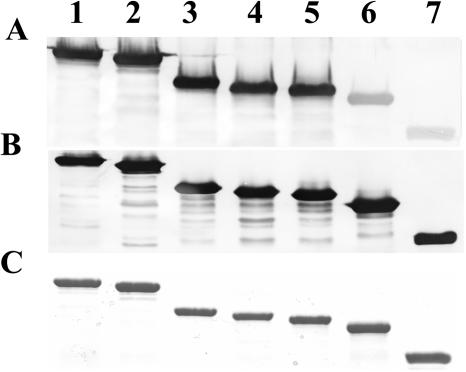
Computer analysis of the deduced amino acid sequence of PlpE with MacVector, version 7.2.2 (Accelrys, Inc., San Diego, Calif.), which employed algorithms such as antigenic index (47), hydrophilicity (38), and surface probability (36), revealed the presence of putative antigenic regions randomly distributed throughout the protein (Fig. 2). To determine whether this was true, we constructed a series of truncated proteins. A total of six plasmid constructs (pSAC30, pSAC31, pSAC32, pSAC63, pSAC64, and pSAC65) carrying the plpE gene with various deletions were made (Table 2). Western blot analysis clearly demonstrated that all of the six truncated forms of rPlpE and the full-length protein reacted with hyperimmune serum from calves immunized with full-length rPlpE. Quantitation of the respective bands on a Western blot (Fig. 3) in which equal amounts of each recombinant protein were resolved on SDS-12.5% PAGE gels demonstrated substantial differences in the intensities of the reactions of hyperimmune serum with the truncated recombinant proteins (Table 2). There was no appreciable difference in the intensity of the reaction between rPlpE (lane 1) and pSAC63 (rPlpEΔN28) (lane 2), lacking the N-terminal 28 amino acids. There were also no differences in the intensity of binding between rPlpE and mutants carrying 86 (rPlpEΔC86), 96 (rPlpEΔC96), and 106 (rPlpEΔC106) amino acid deletions on the C terminus of PlpE (lanes 3, 4, and 5, respectively). In contrast, the binding capacity of antibodies to mutants carrying deletions on their N termini decreased with increasing deletions. The reactivity to pSAC64 (rPlpEΔN76) (lane 6), which carries a deletion of 76 amino acids on the N terminus, decreased to 28%, a reduction of 72% compared to rPlpE. Further deletion of the N terminus, as seen with pSAC65 (rPlpEΔN150) (lane 7), reduced the binding capacity of immunoglobulins to the truncated protein to 12.5%. These findings clearly indicate that the region between residues 28 and 76 from the N terminus of PlpE carries a stretch of amino acids with possible epitope(s) that may be responsible for inducing the immune response elicited when rPlpE is used as a vaccine.
FIG. 2.
Predicted features of the deduced amino acid sequence of PlpE showing regions that are potentially antigenic, hydrophilic, and surface exposed and secondary structures.
FIG. 3.
Nested deletion mutants were tested for binding capacity of anti-rPlpE antibodies from bovine and murine sources. 1, rPlpE; 2, rPlpEΔN28; 3, rPlpEΔC86; 4, rPlpEΔC96; 5, rPlpEΔC106; 6, rPlpEΔN76; 7, rPlpEΔN150. (A) Anti-PlpE bovine hyperimmune serum; (B) anti-His-tagged mouse monoclonal antibody; (C) Coomassie stain.
Fine mapping of epitopes on PlpE.
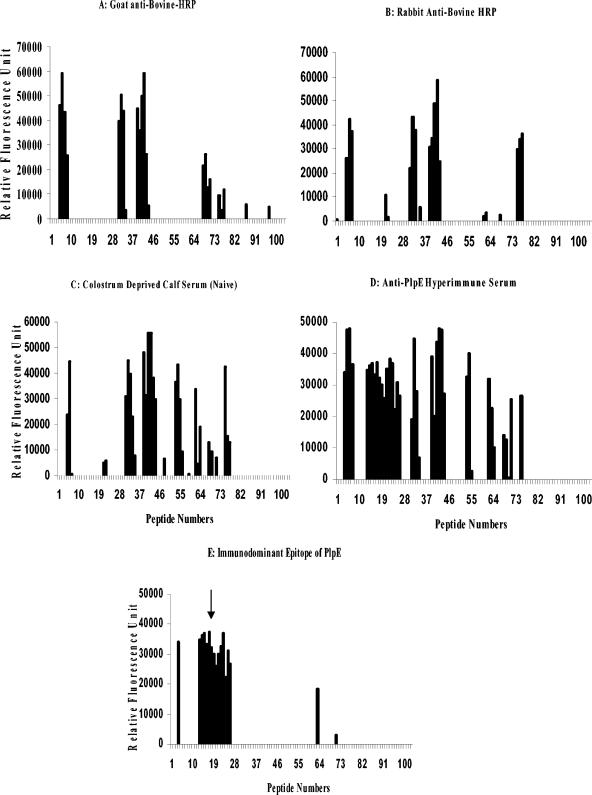
To further localize epitopes on PlpE, an array of overlapping peptides was probed with immune sera. A total of eight discrete highly reactive regions were identified when the peptide array was sequentially probed as described (Fig. 4). HRP-conjugated goat and rabbit anti-bovine secondary antibodies were reactive to regions 1, 3, 4, 7, and 8 (Fig. 4A and B, respectively). When naive serum from a colostrum-deprived newborn calf was used to test the peptide array, regions 5 and 6 were identified in addition to those already mentioned (Fig. 4C). When antibodies obtained from calves that were immunized with rPlpE, live M. haemolytica, or natural exposure to M. haemolytica were used to probe the peptide array, all eight of the regions were found to be highly reactive (Fig. 4D). When the nonspecific regions, i.e., 1, 3, 4, 5, 6, 7, and 8 were subtracted from those that reacted with hyperimmune sera produced against PlpE, region 2 (R2), corresponding to amino acids 37 to 79 of the PlpE sequence, remained (Fig. 4E) the immunodominant region of PlpE.
FIG. 4.
Overlapping 13-mer peptides spanning PlpE were sequentially probed with a variety of antibodies. (A) Goat anti-bovine-HRP; (B) rabbit anti-bovine-HRP; (C) colostrum-deprived calf serum (naive); (D) bovine anti-PlpE hyperimmune serum; (E) immunodominant epitope (R2) identified by subtracting the background, i.e., panels A, B, and C from panel D. The clusters of peaks also known as regions (R) are numbered from left to right as they appear in panel D (R1, peptides 5 to 7; R2, peptides 13 to 26; R3, peptides 31 to 34; R4, peptides 39 to 44; R5, peptides 53 to 55; R6, peptides 62 to 64; R7, peptides 68 to 71; R8, peptides 75 to 77). The arrow shows the immunodominant epitope region (R2) of the PlpE protein.
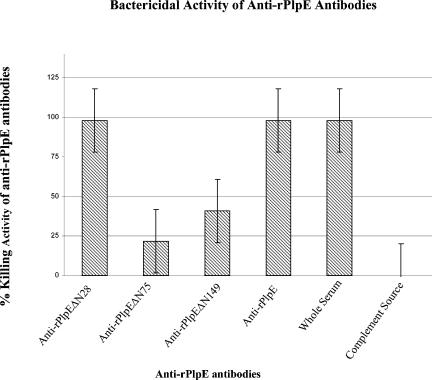
Bactericidal activities of anti-PlpE antibodies.
To determine whether immune sera produced against the purified rPlpE would have complement-mediated cell killing activity, hyperimmune sera from calves immunized with rPlpE were tested for their ability to promote complement-mediated killing of the homologous strain M. haemolytica 89010807N. Sera from calves vaccinated with rPlpE exhibited bactericidal activity of 98 to 100% in the presence of a complement source (Fig. 5).
FIG. 5.
Complement-mediated bacterial killing activity of anti-PlpE antibodies purified by affinity columns with intact rPlpE, deletion derivatives of rPlpE as ligands, and whole serum from a calf that was immunized with rPlpE and from a colostrum-deprived newborn calf.
Complement-mediated cell killing activities of anti-rPlpE antibodies that were purified on rPlpEΔN28, rPlpEΔN76, rPlpEΔN150, or rPlpE affinity columns confirmed the results of the binding studies described above (Fig. 5 and Table 2). There was no difference in the complement-mediated cell killing activity of anti-rPlpE antibodies purified on rPlpEΔN28 and rPlpE affinity columns and that exhibited by the whole anti-rPlpE antiserum, in that all showed almost 100% killing activity (Fig. 5). The flowthrough from each of the above purifications had a bactericidal activity that was significantly reduced to 17 to 21% (P < 0.00002). On the other hand, the complement-mediated bactericidal activity of anti-rPlpE antibodies purified on rPlpEΔN76 and rPlpEΔN150 affinity columns were only 25 and 40%, respectively, which is significantly lower (P < 0.0001) than those exhibited by anti-rPlpE antibodies purified by rPlpEΔN28 and rPlpE affinity columns. The flowthrough sera depleted by rPlpEΔN76 and rPlpEΔN150 affinity columns showed a killing activity of ≥65%, demonstrating that the absorption did not significantly reduce (P > 0.05) the bactericidal activity of anti-PlpE sera. These results indicate that complement-mediated bactericidal activity is associated with anti-rPlpE antibodies that are directed against epitope located in R2.
DISCUSSION
Numerous attempts have been made previously to develop efficacious vaccines against M. haemolytica. These include live M. haemolytica (2, 6, 7, 9, 11, 19, 21, 33, 50), killed M. haemolytica cells (10, 20, 22, 23, 52), components or fractions of M. haemolytica cells (17, 24, 27, 28, 35, 49, 53-55), and commercial vaccines (57, 58). While immunity to M. haemolytica appears to require anti-LKT and anti-surface antigen antibodies (55), partial protection could be afforded to cattle experimentally vaccinated with M. haemolytica preparations that did not contain LKT (21, 40). The partial protection against challenge was not attributed to any specific protein until Pandher et al. (45) cloned the plpE gene and showed that absorption of anti-PlpE sera with E. coli expressing the PlpE protein could substantially reduce complement-mediated bactericidal activity.
It was previously shown that vaccination with rPlpE enhances resistance to M. haemolytica challenge and augments commercial vaccine-induced immunity, suggesting that this is an important surface antigen (15). Epitope mapping of the PlpE protein by measuring the binding of anti-PlpE antibodies to truncated rPlpE molecules demonstrated that the major epitopes of this protein lie within the N terminus between amino acids 28 and 76. This region of the polypeptide contains eight imperfect tandem repeats of the hexapeptide QAQNAP (45). Further evaluation by peptide array analysis demonstrated that R2, between residues 36 and 76, is the major epitopic region. The use of deletion mutants to adsorb anti-rPlpE antibodies further demonstrated that antibodies to R2 are involved in complement-mediated killing of M. haemolytica. Taken together, these results provide solid evidence that R2 of PlpE is surface exposed, immunodominant, and important in stimulating antibodies capable of killing M. haemolytica.
In silico analysis of the deduced amino acid sequence of R2 with algorithms such as Parker's antigenicity (47), Kyte-Doolittle hydrophilicity (38), surface probability (36), and Chou Fasman secondary structure indices (13) demonstrated that this stretch of amino acids has a moderately high antigenicity, is fairly hydrophilic, contains a fairly high number of amino acids with very high surface probability, and is characterized by a series of turns associated with helices and sheets, respectively, all of which are strong indicators of a region that is potentially highly immunogenic (Fig. 2). Repeats in OMPs are widely distributed and perform vital functions in many bacteria. Some serve as adhesins (25, 26, 56). Others perform functions such as mediation of adhesion, infection, and transmission of the organism, as well as contributing to protective immunity (25, 30, 37, 39, 41), and still others confer multidrug resistance (29). The repeats that constitute PlpE R2 share little or no sequence homology with the proteins mentioned above. They do, however, exhibit a similar architectural design, being comprised of amino acids which may not be identical but are similar in their properties, such as charge, hydrophilicity, and surface probability. Some, such as R2 in PlpE, enhance resistance when given as immunogens.
The use of an intact recombinant protein or a subregion of it as a vaccine or component of a vaccine depends not only on its inherent immunogenic nature but also on its conservation in homologous and closely related strains involved in a disease process. The latter is particularly important in bovine respiratory disease in which M. haemolytica S1, S2, and S6 are associated with the disease, albeit to different degrees (1, 51). Previously, whole-cell lysates from 11 serotypes of M. haemolytica were probed by Western blotting with anti-PlpE sera to demonstrate the presence of the PlpE protein in all of them (45). However, there was no sequence information showing the extent of the conservation of this protein among the different serotypes. PCR amplification and sequencing of the plpE gene from M. haemolytica S1 and S6, the two serotypes that play the major roles in M. haemolytica-induced bovine respiratory disease indicated that the nucleotide sequences were identical (5). This suggests that a single peptide, viz., R2, can be used as a vaccine efficacious against two serotypes usually implicated in the bovine respiratory disease complex. We are currently sequencing plpE genes from a larger number of the aforementioned serotypes collected from different geographical areas over an extended period of time to determine similarities among various isolates within S1 and other serotypes, especially in R2.
Acknowledgments
This work was supported in part by grant no. 2002-02232 from the USDA CSREES, National Research Initiative Competitive Grant Program, and by a grant from The Noble Foundation of Ardmore, Okla.
We thank Marie Montelongo and Kayla Ingram for technical assistance and Richard Eberle for critical review of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Al-Ghamdi, G. M., T. R. Ames, J. C. Baker, R. Walker, C. C. Chase, G. H. Frank, and S. K. Maheswaran. 2000. Serotyping of Mannheimia (Pasteurella) haemolytica isolates from the upper Midwest United States. J. Vet. Diagn. Investig. 12:576-578. [DOI] [PubMed] [Google Scholar]
- 2.Aubry, P., L. D. Warnick, C. L. Guard, B. W. Hill, and M. F. Witt. 2001. Health and performance of young dairy calves vaccinated with a modified-live Mannheimia haemolytica and Pasteurella multocida vaccine. J. Am. Vet. Med. Assoc. 219:1739-1742. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz, L. O., B. J. Kennedy, L. A. Novotny, G. Duquesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:2746-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakaletz, L. O., E. R. Leake, J. M. Billy, and P. T. Kaumaya. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955-961. [DOI] [PubMed] [Google Scholar]
- 5.Blackwood, E. R., S. Ayalew, and A. W. Confer. 2002. Molecular and immunological analysis of the outer membrane protein, PlpE, from Mannheimia haemolytica serotypes 1, 2, and 6, abstr. 93P. In R. P. Ellis (ed.), Proceedings of the 83rd Annual Meeting of the Conference of Research Workers in Animal Diseases, St. Louis, Mo. Iowa State University Press, Ames, Iowa.
- 6.Blanchard-Channell, M. T., M. K. Ashfaq, and W. L. Kadel. 1987. Efficacy of a streptomycin-dependent, live Pasteurella haemolytica vaccine against challenge exposure to Pasteurella haemolytica in cattle. Am. J. Vet. Res. 48:637-642. [PubMed] [Google Scholar]
- 7.Brennan, R. E., R. E. Corstvet, and D. B. Paulson. 1998. Antibody responses to Pasteurella haemolytica 1:A and three of its outer membrane proteins in serum, nasal secretions, and bronchoalveolar lavage fluid from calves. Am. J. Vet. Res. 59:727-732. [PubMed] [Google Scholar]
- 8.Bryson, D. G. 1985. Calf pneumonia. Vet. Clin. North Am. Food Anim. Sci. 1:237-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, C. M., F. J. Bester, and D. J. Du Toit. 1984. Factors affecting the immunogenicity of Pasteurella haemolytica in mice. Onderstepoort J. Vet. Res. 51:97-102. [PubMed] [Google Scholar]
- 10.Cardella, M. A., M. A. Adviento, and R. M. Nervig. 1987. Vaccination studies against experimental bovine Pasteurella pneumonia. Can. J. Vet. Res. 51:204-211. [PMC free article] [PubMed] [Google Scholar]
- 11.Catt, D. M., M. M. Chengappa, W. L. Kadel, and C. E. Herren. 1985. Preliminary studies with a live streptomycin-dependent Pasteurella multocida and Pasteurella haemolytica vaccine for the prevention of bovine pneumonic pasteurellosis. Can. J. Comp. Med. 49:366-371. [PMC free article] [PubMed] [Google Scholar]
- 12.Chae, C. H., M. J. Gentry, A. W. Confer, and G. A. Anderson. 1990. Resistance to host immune defense mechanisms afforded by capsular material of Pasteurella haemolytica, serotype 1. Vet. Microbiol. 25:241-251. [DOI] [PubMed] [Google Scholar]
- 13.Chou, P. Y., and G. D. Fasman. 1978. Empirical predictions of protein conformations. Annu. Rev. Biochem. 47:251-276. [DOI] [PubMed] [Google Scholar]
- 14.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober. 1994. Current protocols in immunology, vol. 1. John Wiley & Sons, Inc., Somerset, N.J.
- 15.Confer, A. W., S. Ayalew, R. J. Panciera, M. Montelongo, L. C. Whitworth, and J. D. Hammer. 2003. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine 21:2821-2829. [DOI] [PubMed] [Google Scholar]
- 16.Confer, A. W., R. W. Fulton, K. D. Clinkenbeard, and B. A. Driskel. 1998. Duration of serum antibody responses following vaccination and revaccination of cattle with non-living commercial Pasteurella haemolytica vaccines. Vaccine 16:1962-1970. [DOI] [PubMed] [Google Scholar]
- 17.Confer, A. W., B. A. Lessley, R. J. Panciera, R. W. Fulton, and J. A. Kreps. 1985. Serum antibodies to antigens derived from a saline extract of Pasteurella haemolytica: correlation with resistance to experimental bovine pneumonic pasteurellosis. Vet. Immunol. Immunopathol. 10:265-278. [DOI] [PubMed] [Google Scholar]
- 18.Confer, A. W., R. D. McCraw, J. A. Durham, R. J. Morton, and R. J. Panciera. 1995. Serum antibody responses of cattle to iron-regulated outer membrane proteins of Pasteurella haemolytica A1. Vet. Immunol. Immunopathol. 47:101-110. [DOI] [PubMed] [Google Scholar]
- 19.Confer, A. W., R. J. Panciera, R. E. Corstvet, J. A. Rummage, and R. W. Fulton. 1984. Bovine pneumonic pasteurellosis: effect of culture age of Pasteurella haemolytica used as a live vaccine. Am. J. Vet. Res. 45:2543-2545. [PubMed] [Google Scholar]
- 20.Confer, A. W., R. J. Panciera, R. W. Fulton, M. J. Gentry, and J. A. Rummage. 1985. Effect of vaccination with live or killed Pasteurella haemolytica on resistance to experimental bovine pneumonic pasteurellosis. Am. J. Vet. Res. 46:342-347. [PubMed] [Google Scholar]
- 21.Confer, A. W., R. J. Panciera, M. J. Gentry, and R. W. Fulton. 1986. Immunologic response and resistance to experimentally induced pneumonic pasteurellosis in cattle vaccinated with various dosages of lyophilized Pasteurella haemolytica. Am. J. Vet. Res. 47:1853-1857. [PubMed] [Google Scholar]
- 22.Confer, A. W., R. J. Panciera, M. J. Gentry, and R. W. Fulton. 1987. Immunologic response to Pasteurella haemolytica and resistance against experimental bovine pneumonic pasteurellosis, induced by bacterins in oil adjuvants. Am. J. Vet. Res. 48:163-168. [PubMed] [Google Scholar]
- 23.Confer, A. W., J. C. Wright, J. M. Cummins, R. J. Panciera, and R. E. Corstvet. 1983. Use of a fluorometric immunoassay to determine antibody response to Pasteurella haemolytica in vaccinated and nonvaccinated feedlot cattle. J. Clin. Microbiol. 18:866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlon, J. A., P. E. Shewen, and R. Y. Lo. 1991. Efficacy of recombinant leukotoxin in protection against pneumonic challenge with live Pasteurella haemolytica A1. Infect. Immun. 59:587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2003. Characterization of the functional domain of major surface protein 1a involved in adhesion of the rickettsia Anaplasma marginale to host cells. Vet. Microbiol. 91:265-283. [DOI] [PubMed] [Google Scholar]
- 26.de La Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, S. D. Rodriguez, M. A. Garcia, and K. M. Kocan. 2001. Evolution and function of tandem repeats in the major surface protein 1a of the ehrlichial pathogen Anaplasma marginale. Anim. Health Res. Rev. 2:163-173. [PubMed] [Google Scholar]
- 27.Donachie, W., C. Burrells, and A. M. Dawson. 1983. Specificity of the enzyme-linked immunosorbent assay (ELISA) for antibodies in the sera of specific pathogen-free lambs vaccinated with Pasteurella haemolytica antigens. Vet. Microbiol. 8:199-205. [DOI] [PubMed] [Google Scholar]
- 28.Donachie, W., A. D. Sutherland, and G. E. Jones. 1986. Assessment of immunity to Pasteurella haemolytica in sheep by in vitro methods. Dev. Biol. Stand. 64:63-69. [PubMed] [Google Scholar]
- 29.Eda, S., H. Yoneyama, and T. Nakae. 2003. Function of the MexB efflux-transporter divided into two halves. Biochemistry 42:7238-7244. [DOI] [PubMed] [Google Scholar]
- 30.Emsley, P., I. G. Charles, N. F. Fairweather, and N. W. Isaacs. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90-92. [DOI] [PubMed] [Google Scholar]
- 31.Frank, G. H. 1989. Pasteurellosis of cattle. Academic Press Limited, London, United Kingdom.
- 32.Frank, G. H. 1986. The role of Pasteurella haemolytica in the bovine respiratory disease complex. Vet. Med. 81:838-846. [Google Scholar]
- 33.Frank, G. H., R. E. Briggs, R. W. Loan, C. W. Purdy, and E. S. Zehr. 1994. Serotype-specific inhibition of colonization of the tonsils and nasopharynx of calves after Pasteurella haemolytica serotype A1 after vaccination with the organism. Am. J. Vet. Res. 55:1107-1110. [PubMed] [Google Scholar]
- 34.Gerlach, G. F., C. Klashinsky, S. Rossi-Campos, A. A. Potter, and P. J. Wilson. 1993. Molecular characterization of a protective outer membrane lipoprotein (OmlA) from Actinobacillus pleuropneumoniae serotype 1. Infect. Immun. 61:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilmour, N. J., W. B. Martin, J. M. Sharp, D. A. Thompson, P. W. Wells, and W. Donachie. 1983. Experimental immunisation of lambs against pneumonic pasteurellosis. Res. Vet. Sci. 35:80-86. [PubMed] [Google Scholar]
- 36.Janin, J., S. Wodak, M. Levitt, and B. Maigret. 1978. Conformation of amino acid side-chains in proteins. J. Mol. Biol. 125:357-386. [DOI] [PubMed] [Google Scholar]
- 37.King, A. J., G. Berbers, H. F. van Oirschot, P. Hoogerhout, K. Knipping, and F. R. Mooi. 2001. Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology 147:2885-2895. [DOI] [PubMed] [Google Scholar]
- 38.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 39.Lindmark, H., K. Jacobsson, L. Frykberg, and B. Guss. 1996. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect. Immun. 64:3993-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacDonald, J. T., S. K. Maheswaran, J. Opuda-Asibo, E. L. Townsend, and E. S. Thies. 1983. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet. Microbiol. 8:585-599. [DOI] [PubMed] [Google Scholar]
- 41.Mooi, F. R., H. van Oirschot, K. Heuvelman, H. G. van der Heide, W. Gaastra, and R. J. Willems. 1998. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect. Immun. 66:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton, R. J., R. J. Panciera, R. W. Fulton, G. H. Frank, S. A. Ewing, J. T. Homer, and A. W. Confer. 1995. Vaccination of cattle with outer membrane protein-enriched fractions of Pasteurella haemolytica and resistance against experimental challenge exposure. Am. J. Vet. Res. 56:875-879. [PubMed] [Google Scholar]
- 43.Mosier, D. A., K. R. Simons, A. W. Confer, R. J. Panciera, and K. D. Clinkenbeard. 1989. Pasteurella haemolytica antigens associated with resistance to pneumonic pasteurellosis. Infect. Immun. 57:711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novotny, L. A., J. A. Jurcisek, M. E. Pichichero, and L. O. Bakaletz. 2000. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect. Immun. 68:2119-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandher, K., A. W. Confer, and G. L. Murphy. 1998. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infect. Immun. 66:5613-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandher, K., G. L. Murphy, and A. W. Confer. 1999. Identification of immunogenic, surface-exposed outer membrane proteins of Pasteurella haemolytica serotype 1. Vet. Microbiol. 65:215-226. [DOI] [PubMed] [Google Scholar]
- 47.Parker, J., D. Guo, and R. Hodges. 1986. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 25:5425-5432. [DOI] [PubMed] [Google Scholar]
- 48.Peeters, C. C., I. J. Claassen, R. Schuller, G. F. Kresten, E. M. van der Voort, and J. T. Poolman. 1999. Immunogenicity of various presentation forms of PorA outer membrane protein of Neisseria meningitidis in mice. Vaccine 17:2702-2712. [DOI] [PubMed] [Google Scholar]
- 49.Potter, A. A., A. B. Schryvers, J. A. Ogunnariwo, W. A. Hutchins, R. Y. Lo, and T. Watts. 1999. Protective capacity of the Pasteurella haemolytica transferrin-binding proteins TbpA and TbpB in cattle. Microb. Pathog. 27:197-206. [DOI] [PubMed] [Google Scholar]
- 50.Purdy, C. W., C. W. Livingston, Jr., G. H. Frank, J. M. Cummins, N. A. Cole, and R. W. Loan. 1986. A live Pasteurella haemolytica vaccine efficacy trial. J. Am. Vet. Med. Assoc. 188:589-591. [PubMed] [Google Scholar]
- 51.Purdy, C. W., R. H. Raleigh, J. K. Collins, J. L. Watts, and D. C. Straus. 1997. Serotyping and enzyme characterization of Pasteurella haemolytica and Pasteurella multocida isolates recovered from pneumonic lungs of stressed feeder calves. Curr. Microbiol. 34:244-249. [DOI] [PubMed] [Google Scholar]
- 52.Purdy, C. W., D. C. Straus, R. J. Sutherland, and J. R. Ayres. 1996. Efficacy of a subcutaneously administered, ultraviolet light-killed Pasteurella haemolytica A1-containing vaccine against transthoracic challenge exposure in goats. Am. J. Vet. Res. 57:1168-1174. [PubMed] [Google Scholar]
- 53.Rajeev, S., S. A. Kania, R. V. Nair, J. T. McPherson, R. N. Moore, and D. A. Bemis. 2001. Bordetella bronchiseptica fimbrial protein-enhanced immunogenicity of a Mannheimia haemolytica leukotoxin fragment. Vaccine 19:4842-4850. [DOI] [PubMed] [Google Scholar]
- 54.Shewen, P. E., C. W. Lee, A. Perets, D. C. Hodgins, K. Baldwin, and R. Y. Lo. 2003. Efficacy of recombinant sialoglycoprotease in protection of cattle against pneumonic challenge with Mannheimia (Pasteurella) haemolytica A1. Vaccine 21:1901-1906. [DOI] [PubMed] [Google Scholar]
- 55.Shewen, P. E., and B. N. Wilkie. 1988. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can. J. Vet. Res. 52:30-36. [PMC free article] [PubMed] [Google Scholar]
- 56.Shimoji, Y., Y. Ogawa, M. Osaki, H. Kabeya, S. Maruyama, T. Mikami, and T. Sekizaki. 2003. Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J. Bacteriol. 185:2739-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srinand, S., S. L. Hsuan, H. S. Yoo, S. K. Maheswaran, T. R. Ames, and R. E. Werdin. 1996. Comparative evaluation of antibodies induced by commercial Pasteurella haemolytica vaccines using solid phase immunoassays. Vet. Microbiol. 49:181-195. [DOI] [PubMed] [Google Scholar]
- 58.Srinand, S., S. K. Maheswaran, T. R. Ames, R. E. Werdin, and S. L. Hsuan. 1996. Evaluation of efficacy of three commercial vaccines against experimental bovine pneumonic pasteurellosis. Vet. Microbiol. 52:81-89. [DOI] [PubMed] [Google Scholar]
- 59.Thomson, R. G., S. Chandler, M. Savan, and M. L. Fox. 1975. Investigations of factors of probable significance in the pathogensis in cattle. Can. J. Comp. Med. 39:194-207. [PMC free article] [PubMed] [Google Scholar]
- 60.Turbyfill, K. R., J. A. Mertz, C. P. Mallett, and E. V. Oaks. 1998. Identification of epitope and surface-exposed domains of Shigella flexneri invasion plasmid antigen D (IpaD). Infect. Immun. 66:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Ley, P., J. van der Biezen, R. Sutmuller, P. Hoogerhout, and J. T. Poolman. 1996. Sequence variability of FrpB, a major iron-regulated outer-membrane protein in the pathogenic neisseriae. Microbiology 142:3269-3274. [DOI] [PubMed] [Google Scholar]
- 62.Webb, D. C., and A. W. Cripps. 2000. A P5 peptide that is homologous to peptide 10 or OprF from Pseudomonas aeruginosa enhances clearance of nontypeable Haemophilus influenzae from acutely infected rat lung in the absence of detectable peptide-specific antibody. Infect. Immun. 68:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yates, W. D. 1982. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can. J. Comp. Med. 46:225-263. [PMC free article] [PubMed] [Google Scholar]