Abstract
The heparin-binding hemagglutinin (HBHA) of Mycobacterium tuberculosis is a surface-expressed adhesin that can affect binding to host cells via a unique, methylated, carboxyl-terminal, lysine-, alanine-, and proline-rich repeat region. It has been implicated in extrapulmonary dissemination of M. tuberculosis from the lung following the initial infection of the host. To assess the vaccine potential of this protein, purified preparations of HBHA were emulsified in a dimethyldioctadecylammonium bromide-monophosphoryl lipid A adjuvant and tested for the ability to reduce M. tuberculosis infection in the mouse aerosol challenge model for tuberculosis. The HBHA-containing vaccine gave a ∼0.7-log reduction in CFU in both mouse lungs and spleens compared to adjuvant controls 28 days following challenge. Although a notable level of serum antibody to HBHA was elicited after three immunizations and the antibodies were able to bind to the surface of M. tuberculosis, passive immunization with monoclonal antibodies directed against HBHA did not protect in the challenge model. Compared to adjuvant controls, an elevated gamma interferon response was generated by splenic and lymph node-derived T cells from immunized mice in the presence of macrophages pulsed with purified HBHA or infected with live M. tuberculosis, suggesting that the effective immunity may be cell mediated. Efforts to construct effective recombinant HBHA vaccines in fast-growing Mycobacterium smegmatis have been unsuccessful so far, which indicates that distinctive posttranslational modifications present in the HBHA protein expressed by M. tuberculosis are critical for generating effective host immune responses. The vaccine studies described here demonstrate that HBHA is a promising new vaccine candidate for tuberculosis.
The heparin-binding hemagglutinin (HBHA) of mycobacteria was originally identified as a lectin-like factor found in extracts of Mycobacterium tuberculosis cells that agglutinates erythrocytes (13). Hemagglutination and attachment of M. tuberculosis to epithelial cells in vitro were specifically inhibited by sulfate-containing sugars, such as heparin, implying that HBHA is a bacterial adhesion. Construction of an M. tuberculosis mutant lacking the hbhA gene confirmed that HBHA is involved in attachment (14) and invasion of epithelial cells but not macrophages (16). Infection studies with mice also demonstrated that the hbhA deletion mutant was defective in extrapulmonary dissemination from the lung, implicating HBHA in the secondary stages of tuberculosis pathogenicity in the host. The affinity of HBHA for heparin was used to develop a method for purification of the 28-kDa protein from M. tuberculosis and Mycobacterium bovis BCG culture filtrates, as well as from cell extracts (12). Two monoclonal antibodies (MAbs) have been identified, MAb E4, which reacts with both HBHA purified from bacteria and recombinant His-tagged HBHA expressed in Escherichia coli (rEC-HBHA), and MAb D2, which reacts only with native HBHA (nHBHA) (4). Although both MAbs recognize a Lys-Ala-Pro-rich repeat region at the C terminus of HBHA, methylation of the lysine residues is required for reactivity with MAb D2 (17). This unusual methylation of lysines is also found in laminin-binding proteins expressed by Mycobacterium leprae (20, 24) and other mycobacteria (18). T-cell responses to HBHA may have a role in protection against disease, since healthy subjects infected with M. tuberculosis produce significant levels of HBHA-specific gamma interferon (IFN-γ), while patients with active tuberculosis do not (11). The more recent finding that human immune responses to HBHA following infection with M. tuberculosis are more dominant for native HBHA than for recombinant E. coli-expressed HBHA indicates that methylation of HBHA is critical for the development of effective T-cell antigenicity (22). For all these reasons, HBHA is a viable candidate for study as a tuberculosis vaccine. In this report, we present the results of a number of immunogenicity and efficacy studies in which the mouse aerosol challenge model of tuberculosis (3) was used.
MATERIALS AND METHODS
Microorganisms.
M. tuberculosis strains H37Ra (= TMC 201) and Erdman (= TMC 107) and the M. bovis BCG Pasteur strain were obtained from the Trudeau Institute, Saranac Lake, N.Y. M. tuberculosis CDC1551 was obtained from the strain collection of the Center for Biologics Evaluation and Research, Food and Drug Administration, and strain HN878 was a gift from C. Barry, National Institute of Allergy and Infectious Diseases. The recombinant Mycobacterium smegmatis pMV3-38 strain was used to express and purify histidine-tagged methylated HBHA (6). All mycobacterial strains were grown in 7H9 broth (Difco, Detroit, Mich.) supplemented with 10% OADC (Becton Dickinson, Cockeysville, Md.) and 0.02% Tween 80 (Sigma Chemical, St. Louis, Mo.) in roller bottles shaken at 120 rpm at room temperature until logarithmic-phase growth was obtained. For purification of HBHA, bacteria were cultured with shaking in 7H9 broth or Long's synthetic medium (Quality Biological, Inc., Gaithersburg, Md.).
Vaccine antigens.
Native HBHA was purified from M. tuberculosis H37Ra or M. bovis BCG extracts by heparin-Sepharose chromatography as described by Menozzi et al. (12) or by heparin-Sepharose chromatography followed by high-performance liquid chromatography (HPLC) as described by Masungi et al. (11). Recombinant HBHA was purified from E. coli expressing HBHA by nickel chromatography as previously described and was extensively dialyzed before use in phosphate-buffered saline (PBS) (4). A recombinant M. smegmatis strain expressing a histidine-tagged HBHA was developed as described by Delogu et al. (6). Briefly, the DNA sequence encoding the histidine-tagged HBHA protein was cut with restriction endonucleases from the pET15b-based plasmid (4) and ligated into the pMV206-derived construct (21) in frame with the DNA sequence corresponding to the hbhA putative promoter region. The construct was electroporated in M. smegmatis mc2155, and the HBHA protein was purified from the recombinant strain by using a procedure similar to the E. coli procedure.
Immunizations and tuberculosis challenge studies.
All animal experiments were performed by using protocols approved by the CBER/FDA Institutional Animal Care and Use Committee. Individual C57BL/6 female mice (n = 5) (Jackson Laboratories, Bar Harbor, Maine) were vaccinated subcutaneously three times with 5 μg of either HPLC-purified HBHA (nHBHA) or recombinant HBHA emulsified in 150 μg of dimethyldioctadecylammonium bromide (DDA) (Acros Organics, Morris Plains, N.J.)-monophosphoryl lipid A (MPL) (Avanti Polar-Lipids, Alabaster, Ala.) adjuvant as described by Weinrich-Olsen et al. (26) or with the adjuvant combination alone. Another group of five mice were immunized with live M. bovis BCG, and vaccine efficacy was determined as described by Collins (3). Two weeks after the third immunization, mice were bled, and sera were collected for immunoblot and immunofluorescence studies. Mice were challenged with aerosolized M. tuberculosis Erdman (200 CFU) 4 weeks following the final immunization. Four weeks after challenge, mice were sacrificed, the lungs and spleens were homogenized in PBS containing 0.02% Tween 80, and serial dilutions were plated onto Middlebrook 7H11 agar (Difco). Colonies were counted after 2 to 3 weeks of incubation at 37°C. Samples of lung tissue were fixed in 10% formalin (BioFluids, Rockville, Md.), embedded in paraffin, and processed for histology (Histo-Path of America, Walkersville, Md.). Sections were stained with hematoxylin and eosin or with the Ziehl-Nielsen acid-fast stain for microscopic examination. Data were analyzed by one-way analysis of variance, and significant differences between the means were measured by using Tukey's test. A P value of <0.001 was considered significant.
For the passive antibody studies, individual C57BL/6 female mice (n = 5) were inoculated once intravenously with 250 μg of immunoglobulin G2a (IgG2a) and 250 μg of IgG3 purified from ascitic fluid (Harlan Bioproducts for Science, Indianapolis, Ind.), producing anti-HBHA monoclonal antibodies D2 and E4 (19), or with 250 μg of purified IgG2a and 250 μg of IgG3 from two isotype control monoclonal antibodies. Twenty-four hours after antibody injection, the mice were challenged with aerosolized M. tuberculosis Erdman (either 100 or 500 CFU). Four weeks after challenge, the mice were sacrificed, the lungs, spleens, and livers were homogenized in PBS containing 0.02% Tween 80, and the numbers of CFU were determined as described above.
Cytokine analysis.
Bone marrow-derived macrophages (BMMΦ) were isolated as previously described (5) and were incubated with the M. bovis BCG Pasteur strain at a multiplicity of infection (MOI) of 3:1 for 2 h or pulsed with 20 μg of nHBHA per ml, 20 μg of recombinant HBHA per ml, or 20 μg of recombinant His-tagged HBHA purified from M. smegmatis (rMS-HBHA) per ml for 24 h. The spleens and periaortic and inguinal lymph nodes from five immunized and five control mice (4 weeks postimmunization) were pooled. Single-cell suspensions of splenocytes and lymph node cells were layered over the antigen-pulsed BMMΦ. Following 3 days of coculture, tissue culture supernatants from triplicate samples were harvested, and the amount of IFN-γ was determined by a capture enzyme-linked immunosorbent assay (Pharmingen, San Diego, Calif.).
SDS-PAGE, immunoblots, and fluorescence assays.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (9) by using a 4 to 12% polyacrylamide gradient gel (ISC Bioexpress, Kaysville, Utah); then the gel was stained with Coomassie blue, or proteins were electrotransferred from the gel to a nitrocellulose membrane by the method described by Towbin et al. (23). Monoclonal antibodies D2 and E4 were used at a 1:500 dilution, and sera from C57BL/6 nHBHA- and rMS-HBHA-immunized mice were used at a 1:250 dilution. The antigen-antibody complexes were detected by using alkaline phosphatase-conjugated goat anti-mouse whole-molecule IgG (Sigma), followed by colorimetric visualization with a 5-bromo-4-chloro-3-indolylphosphate (BCIP)—nitroblue tetrazolium detection kit (KPL, Gaithersburg, Md.). For the immunofluorescence assay bacteria were washed with PBS, dried, and fixed on lysine-coated glass slides by using 4% paraformaldehyde. The slides were incubated with pooled anti-HBHA mouse sera at a 1:250 dilution for 2 h at room temperature, washed extensively with PBS, and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (Zymed Laboratories, Inc., San Francisco, Calif.) at a 1:50 dilution for 1 h. The slides were washed well, coverslips were added by using fluoromount (Fisher Scientific, Inc., Pittsburgh, Pa.), and the preparations were viewed with a Nikon Optiphot-2 microscope equipped with a ×100 fluorescent objective and a SPOT RT color digital camera (Diagnostics Inc., Sterling Heights, Mich.).
Reverse transcription-PCR.
For preparation of total RNA, M. tuberculosis strains were grown in 7H9 broth as described above for ∼30 days (stationary growth). In some experiments, M. tuberculosis CDC1551 was cultured for 30 days under low-oxygen conditions by using the protocol of Wayne and Hayes (25) or under nutrient starvation conditions as described by Betts et al. (1). In other experiments, BMMΦ were infected with M. tuberculosis Erdman at an MOI of 2:1 for 6 days as described above for the cytokine assay, or M. tuberculosis Erdman was recovered from mouse lung and spleen tissues 1 year after a low-dose aerosol challenge. Bacteria were harvested, and the pellets were washed with PBS, resuspended in 1,050 μl of RLT buffer (QIAGEN, Valencia, Calif.) containing 0.1% 2-mercaptoethanol, and then transferred to lysing matrix B tubes containing 0.1-mm silica spheres (Qbiogene, Carlsbad, Calif.). The mixture was then homogenized with a FastPrep FP120 instrument for 45 s at speed 6.5. After centrifugation at 8,000 × g for 1 min at 4°C, the supernatant was transferred to a microcentrifuge tube and precipitated with 0.7 volume of ethanol. The RNA was purified by using an RNeasy mini spin column, and the contaminating DNA was removed by on-column DNase I treatment as described by the manufacturer (QIAGEN). Before cDNA synthesis, 0.5 μg of RNA was treated further with 2 U of DNase I (Invitrogen, Carlsbad, Calif.), and all the samples were tested by PCR for the complete removal of DNA. cDNA was then synthesized in a 25-μl (total volume) reaction mixture containing reverse transcriptase buffer, 250 ng of random primers, 1 μl of a 10 mM deoxynucleoside triphosphate mixture, 1 μl of 0.1 M dithiothreitol, and 200 U of SuperScript III reverse transcriptase (Invitrogen). The reaction mixture was incubated at 50°C for 60 min to synthesize cDNA and then at 70°C for 15 min to inactivate the enzyme. The template RNA was removed by incubating the mixture with 2 U of RNase H at 37°C for 20 min. Then 1 μl of cDNA template was used in a PCR to amplify a 600-bp fragment of the HBHA gene with specific primers (forward primer 5′-ATGGCTGAAAACTCGAACAT-3′ and reverse primer 5′-CTACTTCTGGGTGACCTTCTTG-3′) by using a PTC-200 Peltier thermal cycler (MJ Research, Inc., Waltham, Mass.) with one cycle of 2 min at 95°C followed by 35 cycles of 40 s at 95°C, 45 s at 54°C, and 1 min at 72°C and a final extension for 10 min at 72°C.
RESULTS
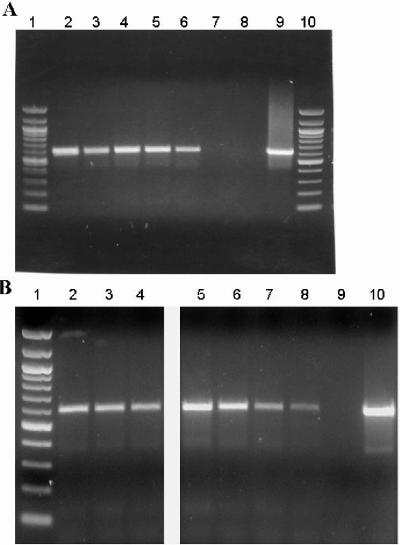
In expression studies, we observed that HBHA was expressed by all M. tuberculosis strains examined to date by reverse transcription-PCR, including the Erdman strain, CDC1551, the Bejing strain HN878, and the avirulent laboratory strain H37Ra, as well as the M. bovis BCG Pasteur vaccine strain (Fig. 1A). M. tuberculosis expresses HBHA during logarithmic and stationary growth in vitro and under environmental stress conditions, including low oxygen and nutrient starvation (Fig. 1B). HBHA was also expressed by M. tuberculosis residing within murine BMMΦ and by bacteria persisting in mouse tissues 1 year following aerosol infection (Fig. 1B). These results are consistent with previous data showing that immune responses to HBHA occur at both early and late stages of human infection (11), and altogether, the evidence suggests that HBHA may be a good candidate for preventive or therapeutic tuberculosis vaccines.
FIG. 1.
Expression of HBHA by M. tuberculosis as determined by reverse transcription-PCR analyses. (A) Total RNA extracted from the M. tuberculosis Erdman strain (lane 2), CDC1551 strain (lane 3), H37Ra strain (lane 4), or HN878 strain (lane 5) or the M. bovis BCG Pasteur strain (lane 6) or BCG Pasteur strain containing a mutation in the HBHA gene (lane 7) was reverse transcribed by using random primers and then combined with gene-specific primers designed to amplify a ∼600-bp segment of HBHA. Lanes 1 and 10 contained molecular weight markers (100-bp ladder). (B) HBHA was expressed in M. tuberculosis CDC1551 after 30 days of growth in media under normal growth conditions (lane 2), under low-oxygen conditions (lane 3), or under nutrient starvation conditions (lane 4). Similarly, HBHA was expressed in the M. tuberculosis Erdman strain in culture (lane 5), after 6 days of growth in BMMΦ in vitro (lane 6), or after 1 year of growth in the lungs (lane 7) and spleen (lane 8) of a C57BL/6 mouse. Lane 1 contained molecular weight markers (100-bp ladder). The same PCR mixture without reverse transcriptase (lane 8 in panel A and lane 9 in panel B) and the same PCR mixture containing DNA instead of RNA (lane 9 in panel A and lane 10 in panel B) were used as controls, and all cDNA preparations were shown to be free of contaminating DNA as described in Materials and Methods.
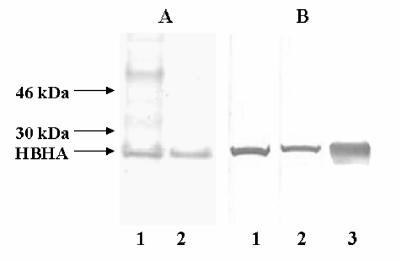
For preparation as a subunit vaccine, HBHA was purified by heparin-Sepharose chromatography or by heparin-Sepharose chromatography followed by high-performance liquid chromatography (producing HPLC-purified HBHA) as previously described (11). Figure 2A shows that the heparin-purified HBHA preparation contained mostly the 28-kDa HBHA protein along with some minor contaminants having molecular masses of about 35 and 70 kDa, while the HPLC-purified material contained only the 28-kDa HBHA band as determined by protein staining with Coomassie blue or with silver (data not shown). Both HBHA preparations were recognized by the anti-HBHA monoclonal antibody D2, which reacted with methylated HBHA (Fig. 2B) (17). The HBHA preparations were emulsified in an adjuvant mixture containing both DDA and MPL and were used to immunize C57BL/6 mice three times over a 6-week period (5 μg of HBHA protein per immunization per mouse).
FIG. 2.
Antigen preparations of HBHA used as vaccines. (A) HBHA purified by heparin-Sepharose gradient chromatography (lane 1) or by subsequent reverse-phase HPLC (lane 2) was analyzed by SDS-PAGE, and gel lanes were stained with Coomassie blue. (B) Immunoblots containing HBHA purified by heparin-Sepharose gradient chromatography (lane 1) or by subsequent reverse-phase HPLC (lane 2), as well as recombinant His-tagged HBHA purified from M. smegmatis (lane 3), were probed with MAb D2, which recognizes methylated HBHA. The positions of the 28-kDa native HBHA protein and molecular mass standards are indicated on the left.
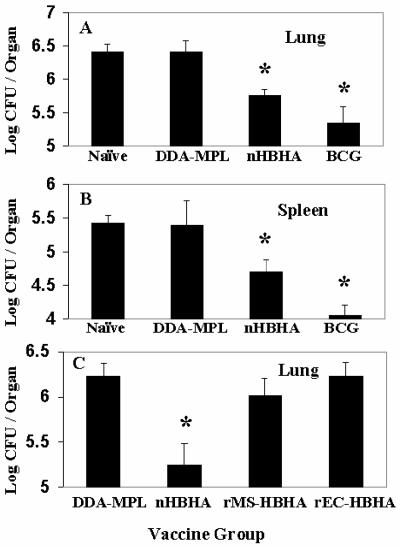
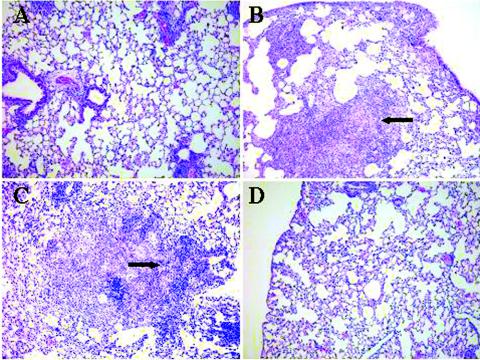
Mice were challenged 4 weeks after a third immunization with HPLC-purified HBHA vaccine with the virulent M. tuberculosis Erdman strain by using the aerosol challenge assay described previously (7). Four weeks following the challenge, the mice (five mice per vaccination group) were sacrificed, the lungs and spleens were removed and homogenized, and dilutions of the homogenates were plated on Middlebrook 7H11 agar in order to determine the numbers of M. tuberculosis CFU in the tissues. Figures 3A and B show that compared with the adjuvant control, the HPLC-purified HBHA vaccine significantly reduced the number of M. tuberculosis cells in both the lungs and spleens by ∼0.7 log (P > 0.001) for both tissues. In comparison, the live M. bovis BCG Pasteur vaccine, which was used as the standard reference vaccine for these assays, gave reductions of 1.1 logs in the lungs and 1.4 logs in the spleen. Histological examination of lung tissues demonstrated that the lung topology of mice vaccinated with HPLC-purified HBHA (Fig. 4A) was similar to that of mice vaccinated with M. bovis BCG (Fig. 4D) and that there were numerous areas of healthy lung tissue compared to the lungs of DDA-MPL-immunized mice (Fig. 4B), in which areas of cellular infiltration and consolidation typical of tuberculosis in mice were observed. In general, lung sections from mice immunized with the HBHA vaccine had more discrete areas of cellular infiltration and fewer lesions.
FIG. 3.
Reduction of M. tuberculosis in mouse tissues following immunization with adjuvant-treated HBHA vaccines. Twenty-eight days following a third vaccination with DDA-MPL adjuvant (DDA-MPL), with HPLC-purified HBHA (nHBHA), or with live BCG vaccine (BCG), C57BL/6 mice were aerogenically challenged with ∼200 CFU of the M. tuberculosis Erdman strain per mouse, and the numbers of viable bacteria in the lungs (A) and spleens (B) were determined 28 days following challenge as previously described (3). (C) By using the immunization and challenge protocol described above, the numbers of M. tuberculosis CFU in the lungs of mice vaccinated with adjuvant only (DDA-MPL), heparin-purified HBHA (nHBHA), recombinant His-tagged HBHA purified from M. smegmatis (rMS-HBHA), or recombinant His-tagged HBHA purified from E. coli (rEC-HBHA) were compared at 28 days following challenge. The asterisks indicate the vaccine groups (each of which contained five mice) in which the bacterial counts were significantly reduced (P < 0.001) compared to the groups that received only adjuvant, as determined by a t test.
FIG. 4.
Histopathological examination of lung tissue (stained with hematoxylin and eosin) from mice 28 days after challenge with M. tuberculosis. Lung sections from mice vaccinated with HPLC-purified HBHA (A), with only the DDA-MPL adjuvant (B), with recombinant His-tagged HBHA purified from M. smegmatis (C), and with BCG vaccine (D) are compared. The arrows indicate areas of dense cellular infiltration and consolidation typical of tuberculosis in mice at 30 days. Magnification, ×100.
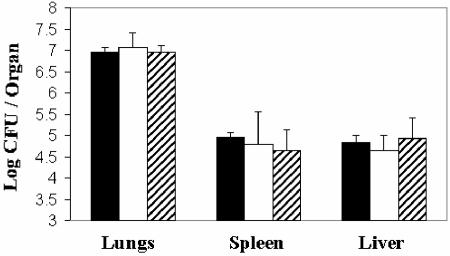
Immunization of mice with the HBHA vaccine elicited antibodies directed against HBHA, as demonstrated by the reaction of sera with the 28-kDa HBHA protein in Western blots (data not shown). Since HBHA is a cell surface protein, an indirect immunofluorescence analysis was performed by using the immunized mouse sera to determine if antibodies recognized HBHA on the surface of M. tuberculosis. As shown in Fig. 5, sera from mice immunized with the HPLC-purified HBHA vaccine contained immunoglobulins which strongly recognized the surface of live M. tuberculosis. Sera from the adjuvant controls were negative in this assay. These observations, together with previous studies which demonstrated that HBHA-specific monoclonal antibodies preincubated with mycobacteria prior to infection can inhibit dissemination of mycobacteria from the lungs of infected mice (16), suggest that antibodies may have a role in protection in the mouse model of tuberculosis. To investigate this hypothesis, mice were inoculated intravenously with 250 μg of each of two HBHA-specific monoclonal antibodies, MAb D2 and MAb E4, 24 h prior to aerosol infection with M. tuberculosis. As shown in Fig. 6, there was no reduction in the colonization of lungs, spleens, or livers from mice immunized with this monoclonal antibody mixture compared with either naïve mice or mice immunized with isotype control monoclonal antibodies.
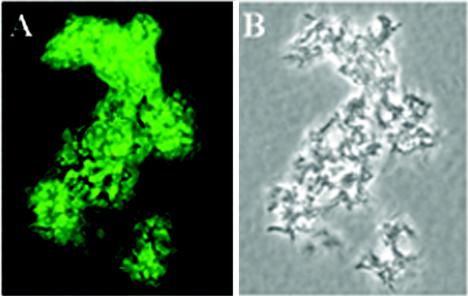
FIG. 5.
Recognition of cell surface antigen on the M. tuberculosis Erdman strain by IgG in sera from mice immunized with HPLC-purified HBHA. Paraformaldehyde-treated bacteria were incubated with pooled mouse sera at a dilution of 1:250, followed by fluorescein isothiocyanate-conjugated anti-mouse IgG. Uniform fluorescence was observed for most bacteria (A) found in a colony of M. tuberculosis visualized by phase-contrast microscopy (B). Fluorescence was visualized by using a Nikon Optiphot-2 microscope with a ×100 phase/fluorescent objective; images were photographed with a SPOT RT digital camera, and the composite was produced by using Adobe Photoshop. Magnification, ×1,000.
FIG. 6.
Effect of passive immunization with anti-HBHA monoclonal antibodies on the infection of mouse tissues with M. tuberculosis following aerosol challenge. C57BL/6 mice (five mice in each group) were inoculated intravenously with 250 μg each of IgG purified from MAb D2 and MAb E4 ascites (open bars) or with 250 μg each of IgG (isotype control) purified from MAb BP-G10 and MAb HB-65 ascites (striped bars) ∼24 h prior to aerosol challenge with 100 CFU of the M. tuberculosis Erdman strain. The bacterial burdens in lungs, spleens, and livers were determined by plating 28 days following challenge and compared with the burdens in mouse tissues that received no MAbs (solid bars). Statistical analyses were performed as described in Materials and Methods.
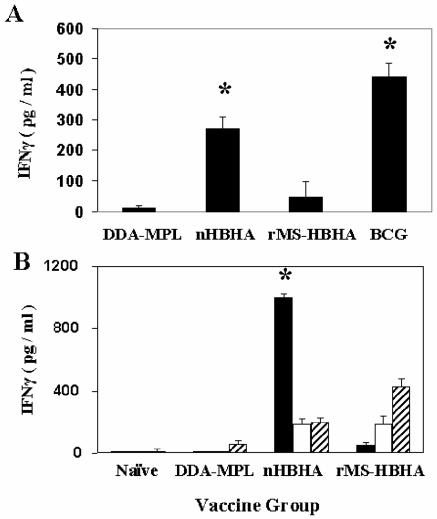
This lack of protection by passive immunization with anti-HBHA antibodies implies that cell-mediated immune responses to HBHA may be responsible for the effective immunity observed in the aerosol challenge studies. To determine if relevant cellular immune responses occur after vaccination with HBHA, splenocytes from immunized mice were incubated with murine BMMΦ infected with M. bovis BCG, and the release of IFN-γ by activated cells was measured. As shown in Fig. 7A, there was a ∼10-fold increase in the amount of IFN-γ released in this ex vivo assay by spleen cells from HBHA-immunized mice compared to cells from naïve mice (data not shown) or mice immunized with only adjuvant. The amount of IFN-γ elicited by splenocytes from BCG-immunized mice is shown in Fig. 7 for comparison. In addition, lymphocytes isolated from draining lymph nodes were restimulated by BMMΦ pulsed with purified HBHA proteins, and the resultant IFN-γ was measured. Lymph node-derived lymphocytes from HBHA-vaccinated mice released approximately eightfold more IFN-γ in the presence of HBHA-stimulated macrophages than cells from naïve or adjuvant control mice released (Fig. 7B). These results demonstrated that cell-mediated immune responses were elicited by the HBHA vaccine, which could help mediate protection against M. tuberculosis infection in the aerosol mouse model.
FIG. 7.
Comparison of the IFN-γ responses generated by immunization of mice with HBHA vaccines. (A) Splenocytes from mice immunized three times with the DDA-MPL adjuvant (DDA-MPL), with heparin-purified HBHA (nHBHA), or with recombinant His-tagged HBHA purified from M. smegmatis (rMS-HBHA) were incubated with murine BMMΦ infected with M. bovis BCG at an MOI of 3:1. Supernatants were collected after 72 h, and the IFN-γ concentration was determined by using a cytokine enzyme-linked immunosorbent assay as described in Materials and Methods. (B) Lymphocytes from periaortic and inguinal lymph nodes from mice immunized as described above for panel A were incubated with murine BMMΦ that were primed with heparin-purified HBHA (solid bars), with recombinant His-tagged HBHA purified from M. smegmatis (open bars), or with recombinant His-tagged HBHA purified from E. coli (striped bars). The IFN-γ concentration was measured as described above. The data represent the pooled responses from five mice, and the error bars indicate the standard deviations of the means. Asterisks indicate the vaccine groups that are statistically different from the adjuvant control.
Although these data indicate that HBHA is a promising new vaccine candidate, the procedure for purification of HBHA from M. tuberculosis or M. bovis BCG is time-consuming, particularly because of the slow growth of the organism, and it is difficult to obtain completely pure preparations of HBHA from mycobacteria. Since HBHA does not produce a level of protection that is equivalent to that produced by the M. bovis BCG vaccine, HBHA may need to be delivered in combination with other antigens for an optimal vaccine. Recent studies demonstrated that a recombinant His-tagged HBHA expressed in E. coli does not effectively protect mice from M. tuberculosis challenge compared with native HBHA purified from M. bovis BCG (22). This finding appeared to be correlated with a lack of methylation of lysine residues on HBHA expressed in E. coli. For these reasons, it would be useful to construct a recombinant HBHA that could be easily purified and properly methylated.
To pursue this goal, several constructs expressing recombinant HBHA in M. smegmatis were developed (6). One of these constructs expressed a recombinant histidine-tagged HBHA protein under the control of the hbhA promoter region of M. tuberculosis that was recognized by MAb D2, which reacted with methylated HBHA (Fig. 2B, lane 3). This M. smegmatis recombinant strain was used to purify methylated HBHA by a rapid and simple procedure. However, as shown in Fig. 3C, immunization of mice with the recombinant His-tagged HBHA purified from M. smegmatis (rMS-HBHA) emulsified in adjuvant provided no protection in the lungs or spleens (data not shown) of M. tuberculosis aerosol-challenged mice. This result is similar to what was observed with recombinant His-tagged HBHA purified from E. coli (Fig. 3C) (22). A bogus heparin-Sepharose preparation purified from a deletion mutant of M. bovis BCG lacking HBHA was used as an immunogen as described above and did not protect mice against challenge. This finding supports the hypothesis that protective immunity was elicited by methylated HBHA and was not due to a contaminant in the native HBHA preparation. The results of a histological examination of lung tissue from rMS-HBHA-immunized mice challenged with M. tuberculosis were similar to the results obtained for the adjuvant control and showed that there were numerous areas of lung consolidation and inflammation (Fig. 4C). Similar to vaccination with native preparations of HBHA, immunization of mice with rMS-HBHA elicited antibodies directed against the 28-kDa band of HBHA, as observed by Western blotting (data not shown). However, as shown in Fig. 7A, there was only a modest increase in the splenic IFN-γ response compared with the response seen in the adjuvant control, when M. bovis BCG-infected BMMΦ were used for antigen presentation. This response was ∼fourfold less than the IFN-γ response observed in splenocytes from mice immunized with native HBHA. Also, as shown in Fig. 7B, purified rMS-HBHA elicited a poor IFN-γ response when it was used to pulse BMMΦ prior to incubation with lymph node cells from mice immunized with either native HBHA or rMS-HBHA. The vaccine preparation containing rMS-HBHA gave results similar to those observed with the ineffective recombinant His-tagged HBHA expressed in E. coli (rEC-HBHA) (Fig. 7B) (22).
DISCUSSION
In this report, we provide evidence that HBHA, which has been shown to have a role in M. tuberculosis infectivity and extrapulmonary dissemination, can elicit an effective immune response that reduces disease in the mouse aerosol challenge model of tuberculosis. Both HBHA purified by heparin affinity chromatography and HBHA purified by heparin affinity chromatography followed by high-performance liquid chromatography offered protection against challenge with virulent M. tuberculosis. Effective immunity was elicited in the presence of the DDA-MPL combined adjuvant, which, by itself, had no protective effect in the challenge model of tuberculosis. To date, comparisons with other adjuvants have not been pursued. Both humoral and cell-mediated immune responses were observed following immunization with HBHA vaccine. Comparison of bacterial colonization of tissues, histopathological examination of lungs, and cytokine analysis in which purified HBHA was used to prime antigen-presenting cells indicated that HBHA elicits an effective immune response. The ex vivo cytokine assay in which murine BMMΦ were used as presenting cells and release of IFN-γ was measured by splenocytes or lymph node-derived lymphocytes from immunized animals indicated that native HBHA vaccines elicit T cells that recognize HBHA epitopes that are presented by live mycobacteria and by purified HBHA antigen. Further studies on T-cell-mediated immunity to HBHA and its role in protection will require identification of T-cell epitopes and construction of properly methylated peptides that mimic the presentation of native HBHA. This may not be trivial, as demonstrated by the differences in immunity elicited by recombinant forms of HBHA and native HBHA which had been properly modified by posttranslational events (17).
In the studies reported here, immunization of mice with HBHA vaccine induced antibodies to HBHA that recognized the 28-kDa protein on Western blots, as well as antigen on the surface of M. tuberculosis. These findings and previous work which demonstrated that mycobacteria coated with anti-HBHA antibodies prior to intranasal infection can reduce extrapulmonary dissemination of the bacteria in the mouse suggest that antibodies directed against HBHA may have a role in protection (16). To investigate this possibility, IgG was purified from two hybridomas (D2 and E4) (19) that react with the C-terminal lysine-rich repeat domain of HBHA and was used to passively immunize mice prior to aerosol challenge with the M. tuberculosis Erdman strain. No protection was observed; however, it is possible that the monoclonal antibodies used here are not sufficient for protection since there is no evidence that they are bactericidal or are found in the lung. Further studies could be pursued by using polyclonal anti-HBHA sera or by focusing on mucosal immunity and the role of IgA (8).
The purified preparations of HBHA contained minor contaminants, some of which elicited a humoral immune response, as observed by Western blotting with immune mouse sera. Since it is difficult to purify HBHA to complete homogeneity from mycobacterial preparations and since bacterial culture and purification of substantial quantities of pure HBHA can take several weeks, construction of recombinant HBHA vaccines was pursued. Other studies have shown that rEC-HBHA purified by nickel chromatography does not elicit protective immunity in tuberculosis challenge studies with mice (22). This finding has been correlated with the finding that the lysine residues present in the C-terminal repeat region of HBHA are not methylated in rEC-HBHA, likely due to the lack of the proper methyl transferases (17). For similar reasons, nucleic acid-based HBHA vaccines have proven to be unsuccessful in eliciting a protective immune response in mice (10). In an attempt to obtain an appropriately methylated HBHA, recombinant M. smegmatis strains expressing HBHA were developed (6). The recombinant M. smegmatis strain chosen for study expresses an HBHA (rMS-HBHSA) that is recognized by MAb D2, which reacts with methylated forms of HBHA (4, 17) and can be easily purified by using a fused histidine tag.
Although rMS-HBHA elicited specific antibodies and some HBHA-specific IFN-γ, it did not protect animals in the murine aerosol challenge model of tuberculosis. This could have been due to differences in the T-cell response, and studies are under way to measure specific CD4, CD8, and cytolytic responses induced by immunization with various HBHAs. Differences in the mono- and dimethylation of lysine residues in rMS-HBHA could account for the lack of an effective immune response following aerosol challenge, and methylation of these residues may contribute to the presentation and/or recognition of T-cell epitopes on HBHA. Similar negative results were obtained when BALB/c mice were used in the aerosol model (data not shown), suggesting that production of effective immunity to HBHA is not restricted to a particular mouse strain. An additional recombinant M. smegmatis strain that expresses HBHA via an mpt-64 promoter and lacks a His tag was purified by heparin affinity chromatography and high-performance liquid chromatography. This antigen combined with the DDA-MPL adjuvant also failed to elicit effective immunity in the challenge studies. The studies with M. smegmatis suggest that this mycobacterium may not contain the methyl transferases required for proper methylation of HBHA. A previous study (17) demonstrated that methylation of a recombinant HBHA by M. smegmatis is similar but not identical to methylation of HBHA obtained from M. tuberculosis or M. bovis BCG. Since BCG contains methyl transferases that can properly modify HBHA, as demonstrated by the purification of immunoprotective native HBHA from M. bovis BCG Pasteur, we are pursuing construction of an HBHA recombinant in M. bovis BCG that can be easily purified for further testing in the tuberculosis challenge model.
Some effective vaccines, such as acellular pertussis vaccines, contain antigens that function as bacterial adhesins and contribute to protective immunity (15). Similarly, HBHA in combination with other tuberculosis vaccine candidates (2) may provide synergistic immunity. To pursue this goal, it is likely that an effective recombinant form of HBHA needs to be constructed, and that is the objective of current investigations. Data presented here indicate that further evaluation of HBHA as a candidate tuberculosis vaccine is warranted.
Acknowledgments
We thank Steve Derrick, Amy Li, and Karen Elkins of the Center for Biologics Evaluation and Research, Food and Drug Administration, for advice and assistance with animal experiments; Julie Misplon of the Center for Biologics Evaluation and Research, Food and Drug Administration, for the gift of MAb HB-65; Bob Boykins of the Center for Biologics Evaluation and Research, Food and Drug Administration, for assistance with the purification of HBHA; and Siobhan Cowley of the Center for Biologics Evaluation and Research, Food and Drug Administration, for a critical review of the manuscript. We are grateful to Aharona Glatmann-Freedman of Albert Einstein College of Medicine for providing advice on passive antibody studies of tuberculosis.
This work was supported in part by grants from the National Vaccine Program Office (to M.J.B.) and from the Aeras Global TB Vaccine Foundation (to G.D.).
Editor: J. T. Barbieri
REFERENCES
- 1.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, M. J. 2004. A new generation of tuberculosis vaccines, p. 177-182. In C. A. de Quadros (ed.), Vaccines—preventing disease & protecting health. Pan American Health Organization, Washington, D.C.
- 3.Collins, F. M. 1985. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle 66:267-276. [DOI] [PubMed] [Google Scholar]
- 4.Delogu, G., and M. J. Brennan. 1999. Functional domains present in the mycobacterial hemagglutinin, HBHA. J. Bacteriol. 181:7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delogu, G., and M. J. Brennan. 2001. Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis. Infect. Immun. 69:5606-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delogu, G., A. Bua, C. Pusceddu, M. Parra, G. Fadda, M. Brennan, and S. Zanetti. 2004. Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis. FEMS Microbiol. Lett. 239:33-39. [DOI] [PubMed] [Google Scholar]
- 7.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatman-Freedman, A., and A. Casadevall. 1998. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin. Microbiol. Rev. 11:514-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 10.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masungi, C., S. Temmerman, J. P. Van Vooren, A. Drowart, K. Pethe, F. D. Menozzi, C. Locht, and F. Mascart. 2002. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 185:513-520. [DOI] [PubMed] [Google Scholar]
- 12.Menozzi, F. D., R. Bischoff, E. Fort, M. J. Brennan, and C. Locht. 1998. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc. Natl. Acad. Sci. USA 95:12625-12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menozzi, F. D., J. H. Rouse, M. Alavi, M. Laude-Sharp, J. Muller, R. Bischoff, M. J. Brennan, and C. Locht. 1996. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 184:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller-Ortiz, S. L., A. R. Wanger, and S. J. Norris. 2001. Mycobacterial protein HbhA binds human complement component C3. Infect. Immun. 69:7501-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olin, P., H. O. Hallander, L. Gustafsson, E. Reizenstein, and J. Storsaeter. 2001. How to make sense of pertussis immunogenicity data. Clin. Infect. Dis. 33:S288-S291. [DOI] [PubMed] [Google Scholar]
- 16.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of Mycobacterium tuberculosis is required for extrapulmonary dissemination. Nature 412:190-194. [DOI] [PubMed] [Google Scholar]
- 17.Pethe, K., P. Bifani, H. Drobecq, C. Sergheraert, A. S. Debrie, C. Locht, and F. D. Menozzi. 2002. Mycobacterial heparin-binding hemagglutinin and laminin-binding protein share antigenic methyllysines that confer resistance to proteolysis. Proc. Natl. Acad. Sci. USA 99:10759-10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pethe, K., V. Puech, M. Daffe, C. Josenhans, H. Drobecq, C. Locht, and F. D. Menozzi. 2001. Mycobacterium smegmatis laminin-binding glycoprotein shares epitopes with Mycobacterium tuberculosis heparin-binding haemagglutinin. Mol. Microbiol. 39:89-99. [DOI] [PubMed] [Google Scholar]
- 19.Rouse, D. A., S. L. Morris, A. B. Karpas, J. C. Mackall, P. G. Probst, and S. D. Chaparas. 1991. Immunological characterization of recombinant antigens isolated from a Mycobacterium avium lambda gt11 expression library by using monoclonal antibody probes. Infect. Immun. 59:2595-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimoji, Y., V. Ng, K. Matsumura, V. A. Fischetti, I. Ramshaw, and A. Rambukkana. 1999. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sci. USA 96:9857-9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 22.Temmerman, S., K. Pethe, M. Parra, S. Alonso, C. Rouanet, T. Pickett, A. Drowart, A.-S. Debrie, G. Delogu, F. D. Menozzi, C. Sergheeraert, M. J. Brennan, F. Mascart, and C. Locht. 2004. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding haemagglutinin. Nat. Med. 10:935-941. [DOI] [PubMed] [Google Scholar]
- 23.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal Pessolani, M. C., M. A. Marques, V. M. Reddy, C. Locht, and F. D. Menozzi. 2003. Systemic dissemination in tuberculosis and leprosy: do mycobacterial adhesins play a role? Microbes Infect. 5:677-684. [DOI] [PubMed] [Google Scholar]
- 25.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinrich Olsen, A., L. A. van Pinxteren, O. L. Meng, R. P. Birk, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]