Supplemental Digital Content is available in the text
Abstract
The strategy for treating small borderline malignant pancreatic neoplasms—such as neuroendocrine tumor (NET) and solid pseudopapillary neoplasm (SPN)—is surgical resection. However, pancreatic resection of these lesions still causes significant morbidity. We evaluated the safety and efficacy of EUS-guided ethanol ablation to treat small solid pancreatic neoplasms.
A total of 8 patients with small borderline malignant pancreatic neoplasms and co-morbidities who refused surgery were included. We identified 2 cases of nonfunctioning NET, 3 cases of insulinomas, 1 case of gastrinoma, and 2 cases of SPN. EUS-guided ethanol ablation was performed, and treatment outcomes were assessed with clinical symptom, hormone assay, and imaging study.
The mean tumor diameter was 15 mm (range, 7–29 mm), and the median volume of injected ethanol was 2.8 mL (range, 1.2–10.5 mL). There was 1 severe acute pancreatitis after EUS-guided ethanol ablation with 20-gauge CPN needle. During follow-up (median 16.5 months), 6 patients achieved treatment success; however, 2 patients (1 nonfunctioning NET and 1 SPN) still had persistent tumors. The patient with persistent SPN underwent surgical resection and the histopathological results showed peripancreatic infiltration with perineural invasion. Among 6 patients who achieved initial treatment success, 1 patient experienced tumor recurrence within 15 months and underwent repeated EUS-guided ethanol ablation.
In conclusion, EUS-guided ethanol ablation therapy is a promising option for patients with small solid pancreatic neoplasm. Multiple sessions or surgical interventions may be required if there is a recurrent or persistent mass, and procedure-related adverse events must be carefully monitored.
INTRODUCTION
With the availability of advanced imaging modalities, an increasing number of small pancreatic solid neoplasms are now being detected.1 The natural history of small pancreatic solid neoplasms other than pancreatic cancer has been poorly studied and still remains uncertain.2 In the case of pancreatic neuroendocrine tumor (NET), the WHO classification identifies pancreatic NET as 3 different degrees of tumor malignancy based on Ki-67 index and mitotic count.3,4 Solid pseudopapillary neoplasms (SPN) are classified into SPN with borderline malignant potential and solid pseudopapillary carcinoma.5 It is still controversial whether tumor size reflects the malignancy potential of these pancreatic neoplasms.4,6 These tumors show heterogeneous clinical and biological behaviors regardless of tumor size, thus, the strategy for treating these small borderline malignant pancreatic neoplasms is a surgical resection.7 However, pancreatic resection of these lesions still causes significant morbidity. EUS-guided injection of cytotoxic agents into a cyst is considered safe, effective, and avoids the risks associated with surgical resection.8–10 Only a few attempts to use ethanol ablation to treat pancreatic solid tumors have been reported.11,12 In our present study, we evaluated the safety and efficacy of EUS-guided ethanol ablation to treat small solid pancreatic neoplasms, including NET and SPN.
MATERIALS AND METHODS
Patients with small borderline malignant pancreatic neoplasms and co-morbidities who refused surgery were included in our study cohort. Since EUS-guided ethanol ablation therapy has the risk of local recurrence and metastasis, we did not enroll the patients with overt carcinoma with peripancreatic invasion. Informed consent was obtained before the procedure. A total of 8 patients were enrolled from 3 different centers between May 2009 and October 2013. The median patient age was 55 years (range: 20–99 years), and 4 male patients were enrolled (Table 1). We identified 2 cases of nonfunctioning NET, 3 cases of insulinomas, 1 case of gastrinoma, and 2 cases of SPN. Functioning NET was diagnosed based on the histopathological results, elevated hormone levels, and the presence of associated symptoms. Nonfunctioning NET and SPN were diagnosed using the histopathological results. In general, SPN shows the presence of a heterogeneously enhanced solid and cystic mass.6 However, small SPNs <3 cm usually appear as purely solid tumor.13 Two cases of SPN included in this study were pure solid masses without cystic component.
TABLE 1.
Clinical Information and Treatment Results for the Study Cohort (n = 8)
EUS-guided ethanol ablation was performed using linear array EUS (GF-UCT140/240/260; Olympus Optical, Tokyo, Japan). Tumor size was measured as the longest diameter on real-time EUS imaging. In 1 patient, contrast-enhanced harmonic EUS (CEH-EUS) with SonoVue (Bracco, Inc., Milan, Italy) was performed to obtain additional information before and after ablation therapy. Puncture was then performed using a 22- or 19-gauge conventional needle (EchoTip; Cook Endoscopy, Winston-Salem, NC) or 20-gauge celiac plexus neurolysis (CPN) needle (EchoTip Ultra Celiac Plexus Neurolysis; Cook Endoscopy) with discretion of attending operator. After puncturing the lesion, 99% ethanol was injected as the needle was gently withdrawn from deep within the tumor. The injection was finished when hyperechoic blush was seen inside the whole tumor, and the needle was retrieved promptly without flushing out. The entire procedure was performed under complete EUS guidance (Supplemental digital content). Treatment outcomes were evaluated during the follow-up period using radiologic or EUS imaging, and hormone-related symptoms were monitored. Treatment success was defined as complete ablation of the tumor on imaging or the absence of hormone-related symptoms. The present study was approved by Institutional Review Board of Asan Medical Center, Ulsan University College of Medicine (IRB No. 2013-0399).
RESULTS
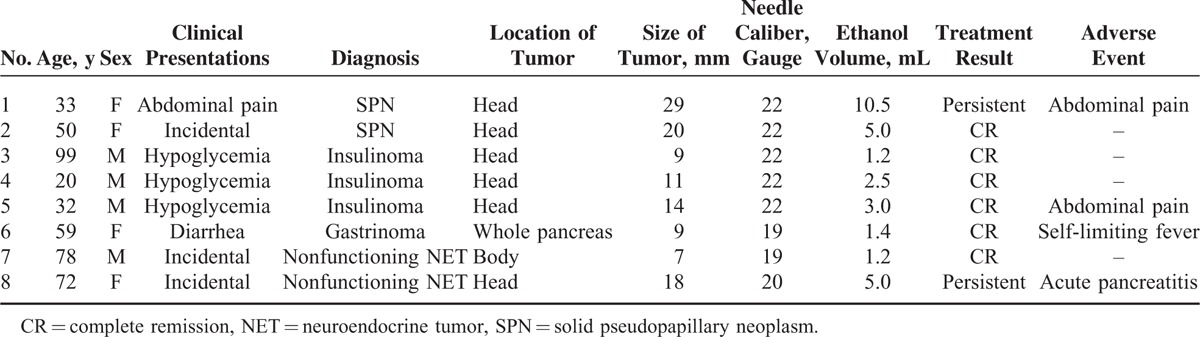
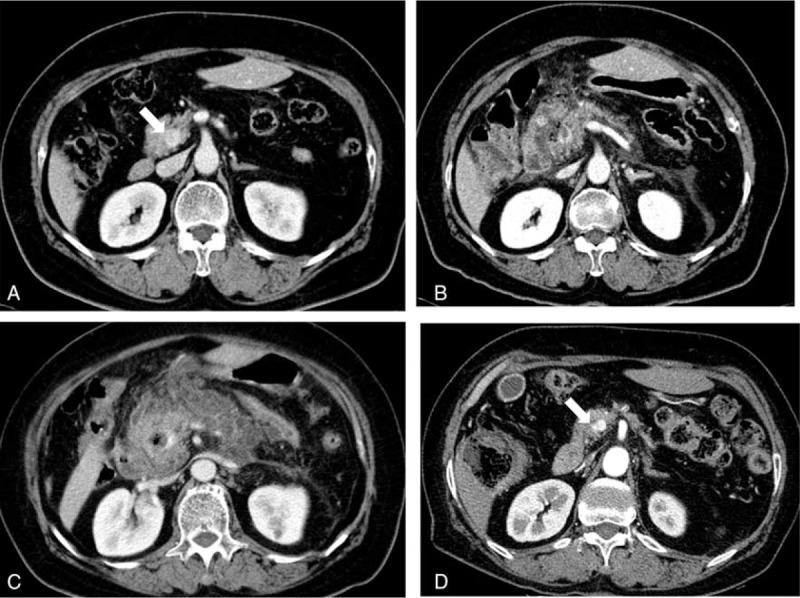
The mean tumor diameter in our study cohort was 15 mm (range, 7–29 mm), and the median volume of injected ethanol was 2.8 mL (range, 1.2–10.5 mL). Three mild adverse events were reported after the procedure: 2 abdominal pains and 1 self-limiting fever. These symptoms were improved promptly. Severe acute pancreatitis developed in 1 patient who received EUS-guided ethanol ablation using a 20-gauge CPN needle (patient no. 8 in Table 1). Serum amylase and lipase levels were elevated to 2027 and 1783 U/L, respectively, at 1 day after treatment. This patient was conservatively managed and hospitalized for 36 days (Figure 1). The median follow-up period was 16.5 months (range, 5.4–55.3 months). After treatment, 6 patients achieved treatment success; however, 2 patients still had persistent tumors. Four patients with functioning NETs reported complete relief from tumor-related symptoms. The patient with insulinoma (no. 3 in Table 1) demonstrated early homogenous enhancement of the tumor on CEH-EUS before ethanol ablation therapy (Figure 2A). After treatment, no enhancement was noted inside the tumor on CEH-EUS (Figure 2B).
FIGURE 1.
(A) Computed tomography (CT) scan showing an 18-mm strong enhancing mass in the head of the pancreas (white arrow). (B) Acute pancreatitis developed 1 d after ethanol ablation therapy. Complicated loculated fluid and air are present in the head of the pancreas, adjacent to the enhancing mass. (C) CT scan taken 10 d after treatment showing aggravated peripancreatic infiltration. (D) CT scan taken 1 y after treatment showing that the peripancreatic infiltration had nearly disappeared. The enhancing mass still persisted in the head of the pancreas, although the volume of the mass had decreased (white arrow).
FIGURE 2.
(A) Early homogenous enhancing insulinoma on contrast-enhanced harmonic EUS (CEH-EUS) before ethanol ablation therapy. (B) After treatment, no enhancement inside the tumor could be observed on CEH-EUS.
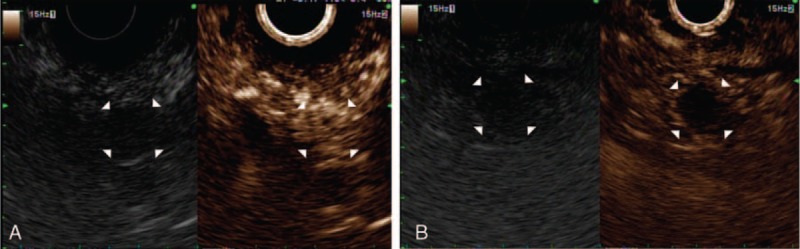
One of the patients with persistent hypoglycemic symptoms (no. 5 in Table 1), who was initially diagnosed with insulinoma in the uncinate process of the pancreas on EUS imaging, underwent surgical resection. However, intra-operatively, the uncinate process lesion could not be found. The resected specimen was not tumorous. During the postoperative stay, peripancreatic fluid collected and the patient was conservatively managed. Hypoglycemic attacks persisted and resulted in progressive bilateral lower limb weakness that eventually led to paraplegia. Three months later, he was referred to our institution, and a well-defined hypoechoic lesion in the head of pancreas was detected using EUS (Figure 3). EUS-guided ethanol ablation was performed twice at an interval of 1 month. After the procedure, there has been significant improvement in general condition, and the patient's paraplegia has also improved. There have been no episodes of hypoglycemia in last 6 months after the treatment.
FIGURE 3.
A 32-y-old male patient with hypoglycemia was diagnosed with insulinoma. A well-defined hypoechoic lesion in the head of pancreas is shown on EUS.
The nonfunctioning NET tumor was almost completely ablated in patient no. 7 (Table 1). However, the tumor recurred in this patient within 15 months and required repeated EUS-guided ethanol ablations. In another patient with nonfunctioning NET (no. 8 in Table 1), the tumor was persistent and received follow-up examination without further interventions. In 2 patients with SPN, the tumor was almost completely ablated in 1 patient (no. 2 in Table 1; Figure 4), but the other patient demonstrated persistent SPN (no. 1 in Table 1). This patient underwent surgical resection 1 year later and the histopathological results showed peripancreatic infiltration with perineural invasion. The patient received follow-up examinations for 3 years without recurrence.
FIGURE 4.
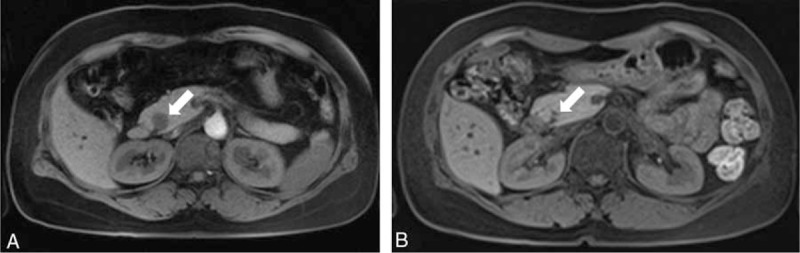
(A) Magnetic resonance imaging (MRI) showing a 20-mm well-demarcated solid mass in the head of the pancreas. The tumor was diagnosed as a solid pseudopapillary neoplasm on EUS-guided fine-needle biopsy. (B) The tumor had almost disappeared after ethanol ablation therapy.
DISCUSSION
EUS-guided ethanol ablation therapy achieved a treatment success rate of 75% in our current cohort. A recent study reported a complete surgical resection rate of 87% for small NET; however, 44% of those patients experienced adverse perioperative events.7 Despite advances in surgery, the perioperative morbidity of pancreatic resection is still high, even in large-volume centers. The severity and incidence of adverse events during EUS-guided ethanol ablation therapy may be less than surgery, but 1 notable procedure-related adverse event developed in present study. Previously, 4 case studies reported that EUS-guided ethanol ablation is a safe and effective treatment for insulinoma that does not demonstrate adverse events.11,12,14,15 Because severe adverse events can occur during EUS-guided ethanol ablation therapy, indiscriminate treatment should be avoided and the procedure must be carefully performed.
No guidelines for optimal alcohol dosage or type of injection needle have been established. Here, severe pancreatitis occurred due to peripancreatic ethanol leakage. A 20-gauge CPN needle was used, which has multiple side holes that allow the solution to be spread over a large area. Prior to treatment, we expected the CPN needle to enable the ethanol to be evenly spread throughout the lesion. During ethanol injection, a hyperechoic blush from the tip of needle was seen on EUS; however, ethanol escaping from the side holes could not be identified. The multiple side holes of the CPN needle resulted in ethanol leakage and pancreatitis. Injecting a smaller volume of ethanol than estimated for the tumor volume could have prevented procedure-related adverse events. Assuming that the tumor was spherical, the estimated tumor volume was approximately 3 mL in the patient who developed postprocedural pancreatitis. The total volume of injected ethanol was 5 mL, and extra ethanol could have spilled into the peripancreatic area. We thus recommend using small aliquots, careful intratumor injection using a single-hole needle, and accurate targeting in order to minimize postprocedural pancreatitis when using ethanol ablation.12
EUS is the most sensitive imaging technique for identifying small pancreatic masses, though sensitivity is operator-dependent.16 In 1 of our current patients with insulinoma, misguided EUS imaging led to poor localization, surgical failure, significant postoperative morbidity, and hypoglycemia-related paraplegia. Nonsurgical treatment is sometimes required when the patient presents with comorbidities, refuses surgery, or if tumor localization is difficult. In such clinical situations, ethanol ablation therapy should be initially considered prior to surgical resection. In functioning NETs, tumor location can be confirmed by symptom improvement following ethanol ablation therapy.
Evaluation of treatment outcome with imaging only seems to be inappropriate in nonfunctioning NET and SPN. The echodensity and echo patterns of tumors may change on conventional EUS after treatment.12 However, NET and SPN can be clearly delineated on CEH-EUS. The enhancement patterns of tumors change after ethanol ablation therapy.
The major concerns of local ablation therapy are recurrence and the risk of metastasis. Here, tumors remained persistent in 2 of our patients after ethanol ablation therapy. One patient with SPN underwent surgical resection. The histopathological results of the surgical specimen confirmed local invasion, and the patient received follow-up examinations for 3 years without recurrence. The other patient received follow-up examinations without further treatment due to old age, and the tumor demonstrated no significant changes for 4 years. Among 6 patients who achieved treatment success, 1 instance of recurrence occurred within 15 months after treatment. Since there were no distant metastases, the patient underwent EUS-guided ethanol ablation again. Repeated complementary ethanol ablation therapy or surgery could be applied to patients with recurrent or persistent tumors.
In conclusion, EUS-guided ethanol ablation therapy is a promising option for patients with small solid pancreatic neoplasm and could be used to complement surgery. To minimize procedure-related adverse events, we recommend using small aliquots and careful intratumor injection using a single-hole needle under accurate targeting. Multiple sessions or surgical interventions may be required if there is a recurrent or persistent mass, and procedure-related adverse events must be carefully monitored.
Supplementary Material
Footnotes
Abbreviations: CEH-EUS = contrast-enhanced harmonic EUS, CPN = celiac plexus neurolysis, CT = computed tomography, MRI = magnetic resonance imaging, NET = neuroendocrine tumor, SPN = solid pseudopapillary neoplasm.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol 2012; 19:2932–2936. [DOI] [PubMed] [Google Scholar]
- 2.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2013; 98:4784–4789. [DOI] [PubMed] [Google Scholar]
- 3.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006; 449:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik WH, Ryu JK, Song BJ, et al. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. J Korean Med Sci 2013; 28:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP 2006; 7:131–136. [PubMed] [Google Scholar]
- 6.Kim CW, Han DJ, Kim J, et al. Solid pseudopapillary tumor of the pancreas: can malignancy be predicted? Surgery 2011; 149:625–634. [DOI] [PubMed] [Google Scholar]
- 7.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011; 146:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011; 140:172–179. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WY, Li ZS, Jin ZD. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol 2013; 19:3397–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc 2008; 67:636–642. [DOI] [PubMed] [Google Scholar]
- 11.Jurgensen C, Schuppan D, Neser F, et al. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc 2006; 63:1059–1062. [DOI] [PubMed] [Google Scholar]
- 12.Levy MJ, Thompson GB, Topazian MD, et al. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc 2012; 75:200–206. [DOI] [PubMed] [Google Scholar]
- 13.Baek JH, Lee JM, Kim SH, et al. Small (< or =3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology 2010; 257:97–106. [DOI] [PubMed] [Google Scholar]
- 14.Muscatiello N, Salcuni A, Macarini L, et al. Treatment of a pancreatic endocrine tumor by ethanol injection guided by endoscopic ultrasound. Endoscopy 2008; 40 Suppl 2:E258–E259. [DOI] [PubMed] [Google Scholar]
- 15.Vleggaar FP, Bij de Vaate EA, Valk GD, et al. Endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma. Endoscopy 2011; 43 Suppl 2:E328–E329. [DOI] [PubMed] [Google Scholar]
- 16.Puli SR, Kalva N, Bechtold ML, et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: a systematic review and meta analysis. World J Gastroenterol 2013; 19:3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


