Abstract
The successful implementation of prevention programs for mother-to-child human immunodeficiency virus (HIV) transmission has dramatically reduced the prevalence of infants infected with HIV while increasing that of HIV-exposed uninfected (HEU) children. Neuropsychological assessments indicate that HEU children may exhibit differences in neurodevelopment compared to unexposed children (HUU). Pathological mechanisms leading to such neurodevelopmental delays are not clear. In this observational birth cohort study we explored the integrity of regional white matter microstructure in HEU infants, shortly after birth.
Microstructural changes in white matter associated with prenatal HIV exposure were evaluated in HEU infants (n = 15) and matched controls (n = 22) using diffusion tensor imaging and tract-based spatial statistics. Additionally, diffusion values were extracted and compared for white matter tracts of interest, and associations with clinical outcomes from the Dubowitz neonatal neurobehavioral tool were investigated.
Higher fractional anisotropy in the middle cerebellar peduncles of HEU compared to HUU neonates was found after correction for age and gender. Scores on the Dubowitz abnormal neurological signs subscale were positively correlated with FA (r = 0.58, P = 0.038) in the left uncinate fasciculus in HEU infants.
This is the first study to present data suggesting that prenatal HIV exposure without infection is associated with altered white matter microstructural integrity in the neonatal period. Longitudinal studies of HEU infants as their brains mature are necessary to understand further the significance of prenatal HIV and antiretroviral treatment exposure on white matter integrity and neurodevelopmental outcomes.
INTRODUCTION
The human immunodeficiency virus (HIV) remains one of the most severe global public health issues, with an estimated 35 million people living with HIV in 2013.1–3 Sub-Saharan Africa, especially southern Africa, remains the global epicenter of the HIV epidemic, accounting for 70% of the people living with HIV worldwide.3 The successful implementation of prevention of mother-to-child programs has dramatically reduced the rate of vertical transmission while increasing the number of HIV-exposed uninfected (HEU) children being born.4–6 Subtle deficits in cognition, motor function, language, and behavior have been observed among HEU children compared to HIV-unexposed uninfected (HUU) controls;7,8 however, there remains lack of consistency in outcomes of studies exploring the neurodevelopmental outcome of HEU children.9,10
Neuroimaging studies using quantitative magnetic resonance imaging techniques are contributing to the improved understanding of the underlying biology of the primary effects of HIV infection on the developing brain. White matter hyper-intensities, subtle white matter microstructural and neurochemical abnormalities, and reduced white matter volume have been reported in older children and adults with HIV infection.11–13 In addition, white matter microstructural alterations have been observed in prenatally infected adolescents in brain areas including the superior longitudinal fasciculus, cingulum, corpus callosum, external capsule, middle cerebral peduncles, and basal pons.11,14 However, the effect of prenatal HIV exposure on white matter microstructure utilizing diffusion tensor imaging (DTI) in HEU infants has not been previously documented.
To address these gaps in the understanding of the effects of prenatal HIV exposure on white matter microstructural integrity and neurobehavioral outcome of HEU infants, we conducted this study in South African neonates.15 Using a cohort from a peri-urban community in the Western Cape, the present study investigated whether the impact of prenatal HIV exposure on early white matter microstructural integrity may be discernible in neonates and whether associations with early neurobehavioral measures are present. Based on the literature in children and adults with HIV infection, we hypothesized that disruptions in white matter integrity would occur (even if more subtly) in similar areas including association and projection pathways and in the cerebellum and that these changes would be associated with infant neurobehavior.
METHODS
Study Population
This is a nested observational substudy of infants enrolled in a larger population-based birth cohort study, the Drakenstein Child Health Study (DCHS).16,17 The DCHS is located in the Drakenstein area in Paarl, a peri-urban area in the Western Cape province of South Africa. The local community of ∼200,000 people is of low socioeconomic status, live in informal housing or crowded conditions, and have high levels of unemployment. Infectious diseases including pneumonia, HIV, and tuberculosis are common. The area has a well-established free healthcare system, where ∼90% of women access public sector antenatal care and child health services.
Participants
The DCHS recruited >1000 pregnant women in their second trimester attending 2 primary health clinics serving different populations—TC Newman (serving a mixed race population) and Mbekweni (serving a black African population).16,17
We recruited pregnant women at 20 to 24 weeks gestation, obtained written informed consent, and collected background data as per the DCHS protocol.16,17 We confirmed the HIV status of mothers antenatally utilizing standard HIV testing algorithms and available laboratory tests.18 We confirmed the HIV status of the infants by testing postnatally using the qualitative polymerase chain reaction (PCR) technique. Infants who tested positive on the PCR testing had their HIV status confirmed using quantitative HIV viral load testing.
The study collected measures of potential confounding factors. The Alcohol, Smoking and Substance Screening Test (ASSIST) was used to assess maternal substance abuse.17–20 Following this initial screen, prenatal alcohol exposure was further defined by a history of moderate-severe alcohol use in any of the pregnancy trimesters. Maternal cigarette smoking status was further documented using the Fagerström Test for Nicotine Dependence.21
Objective measures of maternal substance use were also included.16,17 Maternal urine cotinine was measured antenatally and at the time of birth to detect and quantify current smoking status. In addition, maternal urine samples were tested antenatally with rapid urine dipstick testing for recent use of common illicit substances, including methamphetamines, cocaine, cannabis, methaqualone, and opiates.
Following birth, mother–child pairs identified through the HIV-testing approach were included for study unless mothers had a positive urine screen for illicit drugs of abuse (any group), the infants were premature (<36 weeks), or had low APGAR scores (<7 at 5 minutes), and/or history of neonatal ICU admission for hypoxic ischemic encephalopathy or other significant neonatal complications. Infants were also excluded from this study if they had an identified genetic syndrome or congenital abnormality. In this nested substudy, 39 infants were assessed: 15 HEU infants and 24 matched HUU controls. No infants who were identified in the antenatal visit for inclusion were lost by the time of scanning and none refused consent for scanning.
In this study we imaged 2 to 4-week-old infants during natural (ie unsedated) sleep. Earplugs and mini-muffs were used for ear protection, a pulse oximeter was used to monitor pulse and oxygenation, and a qualified neonatal nurse or pediatrician was present with the infant during the imaging session. At the time of scanning, basic infant anthropometry was acquired, including length, weight, and occipito-frontal head circumference. The Dubowitz neurobehavioral scale, a measure of neonatal neuromotor and neurobehavior status, was used to study early neurological and neurobehavioral changes and to identify potential associations with neuroimaging findings.22 The score is based on the distribution of the scores for each item in the population of low-risk term infants, and the optimality score is obtained by summating the optimality scores of individual items. Together, the examination can be used to detect abnormal neurological signs associated with specific patterns of lesions observed on brain imaging. For this study, the “behavior” cluster, which includes items scoring irritability, cry, consolability, alertness, visual and auditory orientation and eye movements, and the “abnormal signs” cluster, which focuses on posture, tremor, and startle items, were of particular interest.
The DCHS was approved by the Faculty of Health Sciences human research ethics committees of the University of Cape Town and Stellenbosch University in South Africa, as well as by the Western Cape Department of Health Provincial Research Committee. This substudy was independently reviewed and approved as HREC 525/2012. As above, mothers provided written informed consent for participation in the study.
DTI Acquisition
White matter microstructure can be characterized in vivo with diffusion tensor imaging (DTI), a noninvasive technique that utilizes the intrinsic directionality of water diffusion along fiber pathways to provide highly specific anatomical information.23 The most widely used index is fractional anisotropy (FA). This represents orientation-dependent variation in the diffusivity of water and reflects a number of microstructural properties such as degree of myelination, axon diameter, fiber coherence, and fiber tracking density.24,25 Other reported indices include mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). MD represents a measure of average diffusivity. Alterations in MD may indicate a decrease in cellular density, myelin degradation, or an increase in the extra- and/or intracellular volumes.24 Organized neural microstructure associated with improved cognition and behavior are typically associated with higher FA values and lower MD values. White matter microstructural pathology, however, is more generally associated with lower FA and higher MD values.23,24 AD represents diffusivity along the axonal structure, typically reflecting axonal membrane integrity and fiber coherence.26 AD may be higher when there is damage to the neurofilaments or axons.27,28 RD typically reflects average perpendicular diffusion and indicates degree of myelination; RD is generally higher with myelin damage or reduced myelination.28,29
Diffusion weighted images were acquired in the transverse plane on a Siemens Magnetom 3T Allegra MRI system using a spin-echo, echo-planar sequence along 45 noncollinear diffusion directions (b-values 0 s/mm2 and 1000 s/mm2, TR/TE = 7900/90 ms, slice thickness 1.6 mm, FOV 160 mm, voxel size 1.3 × 1.3 × 1.6 mm3, 2 averages in anterior-posterior and posterior-anterior orientation, scanning time 6.27 min per average).
Data Processing
Diffusion imaging techniques are highly sensitive to the motion of subjects during scan acquisition. As a result, acquiring diffusion imaging data in infants offers particular logistical and technical challenges. Initially, manual quality control of individual subject data was applied. Only subjects with a minimum of 12 acquisition volumes that were artifact-free were allowed through the data preprocessing step. Subsequently, FMRIB's Diffusion Toolbox and processing streams from Tract-Based Spatial Statistics (TBSS) were used to perform a whole-brain analysis.
Diffusion weighted images from individual subjects were registered to a corresponding b = 0 image. This step was performed in order to correct for distortions resulting from eddy currents as well as movement. Estimation of susceptibility-induced off-resonance field was performed using the FSL top-up tool. Subsequently, a single corrected image was created using the combination of the 2 images. The FSL Brain Extraction Tool was then used for the brain-extraction of images and following this the calculation of diffusion tensors was performed at each voxel. Values for each subject for FA, MD, AD, and RD were then obtained for between group analyses. Diffusion values by regions of interest (ROIs) were extracted, exported, and compared by group using standard SPSS statistical packages as below. Statistical analyses controlled for gender and postnatal age at the time of the scan. Age (in days) was considered particularly critical due to the rapid pace at which white matter maturation evolves in early neonatal life.30
Whole-Brain Tract-Based Spatial Statistics
The standard pipeline for TBSS analysis was applied for statistical analysis. In this study, the FMRIB FA template for adults, provided by FSL was not considered appropriate for neonatal DTI analysis. Thus, each subject was registered to a representative target that was preselected from the control cohort. The subject with the lowest mean warp coefficient from the control cohort was chosen as the target image.
Each FA image was aligned into a standard space and upsampled to 1 × 1 × 1 mm3 voxel size. Next, the average FA image was created and thinned to create a skeletonized mean FA image, which represents the center of all white matter tracts common to the study group. An FA value of 0.2 was used as a threshold for the skeleton. This study was explorative and so we applied a more stringent threshold compared to that of some previous studies of this age group that used a threshold FA value of 0.15. Subsequently, we projected diffusion data onto this skeleton for the statistical analysis.
The FSL's Randomize tool was used to assess voxelwise differences in DTI metrics among the study groups. Specifically, between group variations were investigated with unpaired t tests and correlational analyses, and statistical analyses were corrected for multiple comparisons with threshold-free cluster enhancement. We considered results with a P value of <0.05 as statistically significant.
Data Analysis by Regions of Interest
Following FSL preprocessing, we examined group main effects using extracted data on different diffusion indices by ROI. We applied affine-registration to each brain and a standard FMRIB58_FA template. The white-matter atlas from Johns Hopkins University was used for the extraction of MD values for each subject for the ROIs. ROIs included associative bundles, commissural bundles, projection bundles, and large white matter tracts of the brainstem and cerebellum. Generalized linear models were used to evaluate group differences in diffusion parameters, with neonatal postnatal age (days) at scan time as well as gender as covariates. Results were Bonferroni corrected.
RESULTS
Demographic Characteristics and Anthropometric Measurements
The sample for the present analysis included 15 HEU infants and 24 matched HUU controls (Table 1). The mean (SD, range) age of all infants at scanning was 21.1 (5.83, 11–34) days and 46% were women. There were no significant differences in the mean values for infant age at scanning, gestational age at birth, weight, length, and head circumference for HEU infants compared to HUU infants. Although maternal smoking and alcohol misuse in pregnancy were present in this cohort, there were no significant differences in these prenatal exposures between HEU and HUU groups (Table 1). All HIV-infected mothers were on triple therapy before delivery and had CD4 T lymphocyte counts between 200 and 800 cells/mm3. Undetectable or low levels of viral loads were observed in 80% of HIV-infected mothers, with 2 mothers having viral loads >50,000 copies/mL.
TABLE 1.
Demographic, Anthropometric, and Dubowitz Data of Exposed and Unexposed Infants

Whole-Brain Group Comparisons of Diffusion Parameters
There were no group differences that reached significance in any diffusion parameters on whole-brain analysis. Infant age at scanning was positively correlated with FA and negatively correlated with MD and RD across groups in several white matter tracts, including the internal and external capsule, and the anterior corona radiata.
Comparisons by Regions of Interest
General linear models by ROIs showed significant difference in diffusion parameters by group in major white matter fiber tracts that interconnect cerebellar and brainstem regions. There was significantly higher FA in the middle cerebellar peduncles of HEU infants compared to HUU [F (3,38) = 6.15, P = 0.002] after correction for age and gender (Table 2, Figures 1 and 2).
TABLE 2.
Results of Group Differences in Diffusion Parameters by Regions of Interest. Fractional Anisotropy was Significantly Higher in the Middle Cerebellar Peduncles of Exposed Infants Compared to Controls

FIGURE 1.

White matter tracts superimposed on a 3D brain template: analysis by regions of interest. Diffusion anisotropy was higher in the middle cerebellar peduncles (blue) of HIV-exposed uninfected infants compared to controls as seen in sagittal (A), coronal (B), and axial (C) views. HIV = human immunodeficiency virus.
FIGURE 2.

Variability of FA values by the group in the middle cerebellar peduncle. FA = fractional anisotropy.
Neurobehavioral Measures and Diffusion Parameters
There was a significant difference between the scores on the abnormal neurological signs subscale of the Dubowitz for HEU infants compared to HUU neonates.
Scores on the Dubowitz abnormal neurological signs subscale were positively correlated with FA (r = 0.58, P = 0.038) in the left uncinate fasciculus; negatively correlated with MD (r = −0.58, P = 0.048) and AD (r = −0.61, P = 0.026) in the right inferior cerebellar peduncle; negatively correlated with MD (r = −0.65, P = 0.017) and AD (r = −0.75, P = 0.003) in the left hippocampal cingulum; and negatively correlated with MD (r = −0.66, P = 0.014) in the right hippocampal cingulum of HEU infants (Figure 3).
FIGURE 3.
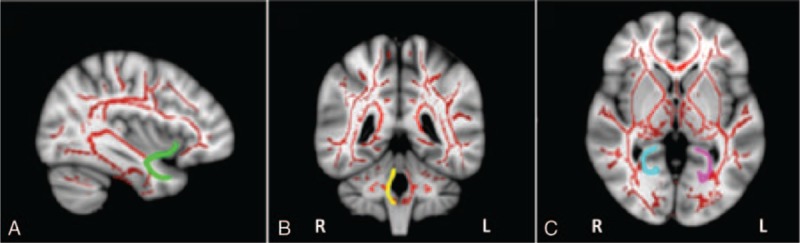
White matter tracts superimposed on a 3D brain template: tracts associated with neurobehavior. Scores on the Dubowitz abnormal neurological signs subscale was positively correlated with FA in the left uncinate fasciculus (green), negatively correlated with MD and AD in the right inferior cerebellar peduncle (yellow), negatively correlated with MD and AD in the left hippocampal cingulum (pink), and negatively correlated with MD in the right hippocampal cingulum (blue) of HIV-exposed uninfected infants, as seen in the sagittal (A), coronal (B), and axial (C) views. AD = axial diffusivity, FA = fractional anisotropy, HIV = human immunodeficiency virus, MD = mean diffusivity.
DISCUSSION
To the best of our knowledge, this is the first time that DTI has been used to investigate white matter microstructural integrity in neonates exposed to HIV, but uninfected at birth. Our study found significantly higher FA in the middle cerebellar peduncles of HEU infants compared to unexposed neonates. In addition, correlational analyses of diffusion parameters with the Dubowitz neurobehavior scores of HEU infants showed abnormal neurological signs cluster was positively correlated with FA in the left uncinate fasciculus. These preliminary results are consistent with and expand on previous studies that found subtle neurodevelopmental effects of prenatal HIV exposure in older children.7,8
Diffuse white matter alterations have been documented in cerebellar pathways of HIV-infected adults and older, specifically in the middle cerebellar peduncles.31,32 These pathways have been implicated in a variety of intellectual and neuropsychological deficits, which are most pronounced in visuospatial, language and memory functions.33 We described higher FA in the middle cerebellar peduncles neonates exposed to prenatal HIV, which suggests that the developing cerebellum may be particularly sensitive to the effects of HIV exposure. Although an elevated FA may indicate increased white matter connectivity,24,25 it has also been postulated as possibly representing microscopic deficits in axonal structures or reductions in axonal diameter, density of axonal packing, and branching by some authors.34,35
Correlational analyses of diffusion parameters with the Dubowitz neurobehavior scores of HEU infants showed associations between the abnormal neurological signs cluster with DTI metrics in several white matter tracts including the uncinate fasciculus, inferior cerebellar peduncles, and hippocampal cingulum. Functions of these networks are believed to include cognition, higher level motor tasks, memory, learning, attention, language, and emotional processing.36–39 These are areas that have been identified as being affected in children with prenatal HIV exposure.7,8 A decrease in AD is usually indicative of axonal damage and might suggest thinner axons due to reduced axonal caliber, less well-ordered axons as a consequence of misguided cell migration during development, or a lower number of axons.28,40,41 In addition, a few studies have documented decreased AD with dysmyelination or demyelination.28,42 Increased MD is normally observed in conditions of reduced membrane density associated with increased volume of extracellular space or with decreased barriers to diffusion in white matter, whereas reduced MD may reflect increased neuronal activity and connectivity.43 Although the clinical significance of the negative correlation between MD and abnormal neurological signs observed in our HEU infants is unknown, we hypothesize that it may relate to abnormal cell proliferation or aberrant pruning. Longitudinal studies of these infants by following up their developmental and behavioral outcomes as they mature may further define the functional or real-world significance of the associations between the abnormal neurological signs and DTI metrics in these tracts.
Infant postconception age at scanning was positively correlated with FA and negatively correlated with MD and RD across both groups in several white matter tracts, including the internal and external capsule, and the anterior corona radiata. These findings are consistent with previous research, which showed a basic pattern of the maturation process in pediatric DTI: an increase in FA and a decrease in MD as a function of age.44 This suggests that the expected increase in FA and decrease in MD should fail to appear in HEU infants in later years if white matter injury is present. A longitudinal follow-up of these infants as their brains mature will help to further understand the significance of prenatal HIV exposure on the development of these white matter tracts.
Although the precise mechanisms underlying potential neurodevelopmental delays in HEU children are not clear, the role of prenatal HIV exposure in neurodevelopment may potentially be understood in terms of the interactions between the immune and central nervous systems. HEU infants have been reported as having up-regulation of pro-inflammatory cytokines at birth when compared to unexposed controls.45 The late prenatal and postnatal period is known to be a time when maximal brain growth as well as processes critical to effective CNS maturation occur. These processes include the maturation and elongation of both axons and dendritic trees and the sensitive migration of neuronal cells to their correct position in the layers of the cortex and may be particularly vulnerable to exposure to higher levels of proinflammatory cytokines and immune activation in general.46,47
A number of limitations to this study should be mentioned. First, our findings were based on a small sample size and so false negative findings cannot be excluded. Other demographic variables that were not controlled for may have influenced results and should be considered in larger samples. In addition, our study used a cross-sectional design and therefore causal inferences remain preliminary. Interpreting how neurobiological and clinical neurodevelopmental differences develop over time will necessitate robust longitudinal follow up and data.
Despite these limitations, we believe there are also some key strengths to our dataset documented here. These include the fact that these neonates were matched for age and gender as well as maternal alcohol use and cigarette smoking during pregnancy. This is the first study to report neurobiological changes in infants prenatally exposed to HIV, but who are uninfected. Cerebral changes are especially intense during the last trimester of gestation and the first several postnatal months30 and structural abnormalities of the brain that manifest at this time may result in motor and cognitive deficits in later years. Imaging during early postnatal weeks may more accurately reflect the specific effects of antenatal HIV exposure on white matter microstructural integrity before postnatal risk factors known to have high prevalence in these high-risk communities additionally negatively affecting brain development.
In conclusion, the results documented in this study suggest that the effects of prenatal HIV exposure can be observed at a neurobiological level in neonates, with altered white matter microstructural integrity of major white matter tracts. The findings are consistent with those reported previously where changes in white matter microstructure in adults and older children with HIV infection were documented. Locating the altered white matter microstructural integrity in these brain regions so early in life may provide better understanding of the underlying mechanisms of neurodevelopmental delays observed in HEU children. Future studies with larger cohorts are more likely to be able to detect specific windows of vulnerability to prenatal HIV exposure on the developing brain. Furthermore, to evaluate the validity of these reported changes, longitudinal studies are needed to investigate the changes in diffusion metrics as these HEU infants age and their brains mature.
Acknowledgments
The authors thank the staff at Paarl Hospital, Mbekweni and TC Newman clinics for their support of the study; and the families and infants who participated in this study. They would like to acknowledge the contributions of Thorkil Plough and Fleur Howells to this project. They would also like to recognize Dr. Katya Mauff, a senior statistician in the UCT department of statistical science, for her involvement in numerous aspects of the statistical analysis.
Footnotes
Abbreviations: DCHS = Drakenstein Child Health Study, DTI = diffusion tensor imaging, FA = fractional anisotropy, HEU = HIV-exposed uninfected, HIV = human immunodeficiency virus, HUU = HIV-unexposed uninfected, M-/A-/RD = mean/axial/radial diffusivity, TBSS = tract-based spatial statistics.
Funding: this study was supported by the Bill and Melinda Gates Foundation [OPP 1017641] and an ABMRF start-up grant. Financial support was also received from the South African MRC, NRF, SAMA and Harry Crossley Foundation.
Author's contributions: all authors in this study have read and approved of the manuscript for submission; have made extensive contribution to the formation, design, collection, analysis, and/or interpretation of data and a contribution to the writing and intellectual content of the article; and acknowledged that they have implemented due care in ensuring the integrity of the work.
KD designed and executed the study and provided input on analysis strategy and results interpretation, and contributed to the preliminary draft of the manuscript and critical evaluation of the manuscript. LT performed statistical analysis, interpreted the results, and wrote the manuscript. AR led the analysis and provided critical review of the manuscript. JF, NK, KN, RW, HZ, and DS all provided input on the overall study design, imaging technique, and provided critical review of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mutevedzi PC, Newell ML. The changing face of the HIV epidemic in Sub-Saharan Africa. Trop Med Int Health 2014; 19:1015–1028. [DOI] [PubMed] [Google Scholar]
- 2.Victoria M, Granich R, Gilks CF, et al. The global fight against HIV/AIDS, tuberculosis, and malaria current status and future perspectives. Am J Clin Pathol 2009; 131:844–848. [DOI] [PubMed] [Google Scholar]
- 3.UN Joint Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic: 2014. Geneva, Switzerland:2014. [Google Scholar]
- 4.Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ 2013; 91:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goga AE, Dinh TH, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health 2014; 69:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman GG, Lilian RR, Bhardwaj S, et al. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. S Afri Med J 2014; 104:235–238. [DOI] [PubMed] [Google Scholar]
- 7.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in kinshasa, democratic republic of the congo. Pediatrics 2008; 122:e123–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014; 26:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abubakar A, Van Baar A, Van de Vijver FJ, et al. Paediatric HIV and neurodevelopment in Sub-Saharan Africa: a systematic review. Trop Med Int Health 2008; 13:880–887. [DOI] [PubMed] [Google Scholar]
- 10.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: The PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013; 32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoare J, Fouche JP, Spottiswoode B, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naïve “slow progressors”. J Neurovirol 2012; 18:205–212. [DOI] [PubMed] [Google Scholar]
- 12.Prado PC, Escorsi-Rosset S, Cervi M, et al. Image evaluation of HIV encephalopathy: a multimodal approach using quantitative MR techniques. Neuroradiology 2011; 53:899–908. [DOI] [PubMed] [Google Scholar]
- 13.Donald KA, Walker KG, Kilborn T, et al. HIV encephalopathy: pediatric case series description and insights from the clinic coalface. AIDS Res Ther 2015; 12:2.DOI 10.1186/s12981-014-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarma MK, Nagarajan R, Keller MA, et al. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage: Clinical 2014; 4:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simbayi LC, Shisana O, Rehle T, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- 16.Stein DJ, Koen N, Donald KA, et al. Investigating the psychosocial determinants of child health in Africa: The Drakenstein Child Health Study. J Neurosci Methods 2015; 70:592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zar HJ, Barnett W, Myer L, et al. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax 2015; 70:592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Department of Health, South Africa, and South African National AIDS Council. Clinical guidelines: PMTCT (prevention of mother-to-child transmission). 2010. [Google Scholar]
- 19.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008; 103:1039–1047. [DOI] [PubMed] [Google Scholar]
- 20.Jackson PB, Williams DR, Stein DJ, et al. Race and psychological distress: the South African Stress and Health Study. J Health Soc Behav 2010; 51:458–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J 1990; 69:763–765. [PubMed] [Google Scholar]
- 22.Dubowitz L, Mercurio E, Dubowitz V. An optimality score for the neurological examination of the term newborn. J Pediatr 1998; 133:406–416. [DOI] [PubMed] [Google Scholar]
- 23.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34:51–61. [DOI] [PubMed] [Google Scholar]
- 24.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am 2006; 16:19–43. [DOI] [PubMed] [Google Scholar]
- 26.Rose J, Vassar R, Cahill-Rowley K, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. NeuroImage: Clinical 2014; 5:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002; 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- 28.Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 2006; 83:392–402. [DOI] [PubMed] [Google Scholar]
- 29.Klawiter EC, Schmidt RE, Trinkaus K, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2011; 55:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois J, Dehaene-Lambertz G, Kulikova S, et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 2014; 276:48–71. [DOI] [PubMed] [Google Scholar]
- 31.Klunder AD, Chiang MC, Dutton RA, et al. Mapping cerebellar degeneration in HIV/AIDS. Neuroreport 2008; 19:1655–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand KS, Wadhwa A, Garg J. A case of cerebellar ataxia associated with HIV infection. J Int Assoc Provid AIDS Care 2014; 13:409–410. [DOI] [PubMed] [Google Scholar]
- 33.Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum 2008; 7:607–610. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002; 15:435–455. [DOI] [PubMed] [Google Scholar]
- 35.Hoeft F, Barnea-Goraly N, Haas BW, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci 2007; 27:11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol 2010; 61:111–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzler-Baddeley C, Jones DK, Steventon J, et al. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci 2012; 32:17612–17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Der Heide RJ, Skipper LM, Klobusicky E, et al. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 2013; 136 (pt 6):1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nir TM, Jahanshad N, Busovaca E, et al. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 2014; 35:975–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiehl TR, Chow EWC, Mikulis DJ, et al. Neuropathologic features in adults with 22q11. 2 deletion syndrome. Cereb Cortex 2009; 19:153–164. [DOI] [PubMed] [Google Scholar]
- 41.Meechan DW, Maynard TM, Tucker ES, et al. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci 2011; 29:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyszka JM, Readhead C, Bearer EL, et al. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 2006; 29:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burzynska AZ, Preuschhof C, Bäckman L, et al. Age-related differences in white matter microstructure: region specific patterns of diffusivity. Neuroimage 2010; 49:2104–2112. [DOI] [PubMed] [Google Scholar]
- 44.Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: application to the study of the developmental brain. J Am Acad Child Adolesc Psychiatry 2007; 46:213–223. [DOI] [PubMed] [Google Scholar]
- 45.Kakkar F, Lamarre V, Ducruet T, et al. Impact of maternal HIV-1 viremia on lymphocyte subsets about HIV-exposed uninfected infants: Protective mechanism or immunodeficiency. BMC Infect Dis 2014; 14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 2009; 35:959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. front. Neuroendocrinol 2012; 33:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]


