Supplemental Digital Content is available in the text
Abstract
Recent functional genomic studies revealed that the oncogenic activity of focally amplified lncRNA on chromosome 1 (FAL1, ENSG00000228126) contributes to tumor growth by p21 repression in human cancers. However, the expression of FAL1 was not investigated in papillary thyroid cancer (PTC).
We aimed to determine if FAL1 was up-regulated in PTC compared to paired contralateral normal thyroid tissues, and to investigate the potential targets of this lncRNA and its clinicopathological significance in PTC.
We analyzed FAL1 and p21 expression levels in 100 PTC samples and matched normal thyroid tissue by qRT-PCR. Using lncRNA microarray data from the Gene Expression Omnibus (accession no. GSE61763), we explored potential targets of FAL1 by Gene Set Enrichment Analysis, followed by verification by qRT-PCR in our PTC samples. A cross-sectional observational study was conducted to investigate the relationship between patients’ clinicopathological features and FAL1 expression.
FAL1 expression was significantly higher in PTC than in paired normal thyroid tissues (paired t test, P < 0.001). p21 mRNA expression was also increased, not decreased, in PTC, and had no correlation with FAL1 expression (r = 0.0897, P = 0.4002). Gene Set Enrichment Analysis, using publicly available microarray data, indicated that a gene set related to the cell cycle, including E2F transcription factors 1 and 2, and cyclin D1, was coordinately enriched among samples with high FAL1 expression. A volcano plot showed that E2F1, E2F2, and VEGFA mRNAs were increased in the high FAL1 samples. In clinicopathological analyses, multifocality was more frequently observed in PTC patients with high FAL1 (P = 0.018). Multivariate analysis showed that high FAL1 expression increased the risk of multifocality (after adjustment for clinical variables, OR = 4.019, CI = 1.041–11.020, P = 0.043).
FAL1 may have a role in cell-cycle progression and may be associated with aggressive tumor behavior in PTC.
INTRODUCTION
Thyroid cancer of follicular cell origin is the most common endocrine malignancy, and its incidence has been continuously increasing worldwide.1,2 The most effective therapy for differentiated thyroid cancer (DTC) is thyroidectomy, followed by ablation with radioactive iodine, which permits 5-year survival rates above 95% in the USA.3,4 However, the treatment of persistent or recurrent thyroid cancer is problematic because DTC is resistant to conventional chemotherapy or radiotherapy.5–7 As a result, the survival rate among patients with persistent DTC is approximately 60%, which means that a significant proportion of DTCs are life-threatening diseases.8 Even more problematic is that diagnostic markers to predict persistence or recurrence of DTC are not available.4,9
A noncoding RNA (ncRNA) is an RNA molecule that is not translated into a protein.10,11 Nonetheless, ncRNAs are abundant and functionally important and include transfer RNAs, ribosomal RNAs, small nuclear RNAs, microRNAs, piwi-interacting RNAs, and long ncRNAs (lncRNAs).12 By definition, lncRNAs are nonprotein-coding transcripts that are longer than 200 nucleotides.13,14 lncRNAs may have an important role in the development of thyroid cancers. For example, the papillary thyroid cancer (PTC) susceptibility candidate 2 (PTSC2) gene encodes a lncRNA that is down-regulated in PTC, affecting the expression of genes functionally related to the cell cycle and cancer.15 In addition, the polymorphism rs944289 on the PTC susceptibility candidate 3 (PTSC3) gene, which also encodes a lncRNA, predisposes to PTC, indicating that PTSC3 functions as a tumor suppressor.16 Moreover, the BRAF-activated lncRNA (BANCR) is up-regulated in PTC compared to matched normal tissue, and BANCR over-expression induces cell proliferation via autophagy regulation.17
Functional genomic studies recently revealed that the oncogenic activity of focally amplified lncRNA on chromosome 1 (FAL1, ENSG00000228126) contributes to the repression of p21 expression in different human cancers such as ovarian cancers.18,19 Mechanistically, FAL1 binds to the BMI1 proto-oncogene (BMI1), a component of the polycomb repressive complex 1 (PRC1). This direct interaction between FAL1 and BMI1 increases BMI1 stability, changes the levels of H2AK119 ubiquitination, and finally represses the expression of a wide range of genes, such as cyclin-dependent kinase inhibitor 1A (CDKN1A, p21, Cip1), Fas cell surface death receptor (FAS), BTG family, member 2 (BTG2), tumor protein p53 inducible protein 3 (TP53I3), F-box and WD repeat domain containing 7 (FBXW7), and cytoplasmic FMR1 interacting protein 2 (CYFIP2).19 Among those targets, CDKN1A, also called p21, inhibits cyclin-dependent kinase (CDK) 1, 2, and 4/6 complexes, thereby inhibiting cell cycle progression at the G1/S transition.20 In untransformed cells, inactivation of CDK4/6 results in de-phosphorylation of retinoblastoma protein (RB), the first tumor suppressor to be identified. De-phosphorylated RB binds to E2F transcription factors (E2F) such as E2F1, E2F2, and E2F3a, which represses E2F transcriptional activity.21 Taken together, the results suggest that repression of p21 expression by FAL1 may increase CDK activity, promote RB phosphorylation and E2F transactivation, and finally promote the G1/S transition.
However, the role of FAL1 expression has never been studied in PTC. Therefore, we have investigated FAL1 expression in PTC and in paired contralateral normal thyroid tissues. In addition, we explored potential targets of FAL1 in PTC using Gene Set Enrichment Analysis and analyzed the clinicopathological significance of this lncRNA in patients with PTC.
METHODS
Study Subjects and Clinical Data
The study enrolled 100 patients who underwent thyroidectomy for PTC between July and October 2014 at Yonsei Cancer Center (Seoul, South Korea). Samples were collected from the central part of the cancer and contralateral histologically normal tissue. Thyroid samples were examined microscopically immediately after surgery and stained with hematoxylin–eosin. On histological examination, >80% of the cells from the central part of the cancer were thyroid cancer cells. The study protocol was approved by the Institutional Review Board of Severance Hospital, and all patients provided informed consent before study participation.
Cell Culture and Plasmid
Human thyroid cancer cell lines BCPAP, 8505C, C643, HTH63, and SW1736 were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA). The cell lines TPC-1 and FTC-133 were cultured in high-glucose DMEM (Invitrogen) with 10% FBS at 37°C in a humidified incubator with 5% CO2. To generate plasmid expressing FAL1, sense (5′-CTC GGA TCC GCG CAT CTC CTA CGG CCT CCA GGA CAG AGG-3′) and anti-sense (5′-CGA GCG GCC GCA GAC ATC CAA GTG TCC TGT GTA ATA GGC TG-3′) primers were used. PCR was performed by the standard PCR protocol (PrimeSTAR® HS Polymerase Takara Bio, Shiga, Japan). The PCR product was cloned into pcDNA3.1 vector (Thermo Fisher Scientific, Waltham, MA).
RNA Isolation, Quantitative RT-PCR, and BRAF Sequencing
Total RNA from thyroid tissue and cells was extracted using TRIzol reagent according to the manufacturer's protocol (Invitrogen). Total RNA (2 μg) was converted into cDNA with Superscript II reverse transcriptase (Invitrogen) and used in quantitative RT-PCR (qRT-RCR) on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). The SYBR green RT-PCR kit (Life Technologies) and the Solg 2X PCR Smart Mix kit (Solgent, Daejeon, South Korea) were used. The primers were as follows: FAL1, 5′-GCA AGC GGA GAC TTG TCT TT-3′ and 5′-TTG AAC TCC TGA CCT CGT GA-3′; p21 (CDKN1A), 5′-CCT CAT CCC GTG TTC TCC TTT-3′ and 5′-GTA CCA CCC AGC GGA CAA GT-3′; E2F transcription factor 1 (E2F1), 5′-GGA CCT GGA AAC TGA CCA TCA G-3′ and 5′-CAG TGA GGT CTC ATA GCG TGA C-3′; E2F transcription factor 2 (E2F2), 5′-CGT CCC TGA GTT CCC AAC C-3′ and 5′-GCG AAG TGT CAT ACC GAG TCT T-3′; vascular endothelial growth factor A (VEGFA), 5′-AGG GCA GAA TCA TCA CGA AGT-3′ and 5′-AGG GTC TCG ATT GGA TGG CA-3′; U6, 5′-CTC GCT TCG GCA GCA CA-3′ and 5′-AAC GCT TCA GGA ATT TGC GT-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GCC GTC TAG AAA AAC CTG CC-3 and 5′-ACC ACC TGG TGC TCA GTG TA-3′. Relative expression was calculated by the StepOne™ Real-Time PCR System (Applied Biosystems). To detect BRAFV600E mutation, BRAF exon 15 was amplified by PCR with the following primers: forward 5′-TGT TAC CCA GTG GTG TGA GG-3′ and reverse 5′-CAT CTG ACT GAA AGC TGT ATG GA-3′. The PCR products were sequenced on an ABI 3730XL DNA Analyzer with BigDye® Terminator v3.1 cycle sequencing kits (Applied Biosystems).
Western Blot Analysis and Immunohistochemistry
Western blot analysis was performed according to previously described protocols with commercially available antibodies: p21 mouse monoclonal antibody (sc-6246, Santa Cruz Biotechnology, Inc., Dallas, TX) and anti-β-Actin Antibody (#4967, Cell Signaling, Danvers, MA).5 The signal intensities on Western blots were quantified by ImageJ software. Immunohistochemical staining for cyclin D1 was performed on 10 PTC samples with the lowest and 10 with the highest FAL1 expression ratios compared to internal control (GAPDH) by the protocols previously described.5 The primary antibody in our IHC study was rabbit anti-human cyclin D1 monoclonal antibody (Thermo Scientific, Fremont, CA).
Bioinformatics and Statistical Analyses
The secondary mRNA structure of FAL1 was predicted using the web server CentroidFold (http://www.ncrna.org/centroidfold).22 lncRNA microarray data (accession no. GSE61763, 15 corresponding normal and malignant tissues) were obtained from the Gene Expression Omnibus (GEO) of NCBI and subjected to Gene Set Enrichment Analysis.23 Analysis of public repository data for p21 expression in thyroid cancers was performed using the Human Protein Atlas (http://www.proteinatlas.org/). Statistical analysis was performed using Prism (GraphPad Software, San Diego, CA) or SPSS version 18.0 for Windows (SPSS, Chicago, IL). All P values are 2-sided.
RESULTS
Expression of FAL1 in PTC FAL1 is a lncRNA-coding gene located in a focal amplicon on chromosome 1q21.2. The coding region of FAL1 starts from 150,515,757 and ends at 150,518,032 on chromosome 1 and has 2 exons of 306 bp and 260 bp (Supplementary Figure 1A). The predicted RNA secondary structure of FAL1 suggests the presence of functional “modules” (Supplementary Figure 1B). Based on this structural prediction, Hu et al19 postulated that FAL1 interacts with other nucleotides and proteins.
To determine if FAL1 expression was increased in PTC, we performed qRT-PCR using cDNA from PTC and paired normal thyroid tissues (n = 100). As shown in Figure 1A, FAL1 expression was significantly higher in PTC than in paired normal thyroid tissues (paired t test, P < 0.001). Because FAL1 was reported to repress p21 expression in cancer through the BMI1 proto-oncogene (BMI1) and ring finger protein 2 (RNF2, Ring1B),19 we compared p21 mRNA expression between the 2 thyroid tissue types. Contrary to that reported for other cancers, p21 mRNA expression was increased, not decreased, in PTC (paired t test, P < 0.0001; Figure 1B), suggesting that p21 expression is not negatively regulated by FAL1 in PTC. In addition, we found no evidence of a correlation between FAL1 and p21 expression in PTC (Pearson r = 0.0897, P = 0.4002; Supplementary Figure 2A). This finding was supported by qRT-PCR using cDNA from thyroid cancer cell lines, which also indicated the absence of a negative relationship between FAL1 and p21 expression (Supplementary Figure 2B). The increased p21 mRNA expression raised the possibility that p21 mRNA levels might not reflect precisely p21 protein levels. To verify p21 expression at the protein level, we conducted Western blot analyses using PTC samples showing high FAL1 expression. Consistently, p21 expression was not lower in these PTC samples than in matched normal tissues (Figure 1C and D). Furthermore, immunohistochemical staining data from Human Protein Atlas indicated that p21 expression is easily observable in PTCs but not in Follicular neoplasm (FN), supporting the notion that p21 is active in PTC tumor cells (Supplementary Figure 2C and D).
FIGURE 1.
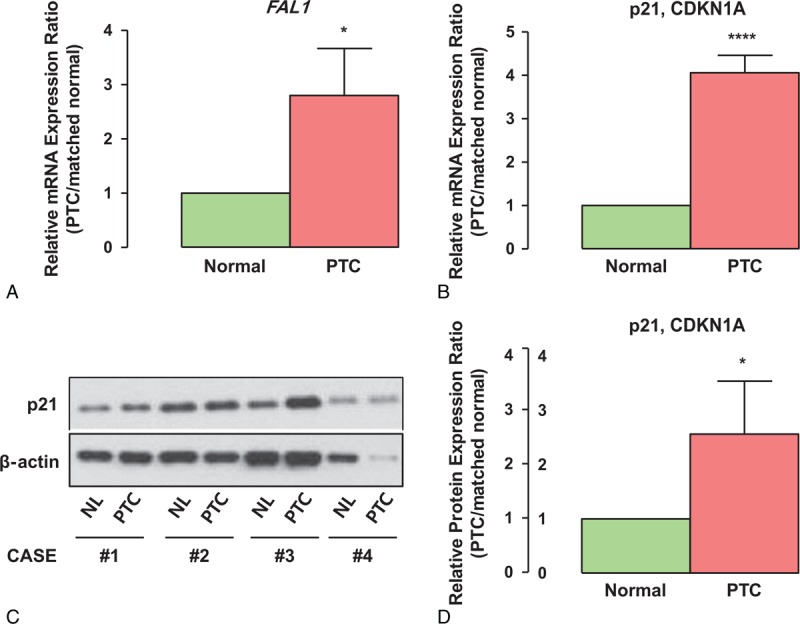
Relative RNA expression FAL1 in PTC. (A, B) Relative RNA-expression ratios of FAL1 and p21 between PTC and paired normal thyroid tissue (n = 100). Average ratios were compared with the 1-sample t test. ∗P < 0.05, ∗∗∗∗P < 0.0001. The experiments were performed in triplicate and repeated 3 times. (C) Comparisons of p21 protein expression status between PTC with high FAL1 expression and paired normal thyroid tissue. (D) Quantitative analysis of p21 expression assessed by ImageJ. The experiments were repeated 3 times. ∗P < 0.05. PTC = papillary thyroid cancer.
Clinicopathological Implications of FAL1 Expression in PTC
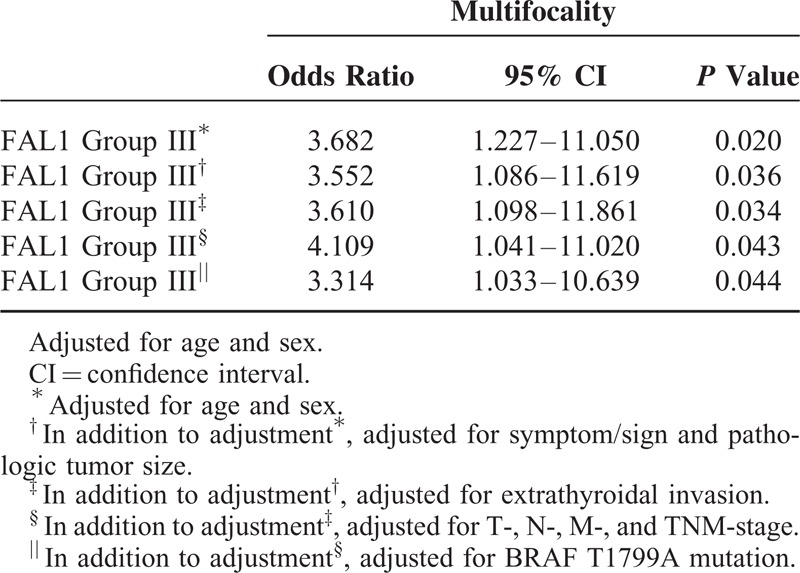
To understand the clinicopathological significance of FAL1 expression, we compared the 30 patients with the highest FAL1 expression to the 30 with the lowest FAL1 expression (Table 1). No association was found for age, sex, tumor size, or tumor stage. Remarkably, patients with high expression were more likely to have multifocality (P = 0.018). Univariate analyses (Supplementary Table 1) and multivariate analyses indicated that high FAL1 expression increased the risk of multifocality (after adjustment for clinical characteristics, OR = 4.019, CI = 1.041–11.020, P = 0.043; Table 2). Taken together, our qRT-PCR data and analysis of clinical data suggest that high FAL1 expression may affect tumor behavior and generate aggressive features of PTC.
TABLE 1.
Clinicopathological Characteristics of Patients with Papillary Thyroid Cancer According to Relative FAL1 Expression
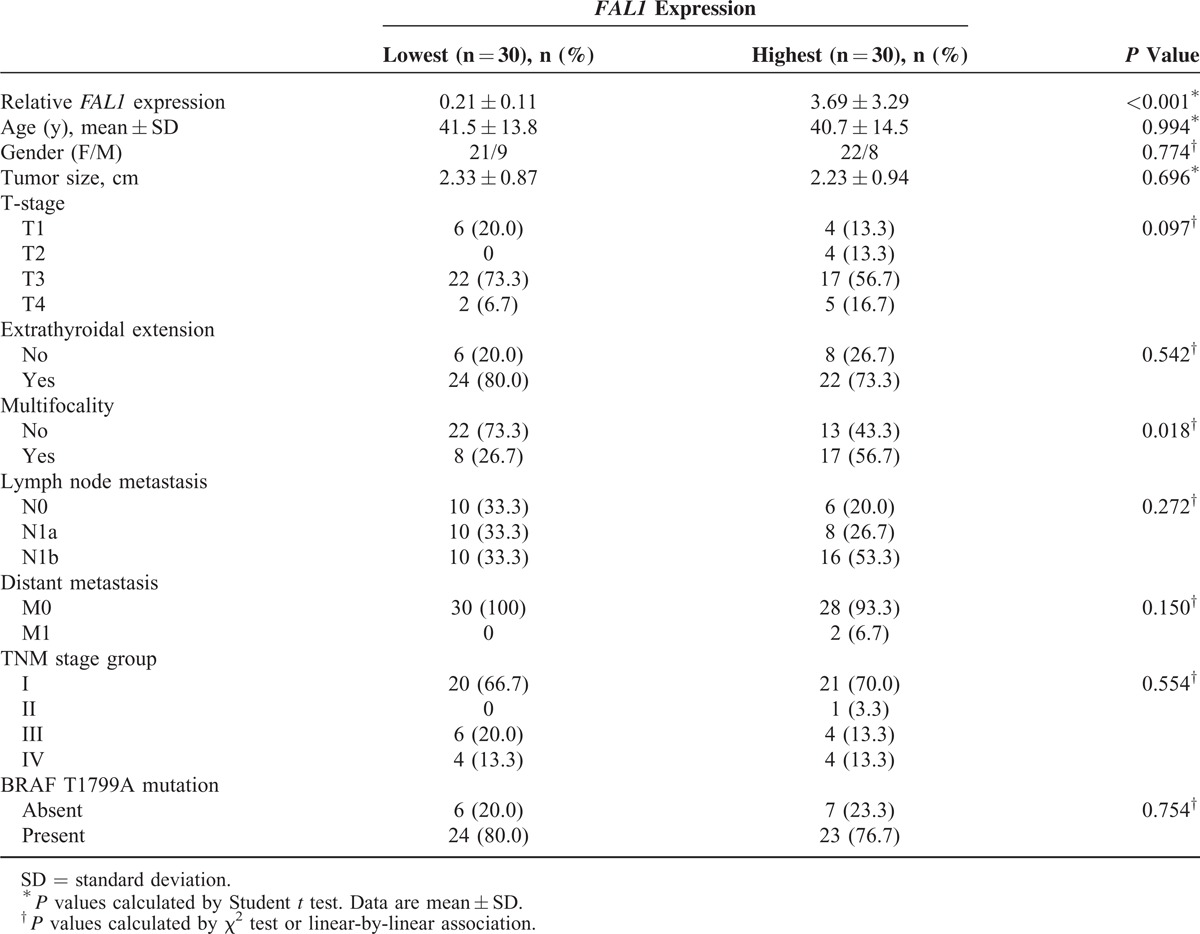
TABLE 2.
Multivariate Analysis of the Association of Multifocality with Highest FAL1 Expression
Discovery of FAL1 Target Genes
Because we did not find a negative relationship between FAL1 and p21, we aimed to find new FAL1 targets that promote tumor aggressiveness. We therefore performed Gene Set Enrichment Analysis. This analysis indicated that a gene set related to the cell cycle, including genes encoding E2F1, E2F2, and cyclin D, was coordinately enriched in FAL1 up-regulation group (Figure 2A). In addition, the mTOR signaling pathway was also related to FAL1 up-regulation (Supplementary Figure 3A). A volcano plot also presented that E2F1, E2F2, cyclin D1, cyclin D2, and VEGFA expression were related to FAL1 up-regulation (Figure 2B). To determine if these findings were reproducible in our own set of 100 PTCs, we performed qRT-PCR using cDNA from the 10 samples with the highest and 10 with the lowest FAL1 expression. Compatibly, mRNA expression ratios between PTC and matched normal thyroid tissue were higher for E2F1, E2F2, and VEGFA among samples with high FAL1 expression (Figure 2C). Furthermore, immunohistochemical staining also showed cyclin D1 up-regulation among PTC samples with the highest FAL1 expression (Figure 2D). To verify the FAL1 targets in the GSEA and qRT-PCR data, we transfected TPC1 (RET/PTC1 rearrangement) and BCPAP (BRAFV600E) thyroid cancer cell lines with the pcDNA3.1-FAL1 plasmid (Figure 3A). Consistently with the GSEA and qRT-PCR data, we confirmed that E2F1, E2F2, VEGFA, and cyclin D1 were significantly induced by FAL1 overexpression in both cell lines (Figure 3B–E).
FIGURE 2.
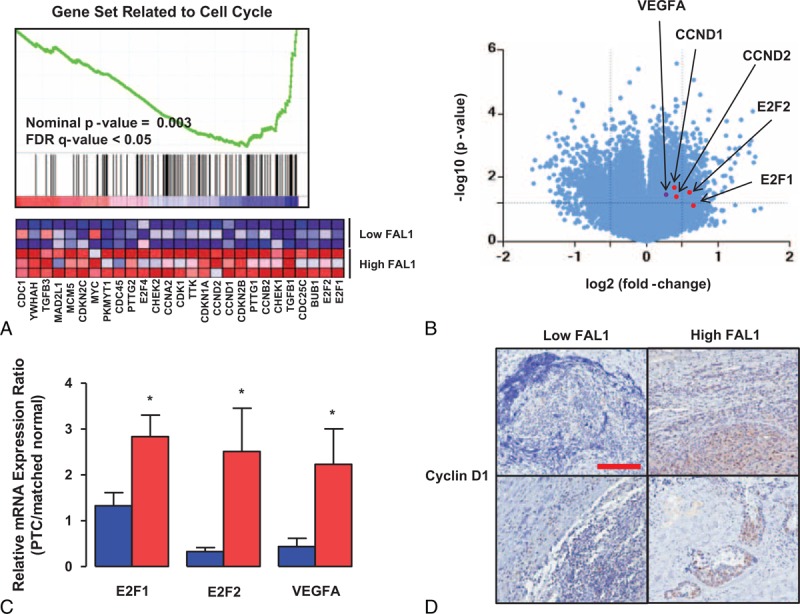
Relation of FAL1 expression with cell-cycle regulators. (A) Gene Set Enrichment Analysis revealed that samples (GSE61763) with high FAL1 expression ratio (FAL1/GAPDH) had up-regulation of genes involved in the cell cycle. (B) Volcano plot of the same data showing protein log2 fold changes (on the X axis) and the corresponding tempered log2P values (on the Y axis). (C) mRNA expression ratios for possible FAL1 target genes in PTC samples with the lowest (blue) and highest (red) FAL1 expression in our study population (each group, n = 10). Average ratios were compared with the unpaired t test with Welch correction. All data are means ± SEM. ∗P < 0.05. The experiment was performed in triplicate and repeated 3 times. (D) Representative photomicrographs of immunohistochemical staining for cyclin D1 among PTC samples with low or high FAL1 expression (original magnification, ×40; scale bar, 300 μm). PTC = papillary thyroid cancer, SEM = standard error of mean.
FIGURE 3.
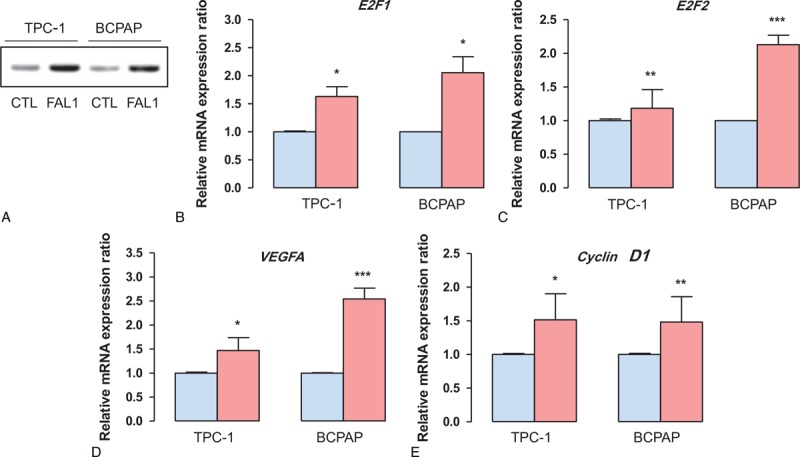
Induction of cell-cycle regulators by FAL1 overexpression in thyroid cancer cell lines. (A) TPC1 (PTC cell line harboring RET/PTC1 rearrangement) and BCPAP (PTC cell line harboring BRAFV600E mutation) cells transfected with pcDNA3.1-FAL1 plasmid for 48 h. (B–E) mRNA expression ratios of potential FAL1 target genes in control (CTL, blue) and FAL1 overexpressed (red) TPC1 and BCPAP cells. Average ratios were compared using the unpaired t test with Welch correction. All data are means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. The experiment was performed in triplicate and repeated 3 times. PTC = papillary thyroid cancer, SEM = standard error of mean.
In fact, a gain of 1q (SCNA-low-1q-amp) is a causative genetic event of FAL1 up-regulation. To investigate the association of SCNA-low-1q-amp with cell cycle regulators, we performed GSEA using TCGA (The Cancer Genome Atlas, http://cancergenome.nih.gov/) data. Remarkably, high expression of E2F1 in PTC was significantly correlated with coordinately enrichment of positional gene set related to chromosome 1q21 which is the coding region of FAL1 (Supplementary Figure 3B). This correlation of E2F1 expression with positional gene set of Chr. 1q21 suggested that 1q amplification might be related to high E2F1 expression.
DISCUSSION
Given the increase in thyroid cancer incidence,1 the proper management of PTC has become a serious concern.24 Although PTC has a favorable outcome, a small proportion of PTC can progress and finally undergo fatal outcomes.4,25 In fact, to improve the clinical outcome of recurrent or persistent PTC, novel therapeutic modalities such as small molecular inhibitors have been investigated rigorously.26,27 Moreover, in line with the development of new therapeutic modalities, novel biomarkers to predict clinical outcomes have also been explored.28–32
Recently, several lncRNAs were reported to be genetic predisposition factors or disease progression markers for PTC.33 For example, loss of PTCS2 and PTCS3, as the names indicate, predisposes to PTC.15,16 Loss of heterozygosity in genomic regions harboring NAMA (ncRNA associated with MAP kinase pathway and growth arrest) or up-regulation of BANCR contributes to tumor progression.17,34 In this study, we investigated the expression of FAL1 in PTC, as this lncRNA is increased in various cancers such as breast, colon, and ovarian cancers by somatic copy-number alterations (SCNAs).19 Compatible with this previous report, our qRT-PCR data indicate that FAL1 is up-regulated in PTC compared to paired contralateral normal thyroid tissues. However, in contrast to the previous report, our data did not show a negative relationship between FAL1 and p21 in PTC, indicating that the regulatory target of FAL1 in PTC might be different from that in other cancers. This possibility is supported by the observation that lncRNAs are expressed in a tissue-specific manner.35 In fact, high mRNA expression of p21 was not expected. However, in other types of cancer such as colon, breast, and ovary cancers, p21 positive tumors presented less aggressive tumor behaviors.36–38 The biological relevance and prognostic value of p21 in PTC will be necessary to elucidate in future studies.
To understand the clinicopathological significance of FAL1 expression in PTC, we analyzed the association between FAL1 expression levels and the patients’ characteristics. Interestingly, these analyses showed that FAL1 expression increases the risk of tumor multifocality. In fact, multifocality is a predictor of aggressiveness such as intrathyroidal metastasis or disease recurrence.39,40 Supporting this idea, we found that a gene set related to the cell cycle was coordinately enriched in high FAL1 expression group and that 3 genes from this set, namely, E2F1, E2F2, and VEGFA, were up-regulated in our PTC samples. Moreover, PTC with high E2F1 expression showed coordinately up-regulated expression of genes located on Chromosome 1q21 expression, suggesting 1q amplification might be related to high E2F1 expression.
E2F1 and E2F2 are members of the E2F family of transcription factors and bind specifically to RB, indicating that these transcription factors might function as tumor suppressors.41,42 However, as they function in a cell-cycle-dependent manner, E2F1- and E2F2-dependent transactivation induces the expression of genes related to cell-cycle progression.43,44 Compatibly, our qRT-PCR and immunohistochemical staining data clearly show that PTC with high FAL1 expression have not only high E2F1 and E2F2 mRNA expression but also high VEGFA mRNA and high cyclin D1 protein levels. VEGFA and cyclin D1 are important molecular markers of cellular proliferation in PTC.45 In fact, most lncRNAs including FAL1 are located in the nucleus and work as chromosome modifiers, ribonucleoprotein components, miRNA sponges, or mRNA stabilizers.46 It was previously shown that FAL1 associates with BMI1 and regulates its stability to repress p21.19 In line with these actions of this lncRNA, we postulate that FAL1 in PTC integrates with protein complexes such as chromatin-modifying complexes or ribonucleoprotein components. Therefore, our future work should investigate a possible role of FAL1 in cell-cycle regulation.
Recently, several small molecular inhibitors, such as vemurafenib, dabrafenib, and trametinib, have been developed to treat recurrent or persistent PTC. However, because de novo and acquired drug resistance has emerged, new investigations are needed to determine the therapeutic efficacy of broad-range RAF inhibitors or combination therapies employing 2 drugs with different action mechanisms.5,47–49 Although the study of lncRNA is in its infancy, we are cautiously anticipating that lncRNAs such as FAL1 will become a new therapeutic target. Unfortunately, to the best of our knowledge, lncRNA inhibitors have not yet been used in clinical practice. However, strategies for therapeutic manipulation of lncRNAs have been developed. For example, targeting natural antisense transcripts (NATs) with single-stranded therapeutic oligopeptides termed “antagoNAT” can cause mRNA de-repression.50,51
The limitations of our study include its retrospective nature and the small number of study patients. In addition, the mechanism by which FAL1 induces mRNA expression of E2F1, E2F2, VEGFA, and cyclin D remains to be elucidated.
In summary, PTC samples had increased expression of FAL1 compared to paired normal thyroid tissues. Up-regulation of FAL1 is related to an increase in expression of genes such as E2F1, E2F2, VEGFA, and cyclin D1, which can promote cellular proliferation and make the tumor more aggressive. In the clinical setting, up-regulation of FAL1 is associated with aggressive tumor features such as multifocality. Taken together, our data suggest that FAL1 is a potential biomarker to predict poor clinical outcomes and a molecular target for the treatment of aggressive thyroid cancer.
Supplementary Material
Footnotes
Abbreviations: BANCR = BRAF-activated lncRNA, BCPAP = BCPAP cell, BMI1 = BMI1 proto-oncogene, BTG2 = BTG family member 2, CDKN1A = cyclin-dependent kinase inhibitor 1A, CYFIP2 = cytoplasmic FMR1 interacting protein 2, DTC = differentiated thyroid cancer, E2F1 = E2F transcription factor 1, E2F2 = E2F transcription factor 2, FAL1 = focally amplified long noncoding on chromosome 1, FAS = Fas cell surface death receptor, FBXW7 = F-box and WD repeat domain containing 7, GEO = Gene Expression Omnibus, GSEA = Gene Set Enrichment Analysis, H2AK119 = Histone H2A (Lys119), IHC = immunohistochemical, lncRNA = long noncoding ribonucleic acid, LOH = loss of heterozygosity, mTOR = mechanistic target of rapamycin, NAMA = noncoding RNA associated with MAP kinase pathway and growth arrest, NCBI = National Center for Biotechnology Information, PRC1 = polycomb repressive complex 1, PTC = papillary thyroid cancer, PTMC = papillary thyroid microcarcinoma, PTSC2 = papillary thyroid cancer susceptibility candidate 2, PTSC3 = papillary thyroid cancer susceptibility candidate 3, RB = retinoblastoma protein, Ring1B = ring finger protein 2, RT-PCR = reverse transcription polymerase chain reaction, SCNAs = somatic copy-number alterations, TP53I3 = tumor protein p53 inducible protein 3, TPC1 = TPC1 cell, VEGFA = vascular endothelial growth factor A.
SJ and JL contributed equally to this work.
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Korean Government (MEST) (2014R1A1A2059343).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006; 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 2.Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014; 99:E276–E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 2002; 26:879–885. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Jeong S, Park JH, et al. Aberrant expression of COT is related to recurrence of papillary thyroid cancer. Medicine (Baltimore) 2015; 94:e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Seol MY, Jeong S, et al. A metabolic phenotype based on mitochondrial ribosomal protein expression as a predictor of lymph node metastasis in papillary thyroid carcinoma. Medicine (Baltimore) 2015; 94:e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012; 97:1250–1257. [DOI] [PubMed] [Google Scholar]
- 8.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 1990; 71:414–424. [DOI] [PubMed] [Google Scholar]
- 9.Vasko V, Espinosa AV, Scouten W, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA 2007; 104:2803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgs PG, Lehman N. The RNA world: molecular cooperation at the origins of life. Nat Rev Genet 2015; 16:7–17. [DOI] [PubMed] [Google Scholar]
- 11.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet 2014; 15:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011; 12:861–874. [DOI] [PubMed] [Google Scholar]
- 13.Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009; 458:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014; 15:7–21. [DOI] [PubMed] [Google Scholar]
- 15.He H, Li W, Liyanarachchi S, et al. Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J Clin Endocrinol Metab 2015; 100:E164–E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jendrzejewski J, He H, Radomska HS, et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci USA 2012; 109:8646–8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Guo Q, Zhao Y, et al. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett 2014; 8:1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sati S, Ghosh S, Jain V, et al. Genome-wide analysis reveals distinct patterns of epigenetic features in long non-coding RNA loci. Nucleic Acids Res 2012; 40:10018–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Feng Y, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell 2014; 26:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 2005; 65:3980–3985. [DOI] [PubMed] [Google Scholar]
- 21.Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 2002; 3:11–20. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Hamada M, Asai K, et al. CENTROIDFOLD: a web server for RNA secondary structure prediction. Nucleic Acids Res 2009; 37:W277–W280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 2014; 371:1765–1767. [DOI] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007; 246:375–381.discussion 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014; 384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372:621–630. [DOI] [PubMed] [Google Scholar]
- 28.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013; 309:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 2014; 32:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015; 33:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi X, Liu R, Qu S, et al. Association of TERT promoter mutation 1,295,228 C > T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J Clin Endocrinol Metab 2015; 100:E632–E637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Chen G, Sheng C, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer 2015; 22:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan X, Zhang H, Wang Z, et al. Genome-wide analysis of long noncoding RNA expression profile in papillary thyroid carcinoma. Gene 2015; 569:109–117. [DOI] [PubMed] [Google Scholar]
- 34.Yoon H, He H, Nagy R, et al. Identification of a novel noncoding RNA gene, NAMA, that is downregulated in papillary thyroid carcinoma with BRAF mutation and associated with growth arrest. Int J Cancer 2007; 121:767–775. [DOI] [PubMed] [Google Scholar]
- 35.Tsoi LC, Iyer MK, Stuart PE, et al. Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol 2015; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.el-Deiry WS, Tokino T, Waldman T, et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res 1995; 55:2910–2919. [PubMed] [Google Scholar]
- 37.Caffo O, Doglioni C, Veronese S, et al. Prognostic value of p21 (WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res 1996; 2:1591–1599. [PubMed] [Google Scholar]
- 38.Barboule N, Mazars P, Baldin V, et al. Expression of p21WAF1/CIP1 is heterogeneous and unrelated to proliferation index in human ovarian carcinoma. Int J Cancer 1995; 63:611–615. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Sohn SY, Jang HW, et al. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg 2013; 37:376–384. [DOI] [PubMed] [Google Scholar]
- 40.Lee YA, Jung HW, Kim HY, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab 2015; 100:1619–1629. [DOI] [PubMed] [Google Scholar]
- 41.Neuman E, Sellers WR, McNeil JA, et al. Structure and partial genomic sequence of the human E2F1 gene. Gene 1996; 173:163–169. [DOI] [PubMed] [Google Scholar]
- 42.Yang R, Muller C, Huynh V, et al. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol Cell Biol 1999; 19:2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong HJ, Yu HJ, Hong S, et al. Interaction and functional cooperation of the cancer-amplified transcriptional coactivator activating signal cointegrator-2 and E2F-1 in cell proliferation. Mol Cancer Res 2003; 1:948–958. [PubMed] [Google Scholar]
- 44.Chafin CB, Regna NL, Caudell DL, et al. MicroRNA-let-7a promotes E2F-mediated cell proliferation and NFkappaB activation in vitro. Cell Mol Immunol 2014; 11:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo YS, Li S, Song JH, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab 2006; 91:3667–3670. [DOI] [PubMed] [Google Scholar]
- 46.Knoll M, Lodish HF, Sun L. Long non-coding RNAs as regulators of the endocrine system. Nat Rev Endocrinol 2015; 11:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 2013; 3:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010; 467:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov 2013; 12:433–446. [DOI] [PubMed] [Google Scholar]
- 51.Modarresi F, Faghihi MA, Lopez-Toledano MA, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol 2012; 30:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


