Supplemental Digital Content is available in the text
Abstract
Diabetic ketoacidosis (DKA) is a life-threatening acute complication of diabetes mellitus and the novel systemic inflammation marker platelet-to-lymphocyte ratio (PLR) may be associated with clinical outcome in patients with DKA. This study aimed to investigate the utility of PLR in predicting 90-day clinical outcomes in patients with DKA.
Patient data exacted from the Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC II) database was analyzed. A cutoff value for PLR of 267.67 was determined using Youden index (P < 0.05) and used to categorize subjects into a high PLR group and a low PLR group. The hazard ratios (HRs) and 95% confidence intervals (CIs) for DKA were calculated across PLR. Clinical outcomes in our study were defined as intensive care unit (ICU) 90-day readmission and all-cause mortality.
A total of 278 ICU admissions were enrolled and stratified by cutoff value of PLR. The incidence of readmission and mortality was 17.8% in the high PLR group, significantly higher than 7.4% in the low PLR group. In the multivariable model, after adjusting for known confounding variables including clinical parameters, comorbidities, laboratory parameters, the HRs for DKA were 2.573 (95% CI 1.239–5.345; P = 0.011), 2.648 (95% CI 1.269–5.527; P = 0.009), and 2.650 (95% CI 1.114–6.306; P = 0.028), respectively. The Kaplan–Meier survival curve showed that a high PLR level was associated with a higher risk for 90-day outcomes in patients with DKA.
The authors report that higher PLR presents a higher risk for 90-day incidence of readmission and mortality in patients with DKA. It appears to be a novel independent predictor of 90-day outcomes in critically ill DKA patients in ICU units.
INTRODUCTION
Diabetic ketoacidosis (DKA) is a life-threatening acute complication of diabetes mellitus (DM) and is characterized by uncontrolled hyperglycemia, acidosis, and high concentrations of ketone bodies. Diabetic ketoacidosis occurs in patients with both type 1 and type 2 diabetes, for which type 1 and type 2 diabetes is responsible for 66% and 34% patients, respectively.1,2 It accounted for approximately 140,000 hospitalizations in the US in 2009 and a worldwide increase over the years has been reported.3–5 Despite the development of improved treatment, DKA remains at a high incidence of recurrence and a leading cause of mortality among patients with DM, resulting in an elevated burden for patients, hospitals, and healthcare providers.6–8 The increased number of patients critically ill with DKA demands more critical care from the intensive care unit (ICU), resulting in resource shortages for patients at high risk of the disease. Therefore, understanding the risk factors is crucial for both diagnosis and treatment of DKA.
Platelet-to-lymphocyte ratio (PLR) is a novel inflammatory marker, which has been demonstrated to be a predictor of various cardiovascular diseases and tumors.9–12 Previous studies have shown that DKA was associated with an inflammatory response in the hyperglycemic state.13,14 Given that PLR measurement is inexpensive and routinely tested in the hospital environment, PLR may be an ideal predictor for clinical outcomes in patients with DKA.
In this study, we conducted a longitudinal population analysis to investigate the utility of PLR in predicting the 90-day clinical outcomes in patients with DKA.
MATERIALS AND METHODS
The Database
The Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC II) database contains physiologic signals, laboratory examination, and comprehensive clinical data from patients at Beth Israel Deaconess Medical Center.15 Data were collected between 2001 and 2008 from a variety of ICU units (medical, surgical, coronary care, and neonatal) and maintained by the Laboratory for Computational Physiology at the Massachusetts Institute of Technology (MIT). To protecting patient privacy, all personal data were deidentified and every patient was identified by an integer number called a subject ID. The database was freely available for researchers after completion of NIH web-based training course named “Protecting Human Research Participants” (Our certification number: 1605699).
Study Design
To investigate the prognostic significance of PLR for predicting outcomes of patient with DKA, a longitudinal population from MIMIC II database was included. This retrospective cohort consisted of individuals who met a diagnosis of DKA and were admitted to ICU units in the Beth Israel Deaconess Medical Center, from 2001 to 2008. The research protocol of the study was approved by the Ethics Committee of Beth Israel Deaconess Medical Center.
Population Selection and Definitions
A total of 32,535 ICU patients were recorded in the MIMIC II database, classified by International Classification of Disease 9 code categories. We used primarily International Classification of Disease 9 code 250.1x to identify DKA patients, based on the criteria of the American Diabetes Association (ADA): plasma glucose > 13.9 mmol/l (250 mg/dL), arterial pH < 7.3 and a bicarbonate level <18 mEq/l, with ketonuria.
Subjects who met the following criteria were excluded: age <18 years; missing data of more than 5%, or lack of PLR data; readmission; subjects with a history of hematological disease or any other known potential causes of hematologic disorder; subjects who died before ICU stay.
Date Extraction
Patient data was exacted from MIMIC II (version 2.6) using structure query language with pgAdmin PostgreSQL tools (version 1.20.00), including patient identifiers, clinical parameters, laboratory parameters, and scoring systems.
There are 3 identifiers associated with any given patient:
Subject ID: an integer represents a particular patient, which can identify readmission of the same patient.
Hospital admission ID: an integer number identifying a particular admission to the hospital. Because the patient might be admitted to hospital several times, each patient (subject ID) may have multiple hospital admission IDs.
Intensive care unit stay ID: an integer number identifying a particular ICU unit stay record. When patients enter or leave a new care unit (eg, medical, surgical, coronary, trauma, cardiac surgery care units), a new ICU stay ID will be created.
According to the patient identifier system, we can obtain the hospital record of a particular patient from 2001 to 2008 at Beth Israel Deaconess Medical Center.
A clinical parameters record was conducted in the first 24 hours after patient admission. Physiologic information (heart rate, respiratory rate, systolic blood pressure, and diastolic blood pressure) was measured by bedside monitors. Age, sex, the length of stay in hospital, and readmission record were also recorded in database queried according patient identifiers. The date of death for patients who died in the hospital is taken to be the date of discharge. For other patients, date of death was obtained from social security death records from the US government.
Platelet count and lymphocytes were obtained from the same blood sample. The laboratory measurements included platelet, white blood cell, lymphocyte, neutrophil, creatinine, blood urea nitrogen, serum potassium, serum sodium, serum pH, partial pressure of carbon dioxide, partial pressure of oxygen, serum glucose, and urine ketone. All factors extracted were in the first 24 hours after patient admission.
Severity of illness scores was also recorded and calculated for patients, including Simplified Acute Physiology Score, Sequential Organ Failure Assessment, and Glasgow Coma Scale. In addition, the Elixhauser comorbidity score was used as a comorbidity estimate.
Statistical Analysis
The outcomes defined in our study were ICU 90-day readmission with a secondary ketoacidosis and all-cause mortality. Platelet-to-lymphocyte ratio was defined as the ratio of absolute platelet count divided by the absolute lymphocyte count. Urine ketone was classified according to concentration, as negative (negative laboratory result), low (negative to 50 mg/dL), moderate (50 to 150 mg/dL), and high (over 150 mg/dL). An optimal cutoff value for PLR of 267.67 was determined using Youden index (best pair of sensitivity and specificity for 90-day outcomes from a receiver operating characteristic curve),16,17 to categorize both high and low PLR groups. Platelet-to-lymphocyte ratio was treated as a categorical variable (≤267.67 or >267.67).
In this longitudinal population, the hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes were calculated using multivariable Cox proportional hazards regression after adjusting for known confounding variables across PLR. In addition, Kaplan–Meier survival curve was applied to describe the incidence of outcomes during 90 days, stratified by cutoff value of PLR.
Continuous variables were summarized as mean ± standard deviation (SD) and median (interquartile range [IQR]), respectively. The categorical variables were displayed as counts or percentages (%). The characteristics of the study population in two groups were compared using Student t test or nonparametric Wilcoxon test for continuous variables and χ2 test for categorical variables. All P-values are 2-sided and a P value of <0.05 was considered statistically significant. Analyses were performed in SPSS version 20.0 (SPSS, Chicago, IL).
RESULTS
Subject Characteristics
Patient records from 32,535 subjects who underwent ICU treatment in the Beth Israel Deaconess Medical Center were initially extracted from the MIMIC II database. After exclusion of individuals who did not meet the inclusion criteria (Figure 1), 278 eligible subjects were enrolled, including 128 men and 150 women, with a mean age of 49.1 ± 17.2 years and 46.2 ± 18.9 years, respectively. There were 33 subjects excluded because of data missing, and no difference was found between them and included individuals in characteristics.
FIGURE 1.
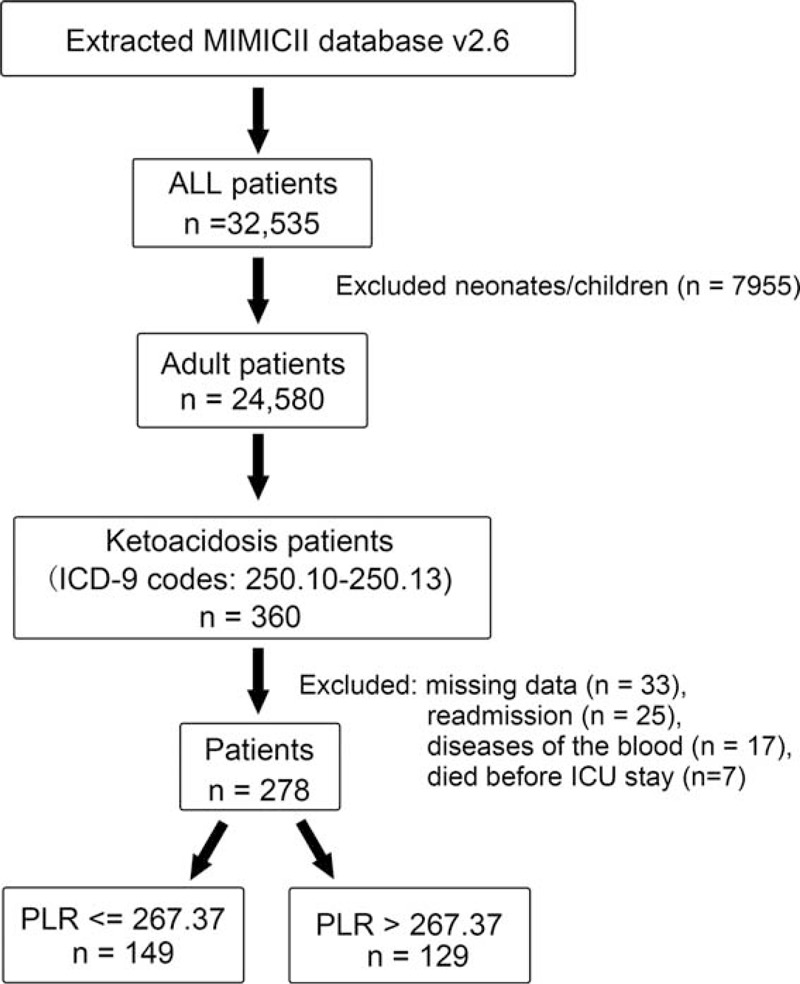
Study flow diagram. A total of 32,535 patients were recorded in Multiparameter Intelligent Monitoring in Intensive Care II database. After exclusion of those individuals who did not meet the inclusion criteria, 278 patients were included.
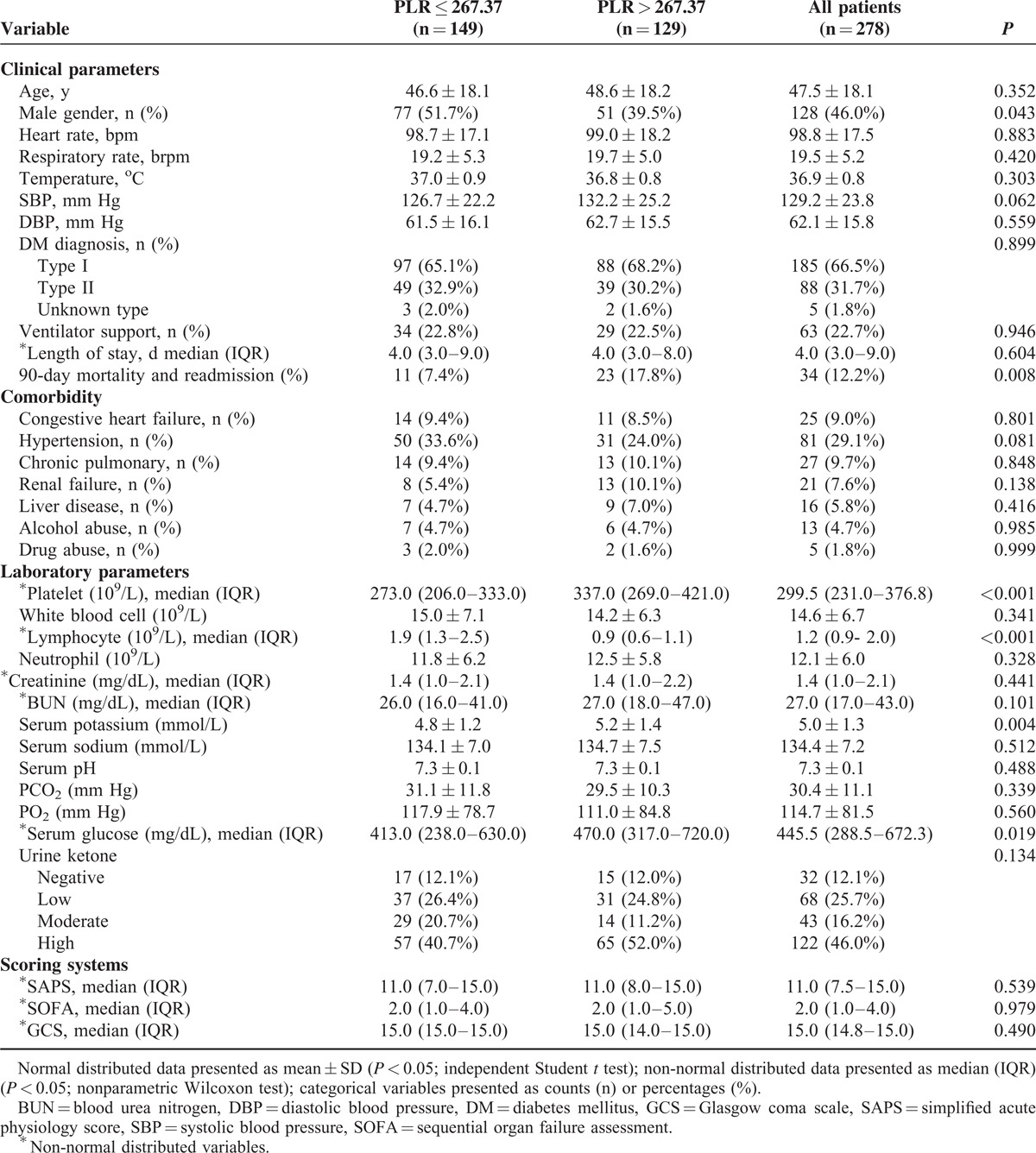
The mean value of PLR was 315.6 ± 256.9 for the study subjects. According to the cutoff value of PLR, 149 (53.6%) subjects were included into the low PLR group (PLR ≤ 267.37), whereas the remaining 129 (46.4%) subjects were in the high PLR group (PLR > 267.37). Patients with PLR ≤ 267.37 had a median PLR of 155.5 (109.6–202.2), whereas those with PLR > 267.37 had a median PLR of 392.0 (333.6–561.2). Table 1 shows the characteristics of study subjects classified by PLR cutoff value. Subjects with higher PLR exhibited higher incidence of readmission and mortality in 90 days. Platelet counts, serum potassium, and glucose were significantly higher, whereas the lymphocyte count was lower among subjects with a higher PLR value.
TABLE 1.
Characteristics of Patients With ketoacidosis, Stratified by Cutoff of Platelet to Lymphocyte Ratio
Risk Factor Analysis for Diabetic Ketoacidosis Patients in the 90-day Outcomes
To identify whether PLR plays a causal role in the incidence of readmission and in mortality, Cox proportional hazards regression analyses were performed and Kaplan–Meier survival curve was generated. As shown in Table 2 by the univariate analysis, age, ventilator support, congestive heart failure, PLR > 267.37 and serum potassium were all significantly associated with readmission and mortality. Patients with PLR > 267.37 were more likely to readmit and die within 90 days (HR 2.551; 95% confidence interval [CI] 1.244–5.234; P = 0.011). After adjusting for clinical parameters, comorbidities and laboratory parameters, a PLR > 267.37 still showed an independent association with outcomes (Table 3). The HRs of PLR were 2.573 (95% CI 1.239–5.345; P = 0.011), 2.648 (95% CI 1.269–5.527; P = 0.009), and 2.650 (95% CI 1.114–6.306; P = 0.028), respectively. Hazard ratios of quartile PLR groups and the comparison of platelet, lymphocyte, and PLR for prediction of 90-day outcomes are presented in Supplementary Table 1 and Supplementary Table 2, respectively.
TABLE 2.
Univariate Analysis of the Associations Between 90-day Outcomes and Clinical and Biochemical Characteristics in Patients With ketoacidosis
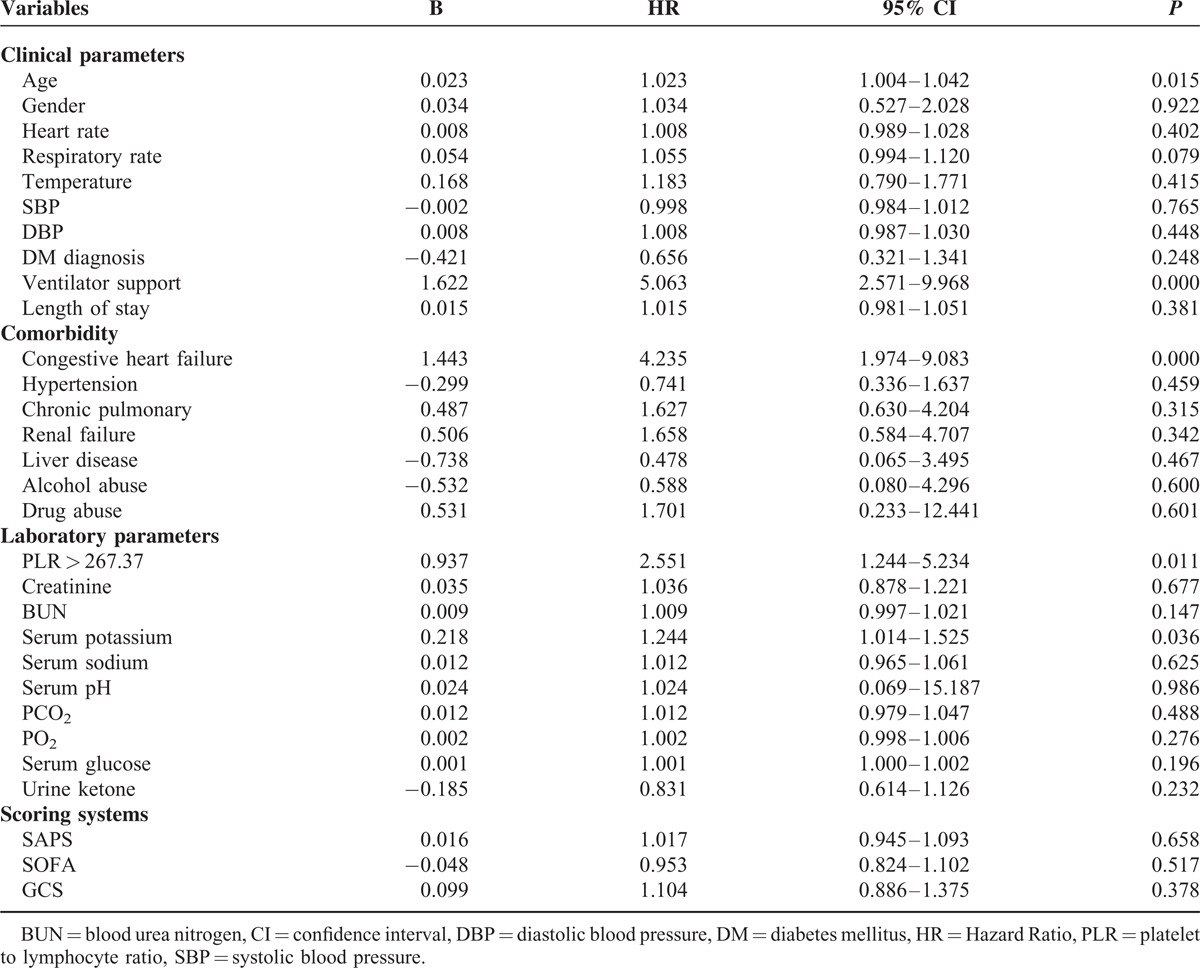
TABLE 3.
Hazard Ratio (95% Confidence Interval) for Platelet to Lymphocyte Ratio in 90-day Readmission and Mortality of Ketoacidosis Patients
Figure 2 shows the cumulative incidence of readmission and mortality status in the 90-day period, stratified by cutoff value of PLR. Details on the correlation of groups with incident are shown in Figure 2. At 90 days, the incidence of outcomes was 7.4% in the low PLR group and 17.8% in the high PLR group, respectively. A high PLR level had a higher risk of short-term outcomes for patients with DKA. In 1-year outcome measures, patient groups with a high PLR value maintained a higher incidence of mortality and readmission, however, there was no statistical significance (17.8% versus 10.7%, P = 0.082).
FIGURE 2.
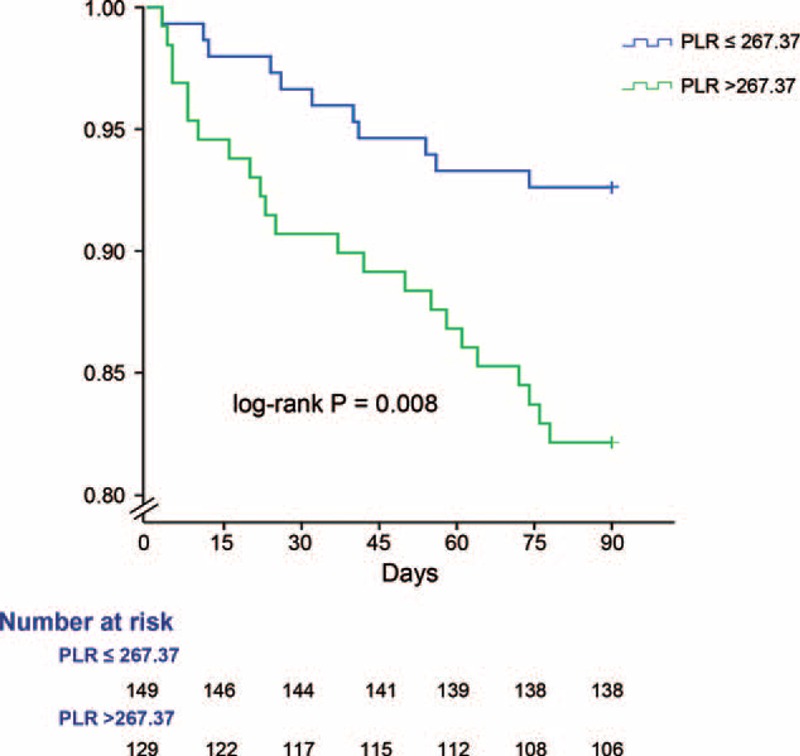
Kaplan–Meier survival curve showing mortality and readmission status of the groups above and below platelet to lymphocyte ratio cutoff.
DISCUSSION
The MIMIC II database has used in several studies focused on critically ill patients.18–20 In our study, we have extracted clinical data from electronic medical records based on the MIMIC II database and presented a potentially interesting relationship between PLR and short-term outcomes for patients with DKA. The enrolled DKA population included 278 subjects which were then stratified by PLR cutoff value into a high PLR group and a low PLR group. By comparing the 2 groups, we found that there was no significant difference in characteristics except serum potassium and glucose levels were higher in the high PLR group. To identify whether PLR is a risk factor actively involved in the readmission and mortality of DKA, we performed Cox proportional hazards regression and generated survival curves based on Kaplan–Meier estimates. We demonstrated that the high PLR level appeared to make a significant contribution to an increased risk of readmission and death in patients with DKA.
At the time of writing, PLR has been a popular topic in various investigational studies and been demonstrated to play a crucial role in several tumor types and in cardiovascular disease.21–24 Data generated from research in these diseases has supported a close association of systemic inflammatory processes with oxidative stress, leading to alterations of platelet and lymphocyte levels.25–27 To our knowledge, our research is the first study aimed at evaluating the association between PLR levels and disease prognosis in critically ill patients with DKA. Previous studies have proposed that DKA is associated with oxidative stress and inflammatory reaction in the hyperglycemic state.13,14 Thus, the underlying mechanism of up-regulated PLR may also be based on the dysfunction of the inflammatory response. Studies have proposed that hyperglycemia may lead to an excessive oxidation reaction in the tricarboxylic acid cycle, leading to an increase in the generation of reactive oxygen species (ROS). As a result, mitochondrial function is impaired during the production of ROS.28–30 Fengming et al showed the dysfunction of mitochondria found in platelets, which lead to lower platelet viability in a rat model of DM and higher platelet counts in patients.31 These findings are consistent with current clinical research, which has demonstrated elevated platelet levels in ketoacidosis patients with type 1 DM32 and moreover, the lymphocyte count was also influenced by systemic inflammation. Studies have reported that leukocytes may generate more ROS in subjects with DM,33 leading to elevated oxidative DNA damage of lymphocytes in the hyperglycemia state.34 As a result, a high proportion of apoptotic lymphocytes have been found both in a rat model and in patients with DM.35 Taken together, these studies illustrate the potential causes of down-regulated lymphocyte levels in patients with DKA patients. In conclusion, the increased PLR might be attributed to an opposing influence of hyperglycemia in platelet and lymphocytes.
Our study has several limitations. First, although the MIMIC II database included more than 30,000 patients, few subjects with DKA met the inclusion criteria and the sample size was small, thus limiting the statistical power in the analysis. This may explain why the high PLR group maintained a higher incidence but with no statistical significance in long-term outcomes. Second, the subjects with DKA in our study were all extracted from ICU electronic records. Patients whose disease was managed in hospital wards or emergency departments were not included in our study. Thirdly, patients’ height data was missing, because the measurement required the patient to be standing, which was not possible for critically ill patients. Therefore, we could not calculate body mass index and adjusted it in the Cox proportional hazard regression analyses.
In conclusion, a higher PLR was shown to represent an increased risk for 90-day incidence of readmission and mortality in patients with DKA. It appears to be a novel independent predictor for short-term outcomes in critically ill patients with DKA undergoing treatment in ICUs.
Supplementary Material
Footnotes
Abbreviations: BUN = blood urea nitrogen, CI = confidence interval, DBP = diastolic blood pressure, DKA = diabetic ketoacidosis, DM = diabetes mellitus, GCS = Glasgow Coma Scale, HR = hazard ratio, ICU = intensive care unit, MIMIC II = Multiparameter Intelligent Monitoring in Intensive Care II, MIT = Massachusetts Institute of Technology, PLR = platelet-to-lymphocyte ratio, ROS = reactive oxygen species, SAPS = Simplified Acute Physiology Score, SBP = systolic blood pressure, SOFA = sequential organ failure assessment.
This work was supported by grants from National Natural Science Foundation of China (81500665), Zhejiang Provincial Natural Science Foundation (Y14H070034), Health Bureau of Zhejiang Province (2010KYB070), Research Foundation of Education Bureau of Zhejiang Province (Y201009942), and Project of New Century 551 Talent Nurturing in Wenzhou.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res 2015; 9:OC01–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra S, Oliver N, Dornhorst A. Diabetic ketoacidosis: not always due to type 1 diabetes. Br Med J 2013; 346:f3501. [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001; 24:131–153. [DOI] [PubMed] [Google Scholar]
- 4.Abdulrahman GO, Amphlett B, Okosieme OE. Trends in hospital admissions with diabetic ketoacidosis in Wales, 1999-2010. Diabetes Res Clin Pract 2013; 100:e7–10. [DOI] [PubMed] [Google Scholar]
- 5.Lombardo F, Maggini M, Gruden G, et al. Temporal trend in hospitalizations for acute diabetic complications: a nationwide study, Italy, 2001–2010. PLoS One 2013; 8:e63675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohiya S, Kreisberg R, Lohiya V. Recurrent diabetic ketoacidosis in two community teaching hospitals. Endocr Pract 2013; 19:829–833. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou SP, Tsiakou AG, Tsioulos DI, et al. A mortality prediction model in diabetic ketoacidosis. Clin Endocrinol (Oxf) 2002; 57:595–601. [DOI] [PubMed] [Google Scholar]
- 8.Zargar AH, Wani AI, Masoodi SR, et al. Causes of mortality in diabetes mellitus: data from a tertiary teaching hospital in India. Postgrad Med J 2009; 85:227–232. [DOI] [PubMed] [Google Scholar]
- 9.Sunbul M, Gerin F, Durmus E, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens 2014; 36:217–221. [DOI] [PubMed] [Google Scholar]
- 10.Azab B, Shah N, Akerman M, et al. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis 2012; 34:326–334. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol 2015; 36:2263–2269. [DOI] [PubMed] [Google Scholar]
- 12.Tian XC, Zeng FR, Wu DH. Platelet-to-lymphocyte ratio: a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol 2015; 36:4935–4936. [DOI] [PubMed] [Google Scholar]
- 13.Erbagci AB, Tarakcioglu M, Coskun Y, et al. Mediators of inflammation in children with type I diabetes mellitus: cytokines in type I diabetic children. Clin Biochem 2001; 34:645–650. [DOI] [PubMed] [Google Scholar]
- 14.Stentz FB, Umpierrez GE, Cuervo R, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004; 53:2079–2086. [DOI] [PubMed] [Google Scholar]
- 15.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000; 101:E215–220. [DOI] [PubMed] [Google Scholar]
- 16.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 17.Schisterman EF, Perkins NJ, Liu A, et al. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology 2005; 16:73–81. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Xu X, Ni H, et al. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One 2014; 9:e95204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghassemi M, Marshall J, Singh N, et al. Leveraging a critical care database: selective serotonin reuptake inhibitor use prior to ICU admission is associated with increased hospital mortality. Chest 2014; 145:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abhyankar S, Leishear K, Callaghan FM, et al. Lower short- and long-term mortality associated with overweight and obesity in a large cohort study of adult intensive care unit patients. Crit Care 2012; 16:R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 2014; 110:2524–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 2013; 109:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014; 23:1204–1212. [DOI] [PubMed] [Google Scholar]
- 24.Akkaya E, Gul M, Ugur M. Platelet to lymphocyte ratio: a simple and valuable prognostic marker for acute coronary syndrome. Int J Cardiol 2014; 177:597–598. [DOI] [PubMed] [Google Scholar]
- 25.Demircelik MB, Kurtul A, Ocek H, et al. Association between platelet-to-lymphocyte ratio and contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Cardiorenal Med 2015; 5:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014; 31:305. [DOI] [PubMed] [Google Scholar]
- 27.Abakay O, Abakay A, Sen HS, et al. The relationship between inflammatory marker levels and pulmonary tuberculosis severity. Inflammation 2015; 38:691–696. [DOI] [PubMed] [Google Scholar]
- 28.Colwell JA, Winocour PD, Halushka PV. Do platelets have anything to do with diabetic microvascular disease. Diabetes 1983; 32:14–19. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414:813–820. [DOI] [PubMed] [Google Scholar]
- 30.Savu O, Sunkari VG, Botusan IR, et al. Stability of mitochondrial DNA against reactive oxygen species (ROS) generated in diabetes. Diabetes Metab Res Rev 2011; 27:470–479. [DOI] [PubMed] [Google Scholar]
- 31.Wu F, Liu Y, Luo L, et al. Platelet mitochondrial dysfunction of DM rats and DM patients. Int J Clin Exp Med 2015; 8:6937–6946. [PMC free article] [PubMed] [Google Scholar]
- 32.Ma SG, Yang LX, Qiu XQ. Assessment of the platelet parameters and serum butyrylcholinesterase activity in type 1 diabetes patients with ketoacidosis. Platelets 2013; 24:544–548. [DOI] [PubMed] [Google Scholar]
- 33.Chibber R, Ben-Mahmud BM, Chibber S, et al. Leukocytes in diabetic retinopathy. Curr Diabetes Rev 2007; 3:3–14. [DOI] [PubMed] [Google Scholar]
- 34.Adaikalakoteswari A, Rema M, Mohan V, et al. Oxidative DNA damage and augmentation of poly (ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem Cell Biol 2007; 39:1673–1684. [DOI] [PubMed] [Google Scholar]
- 35.Otton R, Soriano FG, Verlengia R, et al. Diabetes induces apoptosis in lymphocytes. J Endocrinol 2004; 182:145–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


