Abstract
High-density lipoprotein (HDL) is highly heterogeneous in its size and composition. Till now, the link of HDL subfractions to coronary risk is less clear. We aimed to investigate the associations of HDL subfractions with traditional risk factors (RFs), coronary severity, and outcomes in a cohort of nontreated patients with stable coronary artery disease (CAD).
We prospectively enrolled 591 eligible patients. Baseline HDL subfractions were separated by Lipoprint system. HDL subfractions (large, medium, and small) and HDL-cholesterol (HDL-C) levels were dichotomized into low and high group according to the 50 percentile. Coronary severity was evaluated by SYNTAX, Gensini, and Jeopardy scoring systems. Patients were followed up annually for major adverse cardiovascular events (MACEs). Cox proportional hazards’ models were used to evaluate the risk of HDL subfractions on MACEs.
Patients with high large HDL-C levels had a decreased number of RFs. Significantly, large HDL-C levels were negatively associated with coronary severity assessed by SYNTAX and Gensini score (both P < 0.05). New MACEs occurred in 67 (11.6%) patients during a median 17.0 months follow-up. Moreover, the log-rank test revealed that there was a significant difference between high and low large HDL-C groups in event-free survival analysis (P = 0.013), but no differences were observed in total HDL-C groups and medium or small HDL-C groups (both P > 0.05). In particular, the multivariate Cox-proportional hazards model revealed that high large HDL-C was associated with lower MACEs risk (hazard ratio [95% confidence interval] 0.531 [0.295–0.959]) independent of potential confounders.
Higher large HDL-C but not medium, small, or total HDL-C is associated with lower cardiovascular risk, highlighting the potential beneficial of HDL subfractionation.
INTRODUCTION
Lipoproteins are major mediators of atherosclerosis, which remains the leading cause of death in the world. Despite lowering low-density lipoprotein (LDL)-cholesterol (LDL-C) has convincingly been shown to reduce major adverse cardiovascular events (MACEs), residual cardiovascular risk remains high in a significant number of patients.1,2 In light of the epidemiologic evidence that low high-density lipoprotein (HDL)-cholesterol (HDL-C) was inversely related to coronary outcomes, raising HDL-C has been proposed as a potential therapeutic target.3 However, after the failure of several HDL-C raising drugs including torcetrapib, dalcetrapib, and niacin,4,5 attention is focusing on specific HDL subfractions as possible cardiovascular protectors.6,7
Indeed, HDL is highly heterogenous in its size and composition.8 Recently, several approaches have been developed to more fully capture which HDL subfractions may confer cardiovascular protection. However, few studies have thoroughly evaluated the relationship between HDL subfractions with cardiovascular outcomes till now. Martin et al9 recently reported that low HDL3-C (small HDL-C) subclasses, but not HDL2-C (large HDL-C) was associated with increased long-term hard clinical events in 2 cohorts of secondary prevention. Given the facts that lipid-lowering drugs may have impact on baseline HDL subfractions’ distribution as well as the existing of different HDL separation methods, the link between HDL subfractions and cardiovascular outcomes remained vague.
Thus, in the present study, we aimed to prospectively and comprehensively investigate the association between HDL subfractions (separated by Lipoprint system) and cardiovascular risk including number of risk factors (RFs), coronary severity assessed by SYNTAX, Gensini, and Jeopardy scoring systems, and MACEs in nontreated patients with stable coronary artery disease (CAD).
METHODS
Patients and Methods
The study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (FuWai Hospital and National Center for Cardiovascular Diseases, Beijing, China). Each participant provided written, informed consent before enrollment.
Consecutive Chinese patients scheduled for coronary artery angiography (CAG) because of angina-like chest pain and/or positive treadmill exercise test or clinically suspected CAD at FuWai Hospital (Beijing, China) from October 2012 to January 2015 were considered for this analysis. Due to the potential impact of lipid-lowering drugs on the levels and distribution of HDL subfractions, we prospectively enrolled patients who did not receive lipid-lowering drugs, including all the commonly prescribed medications, such as statins, fibrate, ezetimibe, XueZhiKang, and so forth. The exclusion criteria were as follows: missing detailed laboratory data; with normal or mild coronary lesion (the presence of coronary lesion <50% in all major epicardial artery segment); with acute coronary syndrome (ACS), heart failure (left ventricular ejection fraction <45%), history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG); and significant hematologic disorders, thyroid dysfunction, severe liver and/or renal insufficiency or malignant disease. Therefore, a total of 591 eligible patients were enrolled in the current analysis. The detailed demographic, clinical, hematologic, and angiographic data were collected from all subjects at baseline.
The definition of traditional cardiovascular RFs was as described as our previous study.10 Hypertension was defined as repeated blood pressure measurements ≥140/90 mm Hg (at least 2 times in different environments) or currently taking anti-hypertensive drugs. Diabetes mellitus (DM) was defined as a fasting serum glucose level ≥126 mg/dL in multiple determinations, and/or the current use of medication for diabetes. Dyslipidemia was defined by medical history or the use of lipid-modulating medications in order to reduce lipids or fasting total cholesterol (TC) ≥200 mg/dL or triglyceride (TG) ≥150 mg/dL. Obesity was defined as patients with body mass index (BMI) ≥30 kg/m2. Smoking was ascertained as subjects who had smoked regularly within the previous 12 months.
Biochemical Analyses
Fasting blood samples were collected in precooled EDTA tubes at baseline from each patient. The HDL-C concentration was determined by selective solubilization method (Determiner L HDL, Kyowa Medex, Tokyo, Japan) and the LDL-C concentration was analyzed by a homogeneous method (LDL-C test kit, Kyowa Medex). TC and TG were measured by enzymatic methods. All of the lipid profiles were determined by automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan). The other relating biomarkers were analyzed by standard commercial kits.
HDL Subfraction Analysis
The cholesterol contents of HDL subfractions were determined electrophoretically by the use of high-resolution 3% polyacrylamide gel tubes and the Lipoprint System (Lipoprint HDL System Quantimetrix Corporation, Redondo Beach, CA) according to the manufacturer's instructions as our previously described.11,12 Briefly, 25 μL of blood sample was placed upon the upper part of the high-resolution 3% polyacrylamide gel and then added 300 μL of Lipoprint HDL Loading Gel to each tube. Mix the loading gel with the specimen by inverting the preparation rack several times. After 30 min of photopolymerization in room temperature, electrophoresis was performed for 50 min with 3 mA for each gel tube. After the electrophoresis was completed, the various stained HDL subfractions presented in the sample were identified by their electrophoretic mobility (Rf) using LDL/very low density lipoprotein (VLDL) as the starting reference point (LDL/VLDL = 0) and albumin as the leading reference point (Albumin = 1). The relative area for each HDL subfraction was determined and multiplied by HDL-C concentration of the sample to yield the amount of cholesterol for each band in mg/dL. By this method, HDL was divided into 10 subfractions. Subfractions 1 to 3 represented large HDL particles, subfractions 4 to 7 indicated medium HDL particles, and subfractions 8 to 10 meant small HDL particles. The cholesterol mass (mg/dL) of each lipoprotein subfraction were determined by this assay.
Assessment for the Severity of CAD
The SYNTAX score is used to estimate the extent and severity of CAD through the assessment of the number of angiographically obstructive coronary lesions, their functional effects, locations, and complexity. Depending on several angiographic characteristics, such as the coronary dominance, location at bifurcation, trifurcation, orosteal lesions, tortuosity, calcifications, the content of the thrombus, presence of diffuse disease, and elongated lesions, the lesion was given a corresponding point value, and finally scores of individual lesions were summed to derive the final score.13 The Gensini score was computed by assigning a severity score to each coronary lesion according to the degree of luminal narrowing and the importance of location and the total score equaled the sum of the severity score times the location score for all diseased segments.14 The Jeopardy score was designed to estimate the myocardium at risk or in jeopardy. The coronary arteries were divided into 6 major segments and 2 points were given to each segment >75% stenotic segment.15
Outcome Measure
Follow-up data were obtained via standardized telephone interviews at 6-month intervals conducted by study nurses or cardiologists who were blinded to the aim of this study. If patients reported that they had been hospitalized, appropriate hospital records were consulted. The follow-up time interval (months) was counted from the enrollment till the last traceable hospital outpatient or inpatient record or telephone interview before April 2015. The primary endpoints were the cardiac death, stroke, nonfatal myocardial infarction (MI), postdischarge revascularization (PCI/CABG) due to clinical deterioration or unstable angina (UA). All available relevant data from any reported possible event were collected. Death of a participant was reported by relatives or the general practitioner who treated the participant. Three experienced physicians who were masked to any of the study data independently classified the events.
Statistical Analysis
The values are expressed as the mean ± SD or median with interquartile range (IQR) for the continuous variables and as the number (percentage) for the categorical variables. In the present study, HDL subfractions (large HDL-C, medium HDL-C, and small HDL-C) and total HDL-C was dichotomized into low and high group according to the 50 percentile. To investigate the relation of HDL subfractions to traditional cardiovascular RFs as well as the severity of CAD, Chi-squared statistic tests, Fisher exact test, Student t tests, nonparametric test, and general linear model (with adjustments for age and sex) were applied when appropriate.
The event-free survival rates between high and low HDL subfractions and total HDL-C were estimated by the Kaplan–Meier method and compared by the log-rank test. The effect of high and low HDL subfractions and total HDL-C on the occurrence of MACEs was evaluated using Cox proportional hazards models.
The statistical analysis was performed with SPSS version 19.0 software (SPSS Inc., Chicago, IL). For all analyses, P < 0.05 was considered statistical significant.
RESULTS
Baseline Characteristics
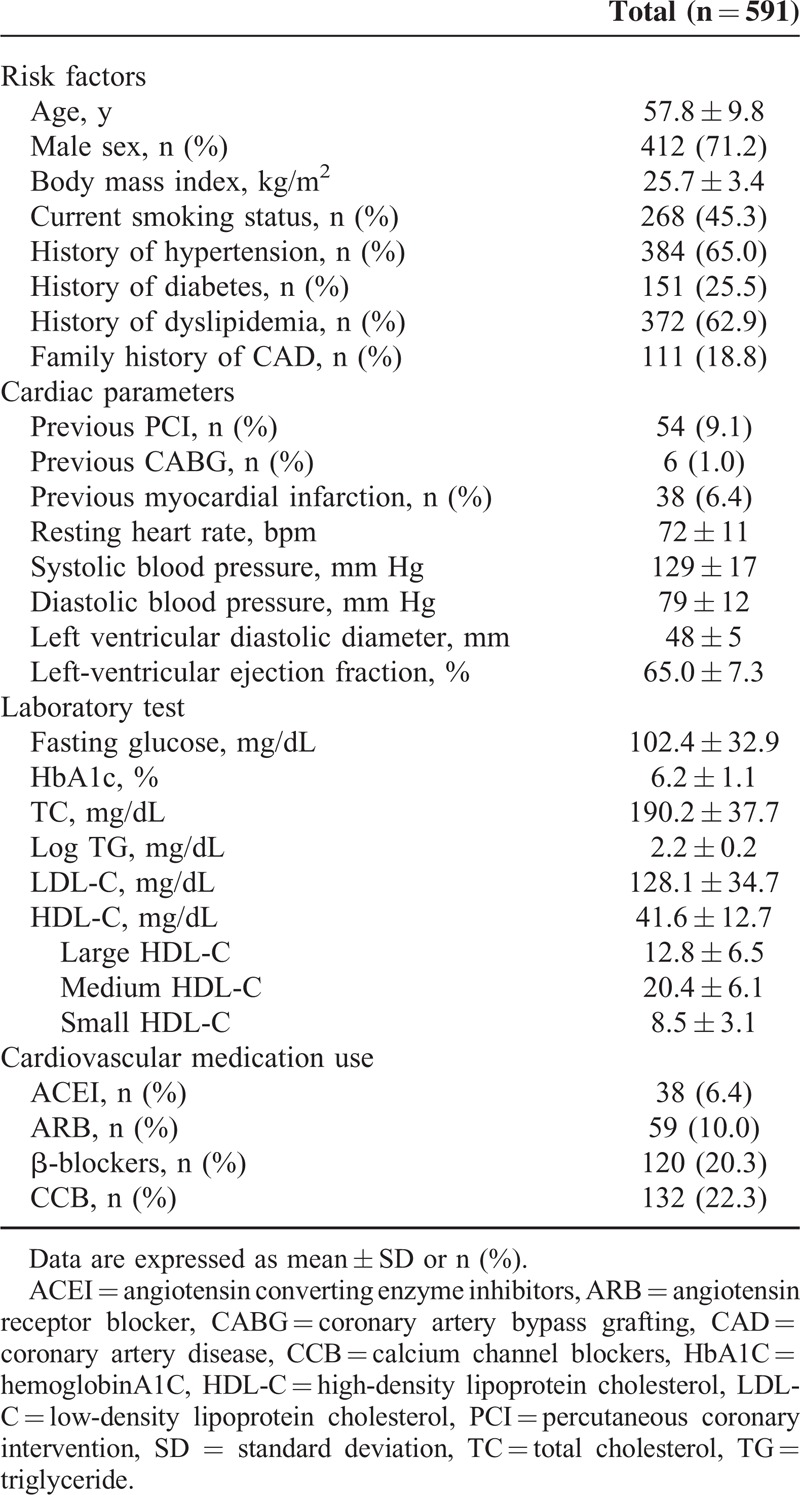
We initially recruited 591 consecutive patients with stable CAD. Baseline characteristics of the enrolled subjects were listed in Table 1. The mean age of the study patients was 57.8 years, and 71.2% were male. The mean HDL-C levels were 41.6 mg/dL. The mean HDL subfractions including large, medium, and small HDL-C levels were 12.8, 20.4, and 8.5 mg/dL, respectively.
TABLE 1.
Baseline Characteristics
HDL Subfractions With the Number of Coronary RFs and Severity of CAD
Initially, we estimated the association between HDL subfractions and traditional RFs in the current analysis. The RFs were comprised of risk age of onset, male, obesity, hypertension, DM, dyslipidemia, smoking, and family history of CAD. As shown in Figure 1, compared with the low total HDL-C, low large and medium HDL-C groups, the corresponding high groups had significantly less number of RFs (4 [3–5] vs 4 [4–5], P = 0.010; 4 [3–5] vs 5 [4–5], P < 0.001; 4 [3–5] vs 4 [4–5], P = 0.019, respectively). However, the high small HDL-C groups had relatively more number of RFs (5 [4–6] vs 4 [3–5], P = 0.004).
FIGURE 1.
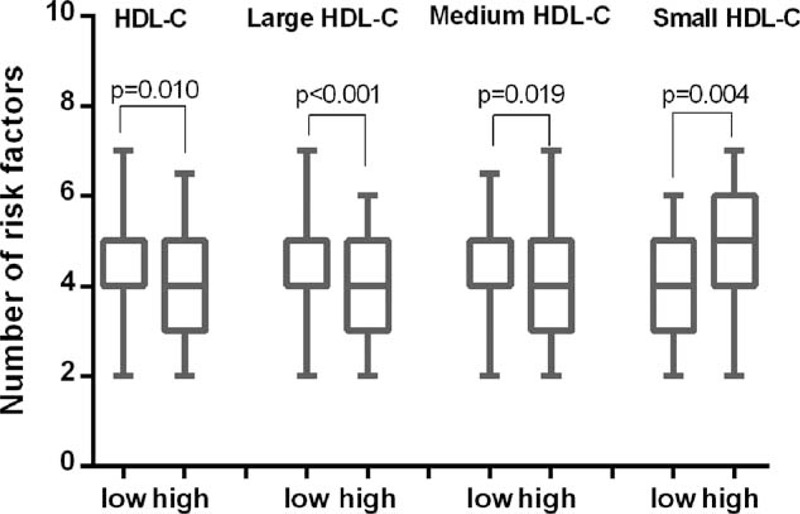
Number of risk factors in high and low groups of HDL-C, large HDL-C, medium HDL-C, and small HDL-C. HDL-C = high-density lipoprotein cholesterol.
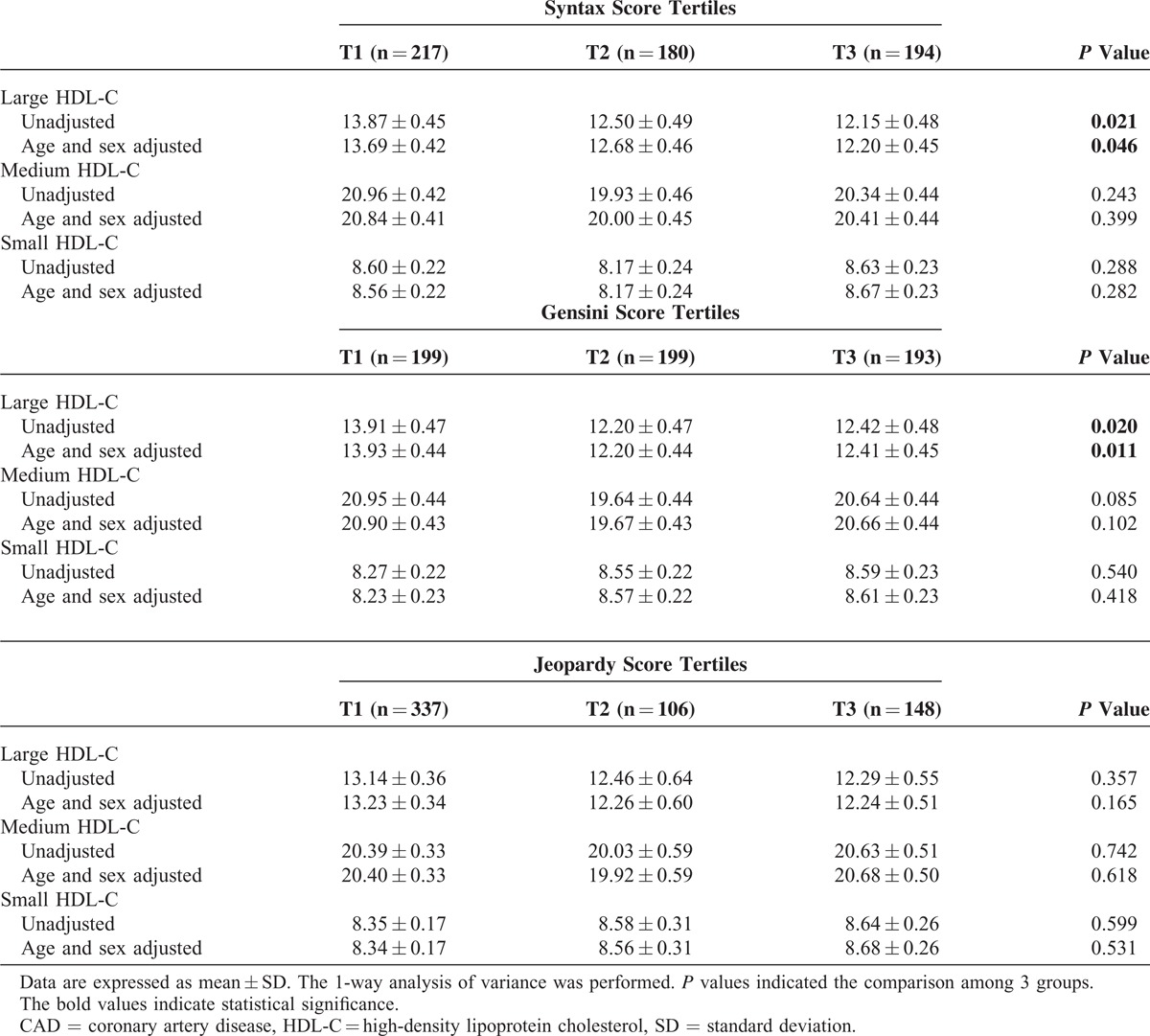
Moreover, we analyzed the relationship between HDL subfractions and the severity of CAD. As indicated in Table 2, large HDL-C levels were markedly decreased across the tertiles of SYNTAX (P = 0.021), and the tertiles of Gensini score (P = 0.020), although no similar results were observed by Jeopardy tertiles (P = 0.357). These relationships were further confirmed after adjusting for age and sex (P = 0.046, P = 0.011, and P = 0.165, respectively). No significant associations were found between medium or small HDL-C and the coronary severity scores (all P > 0.05).
TABLE 2.
Association Between HDL Subfractions and Severity of CAD
Cardiac Events During Follow-Up
The median period of follow-up was 17.0 months. Follow-up data were not obtained in 12 patients for several reasons (declined to participate, wrong addresses, etc.). Sixty-seven patients presented with MACEs during the follow-up period. Of these, twenty-seven (40.3%) developed UA, six (9.0%) suffered nonfatal MI, twenty-seven (40.3%) underwent myocardial revascularization procedures (PCI or CABG) because of clinical deterioration, three (4.5%) had strokes, and four (6.0%) suffered cardiac death. Patients suffered ACS and underwent revascularization procedures were assigned once in the analysis.
HDL Subfractions and MACEs
As depicted in Table 3, the occurrence of MACEs was less frequent in the patient with high versus low large HDL-C levels (8.7% vs 14.1%, P = 0.042). In light of the inter-relationship between HDL subfractions, traditional RFs and occurrence of MACEs, subgroup analysis was performed based on RFs tertiles (RFs < 4, n = 165; RFs = 4, n = 150; RFs > 4, n = 264). As a result, we observed that the relationship between large HDL-C groups and MACEs occurrence was most significant in the bottom RFs tertiles (Figure 2). The Kaplan–Meier analysis demonstrated a significant difference in the event-free survival rate between high and low large HDL-C groups (P = 0.013, Figure 3B). However, no significant difference was observed in high and low total HDL-C, medium, and small HDL-C groups (all P > 0.05, Figure 3A, C–D). The cardiovascular medication use was balanced between patients with and without MACEs (P > 0.05, all).
TABLE 3.
Relationship of HDL Subfractions and Cardiovascular Medication Use With Future Cardiovascular Events
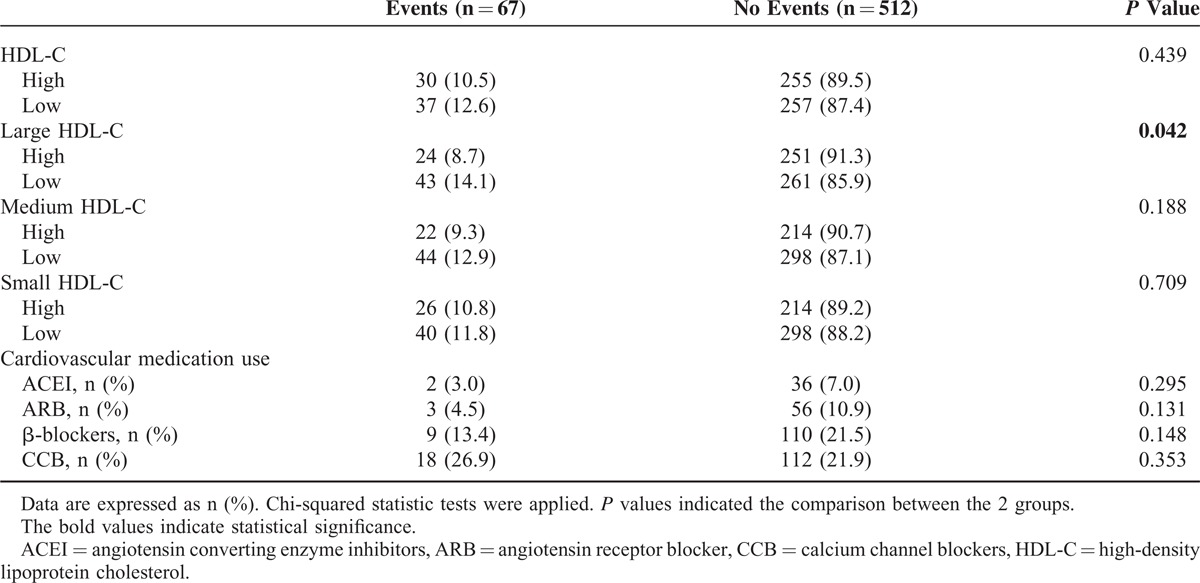
FIGURE 2.
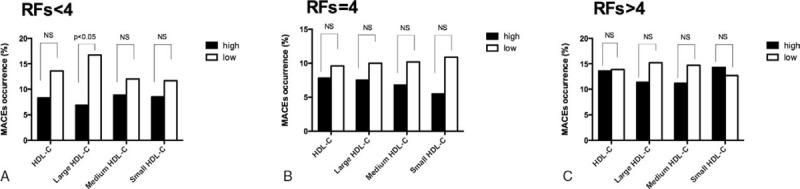
Relationship of HDL subfractions and future cardiovascular events based on the number of risk factors tertiles. HDL = high-density lipoprotein.
FIGURE 3.
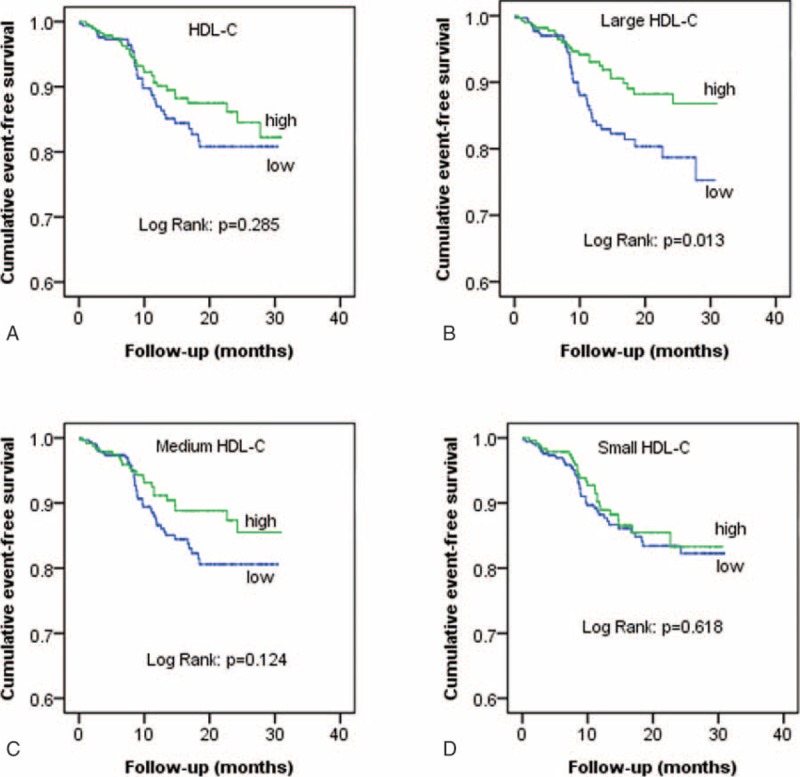
Cumulative event-free survival analysis between high and low groups of HDL-C (A), large HDL-C (B), medium HDL-C (C), and small HDL-C (D). HDL-C = high-density lipoprotein cholesterol.
To test whether large HDL-C was independently related to MACEs, univariate Cox proportional hazard regression analysis was performed in this analysis. Compared with patients with low large HDL-C, those with high large HDL-C was less likely to develop MACEs (hazard ratio [HR] = 0.536, 95% confidence interval [CI]: 0.325–0.885, Figure 4A). After adjusting potential confounders including age, male, BMI, smoke, family history of CAD, previous PCI/CABG, previous MI, systolic blood pressure, LDL-C, TG, and glucose, the predictive value of large HDL-C remained significant in this fully adjusted multivariate Cox regression analysis (multivariate-adjusted HR = 0.531, 95% CI: 0.295–0.959, Figure 4B).
FIGURE 4.
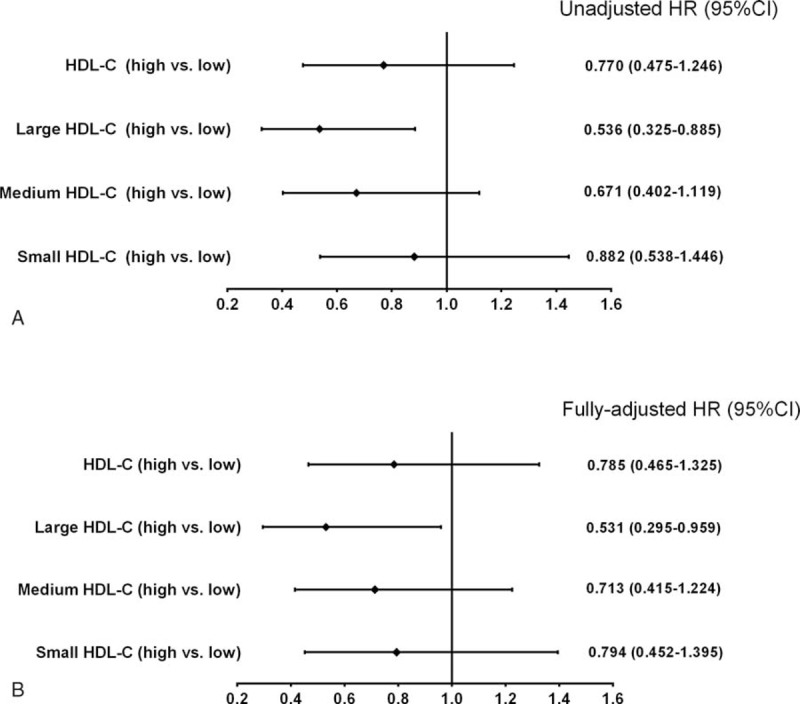
Univariate (A) and multivariate (B) Cox proportional models to reveal the associations of HDL-C, large HDL-C, medium HDL-C, and small HDL-C with future cardiovascular events. HDL-C = high-density lipoprotein cholesterol.
DISCUSSION
In this prospective study of patients with stable CAD, we for the first time comprehensively demonstrated that high large HDL-C was inversely associated with cardiovascular risk including traditional RFs, severity of CAD, and future cardiovascular outcomes. In addition, high large HDL-C was negatively and independently related to the occurrence of MACEs after adjustment for multiple confounders. The present study provided potential evidence for the value of HDL subfractionation in CAD risk assessment.
For the past few years, the inverse relevance between the cholesterol content of HDL and cardiovascular outcomes has been challenged by the failure of several HDL-C-raising drugs along with the genetic studies.16,17 Since then, the focus on HDL has been started to shift away from the HDL-C centric view toward alternative indexes of HDL. The emerging opinion is that the cholesterol concentration in specific HDL subpopulations may be more valuable than the total cholesterol contained in HDL. However, the accurate relationship between HDL subfractions and cardiovascular risk is not well clarified.
Although several cross-sectional as well as prospective studies had made efforts to address this issue, results were not consistent. As early in 1991, Salonen et al18 conducted an analysis involving 1799 men and reported that large HDL-C levels (HDL2-C) were inversely associated with the risk of acute MI and may thus be protective factors, whereas the role of small HDL-C (HDL3-C) remains equivocal. Similarly, in the Quebec Cardiovascular Study covering 1169 French-Canadian men younger than 60 years, large HDL-C (HDL2-C) but not small HDL-C (HDL3-C) was inversely correlated with the incidence of ischemic heart disease.19 However, the Monitored Atherosclerosis Regression Study (MARS) found that greater baseline levels of small HDL-C (HDL3-C) were related to a lower likelihood of coronary artery lesion progression.20 Significantly, the recent study analyzed data from 2 prospective cohorts (the TRIUMPH study of 2465 acute MI patients, and the IHCS study of 2414 patients who underwent coronary angiography), and finally concluded that small HDL-C (HDL3-C) but not HDL-C or large HDL-C (HDL2-C) levels were inversely associated with cardiovascular outcomes.9 Besides that, no predictive value of any HDL subclasses were found in the Atherosclerosis Risk in Communities (ARIC) study.21 Till now, controversy remains unresolved regarding the role of HDL subfractions on cardiovascular risk.
Despite the differences in study designs, studied populations, and primary outcomes, the existing of diverse HDL-C fractionation methodologies may mainly contribute to these inconsistencies. Among the various methods, Lipoprint system, Vertical Auto Profile (VAP) method, 2-dimensional gel electrophoresis and nuclear magnetic resonance (NMR) spectroscopy are most commonly used in clinical research. The Lipoprint system determined 10 HDL subclasses (large HDL-C 1–3, intermediate HDL-C 4–7, and small HDL-C 8–10) by decreasing size and increasing density based on electrophoresis of a liquid loading gel with lipophilic dye in the precast linear polyacrylamide gel (stacking gel and separating gel).22 The VAP separates lipoproteins into HDL2-C and HDL3-C on the base of density using single vertical-spin density gradient ultracentrifugation.9 The 2-dimensional gradient gel electrophoresis can identify 5 major HDL particles (HDLα1–4 and pre-β1) first by charge and then by size,8 while the method of NMR can separate up to 26 HDL-C subpopulations by their differences in magnetic susceptibility.23 Although several methods had been used in HDL-C separation, none of the currently used techniques can be proposed as markedly superior to the others. In the present study, we applied the Lipoprint system, which was a rapid, quantitative method and has been applied in multiple clinical studies.24 Till now, only few studies have applied this method to assess the role of HDL subfractions on cardiovascular risk. Recently, clinical investigations suggested that stable CAD patients presented as higher small HDL subfractions25 and large HDL subfractions were inversely associated with Gensini score.26 In the present study, we prospectively collected patients who did not receive lipid-lowering drugs and found that large HDL-C levels were negatively related to cardiovascular risk including the number of traditional RFs, CAD severity, and clinical outcomes, which extended previous studies and provided evidence for the predictive value of large HDL-C in cardiovascular risk assessment.
There were several limitations of the present study. First, the number of events is relatively small to assess the prognostic value of a potential RF. The conclusions need to be testified by large-scale study in the future. Secondly, we only documented the HDL-C subfractions and traditional medication use at baseline. Although all the studied patients have received secondary prevention since enrollment, the alterations of cardiovascular medications use during the follow up might influence the levels of HDL subfractions and the primary endpoints. Third, the HDL subclassification was performed by Lipoprint system, and the results should be testified by other methodologies.
CONCLUSION
In summary, we enrolled 591 consecutive nontreated patients with angiography proven CAD and found that large HDL-C levels were inversely associated with cardiovascular risk covering traditional RFs, CAD severity assessed by SYNTEX and Gensini score, and clinical outcomes. Moreover, the negative relationship between large HDL-C levels and clinical outcomes was independent of traditional RFs analyzed by Cox proportional hazard models, highlighting the potential value of HDL subfractionation in evaluating cardiovascular risk.
Acknowledgment
We are grateful to the field staff and the participants of our study.
Footnotes
Abbreviations: ACS = acute coronary syndrome, BMI = body mass index, CABG = coronary artery bypass grafting, CAD = coronary artery disease, DM = diabetes mellitus, HDL = high-density lipoprotein, HDL-C = high-density lipoprotein-cholesterol, LDL-C = low-density lipoprotein-cholesterol, MACEs = major adverse cardiovascular events, MI = myocardial infarction, PCI = percutaneous coronary intervention, RFs = risk factors, TC = total cholesterol, TG = triglyceride, UA = unstable angina.
J-JL and YZ have contributed equally.
This work was partly supported by National Natural Science Foundation (81070171 and 81241121), Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), Capital Special Foundation of Clinical Application Research (Z121107001012015), Capital Health Development Fund (2011400302), and Beijing Natural Science Foundation (7131014) awarded to J-JL.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Blacher J, Evans A, Arveiler D, et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens 2010; 24:19–26. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keys A. Alpha lipoprotein (HDL) cholesterol in the serum and the risk of coronary heart disease and death. Lancet 1980; 2:603–606. [DOI] [PubMed] [Google Scholar]
- 4.Tuteja S, Rader DJ. High-density lipoproteins in the prevention of cardiovascular disease: changing the paradigm. Clin Pharmacol Ther 2014; 96:48–56. [DOI] [PubMed] [Google Scholar]
- 5.Fazio S, Linton MF. Elevated high-density lipoprotein (HDL) levels due to hepatic lipase mutations do not reduce cardiovascular disease risk: another strike against the HDL dogma. J Clin Endocrinol Metab 2009; 94:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arsenault BJ, Lemieux I, Després JP, et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis 2009; 206:276–281. [DOI] [PubMed] [Google Scholar]
- 7.Superko HR, Pendyala L, Williams PT, et al. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol 2012; 6:496–523. [DOI] [PubMed] [Google Scholar]
- 8.Rosenson RS, Brewer HB, Jr, Chapman MJ, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem 2011; 57:392–410. [DOI] [PubMed] [Google Scholar]
- 9.Martin SS, Khokhar AA, May HT, et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J 2015; 36:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Li S, Zhu CG, et al. Risk factors, coronary severity, outcome and ABO blood group: a large Chinese Han Cohort Study. Medicine (Baltimore) 2015; 94:e1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xu RX, Li S, et al. Lipoprotein subfractions partly mediate the association between serum uric acid and coronary artery disease. Clin Chim Acta 2015; 441:109–114. [DOI] [PubMed] [Google Scholar]
- 12.Xu RX, Li S, Zhang Y, et al. Relation of plasma PCSK9 levels to lipoprotein subfractions in patients with coronary artery disease. Lipid Health Dis 2014; 13:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav M, Palmerini T, Caixeta A, et al. Prediction of coronary risk by SYNTAX and derived scores: synergy between percutaneous coronary intervention with taxus and cardiac surgery. J Am Coll Cardiol 2013; 62:1219–1230. [DOI] [PubMed] [Google Scholar]
- 14.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606. [DOI] [PubMed] [Google Scholar]
- 15.Califf RM, Phillips HR, III, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol 1985; 5:1055–1063. [DOI] [PubMed] [Google Scholar]
- 16.Kingwell BA, Chapman MJ, Kontush A, et al. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov 2014; 13:445–464. [DOI] [PubMed] [Google Scholar]
- 17.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006; 113:1556–1563. [DOI] [PubMed] [Google Scholar]
- 18.Salonen JT, Salonen R, Seppänen K, et al. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation 1991; 84:129–139. [DOI] [PubMed] [Google Scholar]
- 19.Lamarche B, Moorjani S, Cantin B, et al. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol 1997; 17:1098–1105. [DOI] [PubMed] [Google Scholar]
- 20.Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS). Treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol 1996; 16:697–704. [DOI] [PubMed] [Google Scholar]
- 21.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein (a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001; 104:1108–1113. [DOI] [PubMed] [Google Scholar]
- 22.Tsuzaki K, Kotani K, Sano Y, et al. The relationship between adiponectin, an adiponectin gene polymorphism, and high-density lipoprotein particle size: from the Mima study. Metabolism 2012; 61:17–21. [DOI] [PubMed] [Google Scholar]
- 23.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med 2011; 17:594–603. [DOI] [PubMed] [Google Scholar]
- 24.Hoefner DM, Hodel SD, O’Brien JF, et al. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem 2001; 47:266–274. [PubMed] [Google Scholar]
- 25.Xu RX, Zhang Y, Ye P, et al. Analysis of lipoprotein subfractions in Chinese Han patients with stable coronary artery disease. Heart Lung Circ 2015; 24:1203–1210. [DOI] [PubMed] [Google Scholar]
- 26.Xu RX, Li S, Li XL, et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: a Gensini score assessment. J Clin Lipidol 2015; 9:26–34. [DOI] [PubMed] [Google Scholar]


