Abstract
Although CD3-CD56+NKp44+ natural killer (NKp44+NK) cells have been linked to autoimmune diseases including inflammatory bowel disease, ankylosing spondylitis, and primary Sjogren syndrome, the expansion and role of those cells in patients with rheumatoid arthritis (RA) remain less defined. Here, we investigate the proportion and pathogenesis of NKp44+NK cells in patients with RA. The results show NKp44+NK cells significantly expanded in RA peripheral blood and synovial fluid, which were correlated positively with RA disease activity. They also highly expressed in RA synovial tissues and secreted a high concentration of interleukin-22 (IL-22) in vitro. Further, NKp44+NK cells culture supernatant promoted the proliferation of fibroblast-like synoviocytes (FLS) which was blocked by IL-22 antagonist and AG490. Treated with recombination human IL-22, the proliferation and phosphorylation-STAT3 on RA-FLS increased in a dose-dependent manner and time-dependent manner; the progress of which could be blocked by AG490. The present study clarifies the expansion of NKp44+NK cells in the peripheral blood and synovial fluid of patients with RA, especially in the synovial tissues of RA for the first time. STAT3 is an essential pathway in mediating the effects of IL-22 secreted by NKp44+NK cells on the proliferation of FLS in patients with RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, inflammatory, systemic, autoimmune disorder characterized by destructive polyarticular joint disease, which leads to functional disability, premature mortality, and severe long-term economic consequences.1,2 RA affects approximately 0.5% to 1.0% of the adult population across the globe.3 The current view on RA is that the disease results from a complex interplay among multiple factors including genetic predisposition, environmental triggers, immune status, and stochastic factors.4 Environmental exposure such as smoking leads to the loss of tolerance to self-proteins by environment–gene interactions.5 Thereafter, antigens processed by dendritic cells drive the activation of CD4+ T and B cells in synovial germinal centers or lymph nodes.6 Further, the production of pathogenic antibodies, abnormal activation of cytokine networks, and other molecular products of damage lead to chronic synovitis and progressive bone destruction in joints as well as systemic disorders through migration and local positive feedback mechanisms.7
The main features of RA pathogenesis include massive and persistent synovial proliferation in multiple joints followed by destruction of cartilages and bones.8 As an important component of innate immune system, natural killer (NK) cells characterized as CD3-CD56+ cells have also been implicated in the adaptive immune responses through cytokine and chemokine production or contact-dependent cell–cell interactions during the pathogenesis of RA synovitis.9 However, it remains controversial whether NK cells promote or protect against disease progression in mice with collagen-induced arthritis,10,11 because different subsets of NK cells may play a distinct role in different local microenvironment. High proportion of CD3-CD56+NKp44+ NK cells (NKp44+NK cells), a CD56bright NK cells subset, have been detected in patients with inflammatory bowel disease,12 ankylosing spondylitis,13 and primary Sjogren syndrome14; and these cells play a tissue-protective or a proinflammatory role by overexpression of interleukin 22 (IL-22). Also, the expression of IL-22 receptor 1 has already been identified on the fibroblast-like synoviocytes (FLS) in patients with RA.15 Our previous studies have also showed that a subset of NKp44+NK cells, NK-22 cells, have a significantly greater proportion in patients with RA; and these cells were found to play a role in the pathogenesis of RA.16 In addition, synovial fluid (SF) NK cells express a higher degree of activation markers including CD69 and NKp44 compared to the peripheral blood (PB) NK cells.17 However, the expression of NKp44+NK cells in RA synovial tissue and their mechanism in the pathogenesis of FLS proliferation are still unclear.
The purpose of this study was to investigate not only the proportion of NKp44+NK cells in the SF and PB of patients with RA, but also the expression of those cells in RA synovial tissue. Since NKp44+NK cells could secrete IL-22, the study also aimed to evaluate the IL-22-dependent signal pathway that could promote FLS proliferation.
PATIENTS AND METHODS
Ethics Statement
The study was conducted according to the principles expressed in the Declaration of Helsinki. All samples, clinical data, and demographic data were obtained after patients had given their written consent which was approved by the Institutional Medical Ethics Review Board of Nanfang Hospital (NO. NFEC-20120201).
Antibodies, Reagents, and Kits
Fluorochrome-conjugated mouse immunoglobulin (IgG) anti-human antibodies, including APC-CD56 (B159), FITC-CD3 (HIT3a), PE-NKp44 (p44-8.1), FITC-CD55 (IA10), and APC/FITC/PE-conjugated mouse IgG1 (MOPC-21) or IgG2a (G155-178) isotype-matched control antibodies were purchased from BD Pharmingen (San Jose, CA). 1-Step Fix/Lyse Solution (10×), cell stimulation cocktail (500×) containing phorbol 12-myristate 13-acetate and ionomycin, and human IL-22 platinum Enzyme-linked immunosorbent assay (ELISA) were purchased from eBiosciences (San Diego, CA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), l-glutamine, penicillin, and streptomycin were purchased from GIBCO (Grand Island, NY). Type I collagenase, Triton X-100, methyl thiazolyl tetrazolium (MTT) solution, and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). IL-22 antagonist (142928) and recombinant human IL-22 (rhIL-22) were purchased from R&D Systems (Minneapolis, MN). Tyrphostin AG490, mouse IgG1 anti-human vimentin (RV202), mouse IgG1 anti-human CD68 (KP1), and immunoCruz mouse LSAB staining system (sc-2050) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse IgG anti-human CD56 (RNL-1), rabbit IgG anti-human NKp44 (EPR5060-2), and mouse or rabbit IgG isotype-matched control antibodies (ICIGG1, SP137) were purchased from Abcam (Cambridge, MA). Alexa fluor 488 donkey antimouse IgG antibody, alexa fluor 555 donkey antirabbit IgG antibody, and Hoechst 33342 were purchased from Invitrogen (Carlsbad, CA). Radioimmunoprecipitation assay (RIPA) lysis buffer I, BCA protein assay kit, and polyvinylidene difluoride (PVDF) membranes were purchased from Sangon Biotech (Shanghai, China). Anti-STAT3 mouse monoclonal antibody (mAb, 124H6), anti-phospho-STAT3 (Tyr705) mouse mAb (3E2), and anti-glyceraldehyde phosphate dehydrogenase (GAPDH) rabbit mAb (14C10) were purchased from Cell Signaling Technology (Danvers, MA). Peroxidase AffiniPure goat antimouse or rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The ECL Plus kit was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Patients’ Samples
PB samples from 37 patients with RA and 35 healthy volunteers were collected in ethylenediaminetetraacetic acid anticoagulant tubes. SF samples were isolated from the knee joint of 16 patients with knee osteoarthritis (KOA) or 21 patients with RA who donated their PB samples synchronously during the therapeutic arthrocentesis. Synovial tissue specimens were obtained during the joint replacement surgery from patients with RA and KOA (3 patients each) who also donated their PB and SF samples. All patients with RA and KOA fulfilled the American College of Rheumatology classification criteria for RA and KOA.
Flow Cytometric Analysis
To 100 μL of the whole blood, 10 μL each of anti-CD3/CD56/NKp44 fluorescently labeled antibodies was added and incubated for 30 minutes at 4°C. Red blood cells were then lysed with 2 mL 1-Step Fix/Lyse solution (1×) for 20 minutes. Finally, cells were resuspended in flow stain buffer and analyzed on a FACSAria I (BD Bioscience, San Jose, CA). Isotype-matched control antibodies were simultaneously processed. For SF samples, 1 × 106 cells were collected and handled as described for PB samples except for the lysis of red blood cells.
The separation of NKp44+NK cells from RA SF samples were performed in 1 mL experimental sample with 3 × 107 cells by adding 100 μL each of anti-CD3/CD56/NKp44 antibodies. The manipulation was completed according to the procedure described above. 1 × 105 cells were collected for NKp44+NK cells culture in vitro. The NK cells marked with CD3-CD56+ were also separated from RA SF samples.
The identification procedure of RA FLS incubated with anti-CD55 antibody18 was similar as described above.
Cell Culture and Drug Treatment In Vitro
The separated NK and NKp44+NK cells were cultured in DMEM complete medium which contains 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% carbon dioxide (CO2).16 The cells were treated with cell stimulation cocktail (1×) for 5 hours before the supernatant was collected as previously described.16
FLS were isolated from knee joint synovial tissues as previously described.19 Briefly, synovial tissues were minced into 2 to 3 mm pieces and treated with 2 mg/mL type I collagenase in DMEM for 2 hours at 37°C in 5% CO2. The cells then were dissociated through a nylon mesh, harvested in DMEM complete medium, and cultured at 37°C in 5% CO2. All in vitro experiments were conducted using FLS between passages 4 and 5.
To identify the FLS proliferation, the cells were stimulated with a final concentration of 50% NKp44+NK cells culture supernatant, 50 μg/mL IL-22 antagonist, combination of cells culture supernatant and IL-22 antagonist, different concentrations of rhIL-22 (1, 10, 50, and 100 ng/mL), and negative controls for 24, 48, or 72 hours separately. To clarify the signaling pathways in IL-22 treated FLS, the cells were treated with 50 ng/mL rhIL-22 for 0, 0.25, 0.5, 1, 1.5, 2, 4, and 8 hours. To inhibit the STAT3 signaling pathways, 100 μM AG490 was added 2 hours before stimulation of 50 ng/mL rhIL-22; and the FLS were continually incubated for 0.25, 0.5, 1, 1.5, and 2 hours. At last, the FLS were treated with 100 μM AG490 combined with 50% NKp44+NK cells culture supernatant or 50 ng/mL rhIL-22 for 24, 48, or 72 hours to identify the blocking effect of FLS proliferation.
Immunohistochemical Analysis
Immunohistochemical identification of FLS was performed on 4% paraformaldehyde fixed cells cultured on chamber slides using immunoCruz mouse LSAB staining system.18,19 After the slides were incubated in 0.3% Triton X-100 for 20 minutes and inactivated with 3% peroxide-methanol for 15 minutes at room temperature. The slides were incubated at 37°C with 5% normal goat serum for 30 minutes, with primary antibodies (mouse IgG1 antihuman vimentin/CD68, or normal mouse IgG as negative controls) for 2 hours and then with biotinylated antimouse IgG for 30 minutes. After incubated with horseradish peroxidase-streptavidin complex for 30 minutes at 37°C, the slides were dyed with diaminobenzidine for 10 minutes and counterstained with hematoxylin for 1 minute at room temperature.
Dual-labeling immunofluorescence was performed on 5 μm thick frozen sections.12–14 The sections were incubated in 0.3% Triton X-100 for 20 minutes at room temperature. After blockade with 5% normal goat serum for 30 minutes at 37°C, the primary antibodies (mouse IgG anti-human CD56 and rabbit IgG anti-human NKp44) with 1:50 dilution were added and incubated overnight at 4°C. Isotype-matched control antibodies were used as negative controls. The sections were then incubated with alexa fluor 488 donkey antimouse IgG antibody and alexa fluor 555 donkey antirabbit IgG antibody for 1 hour at 37°C. At last, nuclear counterstaining was performed with Hoechst 33342 for 10 minutes. All images were obtained on an Axio Imager Z1 microscope equipped with an AxioCam digital camera (Zeiss, Jena, Germany).
ELISA and Cell Proliferation Assay
IL-22 concentrations in NK and NKp44+NK cells culture supernatants were determined using Human IL-22 Platinum ELISA according to the manufacturer's protocol.
Cell proliferation was examined by MTT assay.16 A total of 3 × 103 FLS/well were incubated in a 96-well plate for 24 hours. After the intervention, MTT was then added to a final concentration of 0.5 mg/mL, and the FLS were incubated again for 4 hours. At last, 150 μg DMSO was added to the empty wells which were shaken for 10 minutes to fully melt the crystals. Optical density at 490 nm was determined by a Model EL309 microplate reader (Bio-Tek Instruments Inc., Burlington, VT).
Western Blot Analysis
The signaling pathways were detected by western blot analysis.15 A total of 1 × 105 FLS/well were incubated in a 6-well plate for 24 hours. After the intervention, the cells were washed with ice-cold PBS, homogenized on ice in the RIPA lysis buffer I with protease inhibitors, and centrifuged at 10,000 rpm for 10 minutes at 4°C. The total protein concentration was determined using the BCA protein assay kit. Equal amounts of total proteins (30 μg) were separated on a 12% sodium dodecyl sulfate-polyacrylamide gelelectrophoresis and transferred onto a PVDF membrane. After being blocked for 1 hour with Tris buffered saline containing 0.5% Tween 20 and 5% skim milk at room temperature, the membranes were incubated overnight at 4°C with primary antibodies (anti-STAT3 and anti-phospho-STAT3 mouse mAb) diluted to 1/1000. Membranes were then incubated with a 1:5000 dilution of peroxidase AffiniPure goat anti-mouse or rabbit IgG for 1 hour at room temperature. The Western blot was visualized by the ECL Plus kit. Human GAPDH proteins expression was measured as an internal control.
Statistical Analysis
All results had been representative of at least 3 independent experiments. All statistics were calculated with SPSS 16.0 (SPSS Inc., Chicago, IL). Data are presented as mean ± standard deviation (mean ± SD). Student's t-test or one-way analysis of variance followed by Bonferroni or Tambane T2 test was used to evaluate the significance of the differences. The nonnormal distribution measurement data are tested with the Wilcoxon or Kruskal–Wallis rank-sum test. Spearman correlation analysis was performed to evaluate the association between variables. A P < 0.05 was considered statistically significant (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). All P values were 2-tailed.
RESULTS
NKp44+NK Cells Expand in the PB, SF, and Synovial Tissues of Patients With RA
Characteristics of the patients and controls included for flow cytometric analysis are shown in Table 1. The phenotypes of NKp44+NK cells in the PB and SF were analyzed using 3 color flow cytometry (Figure 1A). Compared with healthy volunteer controls, the proportion of NKp44+NK cells in the PB NK cells increased significantly in patients with RA (3.1% ± 2.4% vs 0.5% ± 0.7%; P < 0.001). The substantial increase of these cells was also detected in the SF of these patients, when compared with in the SF of KOA controls (6.6% ± 4.3% vs 0.9% ± 1.1%; P < 0.001) as well as compared with in the PB of matched patients with RA (6.6% ± 4.3% vs 3.1% ± 2.4%; P < 0.001) (Figure 1B).
TABLE 1.
Characteristics of the Patients and Controls Included in Flow Cytometric Analysis
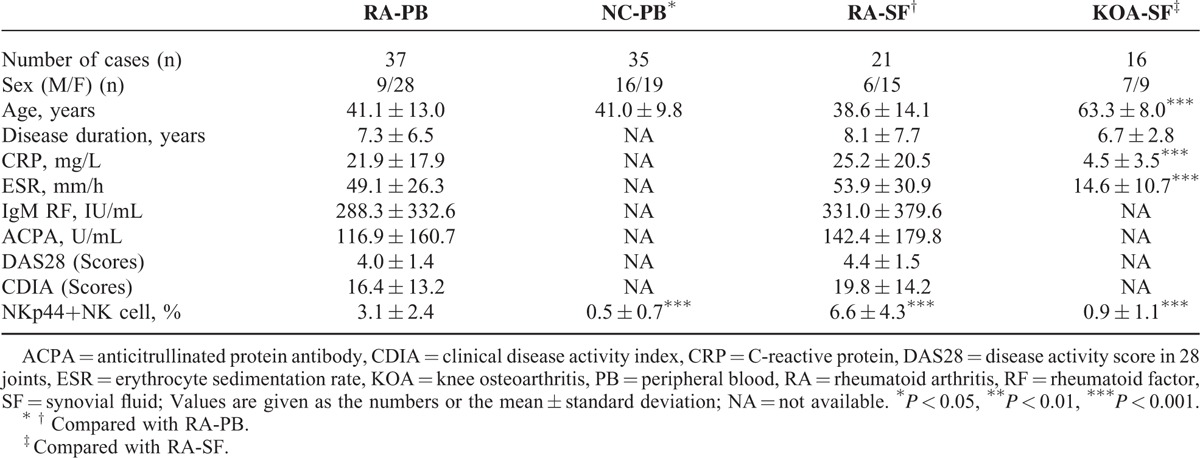
FIGURE 1.
Expansion of NKp44+NK cells in PB, SF, and synovial tissues of patients with RA. (A, B) Cells were prepared from PB and SF of patients with RA, KOA, and normal controls (NC) and were then stained for CD3, CD56, and NKp44. Lymphocytes were defined by the forward and side scatter gates (Gates-1). NK cells were defined by CD3-CD56+ lymphocytes (Gates-2-Q1). The proportion of NKp44+NK cells marked with NKp44-positive NK cells was penned in Gates-3. The representative flow chart (A) and statistical analysis (B) are shown. ∗∗∗P < 0.001. (C) Representative dual-labeling immunofluorescence shows that CD56+ (green) cells express in both RA and KOA synovial tissues, while NKp44+ (red) cells express only in RA. NKp44+NK cells with coexpressed CD56 and NKp44 show a round to oval morphology (white). Original magnifications were ×200. The results represent 3 independent experiments. KOA = knee osteoarthritis, NC = normal controls, NK = natural killer, PB = peripheral blood, RA = rheumatoid arthritis, SF = synovial fluid.
Additionally, the expressions of NKp44+NK cells with CD56+ and NKp44+ examined by dual-labeling immunofluorescence were observed in RA synovial tissues, whereas those cells were hardly expressed in KOA synovial tissues (Figure 1C).
NKp44+NK Cells Expansion Is Correlated With RA Disease Activity
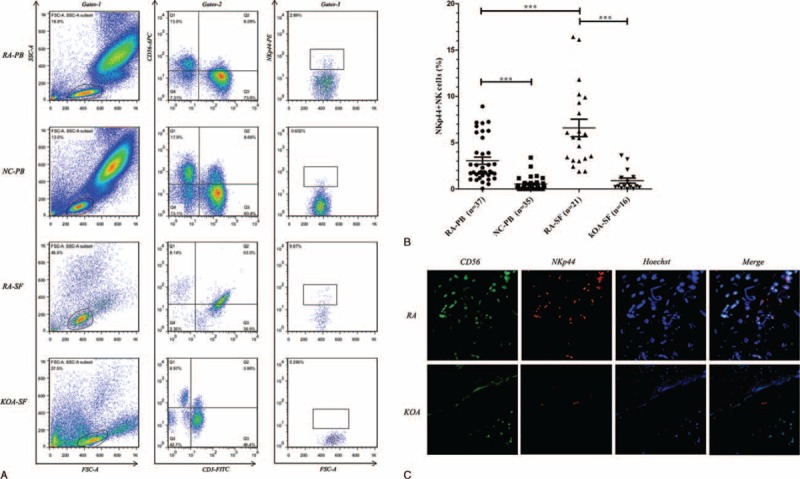
The average scores for disease activity score in 28 joints (DAS28) and clinical disease activity index (CDIA) were 4.0 ± 1.4 and 16.4 ± 13.2 in RA-PB group and 4.4 ± 1.5 and 19.8 ± 14.2 in RA-SF group, respectively (Table 1). The frequency of NKp44+NK cells in the PB of patients with RA positively correlated with the levels of DAS28 (r = 0.886, P < 0.001) (Figure 2A) or CDIA (r = 0.895, P < 0.001) (Figure 2B). Similar in the SF of patients with RA, NKp44+NK cells were also positively correlated with the levels of DAS28 (r = 0.930, P < 0.001) (Figure 2C) or CDIA (r = 0.904, P < 0.001) (Figure 2D).
FIGURE 2.
Elevation of NKp44+NK cells in PB and SF correlates with RA disease activity. Positive correlation was found between the frequency of NKp44+NK cells and levels of DAS28 (A) and CDIA (B) in the PB of patients with RA. Positive correlation was also found between the frequency of NKp44+NK cells and levels of DAS28 (C) and CDIA (D) in the SF of patients with RA. CDIA = clinical disease activity index, DAS28 = disease activity score in 28 joints, NK = natural killer, PB = peripheral blood, RA = rheumatoid arthritis, SF = synovial fluid.
NKp44+NK Cells Cultured In Vitro Secrete High Concentrations of IL-22
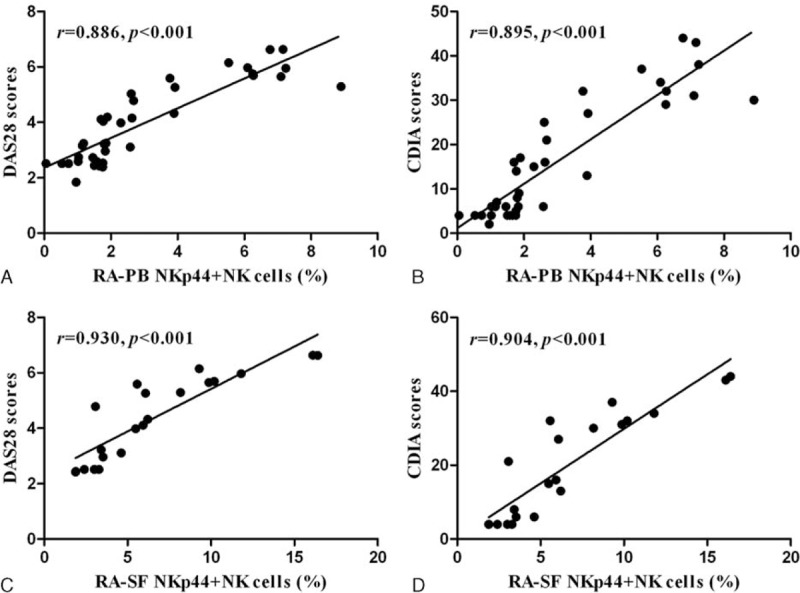
Considering that NKp44+NK cells may provide an innate source of IL-22,13,14 the levels of IL-22 were detected. After treatment with cell stimulation cocktail, the levels of IL-22 in NKp44+NK and NK cell culture supernatants were 5826.5 ± 284.2 and 801.4 ± 158.9 pg/mL (P < 0.001) (Figure 3).
FIGURE 3.
The concentration of IL-22 expresses in the cells culture supernatants. Increased expression of IL-22 in NKp44+NK cell cultured supernatants was observed compared with that of NK cells after stimulation with cell stimulation cocktail (1×) for 5 hours, as determined by ELISA. ∗∗∗P < 0.001. The results are representative of three independent experiments. ELISA = Enzyme-linked immunosorbent assay, IL-22 = interleukin 22, NK = natural killer.
Identification of FLS Cultured In Vitro
Vimentin-positive FLS cells were almost found in all in vitro culture of FLS between passages 4 and 5, while CD68-positive macrophages were barely detected (Figure 4A). The purity of FLS marked with CD55 was nearly 99% according to the flow cytometric analysis (Figure 4B).
FIGURE 4.
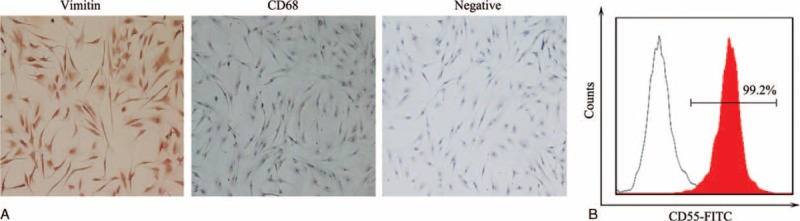
Identification of fibroblast-like synoviocytes (FLS) cultured in vitro. FLS between passages 4 and 5 was used for identification. Vimentin and CD68 were stained in cell climbing slices by immunohistochemical analysis. Vimentin-positive cells were almost found all in vitro culture of FLS, while CD68-positive cells were barely detected (A). Original magnifications were ×100. The purity of FLS marked with CD55 was nearly 99% according to the flow cytometric analysis (B). All results represent three independent experiments.
Effect of NKp44+NK Cells on the Proliferation of FLS via IL-22
FLS was cultured with NKp44+NK cell culture supernatants. Compared with untreated cells, treatment with 50% cell culture supernatants after 24, 48, or 72 hours increased the proliferation of FLS (Figure 5A).
FIGURE 5.
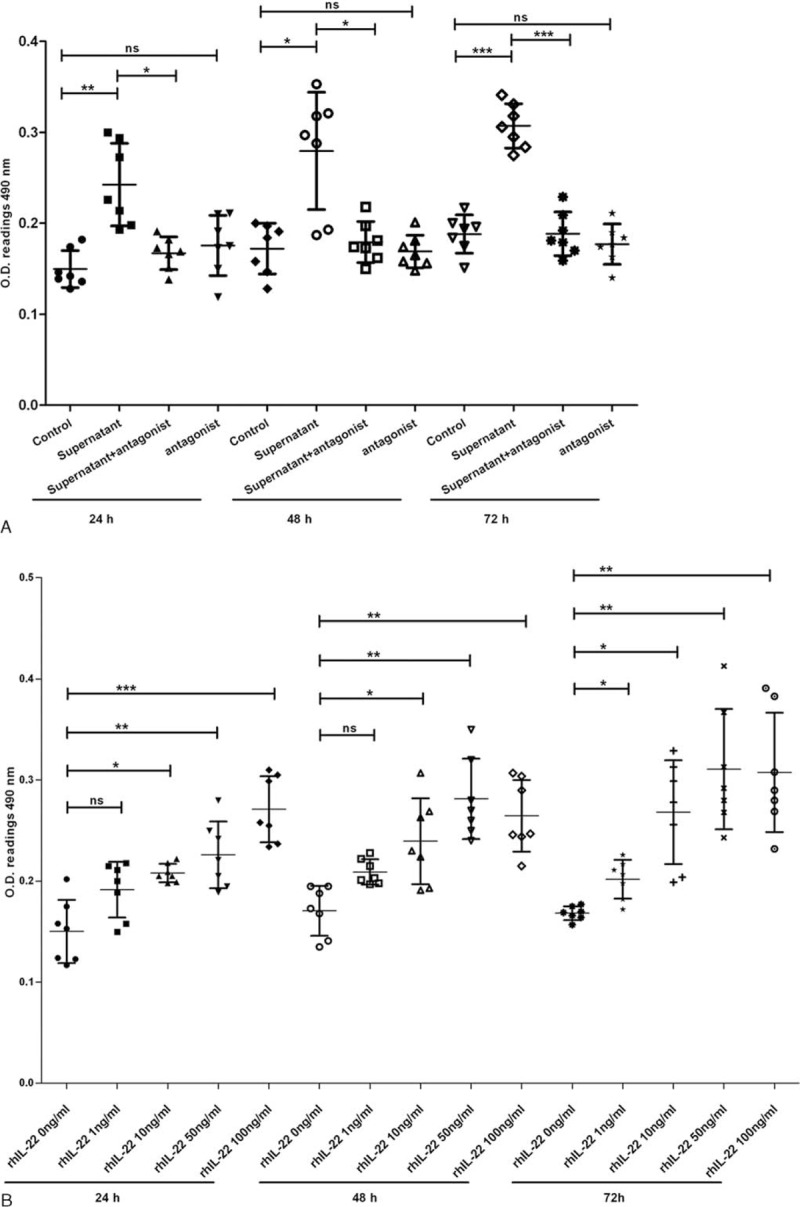
NKp44+NK cells promote the proliferation of FLS via IL-22. The FLS were treated by 50% NKp44+NK cell culture supernatants, 50 μg/mL IL-22 antagonist, combination of both of them, different concentrations of rhIL-22, and negative controls for 24, 48, or 72 hours, respectively. They were then analyzed by MTT assay. NKp44+NK cells culture supernatant promote the proliferation of FLS (A). IL-22 antagonist blocks those promotion process (A). Different concentrations of rhIL-22 promote the proliferation of RA-FLS (B). Values represent mean ± SD of O.D. 490 nm. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, no significant difference. The data are representative of seven independent experiments. FLS = fibroblast-like synoviocytes, IL-22 = interleukin 22, MTT = methyl thiazolyl tetrazolium, NK = natural killer, RA = rheumatoid arthritis, rhIL-22 = recombinant human interleukin 22, SD = standard deviation.
Further, the proliferation of FLS decreased significantly when treated with the combination of 50% NKp44+NK cell culture supernatants and 50 μg/mL IL-22 antagonist compared with individual cell culture supernatants after 24, 48, or 72 hours (Figure 5A). However, no significant differences of the FLS proliferation were found in response to the treatment with or without 50 μg/mL IL-22 antagonist.
Additionally, to clarify the relationship between IL-22 secreted by NKp44+NK cells and FLS proliferation, the stimulation of rhIL-22 on the proliferation of FLS was examined. The FLS proliferation increased with rhIL-22 concentration in a dose-dependent manner, especially treated after 72 hours (Figure 5B).
STAT3 Signaling Pathway Is Activated in the FLS Treated by RHIL-22
Because of the dependency of RA synoviocyte abnormal growth and survival on STAT3-dependent signaling pathway,20 the phosphorylation and total protein of STAT3 were examined in rhIL-22 treated FLS. Western blot analysis showed that 50 ng/mL rhIL-22 promoted the tyrosine phosphorylation of STAT3 from 0 to 8 hours, while the total protein expression of STAT3 had no change (Figure 6A). Further, semiquantitative Western blotting analyzed that tyrosine phosphorylation of STAT3 increased in a time-dependent manner, but the total protein of STAT3 did not change (Figure 6B,C).
FIGURE 6.
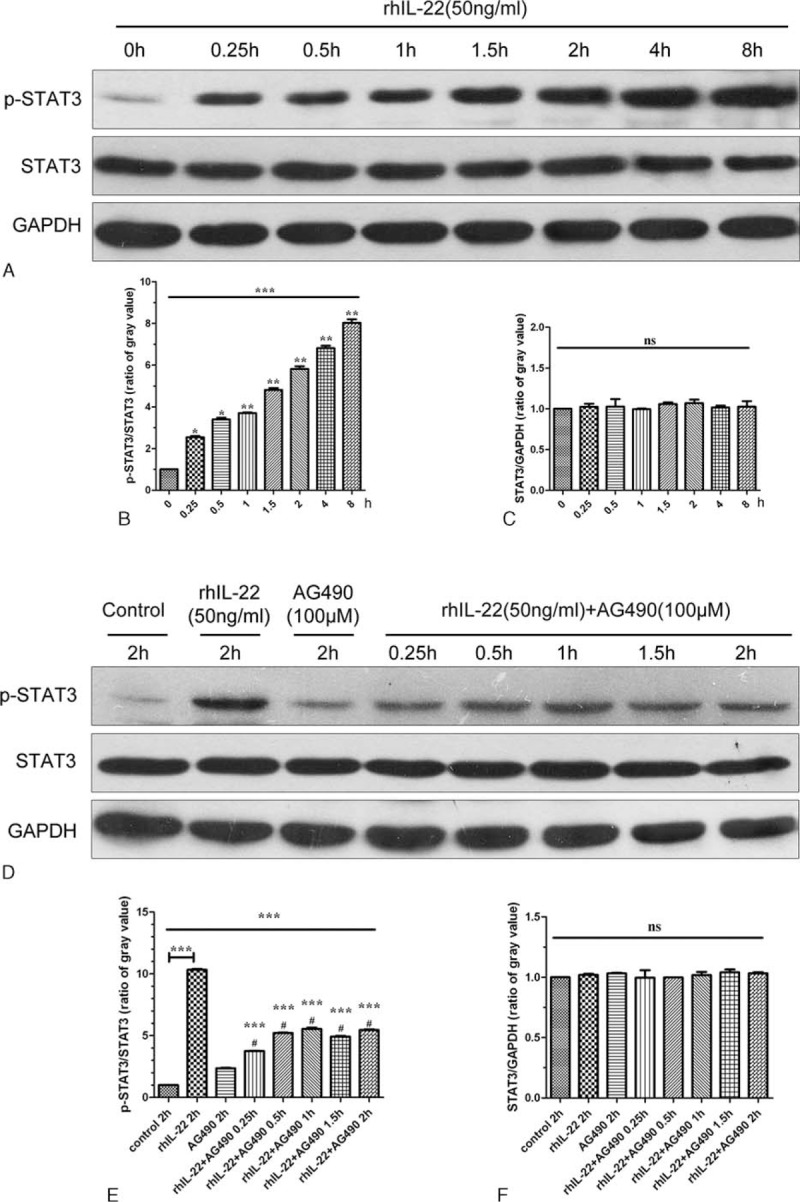
The activation of STAT3 signaling pathway in rhIL-22 treated FLS. The FLS were treated with 50 ng/mL rhIL-22 and/or 100 μM AG490 for different times. The representative Western blot chart and statistical analysis show tyrosine phosphorylation of STAT3 increased in a time-dependent manner when compared with controls (A, B) (∗∗∗P < 0.001). Simultaneously, a significant decrease in rhIL-22 stimulated tyrosine phosphorylation of STAT3 was found after treatment with the combination of AG490 when compared with single rhIL-22 stimulation (D, E) (∗∗∗P < 0.001). However, the total protein of STAT3 did not change when compared with controls (A, C, D, F) (P > 0.05). The results are representative of 3 independent experiments. FLS = fibroblast-like synoviocytes, rhIL-22 = recombinant human interleukin 22.
Additionally, AG490 was used to selectively inhibit SATA3 signaling. A significant decrease in 50 ng/mL rhIL-22 stimulated tyrosine phosphorylation of STAT3 was found after treatment with the combination of 100 μM AG490 from 0.25 to 2 hours when compared with single rhIL-22 stimulation for 2 hours (Figure 6D). Semiquantitative Western blotting revealed the same findings (Figure 6E). The total protein expression of STAT3 also had no change after stimulation (Figure 6D,F).
AG490 Blocks the Proliferation of FLS Promoted by rhIL-22 or NKp44+NK Cell Culture Supernatants
A significant decrease in 50 ng/mL rhIL-22 inducing the FLS proliferation was found after 24, 48, or 72 hours intervention with 100 μM AG490 when compared with intervention without inhibitor (Figure 7A). In addition, AG490 could also inhibit the FLS proliferation significantly after 24, 48, or 72 hours when compared with untreated cells (Figure 7A). Similarly, MTT assay revealed significantly less proliferation in 100 μM AG490-treated than no inhibitor-treated cells when 50% NKp44+NK cell culture supernatants were used together for intervention (Figure 7B). Compared with untreated cells, 100 μM AG490 decreased 50% NKp44+NK cell culture supernatants induced the FLS proliferation (Figure 7B).
FIGURE 7.
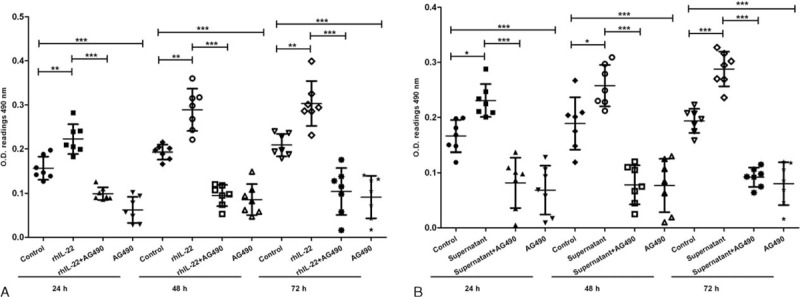
AG490 blocks the proliferation of FLS promoted by rhIL-22 or NKp44+NK cells culture supernatants. The FLS between passages 4 and 5 were treated by 100 μM AG490 combined with 50% NKp44+NK cell culture supernatants or 50 ng/mL rhIL-22 for 24, 48, or 72 hours, respectively. And then, they were analyzed by MTT assay. AG490 blocks the proliferation of RA-FLS interfered by rhIL-22 (A). AG490 blocks the proliferation of RA-FLS which were interfered with NKp44+NK cell supernatant (B). Values represent mean ± SD of O.D. 490 nm. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. The data are representative of seven independent experiments. FLS = fibroblast-like synoviocytes, MTT = methyl thiazolyl tetrazolium, NK = natural killer, RA = rheumatoid arthritis, rhIL-22 = recombinant human interleukin 22, SD = standard deviation.
DISCUSSION
The results of the present study show for the first time that NKp44+NK cells expand in RA synovial tissues. This finding is in agreement with recent report on the expression of NKp44+NK cells in patients with ankylosing spondylitis and primary Sjogren syndrome,13,14 which revealed that those cells also play an important role in adaptive immune responses through the production of IL-22. Although our previous study has showed that NK-22 cells, a subset of NKp44+NK cells, have a significantly greater proportion in the PB and SF of patients with RA,16 no studies have confirmed the expansion of NKp44+NK cells in PB and SF of patients with RA. This study revealed that the proportion of NKp44+NK cells in the PB increased significantly in patients with RA than in healthy controls (3.1% ± 2.4% vs 0.5% ± 0.7%). A substantial increase of these cells was also detected in the SF of patients with RA, when compared with in the SF of KOA controls as well as compared with in the PB of matched patients with RA (6.6% ± 4.3% vs 0.9% ± 1.1%, 6.6% ± 4.3% vs 3.1% ± 2.4%, respectively). Additionally, the elevation of NKp44+NK cells in PB and SF correlates with RA disease activity evaluated by the scores of DAS28 and CDIA. As NKp44 is a member of the natural cytotoxicity receptor family which is expressed following NK-cell activation,9 the expansion of NKp44+NK cells in both RA SF and synovial tissues suggests that those cells may play an important role in the development and progression of local arthritis in RA.
IL-22 is an IL-10 family cytokine member produced by several different cellular sources including Th17, Th22, and NKp44+NK cells.12,21,22 It plays a critical role in the inflammation and proliferation cascade of various autoimmune diseases like RA, primary Sjogren syndrome, and psoriasis.23 A number of studies have revealed that NKp44+NK cells could provide an innate source of IL-22.13,14 In RA, this study confirmed that NKp44+NK cells could secrete high concentrations of IL-22 when compared with NK cells. Previously, Ikeuchi et al15 have reported that IL-22 is also expressed as a proinflammatory cytokine in synovial fibroblasts and macrophages, which promotes inflammatory responses through the expression of IL-22 receptor 1 on FLS in patients with RA. However, it is still unclear whether NKp44+NK cells may interfere with synovial fibroblasts by IL-22 production that contributes to RA pathogenesis. Therefore, the FLS were cultured and treated with NKp44+NK cells culture supernatants and/or IL-22 antagonist. The MTT assay results show NKp44+NK cell culture supernatants promote the proliferation of FLS, and IL-22 antagonist could block that promotion process. Since different concentrations of rhIL-22 promote the proliferation of RA-FLS, it could be concluded that NKp44+NK cells could promote the proliferation of FLS by secreting IL-22. Obviously, other cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-17 also play an important role in the synovitis of patients with RA,24,25 the interaction of those cytokines on the synovitis require further investigation.
Ren et al26 have partly reported the phenomenon that NKp44+NK cells play the role of proliferation and synovium inflammation in RA. However, the mechanism of those cells in the pathogenesis of FLS proliferation is also to be clarified. To understand the mechanism for IL-22-dependent signal pathway in promoting the proliferation of FLS, further analyses were undergone. IL-22 signaling utilizes Jak1 and Tyk2 to propagate downstream phosphorylation signals which include STAT1, STAT3, STAT5, and MAPK signaling pathways.27 Ikeuchi et al15 have investigated that IL-22 can increase the expression of phospho-ERK1/2 and phospho-p38 in synovial fibroblasts, though no selectively signaling pathway inhibitors were used to block those effects. Considering STAT3-mediated signaling is a common pathway shared by the IL-10 cytokine family members,28 the STAT3 signaling pathway was detected by Western blot analysis. The results show that rhIL-22 can activate the STAT3 pathway by increasing the expression of phospho-STAT3. It is precisely because AG490, a selective signaling pathway inhibitor, blocks the proliferation of FLS promoted by rhIL-22 or NKp44+NK cell culture supernatants. Accordingly, the phosphorylation of STAT3 is an essential pathway in mediating the effects of IL-22 on the proliferation of FLS.
There are also several limitations in this study. For example, the number of synovial tissues specimens and SF samples in RA patients is limited. And the healthy volunteers’ synovial tissues specimens and SF samples should be used as controls in an ideal circumstance. In addition, using cultured human NK cell line Nishi and RA-FLS as a coculture model, the results cue that cell-mediated interaction of RA-FLS may be one mechanism by which NK cells influence the local joint inflammation in RA.29 Given that the pathogenesis of NKp44+NK cells in RA is at least partly clarified, it is unclear whether NKp44+NK cells could also influence the local joint inflammation by cross-talk model with RA-FLS or other immunological cells. IL-22 as a proinflammatory cytokine has also been described to promote the inflammatory responses and osteoclastogenesis by the production of cytokine in RA synovial fibroblasts.15,30 Therefore, further studies are necessary to clarify the pathophysiologic role of NKp44+NK cells in RA, such as NKp44+NK cells removal or adoptive transfer experiments in collagen induced arthritis model mice.
CONCLUSION
The present study clarifies the expansion of NKp44+NK cells in the PB and SF of patients with RA, especially in the synovial tissues of RA for the first time. STAT3 is an essential pathway in mediating the effects of IL-22 secreted by NKp44+NK cells on the proliferation of FLS in patients with RA.
Acknowledgments
The authors thank to all the patients and healthy volunteers who kindly donated their peripheral blood, synovial fluid, or synovial tissue. The authors also thank to Doctor Jian Wang and Jun Xiao (Nanfang Hospital) for their help with the acquisition of synovial tissues from patients.
Footnotes
Abbreviations: CDIA = clinical disease activity index, DAS28 = disease activity score in 28 joints, FLS = fibroblast-like synoviocytes, IL-22 = interleukin 22, KOA = knee osteoarthritis, NK = natural killer, PB = peripheral blood, RA = rheumatoid arthritis, RF = rheumatoid factor, SF = synovial fluid.
This study was supported by the Natural Science Foundation of China (No. 81173456 and 81573730) and the Natural Science Foundation of Guangdong Province (No. S2012010009164).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. The Lancet 2009; 373:659–672. [DOI] [PubMed] [Google Scholar]
- 2.Bombardier C, Barbieri M, Parthan A, et al. The relationship between joint damage and functional disability in rheumatoid arthritis: a systematic review. Ann Rheum Dis 2012; 71:836–844. [DOI] [PubMed] [Google Scholar]
- 3.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 2004; 22 (2 Suppl 1):1–12. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Zhou Y, Chen X, et al. A Metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol 2014; 41:1282–1289. [DOI] [PubMed] [Google Scholar]
- 5.Alivernini S, Fedele AL, Cuoghi I, et al. Citrullination: the loss of tolerance and development of autoimmunity in rheumatoid arthritis. Reumatismo 2008; 60:85–94. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Fernandez JL. Antigen presentation by dendritic cells in rheumatoid arthritis. Curr Top Med Chem 2013; 13:712–719. [DOI] [PubMed] [Google Scholar]
- 7.Mcinnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 8.Yamanishi Y, Firestein GS. Pathogenesis of rheumatoid arthritis: the role of synoviocytes. Rheum Dis Clin North Am 2001; 27:355–371. [DOI] [PubMed] [Google Scholar]
- 9.Ahern DJ, Brennan FM. The role of Natural Killer cells in the pathogenesis of rheumatoid arthritis: Major contributors or essential homeostatic modulators? Immunol Lett 2010. [DOI] [PubMed] [Google Scholar]
- 10.Soderstrom K, Stein E, Colmenero P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A 2010; 107:13028–13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo CK, Lam QL, Sun L, et al. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum 2008; 58:2700–2711. [DOI] [PubMed] [Google Scholar]
- 12.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009; 457:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccia F, Accardo-Palumbo A, Alessandro R, et al. Interleukin-22 and IL-22-producing NKp44(+) NK cells in the subclinical gut inflammation of patients with ankylosing spondylitis. Arthritis Rheum 2011. [DOI] [PubMed] [Google Scholar]
- 14.Ciccia F, Guggino G, Rizzo A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren's syndrome. Ann Rheum Dis 2012; 71:295–301. [DOI] [PubMed] [Google Scholar]
- 15.Ikeuchi H, Kuroiwa T, Hiramatsu N, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum 2005; 52:1037–1046. [DOI] [PubMed] [Google Scholar]
- 16.Ren J, Feng Z, Lv Z, et al. Natural killer-22 cells in the synovial fluid of patients with rheumatoid arthritis are an innate source of interleukin 22 and tumor necrosis factor-alpha. J Rheumatol 2011; 38:2112–2118. [DOI] [PubMed] [Google Scholar]
- 17.de Matos CT, Berg L, Michaelsson J, et al. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology 2007; 122:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neidhart M, Seemayer CA, Hummel KM, et al. Functional characterization of adherent synovial fluid cells in rheumatoid arthritis: destructive potential in vitro and in vivo. Arthritis Rheum 2003; 48:1873–1880. [DOI] [PubMed] [Google Scholar]
- 19.Ota F, Maeshima A, Yamashita S, et al. Activin A induces cell proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Rheum 2003; 48:2442–2449. [DOI] [PubMed] [Google Scholar]
- 20.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol 2005; 32:1650–1653. [PubMed] [Google Scholar]
- 21.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 2009; 10:75–82. [DOI] [PubMed] [Google Scholar]
- 22.Trifari S, Kaplan CD, Tran EH, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 2009; 10:864–871. [DOI] [PubMed] [Google Scholar]
- 23.Pan HF, Li XP, Zheng SG, et al. Emerging role of interleukin-22 in autoimmune diseases. Cytokine Growth Factor Rev 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meroni PL, Valesini G. Tumour necrosis factor alpha antagonists in the treatment of rheumatoid arthritis: an immunological perspective. Bio Drugs 2014; 28 Suppl 1:S5–S13. [DOI] [PubMed] [Google Scholar]
- 25.van Hamburg JP, Corneth OB, Paulissen SM, et al. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis 2013; 72:1700–1707. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Zhou Y, Wu H, et al. Role and mechanism of NKp44+NK cells in the proliferation and inflammation of synovium of rheumatoid arthritis patients. Zhonghua Yi Xue Za Zhi 2014; 94:201–203. [PubMed] [Google Scholar]
- 27.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 2002; 277:33676–33682. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev 2014; 13:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen N, Pascal V, Fasth AE, et al. Balance between activating NKG2D, DNAM-1, NKp44 and NKp46 and inhibitory CD94/NKG2A receptors determine NK degranulation towards RA synovial fibroblasts. Immunology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KW, Kim HR, Park JY, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum 2012; 64:1015–1023. [DOI] [PubMed] [Google Scholar]


