Abstract
Gelatin sponge particles are commonly used in the conventional transarterial chemoembolization (c-TACE) as an adjuvant embolizing agent for hepatocellular carcinoma (HCC). However, there are few reports regarding the clinical applications of gelatin sponge microparticles (GSMs) as a main embolizing agent in the treatment of HCC. This retrospective study aim to evaluate the efficacy and safety of patients with Barcelona Clinic Liver Cancer (BCLC) stage B HCC treated with intra-arterial injection of 350 to 560 μm GSMs mixed with anticancer agents.
Twenty-four patients with unresectable BCLC stage B HCC without any prior treatment underwent transarterial chemoembolization with gelatin sponge microparticles (GSMs-TACE) of diameter 350 to 560 μm mixed with lobaplatin. The mixture was injected into tumor-feeding arteries until the sluggish flow in selective artery. Safety was measured by assessing complication rate, and efficacy was reflected by assessing response to mRECIST therapy and overall survival. The survival rate was calculated using the Kaplan–Meier method.
All 24 BCLC stage B HCC patients showed good tolerance to the procedure. The mean follow-up period was 27 months and mean number of TACE treatments per patient was 3.7 sessions (range 1–10) during the follow-up period. Postprocedure complications were mild and treated by symptomatic treatment. Six months and 1 year overall survival rates were 100% and 87.5%, respectively. Overall median survival time was 25 months (95%CI: 21.06–28.95 months).
GSMs-TACE is a safe and effective method for BCLC stage B HCC patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide, with an estimated 692,000 cases per year1 and >50% of them occur in China.2 The Barcelona Clinic Liver Cancer (BCLC) staging system is used as a standard for management of HCC; BCLC links different stages of HCC with appropriate therapeutic strategies.3–5 Transcatheter arterial chemoembolization (TACE) is the standard treatment method for BCLC stage B HCC patients with asymptomatic, large, multifocal HCC with no evidence of vascular invasion or extrahepatic metastasis.5 Different materials were used for embolization worldwide. The combination of iodized oil mixed with anticancer drugs followed by gelatin sponge particles (GSPs) is the most popular techniques.
Lipiodol-TACE was a conventional approach in the treatment of HCC; GSPs were used for many years as an adjuvant embolizing agent followed by lipiodol-TACE.6–9 Generally, 1 mm sized GSPs are used after selective arterial injection of mixture of iodized oil and chemotherapeutic agents to occlude tumor-feeding arteries. There were no standardized and calibrated GSPs available in China until 2006, when variable diameter gelatin sponge microparticals (GSMs) including 150 to 350 μm, 350 to 560 μm, 560 to 710 μm, and 710 to 1000 μm were manufactured by Hanzhou alc Ltd (Gelfoam; Hanzhou alc Ltd, China), and then it became available as an embolic agent. In the present study, we used 350 to 560 μm GSMs as the embolic agent, mixed with lobaplatin to determine the safety and efficacy of this treatment for BCLC stage B HCC patients.
MATERIALS AND METHODS
Patients and Patient's Selection Criteria
This retrospective study was performed between August 2009 and September 2013. This study was approved by the Medical Science Ethical Committee of the hospital and carried out after informed consent was obtained from the patients. We used imaging data and clinical records to evaluate the safety and efficacy of chemoembolization for BCLC stage B HCC patients. All patients received routine preoperative investigations, including liver function tests, α-fetoprotein (AFP), hepatitis serology, chest radiography, abdominal ultrasonography, and abdominal contrast-enhancement CT (CECT) scanning. The diagnosis of hepatocellular carcinoma was based on histologic or cytologic findings, liver tumor on CECT (Figure 1A), and a serum alpha-fetoprotein value exceeding 250 ng per milliliter. Patients were treated by transarterial chemoembolization using gelatin sponge microparticles (GSMs-TACE) mixed with Lobaplatin (Lobaplatin; Hainan Changan International Pharmaceutical, China).
FIGURE 1.
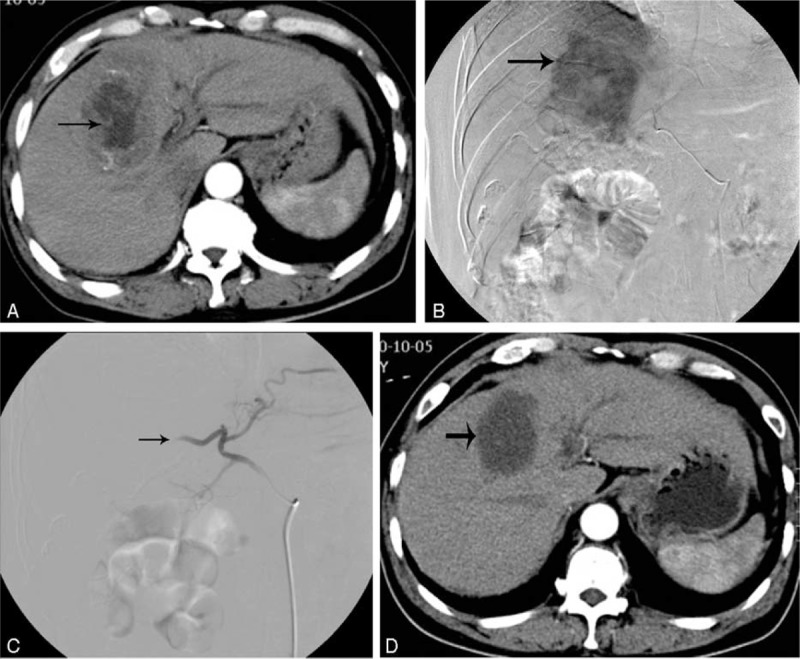
A 52-year-old man with hepatocellular carcinoma. (A) Pre-GSMs-TACE abdominal contrast-enhanced CT shows a tumor mass with 69 mm in diameter (arrow). (B) Arterial phase of hepatic arteriogram shows a large tumor stain before GSMs-TACE. (C) Postembolization image shows complete occlusion of the tumor-feeding vessels. (D) One month after GSMs-TACE abdominal contrast-enhanced CT shows a complete necrosis of tumor mass. CT = computed tomography, GSMs-TACE = transarterial chemoembolization with gelatin sponge microparticles.
The selection criteria were performed as follows: (i) BCLC stage B HCC (asymptomatic, large, or multifocal HCC with no evidence of vascular invasion or extrahepatic metastasis); (ii) no previous treatment for hepatocellular carcinoma; (iii) liver function criteria for enrolment included bilirubin <51 μmol/L and liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) < 270 IU/L). Patients with the following situations were excluded from this study: Porto systemic shunts, encephalopathy, gastrointestinal bleeding, contraindication for hepatic embolization (impaired clotting tests, renal insufficiency/failure, sepsis, and bleeding coagulopathy), and tumor burden >50% of the liver volume.
Chemoembolization Procedure
GSMs-TACE was performed under local anesthesia. The femoral artery was punctured percutaneously. A selective 5-French (5F) catheter was used via 5F sheath to take hepatic angiography to see the tumor-feeding arteries and tumor staining (Figure 1B). Selective catheterization of the tumor-feeding arteries, microcatheter was used if necessary. The treatment regimen comprised of Lobaplatin 20 mg dissolved in 30 mL saline and was mixed with 50 to 100 mg of 350 to 560 μm GSMs. Mixture of GSMs and Lobaplatin was injected into the tumor-feeding arteries under fluoroscopic surveillance slowly. The sluggish flow of the tumor-feeding vessels was the endpoint of embolization. Complete embolization of all the tumor-feeding arteries was performed (Figure 1C). Angiography was performed after embolization to determine the extent of embolization.
Follow-Up Assessments
All patients were followed up by contrast-enhanced CT scans on the 4th day after TACE and every 1 to 2 months during the first 3 months (Figure 1D). Tumor response was evaluated according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST).10 National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 was used to report clinical adverse events (AEs).11 Another session of TACE was performed every 1 to 3 months. No further TACE sessions were performed if one of the following end points was reached: (i) complete devascularization of the tumor; (ii) no further possibility to embolize the tumors; (iii) total resection of the tumor by surgery after TACE; (iv) development of contraindications to TACE. Mean follow-up period was 27 months.
Statistical Analysis
All statistical analyses were performed using software SPSS 16.0 statistical package (SPSS Inc, Chicago, IL). Descriptive statistics (Mean ± SD) were provided when appropriate. Overall survival rates were calculated by the Kaplan–Meier method. Variables with P < 0.05 were defined as statistically significant.
RESULTS
A total of 24 patients were enrolled in the study from August 2009 to September 2013. All GSMs-TACE procedures were technically successful with no technically related complications.
Clinical Profile of Patients
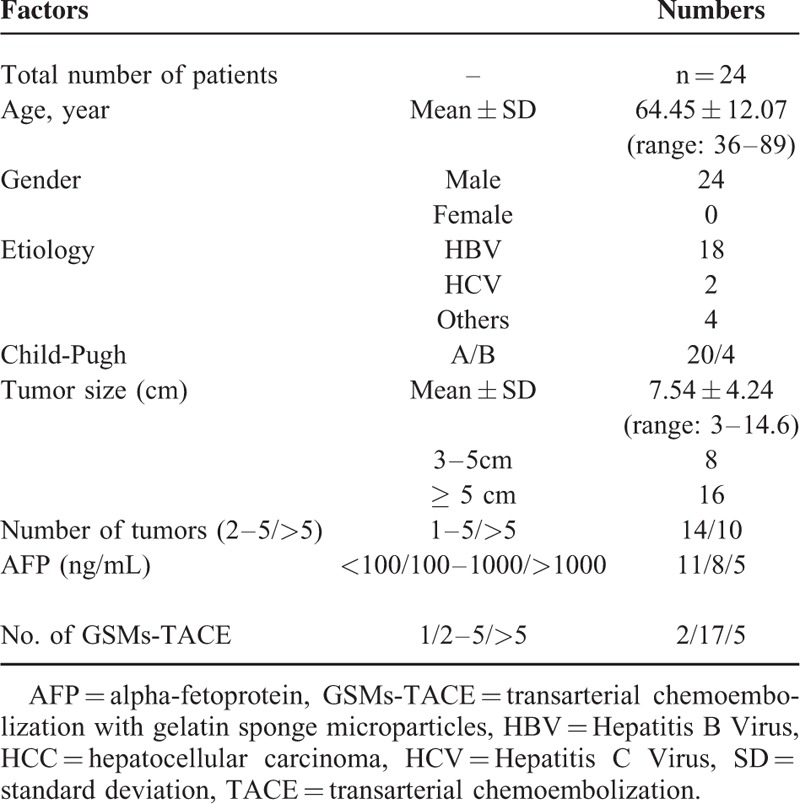
The clinical profile of the study population is depicted in Table 1. A total of 24 patients (all men) had a mean age of (64.45 ± 12.07) years (range: 36–89 years). Hepatitis B virus (HBV) infection was the most common etiological factor of HCC, seen in 18 (75.0%) patients. Hepatitis C virus (HCV) infection was seen in 2 (8.33%) patients and others in 4 (16.67%) patients. 20 (83.33%) patients had Child-Pugh A cirrhosis and 4 (16.67%) had Child-Pugh B. The mean tumor size was (7.54 ± 4.24) cm (range: 3–14.6 cm). Eight (33.33%) patients had tumor size of ≤5 cm, and 16 (66.67%) had tumor size of >5 cm. Number of tumors in 14 (58.33%) patients were <5, whereas number of tumors in 10 (41.67%) patients were >5. Eleven (45.83%) patients had AFP <100 ng/mL, 8 (33.33%) patients had 100 to 1000 ng/mL, and in 5 (20.83%) patients AFP level was >1000 ng/mL.
TABLE 1.
Clinical Characteristic Baseline of 24 HCC Patients
Complications After GSMs-TACE Procedure
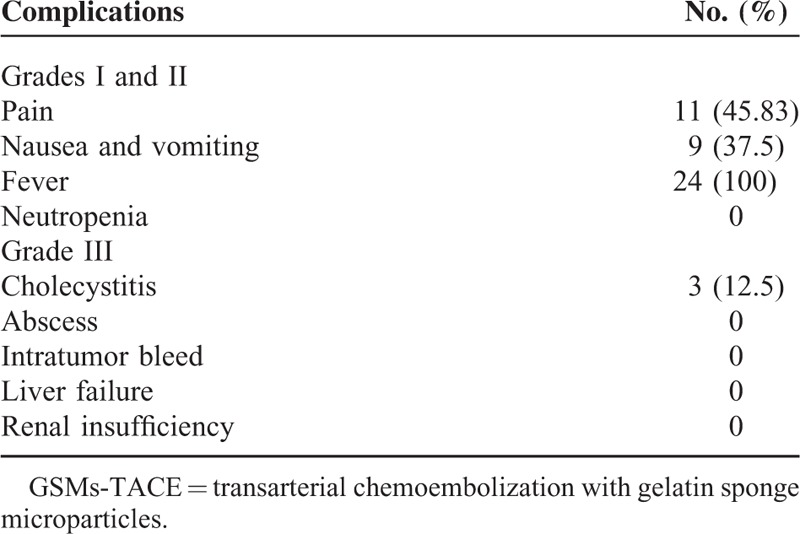
The procedure of TACE was tolerated well by all patients. No complications were encountered during the procedure. Postprocedure complications were mild. Postembolization syndrome was the most common complication which consisted of abdominal pain in 45.83% (11/24) patients, nausea and vomiting in 37.5% (9/24) patients, and fever in 100% (24/24) patients (Table 2). All the symptoms were managed by symptomatic treatment. Three patients had cholecystitis with the incidence of 12.5% (3/24) and recovered in 7 days after treatment with antibiotics and stopping of food and water intake. There were no other serious complications such as liver failure, gallbladder necrosis, liver abscess, cholangitis, or tumor rupture.
TABLE 2.
Complications After GSMs-TACE Procedure
Changes of Serum ALT, AST, ALB, and TBIL After TACE
Liver enzymes presented a statistically significant increase on 4 days postembolization compared to pre-embolization (P < 0.05), that declined to pre-embolization levels at measurements 7 days after each procedure. AST and ALT values were statistically significantly higher on 4 days post-TACE compared to pre-TACE (P<0.05); however, no statistical differences were observed on 7 days (Figure 2A). Also, there is no statistical difference observed of total bilirubin (TBIL) and albumin (ALB) on both 4 and 7 days (Figure 2B and C).
FIGURE 2.
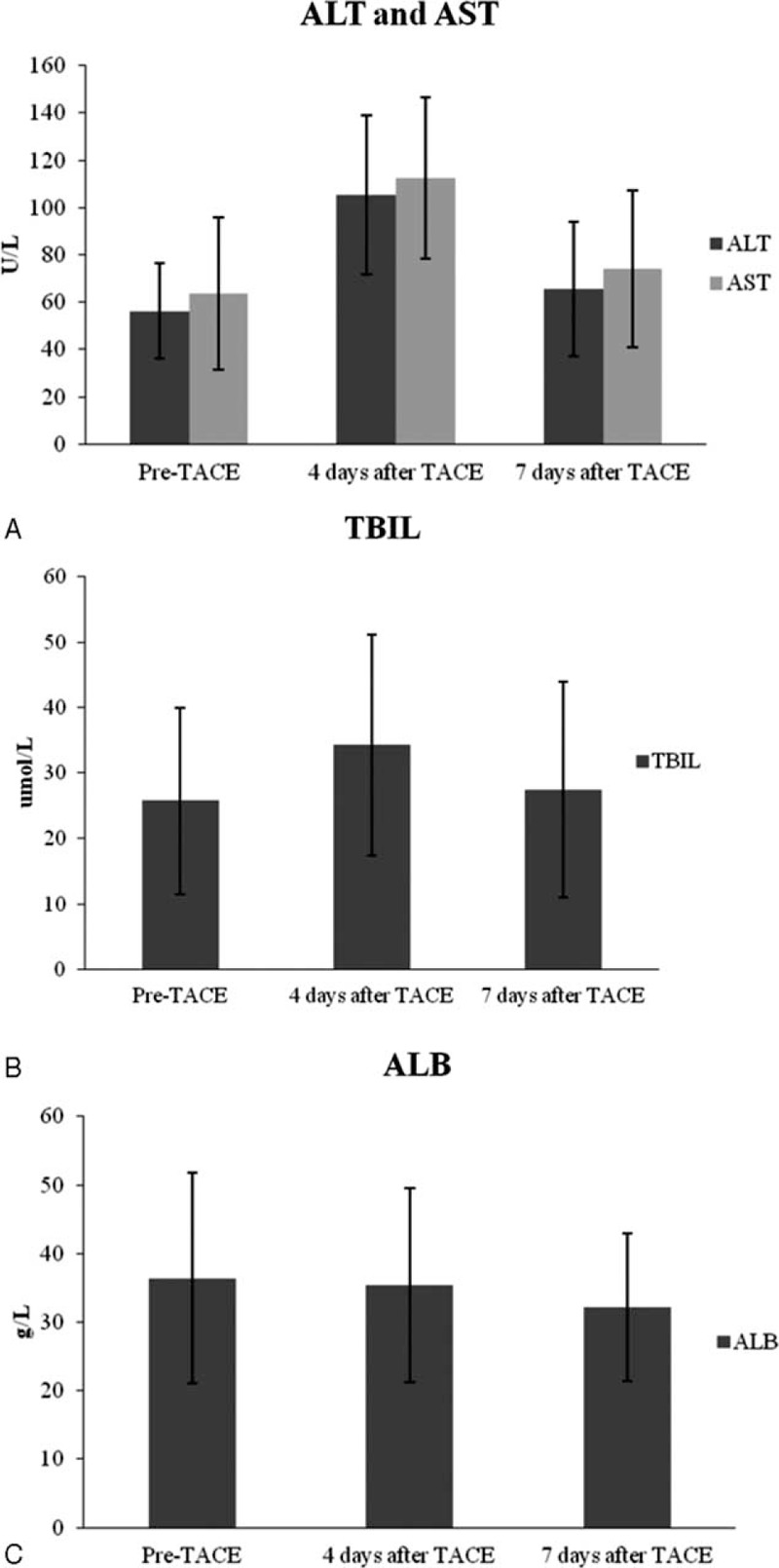
Changes of serum ALT, AST, TBIL, and ALB after TACE. (A) Changes of serum ALT and AST levels at baseline, 4 days and 7 days after GSMs-TACE. (B) Changes of serum TBIL levels at baseline, 4 days and 7 days after GSMs-TACE. (C) Changes of serum ALB levels at baseline, 4 days and 7 days after GSMs-TACE. ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, GSMs-TACE = transarterial chemoembolization with gelatin sponge microparticles, TACE = transarterial chemoembolization, TBIL = total bilirubin.
Tumor Response
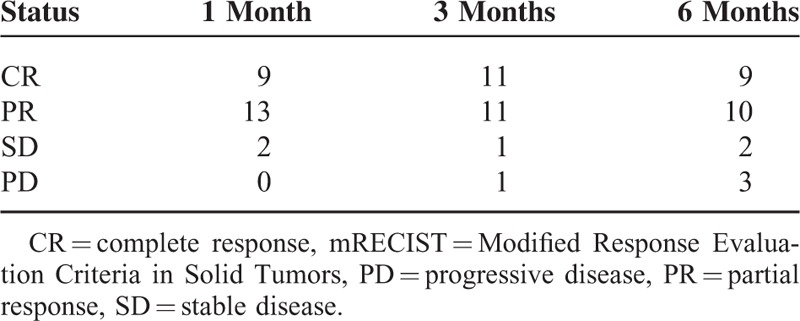
Post-TCAE tumor response evaluation was assessed according to mRECIST, which revealed complete response (CR) in 37.5% (9/24) of patients after 1 month, 45.83% (11/24) of patients after 3 months, and 37.5% (9/24) of patients after 6 months; partial response (PR) in 54.17% (13/24) of patients after 1 month, 45.83% (11/24) of patients after 3 months, and 41.67% (10/24) of patients after 6 months; stable disease (SD) in 8.33% (2/24) of patients after 1 month, 4.17% (1/24) of patients after 3 months, and 8.33% (2/24) of patients after 6 months; and progressive disease (PD) in none (0/24) of patients after 1 month, 4.17% (1/24) of patients after 3 months, and 12.5% (3/24) of patients after 6 months (Table 3).
TABLE 3.
Number of Tumor Response of Patients According to mRECIST
Chemoembolization Procedure and Overall Survival
Patients received a mean of 3.7 (range 1–10) sessions of TACE during the follow-up period. One session in 2 (8.33%) patients, 2 to 5 sessions in 17 (70.83%) patients, and >5 sessions in 5 (20.83%) patients. The overall mean follow-up period was 27 months (range 10–34 months). The mean number of TACE treatments per patient was 3.7 sessions (range 1–10) during the follow-up period. Median survival time was 25 months (95%CI: 21.06–28.95) months. Six months and 1 year survival rates were 100% and 87.5%, respectively (Figure 3).
FIGURE 3.
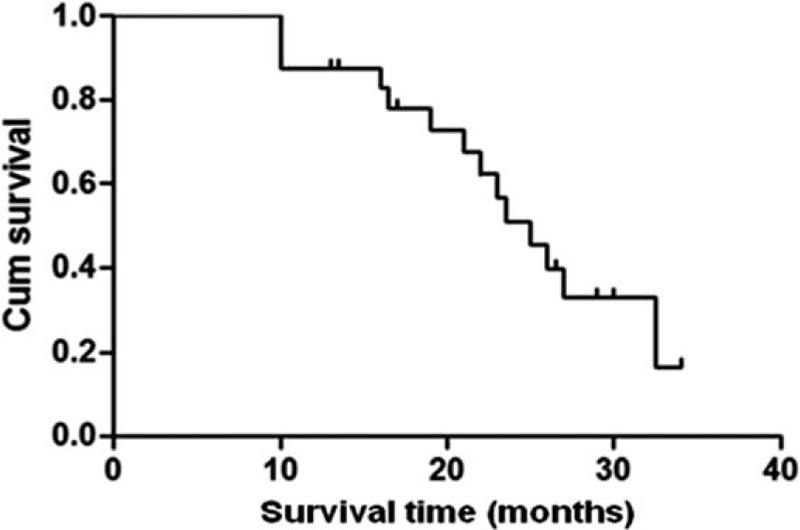
Overall survival curve of 24 BCLC B stage HCC patients after GSMs-TACE. The 6- and 12- month overall survival rates were 100% and 87.5%% respectively. BCLC = Barcelona Clinic Liver Cancer, GSMs-TACE = transarterial chemoembolization with gelatin sponge microparticles, HCC = hepatocellular carcinoma.
DISCUSSION
Gelatin sponge particles are absorbed within certain amount of time12 leading to recanalization of the arteries that is beneficial in minimizing normal tissue damage. Due to this absorbable feature, we embolized the regional tumor arteries and small branches of the liver arteries around the tumor which can possibly become potential collateral tumor-feeding arteries using GSMs. The results showed that this embolization technique was beneficial and increased the rate of tumor necrosis. In this study, we were able to achieve CR 37.5% (9/24) and PR 54.17% (13/24) of 1 month, 45.83% (11/24) and 45.83% (11/24) of 3 months, and 37.5% (9/24) and 41.67% (10/24) of 6 months, respectively. The tumor response rate was 91.67% (22/24) which was higher than previous reports with objective response rate range from 57.9% to 85%.13,14 The high rate of complete response might be one of the reasons that lead to long survival time. In the present study, the mean number of TACE treatments per patient was 3.8 sessions during the mean 27 months follow-up period, and the median survival time was 25 months (95%CI: 21.06–28.95 months), which was much longer compared to patients of BCLC stage B HCC treated with conventional TACE (median survivals of 17.4 months, range 13.9–18.8 months)15 and patients of BCLC stage B HCC treated with yttrium-90 radioembolization (17.2 months, range: 13.5–29.6 months).16
The regional embolization during the procedure of GSMs-ATCE could be the reason of higher rate of tumor response, but conversely, embolization of the tumor-feeding arteries and collateral arteries completely can cause much more damage to normal liver tissue. We observed the levels of liver enzymes which were significantly higher after GSMs-TACE on 4 days compared to pretreatment, and returned to the normal level on 7 days. These results demonstrated that GSMs-TACE did not cause any severe damage of normal liver tissue. It may be related to the absorbable characteristic of GSMs. Severe damage to bile duct is another risk of complete embolization that can lead to cholesteatoma as reported by other studies, whereas in the present study, there was no such complication. Most importantly, there were no severe complications such as liver failure, gallbladder necrosis, liver abscesses, and cholangitis as reported by previous studies.17–20 In this study, all the patients tolerated the procedure well. The general postprocedure complications were minor and self-limiting. Fever was the most common complication. Other complications were abdominal pain, nausea, and vomiting, which were treated with analgesics and antiemetic, respectively.
During TACE, chemotherapeutic drugs play an important role in combination with particles causing maximum damage to cancer cells. Doxorubicin, fluorouracil, mitomycin, and cisplatin are the common chemotherapeutic drugs used worldwide. However, the use of cisplatin causes renal, neurological, and gastrointestinal toxicity. In this study we used lobaplatin (a third-generation platinum analogue to replace cisplatin) mixed with GSMs. Lobaplatin arrests cell cycle in G1, G2 and M phase, and upregulates p53 which stops the proliferation of HCC.21,22 Moreover, lobaplatin has reduced toxicity, better therapeutic index, and higher solubility.23
CONCLUSIONS
In conclusion, GSMs-TACE is a safe and effective palliative procedure. All patients in this study had BCLC stage B HCC. GSMs-TACE prolonged the survival, improved the life quality, and produced clinically negligible long-term impairment of liver function in patients. The main limitation of this study is that it is a retrospective and observational study and with a small population of patients. And also, we only considered a short-term response to TACE. However, these preliminary results were significant and it will be of further interest to evaluate the long-term response to GSMs-TACE.
Footnotes
Abbreviations: AFP = α-fetoprotein, ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinic Liver Cancer, CECT = contrast-enhancement CT, CR = complete response, c-TACE = conventional transarterial chemoembolization, GSMs = gelatin sponge microparticles, GSMs-TACE = transarterial chemoembolization with gelatin sponge microparticles, GSPs = gelatin sponge particles, HBV = Hepatitis B virus, HCC = hepatocellular carcinoma, HCV = Hepatitis C virus, mRECIST = Modified Response Evaluation Criteria in Solid Tumors, PD = progressive disease, PR = partial response, SD = stable disease, TBIL = total bilirubin.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 3.Josep.M Llovet, Concepció Brú, Jordi Bruix Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19:329–338. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010; 30:61–74. [DOI] [PubMed] [Google Scholar]
- 6.Doyon D, Mouzon A, Jourde AM, et al. L’embolisation arterielle hepatique dans les tumeurs malignes du foie. Ann Radiol 1974; 17:593–603. [PubMed] [Google Scholar]
- 7.Yamada R, Sato M, Kawabata M, et al. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983; 148:397–401. [DOI] [PubMed] [Google Scholar]
- 8.Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol 2006; 29:1077–1083. [DOI] [PubMed] [Google Scholar]
- 9.Satoh M, Yamada R. Experimental and clinical studies on the hepatic artery embolization for treatment of hepatoma. Nippon Acta Radiologica 1983; 43:977–1005. [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30:52–60. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. June 14, 2010. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 12.Takayasu K, Arii S, Ikai I, et al. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol 2010; 194:830–837. [DOI] [PubMed] [Google Scholar]
- 13.Nawawi O, Hazman M, Abdullah B. Transarterial embolisation of hepatocellular carcinoma with doxorubicin-eluting beads: single centre early experience. Biomed Imaging Interv J 2010; 6:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song MJ, Park CH, Kim JD, et al. Drug-eluting loaded with doxorubicin versus conventional Lipidol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol 2011; 23:521–527. [DOI] [PubMed] [Google Scholar]
- 15.Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology 2010; 255:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010; 138:52–64. [DOI] [PubMed] [Google Scholar]
- 17.Hu HT, Kim JH, Lee LS, et al. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol 2011; 22:917–923. [DOI] [PubMed] [Google Scholar]
- 18.Lance C, McLennan G, Obuchowski N, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2011; 22:1697–1705. [DOI] [PubMed] [Google Scholar]
- 19.Miyayama S, Yamashiro M, Okuda M, et al. Chemoembolization for the treatment of large hepatocellular carcinoma. J Vasc Interv Radiol 2010; 21:1226–1234. [DOI] [PubMed] [Google Scholar]
- 20.Zhao GS, Zhang YW, Liu Y, et al. Liver abscess occurring after TACE using new-type Gelfoam particles as embolic agent for primary hepatic cancer: report of three cases with literature review. J Intervent Radiol 2013; 22:415–417. [Google Scholar]
- 21.Qiong Wu, Qin SK, Teng FM, et al. Lobaplatin arrests cell cycle progression in human hepatocellular carcinoma cells. J Hematol Oncol 2010; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying Wang, Zheng LW, Ma LW. Lobaplatin inhibits the proliferation of hepatollular carcinoma through P53 apoptosis axis. Hepat Mon 2012; 12 (10 HCC):e6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B, Shan H, Zhu KS, et al. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol 2010; 21:333–338. [DOI] [PubMed] [Google Scholar]


