Abstract
Patients that have suffered a major injury may sustain a period of immunocompromise and altered Th1/Th2 cytokine balance that can predispose them to opportunistic infections. Pseudomonas aeruginosa is frequently a causative organism for nosocomial infections in critically ill patients and is associated with high mortality. We previously mimicked this clinical scenario by challenging mice with P. aeruginosa 5 days after a cecal ligation and puncture (CLP) procedure. Mice that were subjected to CLP had reduced ability to clear bacteria, significantly lower gamma interferon (IFN-γ) concentrations in plasma, and significantly elevated levels of interleukin 10 (IL-10) in plasma in response to the Pseudomonas challenge compared to uninjured control mice. We investigated the significance of the alteration in IFN-γ by administering recombinant IFN-γ to post-CLP mice at the time of Pseudomonas challenge and by challenging IFN-γ knockout (IFN-γ KO) mice with Pseudomonas. Administration of IFN-γ to post-CLP mice attenuated IL-10 secretion and enhanced IL-12 secretion but did not improve bacterial clearance or survival after Pseudomonas challenge. Furthermore, IFN-γ KO mice had significantly higher plasma IL-10 concentrations but did not exhibit impaired bacterial clearance or increased mortality following Pseudomonas challenge. These data indicate that systemic administration of IFN-γ effectively reverses alterations in immune function that are commonly associated with immunosuppression in critically injured mice but does not improve bacterial clearance or survival following Pseudomonas challenge. Further, endogenous IFN-γ does not appear to contribute significantly to early clearance of Pseudomonas bacteremia, nor does it affect the mortality rate after a lethal Pseudomonas challenge.
A major injury may increase susceptibility to infections and sepsis by reducing competence of the innate immune response. Certain early cytokine profiles have been presumed to provide information in regard to the efficacy of the immune response to infection. Increased serum concentrations of interleukin 10 (IL-10) and decreased serum concentrations of gamma interferon (IFN-γ) and IL-12 have been associated with a higher incidence of sepsis and worsened patient outcomes. IL-10 is an anti-inflammatory cytokine primarily derived from T cells and monocytes/macrophages and has been reported to be associated with increased susceptibility to infections (29, 37, 55). IFN-γ is derived primarily from NK cells, T-helper class I (Th1) cells, and to a lesser extent from antigen-presenting cells and has been demonstrated to play a role in resistance to a number of microbial organisms, including viruses, parasites, and intracellular bacteria (49).
IFN-γ has been shown to be important in mediating host resistance to infection with intracellular organisms, but its role in the immune response to extracellular bacteria is less well defined. Pseudomonas aeruginosa is a gram-negative pathogen that frequently causes nosocomial infection and is associated with high mortality rates. Hospital-acquired pneumonia accounts for up to 18% of nosocomial infections (33), with the highest incidence occurring with ventilated patients (8-10). Mortality directly attributable to hospital-acquired pneumonia ranges from 33 to 50% (16, 21). P. aeruginosa is the pathogen most frequently isolated from patients with hospital-acquired pneumonia and is associated with the highest mortality rate of any bacterium (44, 54). Septic shock and multiple organ dysfunction often occur as sequelae to P. aeruginosa pneumonia.
We have previously described a model of postinflammatory immunosuppression in which mice are challenged with an intravenous injection of Pseudomonas 5 days after a sublethal cecal ligation and puncture (CLP) (38). In this model, mice that have been subjected to CLP have increased bacterial colonization of the lung, decreased IFN-γ and IL-12 production, and increased production of IL-10 after the Pseudomonas challenge. In the present study, we used this model to study the importance of the suppressed IFN-γ response in modulating bacterial clearance and resistance to infection-associated mortality. We hypothesized that administration of IFN-γ to post-CLP mice would augment immunity, enhance bacterial clearance, and improve survival after systemic Pseudomonas challenge. The functional significance of IFN-γ in the host response to Pseudomonas infection was further determined by assessing the response of IFN-γ knockout (IFN-γ KO) mice to Pseudomonas infection. We hypothesized that IFN-γ KO mice would have impaired bacterial clearance and increased mortality following systemic Pseudomonas challenge.
METHODS
Mice.
Six- to eight-week-old male wild-type (C57BL/6) and IFN-γ KO (B6.129S7-Ifngtm1Ts/J) mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and were housed in the animal care facility at the University of Texas Medical Branch (Galveston, Tex.). The animals were allowed to acclimate for 1 week prior to use. Animal protocols were approved by the Institutional Animal Care and Use Committee and met National Institutes of Health guidelines for the care and use of experimental animals.
Nonlethal CLP.
Mice were anesthetized with 2.5% isoflurane in an induction chamber and maintained under anesthesia by delivery of isoflurane through a mask. The mice were positioned in dorsal recumbancy, and the ventral abdominal walls were shaved and prepared with 70% isopropanol and 1% iodine. A ventral midline incision (∼1 cm) was made to allow exteriorization of the cecum, and cecal luminal contents were manipulated from the apex to the base of the cecum. The cecum was ligated 1 cm from the apex with 3-0 silk, and a 25-gauge needle was used to penetrate the cecum in a through-and-through fashion. Sham surgery, in which the cecum was exteriorized and manipulated as described but not ligated or punctured, was performed on additional animals.
Administration of IFN-γ to post-CLP mice.
Some groups of mice were given subcutaneous injections of 1 mg (∼8,400 U) of recombinant murine IFN-γ (R&D Systems, Minneapolis, Minn.) at the time of challenge with P. aeruginosa. Preliminary studies were performed to determine a dose of recombinant IFN-γ that would approximate the serum IFN-γ response measured for normal or sham mice after Pseudomonas administration (see Fig. 1A).
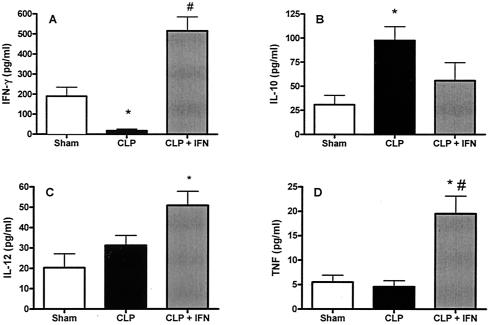
FIG. 1.
Administration of IFN-γ to post-CLP mice attenuated elevated IL-10 production and increased serum concentrations of IL-12 and TNF-α after P. aeruginosa challenge. Mice were subjected to either CLP or sham surgery. Five days later, the mice were given intravenous injections of saline or 5 × 107 CFU of P. aeruginosa and were sacrificed 6 h later for collection of blood samples. Plasma cytokine concentrations were measured by enzyme-linked immunosorbent assay. Graphs show P. aeruginosa-induced plasma concentrations of (A) IFN-γ, (B) IL-10, C) IL-12, and D) TNF-α concentrations in sham and post-CLP mice. *, significantly different from sham group; #, significantly different from CLP group (sham group, n = 4; CLP group, n = 12; CLP + IFN group, n = 14).
Microbiology.
P. aeruginosa (ATCC 19660), a clinical isolate originally obtained from a septic patient, was inoculated into tryptic soy broth and allowed to replicate overnight in a shaking 37°C incubator. The bacteria were pelleted by centrifugation. The supernatant was aspirated, and the pelleted bacteria were washed with 10 ml of sterile 0.9% saline and repelleted. The bacteria were resuspended in saline, and the viable number of CFU in this suspension was determined by culturing serial dilutions overnight on tryptic soy agar. Serial dilutions in sterile 0.9% saline were made to produce the desired bacterial concentration for intravenous injection.
To perform the Pseudomonas challenge, mice were anesthetized with isoflurane and placed in dorsal recumbancy. The penis was extruded, and 0.1 ml of either saline alone (controls) or saline with 5 × 107 CFU (sublethal dose) or 1 × 108 to 4 × 108 CFU (lethal doses) of P. aeruginosa was injected intravenously through the dorsal vein of the penis. The mice were returned to their cages. Some of the mice were observed for mortality during the next 7 days. The remaining mice were sacrificed under isoflurane anesthesia 6 h after Pseudomonas injection for collection of samples.
After euthanasia, lungs were excised aseptically and homogenized in sterile saline at a 1:10 ratio (weight/volume). Serial dilutions of the homogenates were plated on tryptic soy agar and MacConkey's agar and were incubated at 37°C. CFU were counted after 24 h of incubation and recounted after another 24 h. Gram staining and API 20 E biochemical strips (BioMerieux, Hazelwood, Mo.) were used to identify bacterial colonies and to confirm the strain of P. aeruginosa used for injection.
Splenocyte preparation and flow cytometry.
Spleens were aseptically harvested and transferred to six-well culture plates containing RPMI 1640 supplemented with 10% fetal calf serum and antibiotics. Spleens were homogenized and passed through a nylon mesh strainer. Red blood cells were lysed (erythrocyte lysis kit; R&D Systems), and the remaining splenocytes were washed in phosphate-buffered saline (PBS) prior to counting on a hemocytometer. Fluorochrome-conjugated antibodies against CD3, NK1.1, F4/80 (Caltag, Burlingame, Calif.) and CD11c (BD Biosciences) were used to identify T cells, NK cells, macrophages, and dendritic cells, respectively. IL-12 receptor cell surface expression was analyzed using antibodies against the β1 subunit of the IL-12 receptor (BD Biosciences). Splenocytes (106 in 0.1 ml of PBS) were incubated with 0.5 μg of fluorochrome-conjugated antibodies for 30 min at 4°C and washed in PBS, and samples were fixed in 1% paraformaldehyde. Cells were analyzed in a FACSort flow cytometer (Becton Dickinson, Franklin Lakes, N.J.), and specific staining was determined by comparison with appropriate antibody isotype controls.
Quantitation of plasma cytokines.
Enzyme-linked immunosorbent assay kits were used in the sublethal Pseudomonas challenge experiments to assay plasma IL-12 p70 (R&D Systems) and IL-10, IFN-γ, and tumor necrosis factor alpha (TNF-α) (eBiosciences, San Diego, Calif.). In subsequent experiments, plasma cytokines were quantified by multiplex cytokine technology, using the Bio-Plex System (Bio-Rad, Hercules, Calif.) per the manufacturer's instructions. Briefly, plasma was incubated with spectrally addressed polystyrene beads coated with cytokine-specific monoclonal antibodies. After the beads were washed, a second set of fluorochrome-labeled cytokine-specific antibodies were added. The beads were again washed, and cytokine levels were determined by measuring fluorescent signal following laser excitation.
Statistical analyses.
All statistical analyses were performed by using the GraphPad InStat software package. An unpaired, two-tailed Student t test was used to compare CLP and IFN-γ-treated CLP groups. Multiple groups were analyzed by analysis of variance, followed by a Tukey-Kramer multiple comparisons test. Incidences of positive lung culture were compared by chi-square analysis. Statistical analyses of survival curves were performed by using the log rank test. A P value of 0.05 or less was considered significant.
RESULTS
Administration of IFN-γ attenuated the IL-10 response and increased the IL-12 response to an intravenous challenge with a nonlethal dose of P. aeruginosa but did not improve bacterial clearance for post-CLP mice.
Serum IFN-γ concentrations are undetectable in mice 5 days after CLP or sham CLP without further bacterial challenge (38). Mice that were subjected to CLP and 5 days later were challenged with Pseudomonas (5 × 107 CFU, given intravenously) had a significantly lower IFN-γ response than sham CLP mice after challenge with Pseudomonas. IFN-γ was then administered to one group of CLP mice at the time of bacterial challenge to simulate the IFN-γ response seen in sham mice challenged with Pseudomonas (Fig. 1A). The mice were sacrificed 6 h later, and serum cytokine concentrations were determined. Serum IFN-γ was significantly lower after Pseudomonas injection in mice that had been previously subjected to CLP than for sham mice (Fig. 1A). Serum IFN-γ concentrations were significantly higher in CLP mice that received an IFN-γ injection than in untreated CLP mice and were comparable to those in sham controls (Fig. 1A). IL-10 concentrations were increased in post-CLP mice compared to those in sham mice, and this increase was attenuated by IFN-γ administration (Fig. 1B). Concentrations of IL-12 and TNF-α in serum did not differ between sham and CLP mice, but treatment with IFN-γ caused a significant increase in both cytokines in post-CLP mice after Pseudomonas challenge (Fig. 1C and D).
Cell surface expression of the IL-12 receptor protein (IL-12R) was examined by using flow cytometry. IL-12 is an important modulator of the innate response to infection, and IL-12R β1 expression is induced upon lymphocyte activation (18). Expression of the IL-12 receptor β1 subunit protein on the surfaces of CD3+ T cells and NK cells was assessed (Fig. 2). There were significantly more T and NK cells expressing IL-12R in spleens from IFN-γ-treated CLP mice than in those from untreated CLP mice (Fig. 2B and D). In the CD3+ lymphocytes, there was a significant increase in the percentage of cells expressing IL-12R (Fig. 2A) after IFN-γ treatment. In the NK1.1+ lymphocytes, there was no increase in the percentage of cells expressing IL-12R (Fig. 2C), but there was a general increase in the number of NK cells, resulting in an overall increase in the number of IL-12R+ NK cells in the spleens of CLP mice treated with IFN-γ.
FIG. 2.
Administration of IFN-γ caused increased numbers of CD3+ T cells and NK cells expressing IL-12R in post-CLP mice after Pseudomonas challenge. Mice were subjected to either CLP or sham surgery. Five days later, the mice were given intravenous injections of saline or 5 × 107 CFU of P. aeruginosa, and they were sacrificed 6 h later. Spleens were aseptically excised and homogenized. Splenic homogenates were analyzed by flow cytometry after staining for cell surface markers. (A) Percentage of CD3+ T cells expressing IL-12R in control and IFN-γ-treated mice; (B) number of CD3+ IL-12R+ T cells as a percentage of all splenocytes; (C) percentage of NK cells expressing IL-12R; (D) number of NK1.1+ IL-12R+ T cells as a percentage of all splenocytes. *, significantly different from sham group; #, significantly different from CLP group (n = 6 to 10 per group).
To determine if IFN-γ treatment enhances the immune response to Pseudomonas, the bacterial burden in lungs was determined 6 h after Pseudomonas challenge (Fig. 3). All mice that were subjected to CLP prior to the Pseudomonas challenge had bacterial growth from lung tissue. In contrast, only two of the sham CLP mice challenged with Pseudomonas exhibited bacterial growth from lungs (seven of seven CLP mice compared to two of five sham mice; P < 0.05), and the mean number of CFU/lung in these mice was significantly lower than in mice that had been subjected to CLP (P < 0.05). Lung tissue homogenates from CLP mice had approximately 10-fold-greater numbers of bacterial CFU than samples from sham mice (Fig. 3). Treatment of post-CLP mice with IFN-γ at the time of Pseudomonas challenge did not significantly improve bacterial clearance, as indicated by the number of mice exhibiting bacterial growth (eight of eight animals) or number of CFU measured in lung homogenates (Fig. 3).
FIG. 3.
Administration of IFN-γ does not reverse impaired bacterial clearance in mice previously subjected to CLP. P. aeruginosa (5 × 107 CFU) was injected intravenously 5 days after CLP (or sham CLP), and mice were sacrificed 6 h later. Lungs were collected aseptically and homogenized in sterile saline, and serial dilutions were plated on tryptic soy agar. All untreated mice injected with Pseudomonas after CLP had positive lung cultures (seven of seven, versus two of five in the sham-CLP group; P < 0.05), and Pseudomonas growth in mice that had been subjected to CLP was approximately 10-fold higher than that of sham-CLP mice. All mice in the IFN-γ-treated group of CLP mice had positive lung cultures (eight of eight), and IFN-γ administration was not associated with a significant improvement in bacterial clearance. *, bacterial counts significantly different from those of sham group; #, number of animals with positive lung cultures significantly different from that for sham group (n = 5 to 8 per group).
Administration of IFN-γ significantly attenuated the increase in circulating IL-10 concentrations and increased the proinflammatory cytokine response but did not improve bacterial clearance or mortality in post-CLP mice after intravenous challenge with a lethal dose of P. aeruginosa.
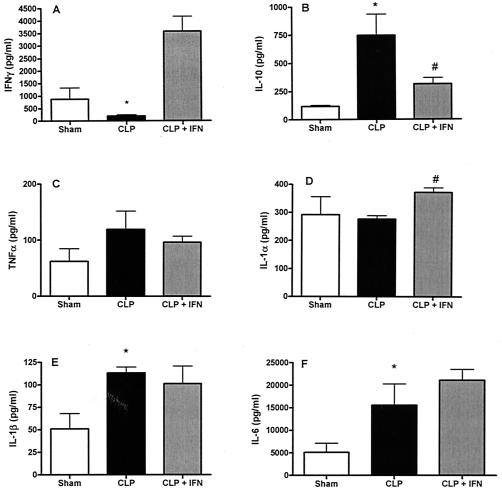
In our initial experiments, IFN-γ administration was associated with a significant effect on the cytokine response and a trend towards improved bacterial clearance in CLP mice after a sublethal Pseudomonas challenge. To further test the efficacy of exogenous IFN-γ in our model of post-CLP immunosuppression, we studied these parameters in post-CLP mice given a dose of Pseudomonas that causes ∼90% mortality. Mice that had been subjected to CLP or sham procedures 5 days earlier were challenged intravenously with P. aeruginosa (4 × 108 CFU) and sacrificed 6 h later for aseptic collection of lung, spleen, and blood samples. CLP caused significant suppression of Pseudomonas-induced IFN-γ concentrations in serum, which was reversed by exogenous IFN-γ administration (Fig. 4A). Serum IL-10 was significantly higher after Pseudomonas injection for mice that had been previously subjected to CLP than for the sham CLP mice (Fig. 4B). Administration of IFN-γ attenuated IL-10 induction, with lower circulating plasma concentrations of IL-10 detected for IFN-γ-treated CLP mice than for untreated CLP mice. At the same time, concentrations of proinflammatory cytokines in plasma were elevated for mice challenged with Pseudomonas after CLP and were relatively unaffected by administration of IFN-γ (Fig. 4C to F). Pseudomonas-induced plasma concentrations of TNF-α, IL-1α, IL-1β, and IL-6 in the CLP mice were all found to be as high as, or higher than, concentrations of those cytokines in plasma of sham-CLP mice. Of those cytokines, only IL-1α differed significantly between the two CLP groups, with higher serum IL-1α levels detected in the plasma of IFN-γ-treated CLP mice.
FIG. 4.
Administration of IFN-γ to CLP mice was associated with decreased serum concentrations of IL-10 but no difference in the proinflammatory cytokine profile induced by administration of a lethal dose of P. aeruginosa. P. aeruginosa (4 × 108 CFU) was injected intravenously 5 days after CLP (or sham CLP), and mice were sacrificed 6 h later. Blood samples were collected for plasma cytokine analysis. (A) Plasma IFN-γ induced by Pseudomonas is significantly lower in mice previously subjected to CLP than with sham CLP animals. Administration of IFN-γ resulted in increased concentrations of circulating IFN-γ in CLP mice. B) Plasma IL-10 induced by Pseudomonas is significantly higher in mice previously subjected to CLP when compared to sham CLP animals. Administration of IFN-γ resulted in a decrease in circulating concentrations of IL-10 in CLP mice. The proinflammatory cytokine response induced by Pseudomonas is as great as, or greater than, that seen in sham CLP animals as determined by plasma concentrations of (C) TNF-α;,(D) IL-1α, (E) IL-1β, and (F) IL-6. Administration of IFN-γ did not affect the proinflammatory cytokine response. *, significantly different from sham group; #, significantly different from CLP group (sham group, n = 4; CLP and CLP + IFN-γ groups, n = 7).
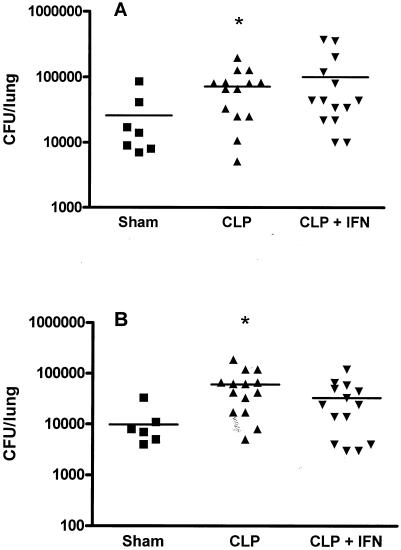
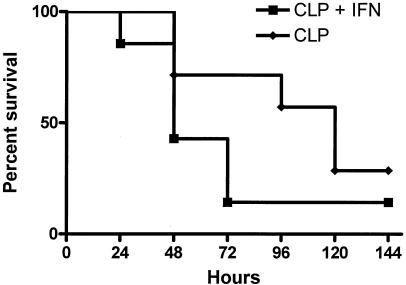
To test the functional significance of IFN-γ treatment, we investigated bacterial clearance in lungs of post-CLP mice treated with IFN-γ following a lethal Pseudomonas challenge. Following challenge with a lethal dose of Pseudomonas, all mice had positive bacterial cultures from the lungs. As at the lower dose of Pseudomonas, post-CLP mice had higher bacterial counts in lungs than sham controls (Fig. 5). Significantly higher levels of total bacterial growth (Fig. 5A) and Pseudomonas growth (Fig. 5B) were observed for post-CLP mice than for sham mice. Exogenous administration of IFN-γ did not improve bacterial burdens in lungs after CLP. Furthermore, there was no observable effect of IFN-γ administration on the mortality rate, with 14% of the IFN-γ-treated CLP mice surviving for 7 days after the Pseudomonas challenge compared to 29% of untreated CLP mice (Fig. 6).
FIG. 5.
Administration of IFN-γ does not reverse impaired clearance of a lethal dose of bacteria in mice previously subjected to CLP. P. aeruginosa (4 × 108 CFU) was injected intravenously 5 days after CLP (or sham CLP), and mice were sacrificed 6 h later. Lungs were collected aseptically and homogenized in sterile saline, and serial dilutions were plated. (A) Total numbers of bacterial CFU in lung homogenates were significantly higher for mice challenged with Pseudomonas after CLP than for sham CLP mice. Exogenous IFN-γ did not improve bacterial clearance. (B) Numbers of Pseudomonas bacterial CFU in lung homogenates were significantly higher for mice challenged with P. aeruginosa after CLP than for sham CLP mice. Exogenous IFN-γ did not improve clearance of the Pseudomonas bacterial challenge. *, significantly different from sham group (sham group, n = 7; CLP and CLP + IFN-γ groups, n = 14).
FIG. 6.
Survival after Pseudomonas challenge for post-CLP mice is not improved by administration of IFN-γ. Mice were subjected to CLP. Five days later, the mice were given intravenous injections of saline or 108 CFU of P. aeruginosa and observed for mortality over the next 7 days. One group of mice was given IFN-γ subcutaneously at the time of Pseudomonas challenge. Mortality was not improved by IFN-γ treatment (no significant difference; n = 7 per group).
IFN-γ KO mice have an increased IL-10 response but little difference in the production of proinflammatory cytokines, bacterial clearance, or survival after a Pseudomonas challenge.
To further investigate the role of IFN-γ in the immune response to Pseudomonas, wild-type and IFN-γ KO mice were challenged with a nonlethal dose of P. aeruginosa (5 × 107 CFU, given intravenously) and sacrificed 6 h later for aseptic collection of lung tissue and blood samples. IFN-γ was present in the plasma of wild-type mice challenged with Pseudomonas but not in that of IFN-γ KO mice (Fig. 7A). IL-10 concentrations in plasma were elevated in IFN-γ KO mice receiving a Pseudomonas challenge compared to those in wild-type mice (Fig. 7B). At the same time, concentrations of TNF-α, IL-1α, IL-1β, and IL-6 in plasma were induced by Pseudomonas in all mice (Fig. 7C to F). Among the proinflammatory cytokines analyzed, only IL-6 was significantly lower in IFN-γ KO mice than in wild-type controls.
FIG. 7.
IFN-γ KO mice exhibit increased serum concentrations of IL-10 but little difference in the proinflammatory cytokine response after Pseudomonas challenge. IFN-γ knockout mice (IFN KO) or wild-type controls (WT) were subjected to an intravenous injection of P. aeruginosa (5 × 107 CFU) and were sacrificed 6 h later. (A) Plasma IFN-γ concentration after Pseudomonas challenge. (B) Significantly higher circulating concentrations of IL-10 were detected after Pseudomonas injection for IFN-γ−/− mice than for wild-type controls. Concentrations of IL-6 (7F) in serum were significantly lower after injection of Pseudomonas into IFN-γ KO mice than for wild-type controls. However, (C) TNF-α, (D) IL-1α, and (E) IL-1β concentrations were similar between the two groups of mice. *, significantly different from other groups; #, significantly different from IFN knockout group (sham group, n = 4; WT and IFN-γ KO groups, n = 7).
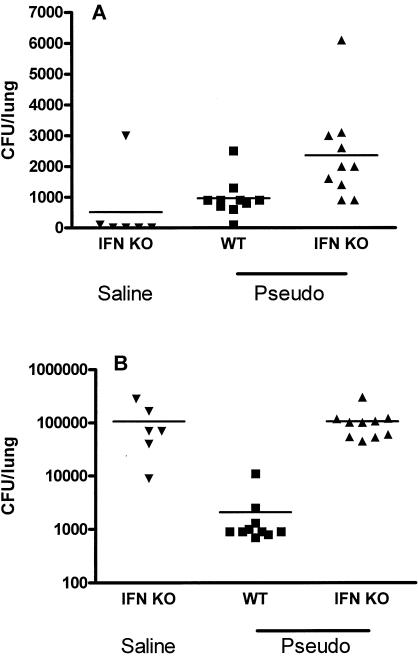
Additional studies were undertaken to measure clearance of Pseudomonas by wild-type and IFN-γ KO mice. Growth of Pseudomonas from the lungs of mice was not significantly different between wild-type and IFN-γ KO mice (Fig. 8A). However, high numbers of gram-positive cocci were cultured from lung samples of all IFN KO mice in both the Pseudomonas challenge group and the saline control group and were significantly higher than those for wild-type mice (Fig. 8B).
FIG. 8.
Loss of IFN-γ activity was not associated with diminished ability to clear a Pseudomonas bacterial challenge but was associated with a high baseline level of endogenous gram-positive bacterial background. IFN-γ knockout mice (IFN KO) or wild-type controls (WT) were subjected to an intravenous injection of P. aeruginosa (5 × 107 CFU) (Pseudo) and were sacrificed 6 h later. Lungs were collected aseptically and homogenized in sterile saline, and serial dilutions were cultured. (A) The number of Pseudomonas CFU in lung homogenates did not differ between wild-type and IFN-γ knockout mice after an intravenous injection of Pseudomonas. (B) Loss of IFN-γ activity was associated with a high background of endogenous gram-positive coccus growth in lung homogenates from animals with, or without, a Pseudomonas bacterial challenge.
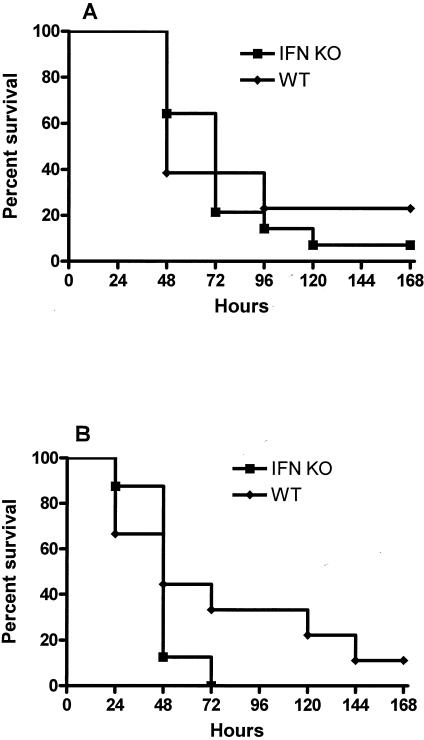
To further determine the functional role of IFN-γ in resistance to Pseudomonas challenge, we challenged IFN-γ KO and wild-type mice with Pseudomonas at two different doses and recorded the mortality rates over the subsequent 7-day period. Loss of IFN-γ had no discernible effect on mortality, with 22% of IFN-γ KO mice and 17% of wild-type mice surviving for 7 days after the low-dose challenge and no IFN-γ KO mice surviving the higher-dose challenge, compared to 11% of wild-type mice (Fig. 9).
FIG. 9.
Survival after intravenous injection of Pseudomonas was not affected by the loss of IFN-γ activity. P. aeruginosa was injected intravenously into IFN-γ KO (IFN KO) or wild-type (WT) mice with no prior injury, and mortality was observed over the next 7-day period. There was no difference between survival of IFN-γ KO mice and that of wild-type mice after challenges of either (A) 1 × 108 CFU or (B) 2 × 108 CFU (no significant difference; n = 18 per group]).
DISCUSSION
Acute survival of patients after a major injury has been greatly improved by advances in fluid resuscitation and physiological support. However, these patients are at risk for development of secondary infections that can impact morbidity and long-term mortality. Immunocompetence may be compromised after a major injury and thus may be a predisposing factor for the development of secondary bacterial and fungal infections. P. aeruginosa is one of the most frequently isolated organisms for patients that have developed nosocomial infections, and it has been associated with a high case fatality rate (3, 34, 50, 60).
In our model of post-CLP immunosuppression, mice that were subjected to CLP had a diminished ability to clear a Pseudomonas challenge administered 5 days after injury. This alteration in bacterial clearance correlated with a suppressed IFN-γ response. Impaired IFN-γ production has been associated with sepsis in patients (45). IFN-γ has a number of effects on inflammatory and antimicrobial immune responses. Up-regulation of major histocompatibility complex class I expression by IFN-γ is important for host response to intracellular pathogens, such as viruses. IFN-γ also up-regulates the expression of class II major histocompatibility complex by antigen-presenting cells, thereby promoting antigen-specific activation of CD4+ T cells (2, 32) and skewing the immune response to a Th1 phenotype. IFN-γ induces production of IL-12 by phagocytes (59), which further drives Th1 differentiation. Conversely, production of IL-12 by macrophages, dendritic cells, and neutrophils after stimulation with lipopolysaccharide or other microbial products (7, 20) promotes secretion of IFN-γ by antigen-stimulated, naive CD4+ T cells and NK cells (26, 31). IFN-γ activates antimicrobial effector functions in macrophages and neutrophils that are manifested by increased receptor-mediated phagocytosis and enhanced microbial killing (2, 13, 19, 25). Furthermore, IFN-γ helps to direct leukocytes to sites of infection by up-regulating the expression of chemokines and adhesion molecules (2, 22, 24, 46). Mice lacking a functional IFN-γ response have been reported to have decreased natural resistance to bacterial, parasitic, and viral infections (4, 23, 40, 52). Humans with loss of functional IFN-γ receptors have an increased susceptibility to infection with viruses and intracellular bacteria but have not been reported to have increased susceptibility to extracellular bacteria (15). Our study indicates that IFN-γ is not a critical factor in the response to systemic Pseudomonas infection. Specifically, treatment of post-CLP mice with IFN-γ did not improve bacterial clearance or survival during subsequent Pseudomonas challenge. Secondly, IFN-γ KO mice did not exhibit impaired resistance to systemic Pseudomonas infection.
Mice that were subjected to CLP also had an increased IL-10 response to Pseudomonas challenge compared to sham mice. Other investigators have reported that IL-10 is measurably elevated in animal models of immunosuppression secondary to trauma, burns, or major surgery (1, 53, 57). Neutralization of IL-10 has been reported to improve resistance to secondary infection in some models of postinflammatory immunosuppression (28, 43, 51). IL-10 is associated with a Th2 cytokine pattern, inhibits proinflammatory cytokine production, and suppresses the antigen-presenting capacity of monocytes and dendritic cells (14, 17, 47, 48). Despite these suppressive activities, IL-10 may promote phagocytic activity in monocytes and macrophages (5) as well as enhancing the cytotoxic activity of NK cells (6). In contrast, Standiford and colleagues reported that CLP-induced elevations in IL-10 caused impairment in alveolar macrophage function and that elevated endogenous IL-10 levels impaired pulmonary clearance of Pseudomonas in a model of post-CLP immunosuppression (43, 51). At the same time, IL-10 plays a protective role in controlling the inflammatory response induced by endotoxin or bacteria (35, 39).
In the present study, administration of IFN-γ at the time of bacterial challenge attenuated the increase in circulating IL-10 concentrations. However, neither bacterial clearance nor mortality was improved in IFN-γ-treated mice. Furthermore, IFN-γ KO mice did not exhibit a diminished ability to clear bacteria or any difference in survival after an intravenous Pseudomonas challenge compared to wild-type mice despite having significantly higher circulating concentrations of IL-10. It is possible that IFN-γ KO mice have compensatory mechanisms that affected their susceptibility to a Pseudomonas challenge. However, our results are compatible with those of a previous investigation, which reported that IFN-γ KO mice had increased susceptibility to localized intratracheal challenges with Klebsiella pneumoniae but were not more susceptible to an intravenous bacterial challenge than wild-type mice (36), suggesting that the host response to systemic infections appeared to be independent of IFN-γ. Furthermore, human patients with genetic deficiencies in IFN-γ protein or receptor have increased susceptibility to mycobacterial infections but no apparent increase in infections due to extracellular bacteria, suggesting that IFN-γ is not an important component of the immune response to bacteria such as Pseudomonas.
Mice that were subjected to CLP had a proinflammatory response to the Pseudomonas challenge that was equal to or greater than that observed with sham controls despite having an increase in IL-10 and a suppression of IFN-γ production. This mix of proinflammatory and anti-inflammatory cytokines is similar to the mixed anti-inflammatory response syndrome reported in some sepsis patients and suggests that inflammatory cells are not unresponsive to stimuli, nor is the inflammatory response generally down-regulated. Rather, it seems that specific immune functions are altered in response to bacterial challenge. We have previously demonstrated that IL-12 production is impaired after CLP or burn injuries (38, 53). IL-12 is an important modulator of the innate immune response to infection. IL-12 strongly induces IFN-γ production and is required for an optimal IFN-γ response to bacterial infections (58). Administration of IFN-γ to mice at the time of Pseudomonas challenge was associated with increased production of proinflammatory cytokines, such as TNF-α, and an increase in IL-12 protein and receptor expression. IL-12 is produced mainly by activated inflammatory cells, including monocytes, macrophages, neutrophils, and dendritic cells (11, 30). These cells appeared to be functional after CLP in respect to mounting a proinflammatory cytokine response, and IFN-γ administration resulted in an increased IL-12 response.
Administration of IFN-γ has been investigated for clinical efficacy in trauma and burn patients (27, 41, 42, 56). Our results, coupled with the findings in previous reports, seem to indicate that systemic administration of IFN-γ alone for prophylaxis, or treatment, of nosocomial infections might not be an effective clinical strategy. Systemic administration of IFN-γ does not appear to result in detectable intrapulmonary concentrations (12), which could be important if IFN-γ functions in local, rather than systemic, immune responses. However, aerosol delivery of IFN-γ is possible and is currently under investigation for some clinical applications. Alternatively, administration of IFN-γ in combination with other cytokine strategies might serve as an avenue for future investigation.
Acknowledgments
This work was supported by grants from the NIH NIGMS and Shriners of North America.
Editor: F. C. Fang
REFERENCES
- 1.Ayala, A., D. L. Lehman, C. D. Herdon, and I. H. Chaudry. 1994. Mechanism of enhanced susceptibility to sepsis following hemorrhage. Interleukin-10 suppression of T-cell response is mediated by eicosanoid-induced interleukin-4 release. Arch. Surg. 129:1172-1178. [DOI] [PubMed] [Google Scholar]
- 2.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 3.Brun-Buisson, C., F. Doyon, J. Carlet, P. Dellamonica, F. Gouin, A. Lepoutre, J. C. Mercier, G. Offenstadt, B. Regnier, et al. 1995. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. JAMA 274:968-974. [PubMed] [Google Scholar]
- 4.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchwald, U. K., H. F. Geerdes-Fenge, J. Vockler, S. Ziege, and H. Lode. 1999. Interleukin-10: effects on phagocytosis and adhesion molecule expression of granulocytes and monocytes in a comparison with prednisolone. Eur. J. Med. Res. 4:85-94. [PubMed] [Google Scholar]
- 6.Carson, W. E., M. J. Lindemann, R. Baiocchi, M. Linett, J. C. Tan, C. C. Chou, S. Narula, and M. A. Caligiuri. 1995. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood 85:3577-3585. [PubMed] [Google Scholar]
- 7.Cassatella, M. A., L. Meda, S. Gasperini, A. D'Andrea, X. Ma, and G. Trinchieri. 1995. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 8.Celis, R., A. Torres, J. M. Gatell, M. Almela, R. Rodriguez-Roisin, and A. Agusti-Vidal. 1988. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest 93:318-324. [DOI] [PubMed] [Google Scholar]
- 9.Craven, D. E., and M. R. Driks. 1987. Nosocomial pneumonia in the intubated patient. Semin. Respir. Infect. 2:20-33. [PubMed] [Google Scholar]
- 10.Craven, D. E., K. A. Steger, and T. W. Barber. 1991. Preventing nosocomial pneumonia: state of the art and perspectives for the 1990s. Am. J. Med. 91:44S-53S. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, and E. Nickbarg. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decker, T., S. Stockinger, M. Karaghiosoff, M. Muller, and P. Kovarik. 2002. IFNs and STATs in innate immunity to microorganisms. J. Clin. Investig. 109:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis, M. 1991. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J. Leukoc. Biol. 49:380-387. [DOI] [PubMed] [Google Scholar]
- 14.de Waal, M. R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorman, S. E., G. Uzel, J. Roesler, J. S. Bradley, J. Bastian, G. Billman, S. King, A. Filie, J. Schermerhorn, and S. M. Holland, S. M. 1999. Viral infections in interferon-gamma receptor deficiency. J. Pediatr. 135:640-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagon, J. Y., J. Chastre, A. Vuagnat, J. L. Trouillet, A. Novara, and C. Gibert. 1996. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA 275:866-869. [PubMed] [Google Scholar]
- 17.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 18.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 19.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 20.Heufler, C., F. Koch, U. Stanzl, G. Topar, M. Wysocka, G. Trinchieri, A. Enk, R. M. Steinman, N. Romani, and G. Schuler. 1996. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 26:659-668. [DOI] [PubMed] [Google Scholar]
- 21.Heyland, D. K., D. J. Cook, L. Griffith, S. P. Keenan, C. Brun-Buisson, et al. 1999. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am. J. Respir. Crit. Care Med. 159:1249-1256. [DOI] [PubMed] [Google Scholar]
- 22.Hou, J., V. Baichwal, and Z. Cao. 1994. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 91:11641-11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 24.Jesse, T. L., R. LaChance, M. F. Iademarco, and D. C. Dean. 1998. Interferon regulatory factor-2 is a transcriptional activator in muscle where it regulates expression of vascular cell adhesion molecule-1. J. Cell Biol. 140:1265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 26.Lederer, J. A., V. L. Perez, L. DesRoches, S. M. Kim, A. K. Abbas, and A. H. Lichtman. 1996. Cytokine transcriptional events during helper T cell subset differentiation. J. Exp. Med. 184:397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livingston, D. H., P. A. Loder, S. M. Kramer, U. E. Gibson, and H. C. Polk, Jr. 1994. Interferon gamma administration increases monocyte HLA-DR antigen expression but not endogenous interferon production. Arch. Surg. 129:172-178. [DOI] [PubMed] [Google Scholar]
- 28.Lyons, A., A. Goebel, J. A. Mannick, and J. A. Lederer. 1999. Protective effects of early interleukin 10 antagonism on injury-induced immune dysfunction. Arch. Surg. 134:1317-1323. [DOI] [PubMed] [Google Scholar]
- 29.Lyons, A., J. L. Kelly, M. L. Rodrick, J. A. Mannick, and J. A. Lederer. 1997. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Surg. 226:450-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071-5079. [PubMed] [Google Scholar]
- 31.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071-5079. [PubMed] [Google Scholar]
- 32.Mach, B., V. Steimle, E. Martinez-Soria, and W. Reith. 1996. Regulation of MHC class II genes: lessons from a disease. Annu. Rev. Immunol. 14:301-331. [DOI] [PubMed] [Google Scholar]
- 33.McEachern, R., and G. D. Campbell, Jr. 1998. Hospital-acquired pneumonia: epidemiology, etiology, and treatment. Infect. Dis. Clin. N. Am. 12:761-779. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery, A. B., M. A. Stager, C. J. Carrico, and L. D. Hudson. 1985. Causes of mortality in patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 132:485-489. [DOI] [PubMed] [Google Scholar]
- 35.Moore, K. W., M. R. de Waal, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 36.Moore, T. A., M. L. Perry, A. G. Getsoian, M. W. Newstead, and T. J. Standiford. 2002. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect. Immun. 70:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muehlstedt, S. G., M. Lyte, and J. L. Rodriguez. 2002. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock 17:443-450. [DOI] [PubMed] [Google Scholar]
- 38.Murphey, E. D., C. Y. Lin, R. W. McGuire, T. Toliver-Kinsky, D. N. Herndon, and E. R. Sherwood. 2004. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock 21:415-425. [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer, A., C. Oberholzer, K. S. Bahjat, R. Ungaro, C. L. Tannahill, M. Murday, F. R. Bahjat, Z. Abouhamze, V. Tsai, D. LaFace, B. Hutchins, L. L. Moldawer, and M. J. Clare-Salzler. 2002. Increased survival in sepsis by in vivo adenovirus-induced expression of IL-10 in dendritic cells. J. Immunol. 168:3412-3418. [DOI] [PubMed] [Google Scholar]
- 40.Pearl, J. E., B. Saunders, S. Ehlers, I. M. Orme, and A. M. Cooper. 2001. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol. 211:43-50. [DOI] [PubMed] [Google Scholar]
- 41.Perl, T. M., L. Dvorak, T. Hwang, and R. P. Wenzel. 1995. Long-term survival and function after suspected gram-negative sepsis. JAMA 274:338-345. [PubMed] [Google Scholar]
- 42.Polk, H. C., Jr., W. G. Cheadle, D. H. Livingston, J. L. Rodriguez, K. M. Starko, A. E. Izu, H. S. Jaffe, and G. Sonnenfeld. 1992. A randomized prospective clinical trial to determine the efficacy of interferon-gamma in severely injured patients. Amer. J. Surg. 163:191-196. [DOI] [PubMed] [Google Scholar]
- 43.Reddy, R. C., G. H. Chen, M. W. Newstead, T. Moore, X. Zeng, K. Tateda, and T. J. Standiford. 2001. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect. Immun. 69:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rello, J., and E. Diaz. 2003. Pneumonia in the intensive care unit. Crit. Care Med. 31:2544-2551. [DOI] [PubMed] [Google Scholar]
- 45.Rigato, O., and R. Salomao. 2003. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock 19:113-116. [DOI] [PubMed] [Google Scholar]
- 46.Rollins, B. J., T. Yoshimura, E. J. Leonard, and J. S. Pober. 1990. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am. J. Pathol. 136:1229-1233. [PMC free article] [PubMed] [Google Scholar]
- 47.Romagnani, S. 1995. Biology of human TH1 and TH2 cells. J. Clin. Immunol. 15:121-129. [DOI] [PubMed] [Google Scholar]
- 48.Romagnani, S. 1995. Biology of human TH1 and TH2 cells. J. Clin. Immunol. 15:121-129. [DOI] [PubMed] [Google Scholar]
- 49.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 50.Seidenfeld, J. J., D. F. Pohl, R. C. Bell, G. D. Harris, and W. G. Johanson, Jr. 1986. Incidence, site, and outcome of infections in patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 134:12-16. [DOI] [PubMed] [Google Scholar]
- 51.Steinhauser, M. L., C. M. Hogaboam, S. L. Kunkel, N. W. Lukacs, R. M. Strieter, and T. J. Standiford. 1999. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J. Immunol. 162:392-399. [PubMed] [Google Scholar]
- 52.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 53.Toliver-Kinsky, T. E., T. K. Varma, C. Y. Lin, D. N. Herndon, and E. R. Sherwood. 2002. Interferon-gamma production is suppressed in thermally injured mice: decreased production of regulatory cytokines and corresponding receptors. Shock 18:322-330. [DOI] [PubMed] [Google Scholar]
- 54.Valles, J., E. Mesalles, D. Mariscal, F. M. del Mar, R. Pena, J. L. Jimenez, and J. Rello. 2003. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 29:1981-1988. [DOI] [PubMed] [Google Scholar]
- 55.van Dissel, J. T., P. van Langevelde, R. G. Westendorp, K. Kwappenberg, and M. Frolich. 1998. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351:950-953. [DOI] [PubMed] [Google Scholar]
- 56.Wasserman, D., J. D. Ioannovich, R. D. Hinzmann, G. Deichsel, G. G. Steinmann, et al. 1998. Interferon-gamma in the prevention of severe burn-related infections: a European phase III multicenter trial. Crit. Care Med. 26:434-439. [DOI] [PubMed] [Google Scholar]
- 57.Woiciechowsky, C., K. Asadullah, D. Nestler, B. Eberhardt, C. Platzer, B. Schoning, F. Glockner, W. R. Lanksch, H. D. Volk, and W. D. Docke. 1998. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med. 4:808-813. [DOI] [PubMed] [Google Scholar]
- 58.Wysocka, M., M. Kubin, L. Q. Vieira, L. Ozmen, G. Garotta, P. Scott, and G. Trinchieri. 1995. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 25:672-676. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida, A., Y. Koide, M. Uchijima, and T. O. Yoshida. 1994. IFN-gamma induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem. Biophys. Res. Commun. 198:857-861. [DOI] [PubMed] [Google Scholar]
- 60.Young, L. S., P. Stevens, and B. Kaijser. 1982. Gram-negative pathogens in septicaemic infections. Scand. J. Infect. Dis. Suppl. 31:78-94. [PubMed] [Google Scholar]