Abstract
The apical organelles in apicomplexan parasites are characteristic secretory vesicles containing complex mixtures of molecules. While apical organelle discharge has been demonstrated to be involved in the cellular invasion of some apicomplexan parasites, including Toxoplasma gondii and Plasmodium spp., the mechanisms of apical organelle discharge by Cryptosporidium parvum sporozoites and its role in host cell invasion are unclear. Here we show that the discharge of C. parvum apical organelles occurs in a temperature-dependent fashion. The inhibition of parasite actin and tubulin polymerization by cytochalasin D and colchicines, respectively, inhibited parasite apical organelle discharge. Chelation of the parasite's intracellular calcium also inhibited apical organelle discharge, and this process was partially reversed by raising the intracellular calcium concentration by use of the ionophore A23187. The inhibition of parasite cytoskeleton polymerization by cytochalasin D and colchicine and the depletion of intracellular calcium also decreased the gliding motility of C. parvum sporozoites. Importantly, the inhibition of apical organelle discharge by C. parvum sporozoites blocked parasite invasion of, but not attachment to, host cells (i.e., cultured human cholangiocytes). Moreover, the translocation of a parasite protein, CP2, to the host cell membrane at the region of the host cell-parasite interface was detected; an antibody to CP2 decreased the C. parvum invasion of cholangiocytes. These data demonstrate that the discharge of C. parvum sporozoite apical organelle contents occurs and that it is temperature, intracellular calcium, and cytoskeleton dependent and required for host cell invasion, confirming that apical organelles play a central role in C. parvum entry into host cells.
Cryptosporidium parvum, an intracellular protozoan parasite, is a major cause of waterborne gastroenteritis throughout the world (12, 24, 32). Immunocompetent subjects infected with C. parvum develop a self-limited watery diarrhea, with symptoms rarely lasting longer than 1 to 2 weeks. However, in immunocompromised individuals, such as patients with AIDS, the disease may be life-threatening (12, 24). The very young and the elderly are also highly susceptible to severe C. parvum infections. Despite the magnitude and severity of C. parvum infections, there is currently no fully effective parasite-specific pharmaceutical therapy (12, 14).
C. parvum is a member of the protistan group of the phylum Apicomplexa (38). The members of this group have complex life cycles involving transmission within and between hosts by specialized cell-invasive stages (generally termed zoites) that show directional gliding motility relative to their anterior-posterior axis. Apical organelles (i.e., rhoptries, micronemes, and dense granules) are vesicular secretory organelles that are present in zoites. Specifically, rhoptries and micronemes are predominantly localized at the apical region and have been implicated in host cell adhesion and entry (5). It has been hypothesized that the apical organelles secrete substances that enable zoites to adhere selectively to and invade host cells, and once within cells, to cause further host cell modifications. The rhoptries and micronemes usually secrete their contents apically, whereas the dense granules release their contents elsewhere on the zoite's surface and are not necessarily apical in position, depending on the stage and species (5).
Like other apicomplexan parasites, C. parvum infects host cells in two steps, attachment and invasion. When an infective C. parvum sporozoite is released from an oocyst in the gastrointestinal tract, it attaches to the apical membrane surface of a host epithelial cell by its apical end and is subsequently encapsulated by a parasitophorous vacuole membrane. At the host cell-parasite interface within the host cell cytoplasm, an electron-dense junction, or dense band, is formed. Thus, the parasitophorous vacuole membrane and the dense band keep the internalized C. parvum intracellular but extracytoplasmic (34). Recent morphological studies have revealed that each C. parvum sporozoite has a single rhoptry and multiple micronemes and dense granules around its apical region (31). Proteins that are localized in the apical region and the surface membrane of C. parvum sporozoites have been intensively studied since they are likely to be involved in parasite motility and host cell invasion. Nevertheless, little is known about the role of apical organelle discharge by C. parvum sporozoites during host cell adhesion and entry.
Regulated secretion has been studied intensively in numerous eukaryotic cells, from protozoa to mammalian cells (16). The activation of intracellular signal transduction, including a rise in cytoplasmic free calcium and the regulation of cytoskeletal filaments, especially microtubules and actin, has been implicated in the machinery of secretory vesicle-plasma membrane fusion, resulting in exocytic discharge of the vesicle contents from cells (7). C. parvum sporozoites contain actin and tubulin, and their cDNA sequences have been determined (1, 6, 30). Using an in vitro model of intestinal cryptosporidiosis, Wiest and colleagues (36) demonstrated that antimicrotubule drugs cannot block excystation but can disrupt the microtubule network within C. parvum sporozoites and inhibit C. parvum infections of host cells. In addition, members of our laboratory used immunogold labeling with antibodies against C. parvum proteins to identify the deposition of parasite proteins in the dense band at the host cell-parasite interface during C. parvum infections of cultured human biliary epithelial cells (i.e., cholangiocytes), suggesting that there is a discharge of parasite proteins into the infected host cell (13, 21). Consequently, we undertook the present study to test the mechanism of apical organelle discharge by C. parvum sporozoites and to examine its association with the host cell invasion of cultured human cholangiocytes. Using a variety of conditions that stimulated or blocked apical organelle secretion, we demonstrated that the discharge of C. parvum sporozoite apical organelle contents is temperature, intracellular calcium, and cytoskeleton dependent and is required for host cell invasion.
MATERIALS AND METHODS
Parasites.
C. parvum oocysts harvested from calves inoculated with an Iowa strain originally obtained from Harley Moon at the National Animal Disease Center (Ames, Iowa) were purchased from a commercial source (Pleasant Hill Farms, Troy, Idaho). Before they were used to infect cells, oocysts were treated with 1% sodium hypochlorite on ice for 20 min and then subjected to an excystation solution consisting of 0.75% taurodeoxycholate and 0.25% trypsin for 30 min at 37°C. After washing with Dulbecco's modified Eagle's medium (DMEM)-F-12 medium (BioWhittaker, Walkersville, Md.), freshly excysted sporozoites were collected by centrifugation at 900 × g for 5 min and then resuspended in a culture medium consisting of DMEM-F-12, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (referred to hereafter as infection medium). The viability of excysted sporozoites was then determined as previously described by others (8). Only those with a viability of >90% were used for all experiments.
Antibodies to C. parvum.
The following antibodies were selected for this study: Y-271, a previously described rabbit antiserum against C. parvum sporozoite proteins, was used to label the whole protein profile of C. parvum sporozoites (39); 4E9, a monoclonal antibody which recognizes a carbohydrate epitope on gp900 and gp40 in sporozoites, was used to label associated microneme proteins of C. parvum sporozoites (10); and CP2, a rabbit polyclonal antibody against the CP2 protein, was chosen to label sporozoite membranous structures (25).
Temperature experiments.
Freshly excysted sporozoites were washed by centrifugation twice with infection medium and then resuspended in infection medium at 107 sporozoites/ml. The sporozoites were then maintained at various temperatures for up to 24 h. After being washed with infection medium, the sporozoites were resuspended in infection medium and then either exposed to cultured cells or processed to measure the viability as described above. Some sporozoites were also fixed for immunofluorescence microscopy. The supernatants were harvested for Western blotting.
Inhibition of cytoskeleton polymerization with colchicine and CD.
Freshly excysted sporozoites were washed twice by centrifugation with infection medium and then resuspended in infection medium at 107 sporozoites/ml. The sporozoites were then pretreated for 30 min with 100 μM colchicine or 1 μg of cytochalasin D (CD)/ml. For infectivity or motility assays, sporozoites were exposed to colchicine and CD at 18°C and then exposed to cultured cells or processed for measurements of the viability or gliding motility after intensive washing with infection medium. For analyses of parasite-associated proteins in the supernatants, sporozoites were treated with these drugs at 37°C in the absence of host cells. Culture medium supernatants were then harvested after centrifugation for Western blotting, and sporozoites were processed for immunofluorescence microscopy.
Manipulation of parasitic intracellular calcium.
BAPTA-AM [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetra-(acetoxymethyl)ester], a highly specific chelator of intracellular Ca2+, and a calcium ionophore, A23187, were obtained from Molecular Probes (Eugene, Oreg.). Freshly excysted C. parvum sporozoites were exposed to 20 μM BAPTA-AM at 18°C for 30 min, and the supernatants were harvested for analysis by Western blotting. After being washed with DMEM-F-12, the sporozoites were either cytospun onto slides for immunofluorescence microscopy or exposed to cultured cholangiocytes. Some sporozoites were also exposed to 25 μM A23187 at 18°C for 30 min. After being washed with DMEM-F-12, the sporozoites were exposed to cultured cells.
Immunofluorescence of C. parvum sporozoites.
C. parvum sporozoites that had been cytospun onto slides were immediately fixed (0.1 M 1,4-piperazinediethanesulfonic acid [pH 6.95], 1 mM EGTA, 3 mM magnesium sulfate [Sigma-Aldrich, Saint Louis, Mo.], and 2% paraformaldehyde) at 37°C for 20 min and then permeabilized with 0.2% (vol/vol) Triton X-100 in phosphate-buffered saline (PBS). Fixed sporozoites were incubated with the Y-271, 4E9, or CP2 antibody to label various components of C. parvum sporozoites, followed by incubation with fluorescein-labeled secondary antibodies (Molecular Probes). Labeled sporozoites were rinsed three times with PBS and once with distilled water, mounted with mounting medium (H-1000; Vector Laboratories, Burlingame, Calif.), and assessed under a Zeiss 510 confocal microscope (Carl Zeiss, Inc., Oberkochen, Germany). With an LSM 510 analysis system provided by Carl Zeiss, Inc., the fluorescence intensity of fluorescein isothiocyanate for C. parvum apical organelle labeling in the apical region of the sporozoites was measured for 200 randomly selected sporozoites for each group.
Electron microscopy.
C. parvum sporozoites were collected by centrifugation and fixed with a primary fixative (100 mM cacodylate [pH 7.4], 2.5% glutaraldehyde) for 1 h. The sporozoites and cells were then postfixed with 1% osmium tetroxide for 1 h and subsequently stained with 1% uranyl acetate overnight at 4°C, dehydrated in a graded series of ethanol, and embedded in Spurrs resin. Each sample was cut into thin sections of 50 nm thick with a Reichert Ultracut S ultramicrotome. Thin sections were collected in ribbons on carbon-reinforced, Formvar-coated slot grids and then stained in Sato lead for 5 min (4, 17, 33). The sections were then observed with a JEOL 1200 electron microscope. The numbers of rhoptries, micronemes, and dense granules in the apical region were measured for 200 randomly selected sporozoites for each group.
For immunogold labeling, cells were grown in 35-mm-diameter culture dishes to 70 to 80% confluence and then exposed to C. parvum sporozoites for 1 h. After three washes with DMEM-F-12 at 37°C, the cells were fixed in 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 h. The samples were processed and embedded in Lowicryl K4 M according to the manufacturer's instructions (Electron Microscopy Sciences, Philadelphia, Pa.). The plastic blocks were cut with a Reichert Ultracut S ultramicrotome, and sections were blocked with PBS containing 10% fetal calf serum. The grids were then incubated with the CP2 antibody diluted in PBS with 10% fetal calf serum. After being washed, the grids were incubated with 10-nm-diameter gold bead-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma-Aldrich) and examined with a JEOL 1200 electron microscope.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Ten-microliter samples of harvested culture medium supernatants were loaded into each lane of a gel, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions, and blotted onto nitrocellulose membranes. The membranes were sequentially incubated with sporozoite-specific antibodies and then 0.2 μg of horseradish peroxidase-conjugated secondary antibody/ml and revealed by use of an enhanced chemiluminescence light substrate (ECL; Amersham, Buckinghamshire, England). As a positive control, freshly excysted C. parvum sporozoites were lysed with a lysis buffer (50 mM Tris-hydrochloride [pH 8.0], 150 mM sodium chloride, 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride, 20 μg of leupeptin/ml, and 20 μg of pepstatin/ml) and sonicated for 30 s. The sporozoite lysates (5 μg of protein) were then loaded into the gels.
Gliding motility assays.
Sporozoites were resuspended in infection medium at 107 sporozoites/ml and then cytospun onto poly-l-lysine-coated glass slides at 1,000 rpm for 3 min at room temperature. The slides were maintained for 5 min at 37°C and were fixed immediately with a solution containing 0.1 M 1,4-piperazinediethanesulfonic acid (pH 6.95), 1 mM EGTA, 3 mM magnesium sulfate (Sigma-Aldrich), and 2% paraformaldehyde at 37°C for 10 min. After membrane permeabilization with 0.2% (vol/vol) Triton X-100 in PBS, the fixed sporozoites were incubated with the Y-271 antibody to label the sporozoites, and short motility trails were monitored by use of a fluorescein-labeled secondary antibody (Molecular Probes). Labeled slides were assessed with a Zeiss 510 confocal microscope (Carl Zeiss, Inc.). The length of the gliding trace of each sporozoite was then measured with an LSM 510 analysis system provided by Carl Zeiss, Inc. Up to 200 randomly selected sporozoites were measured for each group. Gliding motility was expressed in micrometers per second.
Infection of cultured cholangiocytes.
H69 cells (a gift of D. Jefferson, Tufts University, Boston, Mass.) are simian virus 40-transformed human cholangiocytes that were originally derived from a healthy liver that was harvested for transplantation and which have been extensively characterized previously (19). Two in vitro models, an attachment model and an attachment-invasion model, were employed to assay the attachment and invasion of C. parvum to H69 cells, as previously described (11). Briefly, H69 cells were seeded into four-well chamber slides (Becton Dickinson Labware, Franklin Lakes, N.J.) and grown to 70 to 80% confluence. For the attachment model, H69 cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in PBS before exposure to C. parvum sporozoites. In this model, the organism can only attach to the fixed cell surface. For the attachment-invasion model, live cells (without prefixation) were directly exposed to C. parvum sporozoites, thus allowing the organism to both attach to and enter into host cells. Infection with C. parvum was done in a culture medium consisting of DMEM-F-12, 100 U of penicillin/ml, 100 μg of streptomycin (Life Technologies, Carlsbad, Calif.)/ml, and C. parvum sporozoites (106 sporozoites/slide well or culture plate). Inactivated organisms (treated at 65°C for 30 min) were used for sham infection experiments (24). Infection assays (to determine the attachment rate or attachment-invasion rate) were performed after a 2-h incubation of the cells with the parasite by use of an indirect immunofluorescence technique using the Y-271 antiserum as previously described (11). For inhibitory experiments with the CP-2 antibody, 20 μl of the CP-2 antibody/ml was added to the medium at the same time that C. parvum was added. Normal rabbit serum was used as a control.
Statistical analysis.
All values are given as means ± standard errors of the means. Means of groups were compared with Student's t test (unpaired). For multiple comparisons, analyses of variance were performed, with subsequent correction by Bonferroni's method. P values of <0.05 were considered statistically significant.
RESULTS
Apical organelle discharge by C. parvum sporozoites is temperature dependent.
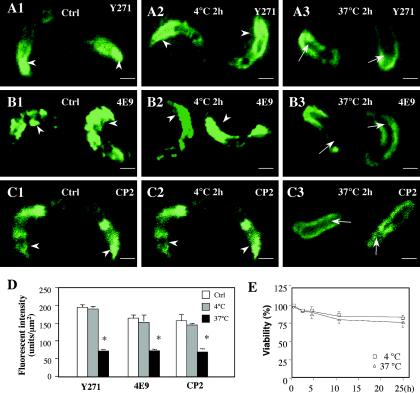
Temperature-dependent apical organelle discharge has been reported for other apicomplexan parasites. To test the effect of temperature on C. parvum apical organelle discharge, we incubated freshly excysted C. parvum sporozoites at 4 or 37°C and then immunofluorescently stained them by using different antibodies against C. parvum. Consistent with previous studies, the Y271, 4E9, and CP2 antibodies showed strong positive reactions at the apical region of control sporozoites (immediately after excystation) (10, 25, 39). The sporozoite membranes and posterior cytoplasm in the control sporozoites also showed positive reactions (Fig. 1A to C). Sporozoites that were maintained at 4°C for 2 h in the culture medium in the absence of host cells showed a similar staining pattern for all of the antibodies to that found in control sporozoites (Fig. 1A to C). However, when C. parvum sporozoites were maintained at 37°C for 2 h in the culture medium in the absence of host cells, they displayed a significant decrease in labeling with Y271, 4E9, or CP2 in the apical region, whereas the membrane labeling remained (Fig. 1A to C). A quantitative analysis showed no difference in fluorescence densities in the apical region for all of the antibodies between freshly excysted sporozoites and sporozoites maintained at 4°C. However, sporozoites maintained at 37°C for 2 h in the absence of host cells showed a significant (P < 0.01) decrease in fluorescence intensity with Y271, 4E9, and CP2 in the apical region compared with that in control or treated (4°C) sporozoites (Fig. 1D). No significant change in the viability of sporozoites was found after incubation in the absence of host cells at 4 or 37°C for up to 24 h (Fig. 1E). These data suggest that a high-temperature treatment of C. parvum sporozoites results in a loss of apical region-associated molecules in the absence of host cells.
FIG. 1.
Labeling of C. parvum sporozoites that were maintained at 4 or 37°C in the absence of host cells. Freshly excysted sporozoites were incubated in culture medium at 4 or 37°C in the absence of host cells for 2 h and then fixed for immunofluorescence staining. Sporozoites collected immediately after excystation were used as a control. (A to C) Fluorescent images of sporozoites stained with antibodies against C. parvum. Y271, a polyclonal antibody which recognizes the whole protein profile of C. parvum sporozoites, showed strong staining in the apical region of control sporozoites (A1, arrowheads) or those that were maintained at 4°C for 2 h (A2, arrowheads), but not in sporozoites that were maintained at 37°C for 2 h (A3, arrows). Both 4E9 (a monoclonal antibody which recognizes microneme-associated gp900 and gp40 proteins) and CP2 (an antibody against membranous structures) also showed strong staining in the apical region of control sporozoites (B1 and C1, arrowheads) or those that were maintained at 4°C for 2 h (B2 and C2, arrowheads), but not in sporozoites that were maintained at 37°C for 2 h (B3 and C3, arrows). (D) Quantitative analysis of fluorescence intensities in the apical region of sporozoites after treatment with antibodies. (E) Viability of C. parvum sporozoites when maintained at 4 or 37°C. Ctrl, control; *, P < 0.05, compared with control or sporozoites maintained at 4°C; error bars, standard errors of the means. Bars = 1 μm.
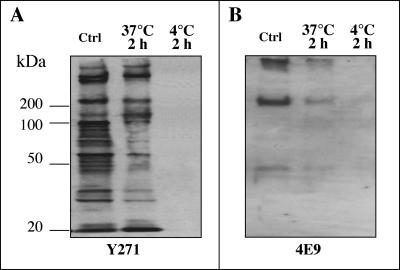
To test that the temperature-induced loss of molecules in the apical region of sporozoites was the consequence of apical organelle discharge and not protein degradation, we used the same antibodies to detect associated molecules in the supernatants. Consistent with previous reports, the positive control (i.e., a whole-cell lysate of freshly excysted sporozoites) showed multiple bands with Y271 (13, 39) and three bands (with molecular masses of 40 kDa, about 200 kDa, and >400 kDa) with 4E9 (10) (Fig. 2). Supernatants from C. parvum sporozoites maintained at 37°C for 2 h showed multiple bands with Y271 and three bands (40 kDa, about 200 kDa, and >400 kDa) with 4E9 (Fig. 2). Supernatants from C. parvum sporozoites maintained at 4°C for 2 h did not show any detectable bands with the three antibodies (Fig. 2). Coupled with the immunofluorescence data described above, these data suggest that an elevated temperature induces the discharge of parasite molecules, resulting in a discharge of apical region-associated molecules in C. parvum sporozoites.
FIG. 2.
Discharge of parasite-associated molecules into the supernatant when C. parvum sporozoites were maintained at 4 or 37°C in the absence of host cells. Freshly excysted sporozoites were incubated in culture medium at 4 or 37°C in the absence of host cells for 2 h, and supernatants were collected for Western blotting. Whole-cell lysates of freshly excysted sporozoites were used as a control. Supernatants from C. parvum sporozoites that were maintained in culture medium at 37°C for 2 h showed multiple bands with Y271 (A) and three bands (40 kDa, about 200 kDa, and >400 kDa) with 4E9 (B). Supernatants from C. parvum sporozoites that were maintained at 4°C for 2 h did not show any detectable bands with both antibodies. Ctrl, control.
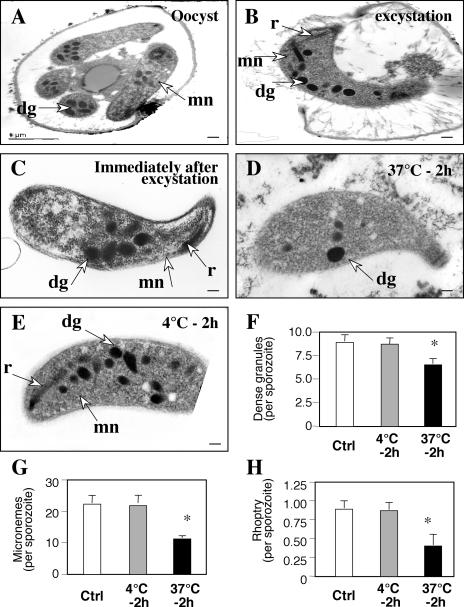
To test whether the discharged parasite proteins did indeed originate from sporozoite apical organelles, we assessed the morphological changes of C. parvum sporozoites by transmission electron microscopy. As shown in Fig. 3A and B, sporozoites before and during excystation showed typical characteristics of apical organelles around the apical region, including a single rhoptry in the apical end and multiple dense granules and micronemes localized throughout the cytoplasm, with a higher density in the apical region. Sporozoites continued to preserve this distribution of apical organelles around the apical region immediately after excystation (Fig. 3C). However, when the sporozoites were maintained in the culture medium for 2 h at 37°C in the absence of host cells, fewer dense granules and micronemes were found in the apical region of sporozoites (Fig. 3D). The single rhoptry in the apical end was detected in only a few of the sporozoites. In contrast, sporozoites maintained at 4°C for 2 h maintained the single rhoptry, the dense granules, and the micronemes in the apical region (Fig. 3E). A quantitative analysis showed a significant decrease in dense granules, micronemes, and rhoptries in sporozoites that were maintained at 37°C for 2 h compared with sporozoites immediately after excystation (control) or that were maintained at 4°C for 2 h (Fig. 3F to H). Taken together, the data suggest that treatment at a higher temperature triggers the discharge of molecules from the apical organelles of C. parvum sporozoites in the absence of host cells.
FIG. 3.
Morphological changes of C. parvum sporozoites after incubation at 4 or 37°C in the absence of host cells, as revealed by transmission electron microscopy. (A and B) Sporozoites before and during excystation showed typical characteristics of a rhoptry (r), dense granules (dg), and micronemes (mn) around the apical region. (C) Sporozoites continued to preserve apical organelles around the apical region of sporozoites immediately after excystation. (D) When the sporozoites were maintained in the culture medium at 37°C for 2 h in the absence of host cells, much fewer dense granules and micronemes were found in the apical region. The single rhoptry in the apical region was detected in only a few of the sporozoites. (E) Sporozoites incubated at 4°C for 2 h preserved the apical organelles in the apical region. (F to H) Quantitative analyses showed a significant decrease in dense granules, micronemes, and rhoptries in sporozoites that were maintained at 37°C for 2 h compared with sporozoites examined immediately after excystation (control) or maintained at 4°C for 2 h. Ctrl, control; *, P < 0.05, compared with control or sporozoites that were maintained at 4°C; error bars, standard errors of the means. Bars = 0.1 μm.
Apical organelle discharge of C. parvum is cytoskeleton and intracellular Ca2+ dependent.
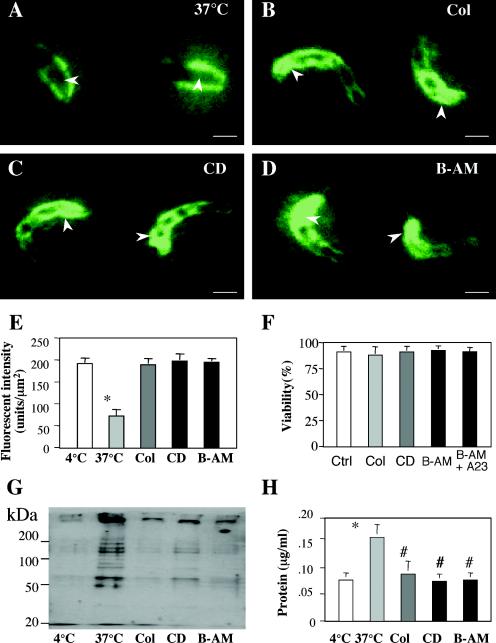
To test whether apical organelle discharge is associated with the parasite cytoskeleton and intracellular Ca2+ in sporozoites, we analyzed culture supernatants and sporozoites at 37°C for apical organelle-associated proteins in the presence or absence of colchicine, CD, and BAPTA-AM by immunofluorescence staining and Western blotting with the Y271 antiserum. Whereas sporozoites maintained at 37°C for 2 h in the absence of host cells showed a decrease in apical region staining (Fig. 4A), sporozoites maintained at 37°C for 2 h in the presence of colchicine, CD, or BAPTA-AM still showed a strong reaction to the antibody in the apical region (Fig. 4B, C, and D). A quantitative analysis showed no difference in fluorescence intensities with Y271 in the apical region between sporozoites that were treated at 4°C and those that were maintained at 37°C in the presence of colchicine, CD, or BAPTA-AM (Fig. 4E). The viability of sporozoites showed no changes after incubation with any of the reagents tested (Fig. 4F). Supernatants from C. parvum sporozoites that were maintained at 37°C for 2 h in the presence of colchicine, CD, or BAPTA-AM showed a significant decrease in detectable bands in Western blots with Y271 compared with those for sporozoites that were maintained at 37°C in the absence of inhibitors (Fig. 4G). Moreover, a significant decrease in the total protein level, as detected by use of the Bradford reagent, in supernatants from sporozoites that were treated with colchicine, CD, or BAPTA-AM was detected compared with that in supernatants from sporozoites maintained at 37°C in the absence of inhibitors (Fig. 4H). These data suggest that apical organelle discharge by C. parvum sporozoites is mediated by cytoskeleton remodeling and intracellular calcium.
FIG. 4.
Effects of colchicine, CD, and BAPTA-AM on discharge of apical organelle contents by C. parvum sporozoites into the supernatants. Freshly excysted sporozoites were incubated in culture medium at 37°C in the presence or absence of CD, colchicine, or BAPTA-AM for 2 h, the supernatants were collected for Western blotting, and the parasites were fixed for immunofluorescence staining. (A to D) Fluorescent images of sporozoites staining with antibody Y271 in the absence of drugs (A) or in the presence of CD (B), colchicine (C), or BAPTA-AM (D). (E) Quantitative analysis of fluorescence intensities in the apical region of sporozoites after treatment with drugs and with antibodies. (F) Viability of C. parvum sporozoites. (G) Western blot detection of parasite-associated proteins in the supernatants after treatment with the reagents. (H) Protein levels in the supernatants after treatment with drugs. *, P < 0.05, compared with sporozoites maintained at 4°C; #, P < 0.05, compared with sporozoites maintained at 37°C; Col, colchicine; B-AM, BAPTA-AM; error bars, standard errors of the means. Bars = 1 μm.
Apical organelle discharge is associated with gliding motility of C. parvum sporozoites.
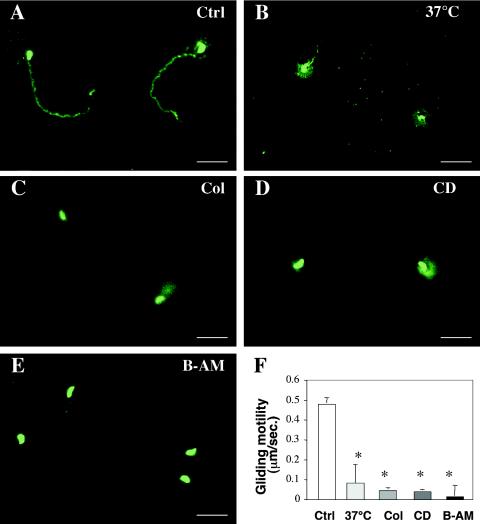
To test whether apical organelle discharge affects sporozoite motility, we assessed the gliding motility of C. parvum sporozoites that were maintained at various temperatures or in the presence or absence of colchicine, CD, or BAPTA-AM. Motility trails were detected by use of an indirect immunofluorescence assay with a polyclonal anti-sporozoite antibody after the incubation of sporozoites on poly-l-lysine-coated glass slides. Long trails were observed behind the parasites when C. parvum sporozoites were maintained for 2 h at 18°C (Fig. 5A) and when freshly excysted sporozoites were used (not shown). However, only short gliding trails were observed for sporozoites that were maintained at 37°C for 2 h (Fig. 5B). Since a significant decrease in sporozoite gliding motility was detected when sporozoites were maintained at 37°C, but not at 18°C, we incubated the sporozoites with colchicine, CD, and BAPTA-AM at 18°C to assess the effects of these drugs on sporozoite gliding motility. No obvious gliding trail was observed for sporozoites that were treated with colchicine, CD, or BAPTA-AM (Fig. 5C to E). A quantitative analysis showed a gliding motility of 0.50 ± 0.04 μm/s for sporozoites that were maintained at 18°C, which was not significantly different from that of freshly excysted sporozoites (0.48 ± 0.04 μm/s; P > 0.05). However, a significant decrease in gliding motility was detected for sporozoites that were maintained at 37°C or treated with colchicine, CD, or BAPTA-AM at 18°C (Fig. 5F), suggesting that the manipulation of apical organelle discharge affects the gliding motility of C. parvum sporozoites.
FIG. 5.
Effects of temperature, colchicine, CD, and BAPTA-AM on gliding motility of C. parvum sporozoites. Freshly excysted sporozoites were incubated in culture medium in the presence or absence of CD, colchicine, or BAPTA-AM for 30 min and then maintained on poly-l-lysine-coated glass slides for 5 min at 37°C. The sporozoites were then fixed, and their gliding trails were detected by immunofluorescence staining with the Y271 antiserum. (A to E) Fluorescent images of sporozoites showing gliding trails when maintained at 18°C (A) or incubated at 37°C (B) or in the presence of CD (C), colchicine (D), or BAPTA-AM (E) at 18°C. (F) Quantitative analysis of gliding motility of C. parvum sporozoites. Ctrl, control (sporozoites incubated at 18°C); *, P < 0.05, compared with control sporozoites maintained at 4°C; Col, colchicine; B-AM, BAPTA-AM; error bars, standard errors of the means. Bars = 5 μm.
Apical organelle discharge of C. parvum is required for parasite host invasion.
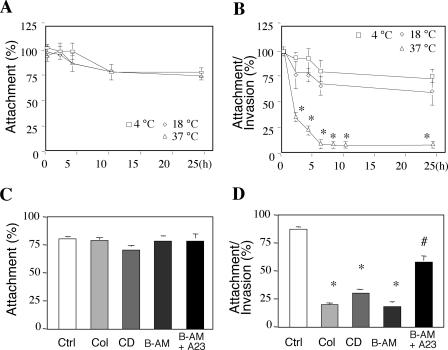
To explore the role of apical organelles in host cell invasion, we exposed sporozoites that were pretreated with various temperatures or drugs to cultured human cholangiocytes to test the effects on host cell infection. When treated sporozoites were exposed to fixed cholangiocytes (a model in which the organism can attach to but not invade the fixed cell surface), no significant difference in attachment rates was found for sporozoites that were maintained at different temperatures before exposure to host cells (Fig. 6A). No decrease in the attachment rate was detected when colchicine- or CD-treated sporozoites were exposed to fixed cholangiocytes compared with that for untreated controls (Fig. 6C). Interestingly, when treated sporozoites were exposed to unfixed cells (a model in which the organism can both attach to and enter host cells), a significant decrease in the invasion rate was detected for sporozoites that were preincubated at 37°C. No decrease in the invasion rate was found for sporozoites that were preincubated at 18°C (Fig. 6B). Since a significant decrease in the invasion rate was detected when sporozoites were maintained at 37°C, but not at 18°C, we incubated sporozoites with colchicine, CD, BAPTA-AM, or A23187 at 18°C and then exposed the parasites to host cells to assess the effects of these drugs on sporozoite cellular invasion. The inhibition of C. parvum sporozoite actin and tubulin polymerization by colchicine and CD resulted in a significant decrease in the invasion rate of C. parvum into cultured cholangiocytes (Fig. 6D). The treatment of C. parvum sporozoites with BAPTA-AM, a highly specific chelator of intracellular Ca2+, also decreased the invasion rate significantly. Moreover, a cotreatment of sporozoites with a Ca2+ ionophore (A23187) in the presence of extracellular Ca2+ restored the infectivity of C. parvum sporozoites (Fig. 6D). Coupled with the observations for prefixed cells, these data suggest that apical organelle discharge by C. parvum sporozoites is required for host cell invasion but not host cell adhesion.
FIG. 6.
Manipulation of C. parvum apical organelle discharge affects parasite host cell attachment and invasion. Freshly excysted C. parvum sporozoites were either incubated in culture medium in the absence of cholangiocytes at various temperatures for 0 to 24 h or incubated in culture medium at 18°C for 30 min in the presence or absence of CD, colchicine, or BAPTA-AM before exposure to host cells. After treatment, the sporozoites were then exposed to host cells to measure infectivity. (A) No significant difference in attachment rates was found for sporozoites incubated at different temperatures. (B) A significant decrease in attachment and invasion rates was detected in sporozoites after incubation at 37°C. (C) No decrease in attachment rates was detected when sporozoites treated with drugs were exposed to fixed cholangiocytes compared with those for untreated controls. (D) Inhibition of C. parvum sporozoite actin and tubulin polymerization by colchicine and CD or depletion of intracellular calcium by BAPTA-AM resulted in a significant decrease in the attachment and invasion rate of C. parvum with cultured cholangiocytes. The cotreatment of sporozoites with a Ca2+ ionophore (A23187) in the presence of extracellular Ca2+ restored the infectivity of C. parvum sporozoites. *, P < 0.05, compared with sporozoites maintained at 4°C; #, P < 0.05, compared with sporozoites treated with BAPTA-AM; Col, colchicine; B-AM, BAPTA-AM; error bars, standard errors of the means.
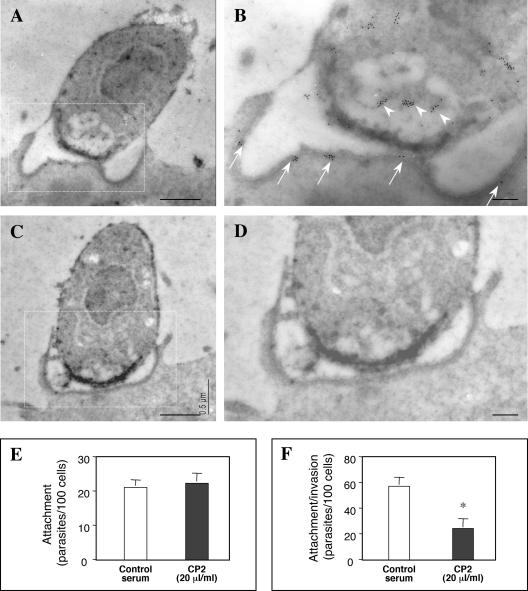
In an effort to confirm the translocation of parasite proteins to the host cell during the invasion process, we performed immunogold labeling of the parasite protein CP2. Several parasites were observed at the internalization stage. Immunoreactive gold particles decorated the invading parasite cytoplasm (Fig. 7B, arrowheads), yet they appeared to accumulate near the apical pole in the region of the anterior vacuole (Fig. 7A and B). In these images, note the accumulation of immunoreactive gold particles to the region of the dense band (Fig. 7B, arrows). Gold particles were not distributed evenly in the region of the dense band; instead, discrete clusters of reactivity were observed. No labeling was found in the controls when the primary antibody was omitted (Fig. 7C and D). Moreover, an infection of cholangiocytes in the presence of the anti-CP2 antibody significantly reduced the invasion of but not attachment to host cells (Fig. 7E and F), suggesting that the CP2 protein is translocated to the host cells at the host cell-parasite interface and may play a critical role during the invasion process.
FIG. 7.
Immunogold labeling of CP2 protein in infected cholangiocytes at the host cell-parasite interface. Panels B and D show a higher magnification of the boxed regions in panels A and C, respectively. The anterior portion of the parasite reveals an accumulation of gold particles in the anterior vacuole region (arrowheads in panel B) as well as at the host-parasite interface (dense band) (arrows in panel B), suggesting the release of parasite factors from the apical region. (C and D) No labeling was found in the controls when the primary antibody was omitted. (E and F) Attachment-invasion assay in the presence of a CP2 antibody. Infection in the presence of an anti-CP2 antibody did not inhibit attachment, yet the invasion of cholangiocytes was significantly inhibited. *, P < 0.05, compared with control serum; error bars, standard errors of the means. Bars = 0.5 μm (A and C) and 0.1 μm (B and D).
DISCUSSION
The major findings reported here relate to the mechanisms by which C. parvum invades host epithelial cells. Using an in vitro model of biliary cryptosporidiosis, we provided several independent lines of evidence linking apical organelle discharge and C. parvum invasion of host cells. We found that (i) the incubation of freshly excysted C. parvum sporozoites at 37°C in the absence of host cells induces the discharge of apical organelles by sporozoites, (ii) the discharge of C. parvum apical organelles is dependent upon the parasite cytoskeleton and intracellular Ca2+, (iii) apical organelle discharge is associated with parasite gliding motility, and (iv) the manipulation of apical organelle discharge of C. parvum sporozoites affects host cell invasion by the parasite. Although a critical role of apical organelle secretion has been well documented for the invasion of host cells by other apicomplexan parasites, our results provide direct evidence that the discharge of C. parvum sporozoite apical organelle contents is temperature, intracellular calcium, and cytoskeleton dependent and is required for host cell invasion.
Rhoptries and micronemes in apicomplexan parasites are secretory organelles that are normally concentrated at the apical region and are poised for release upon contact with host cells during invasion by other parasites in the same phylum, such as Toxoplasma gondii and Plasmodium spp. (26, 32). Therefore, understanding the mechanisms that regulate apical organelle discharge by C. parvum sporozoites is key to understanding the molecular mechanisms of C. parvum invasion. It is well documented that the membrane insertion of intracellular vesicles is a process that is dependent on intracellular signals and requires cytoskeletal elements and associated motor proteins, such as dynein and kinesin (20). Recent studies suggested that the mechanism of apical organelle discharge is conserved among apicomplexan parasites. The discharge of apical organelle contents, especially microneme proteins, has been reported for T. gondii to be temperature, intracellular calcium, and cytoskeleton dependent, which is similar to the exocytic discharge of vesicle contents by mammalian cells (9, 22, 23, 27, 28). Similarly, here we found that the incubation of freshly excysted C. parvum sporozoites in the absence of host cells caused the discharge of proteins at 37°C, but not at lower temperatures. Moreover, apical organelle discharge was blocked by the inhibition of parasite actin and tubulin polymerization and the chelation of intracellular calcium in C. parvum sporozoites. Indeed, a C. parvum calcium transporter (CpATPase 1) has previously been identified which is localized mainly at the sporozoite apical region (37). These results suggest that C. parvum sporozoites, like T. gondii, utilize conserved machinery involving cytoskeletal elements and intracellular calcium to regulate apical organelle discharge. However, the initial trigger for zoite activation and subsequent apical organelle discharge is unclear. It seems that apical organelle discharge in C. parvum begins shortly after excystation and can occur in the absence of host cells. While incubation at 37°C was sufficient for apical organelle discharge in host cell-free cultures, we cannot conclude that this variable alone causes apical organelle discharge, as exogenous factors in the medium may be essential for the initiation of the process.
Many apicomplexan protozoan parasites are also mobile during their infectious life cycle stages by means of gliding motility, a form of substrate-dependent locomotion during which the moving parasite glides over surfaces, utilizing proteins secreted from its apical end. While microfilaments play a critical role in this process, recent studies suggested that apical organelle discharge is also required for the gliding locomotion of T. gondii (15, 27, 28, 35). Forward motility occurs as the translocated adhesion molecules progress posteriorly and are finally capped and cleaved or otherwise detached at the posterior end as new adhesion molecules are added by apical organelle discharge at the anterior end. The gliding motility of C. parvum sporozoites has previously been reported to be associated with a variety of membrane-associated proteins, such as mucin and the gp15/45/60 protein (2, 3, 10, 29). The majority of those proteins have also been identified in the apical organelles of C. parvum sporozoites (2, 3, 10, 29). Here we showed that the manipulation of apical organelle discharge by C. parvum sporozoites affects the parasite's gliding motility. The blockage of apical discharge by the inhibition of parasite actin and tubulin polymerization and the chelation of intracellular calcium in C. parvum sporozoites significantly decreased the gliding motility of the parasite.
Our results also suggest that the apical organelle discharge of C. parvum sporozoites is associated with host cell invasion by the parasite. The incubation of C. parvum sporozoites at 37°C effectively triggered the discharge of apical organelles and resulted in a loss of infectivity. In contrast, the incubation of sporozoites at 4°C showed no discharge of apical organelle contents and did not affect cell invasion. The inhibition of C. parvum apical organelle discharge by the inhibition of actin and tubulin polymerization, as well as by the chelation of intracellular calcium in C. parvum sporozoites, resulted in a loss of the parasite's ability to invade host epithelial cells. Moreover, host cell invasion by C. parvum sporozoites that were treated by intracellular calcium chelation was partially restored by the addition of intracellular calcium by use of the calcium ionophore A23187. These results indicate that only sporozoites that are capable of active apical organelle secretion are competent for cell invasion. One possible explanation for this is that the inhibition of apical organelle discharge inhibits C. parvum sporozoite gliding motility, thus resulting in a loss of infectivity. Parasite gliding motility has been demonstrated to be required for host cell attachment by T. gondii (28). However, we found that a decrease in gliding motility by the use of CD, colchicine, or a calcium chelator showed no effect on the parasite's host cell attachment. This is further supported by a previous report which demonstrated that the inhibition of parasite actin polymerization by CD decreased parasite motility but did not affect host cell attachment (18). It is likely that apical organelle discharge is directly required for C. parvum host cell invasion. Thus, the several processes leading to apical organelle discharge, including temperature, the actin and tubulin cytoskeleton, and intracellular calcium, function together for a successful host-parasite interaction culminating in infection. Morphological studies by electron microscopy revealed a direct connection between the parasite and the host cell during C. parvum sporozoite invasion (21). It was also reported previously that parasite proteins are deposited along the dense-band area within the host cell cytoplasm after C. parvum is internalized into the host cell, which may be a necessary component of the invasion process (13). In further support of this concept, immunoreactivity against CP2 in the region of the dense band was detected: clusters of gold particles were observed in discrete regions along the parasite-host interface as if they were delivered to this region together or clustered in discrete domains of the parasite-host interface. Infection in the presence of the anti-CP2 antibody diminished the infectivity of sporozoites, supporting a functional role not only for this protein, but also for the release of parasite factors to the region of the host cell during the infection process. The 4E9 antibody has also been reported to inhibit C. parvum attachment to and infection of host epithelial cells (10). Taken together, the results show that it is possible that in vivo the intestinal environment triggers sporozoite activation through intracellular signaling pathways including intracellular calcium to stimulate a cytoskeleton-mediated exocytosis response to regulate apical organelle discharge and thus facilitate host cell invasion by the parasite.
In conclusion, our results provide the first direct evidence that the discharge of C. parvum sporozoite apical organelle contents is both intracellular Ca2+ and cytoskeleton dependent and is required for host cell invasion. Further studies should define the regulation of cytoskeleton filament dynamics and their interactions with motor proteins in C. parvum during host cell invasion. The complete dependence of gliding motility and cell invasion on the parasite cytoskeleton and intracellular calcium-associated apical organelle discharge may provide novel targets to prevent infection by this parasite.
Acknowledgments
We thank Pamela S. Tietz and Patrick Splinter for helpful discussions and D. Hintz for secretarial assistance.
This work was supported by National Institutes of Health grants DK57993 and DK24031 (N.F.L.), AI044594 (G.Z.), and AI05786 (H.D.W.) and by the Mayo Foundation (N.F.L.).
Editor: T. R. Kozel
REFERENCES
- 1.Abrahamsen, M. S., T. J. Templeton, S. Enomoto, J. E. Abrahante, G. Zhu, C. A. Lancto, M. Deng, C. Liu, G. Widmer, S. Tzipori, G. A. Buck, P. Xu, A. T. Bankier, P. H. Dear, B. A. Konfortov, H. F. Spriggs, L. Iyer, V. Anantharaman, L. Aravind, and V. Kapur. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304:441-445. [DOI] [PubMed] [Google Scholar]
- 2.Arrowood, M. J., C. R. Sterling, and M. C. Healey. 1991. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J. Parasitol. 77:315-317. [PubMed] [Google Scholar]
- 3.Barnes, D. A., A. Bonnin, J. X. Huang, L. Gousset, J. Wu, J. Gut, P. Doyle, J. F. Dubremetz, H. Ward, and C. Petersen. 1998. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol. Biochem. Parasitol. 96:93-110. [DOI] [PubMed] [Google Scholar]
- 4.Bendayan, M. 1984. Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem. J. 16:85-108. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J., and L. H. Bannister. 2001. Apical organelles of Apicomplexa: biology and isolation by subcellular fractionation. Mol. Biochem. Parasitol. 117:11-25. [DOI] [PubMed] [Google Scholar]
- 6.Bonafonte, M. T., D. Garmon, and J. R. Mead. 1999. Characterization of an alpha-tubulin gene of Cryptosporidium parvum. J. Eukaryot. Microbiol. 46:545-547. [DOI] [PubMed] [Google Scholar]
- 7.Burgoyne, R. D., and A. Morgan. 1993. Regulated exocytosis. Biochem. J. 293:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58:3488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carruthers, V. B., O. K. Giddings, and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 10.Cevallos, A. M., N. Bhat, R. Verdon, D. H. Hamer, B. Stein, S. Tzipori, M. E. Pereira, G. T. Keusch, and H. D. Ward. 2000. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect. Immun. 68:5167-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, X. M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368-379. [DOI] [PubMed] [Google Scholar]
- 12.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 13.Chen, X. M., B. Q. Huang, P. L. Splinter, H. Cao, G. Zhu, M. A. McNiven, and N. F. LaRusso. 2003. Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src. Gastroenterology 125:216-228. [DOI] [PubMed] [Google Scholar]
- 14.Clark, D. P. 1999. New insights in human cryptosporidiosis. Clin. Microbiol. Rev. 12:554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 34:933-939. [DOI] [PubMed] [Google Scholar]
- 16.Erxleben, C., N. Klauke, M. Flötenmeyer, M. P. Blanchard, C. Braun, and H. Plattner. 1997. Microdomain Ca2+-activation during exocytosis in Paramecium cells. Superposition of local subplasmalemmal calcium store activation by local Ca2+ influx. J. Cell Biol. 136:597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiala, J. C., and K. M. Harris. 2001. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. J. Am. Med. Informatics Assoc. 8:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forney, J. R., D. K. Vaughan, S. Yang, and M. C. Healey. 1998. Actin-dependent motility in Cryptosporidium parvum sporozoites. J. Parasitol. 84:908-913. [PubMed] [Google Scholar]
- 19.Grubman, S. A., R. D. Perrone, D. W. Lee, S. L. Murray, L. C. Rogers, L. I. Wolkoff, A. E. Mulberg, V. Cherington, and D. M. Jefferson. 1994. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am. J. Physiol. 266:G1060-G1070. [DOI] [PubMed] [Google Scholar]
- 20.Hamm-Alvarez, S. F., and M. P. Sheetz. 1998. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol. Rev. 78:1109-1129. [DOI] [PubMed] [Google Scholar]
- 21.Huang, B. Q., X. M. Chen, and N. F. LaRusso. 2004. Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: a morphologic study. J. Parasitol. 90:212-221. [DOI] [PubMed] [Google Scholar]
- 22.Lovett, J. L., and L. D. Sibley. 2003. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 116:3009-3016. [DOI] [PubMed] [Google Scholar]
- 23.Lovett, J. L., N. Marchesini, S. N. Moreno, and L. D. Sibley. 2002. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J. Biol. Chem. 277:25870-25876. [DOI] [PubMed] [Google Scholar]
- 24.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 25.O'Hara, S. P., J. R. Yu, and J. J. Lin. 2004. A novel Cryptosporidium parvum antigen, CP2, preferentially associates with membranous structures. Parasitol. Res. 92:317-327. [DOI] [PubMed] [Google Scholar]
- 26.Sam-Yellowe, T. Y. 1996. Rhaptory organellea of the Apicomplexa: their role in host cell invasion and intracellular survival. Parasitol. Today 12:308-316. [DOI] [PubMed] [Google Scholar]
- 27.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304:248-253. [DOI] [PubMed] [Google Scholar]
- 28.Sibley, L. D., S. Hakansson, and V. B. Carruthers. 1998. Gliding motility: an efficient mechanism for cell penetration. Curr. Biol. 8:R12-R14. [DOI] [PubMed] [Google Scholar]
- 29.Strong, W. B., J. Gut, and R. G. Nelson. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68:4117-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulaiman, I. M., A. A. Lal, and L. Xiao. 2002. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J. Parasitol. 88:388-394. [DOI] [PubMed] [Google Scholar]
- 31.Tetley, L., S. M. Brown, V. McDonald, and G. H. Coombs. 1998. Ultrastructural analysis of the sporozoite of Cryptosporidium parvum. Microbiology 144:3249-3255. [DOI] [PubMed] [Google Scholar]
- 32.Tzipori, S., and J. K. Griffiths. 1998. Natural history and biology of Cryptosporidium parvum. Adv. Parasitol. 40:5-36. [DOI] [PubMed] [Google Scholar]
- 33.Ventura, R., and K. M. Harris. 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19:6897-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward, H. D., and A. M. Cevallos. 1998. Cryptosporidium: molecular basis of host-parasite interaction. Adv. Parasitol. 40:151-185. [DOI] [PubMed] [Google Scholar]
- 35.Wetzel, D. M., S. Hakansson, K. Hu, D. Roos, and L. D. Sibley. 2003. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol. Biol. Cell 14:396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiest, P. M., K. L. Dong, J. H. Johnson, S. Tzipori, K. Boeklheide, and T. P. Flanigan. 1994. Effect of colchicine on microtubules in Cryptosporidium parvum. J. Eukaryot. Microbiol. 41:66S. [PubMed] [Google Scholar]
- 37.Zhu, G., and J. S. Keithly. 1997. Molecular analysis of a P-type ATPase gene from Cryptosporidium parvum. Mol. Biochem. Parasitol. 90:307-316. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, G., J. S. Keithly, and H. Philippe. 2000. What is the phylogenetic position of Cryptosporidium. Int. J. Syst. Evol. Microbiol. 50:1673-1678. [DOI] [PubMed] [Google Scholar]
- 39.Zhu, G., M. J. Marchewka, K. M. Woods, S. J. Upton, and J. S. Keithly. 2000. Molecular analysis of a type I fatty acid synthase in Cryptosporidium parvum. Mol. Biochem. Parasitol. 105:253-260. [DOI] [PubMed] [Google Scholar]