Supplemental Digital Content is available in the text
Abstract
The purpose of this study was to examine the relationship between serum ferritin levels and metabolically obese normal weight (MONW) and to determine the appropriate cut-off value of serum ferritin for the prediction of clinical metabolic status in nonobese Korean adults. Data from 9411 participants in the fourth (2008) and fifth (2010) annual Korea National Health and Nutrition Examination Surveys were used in this study. MONW was determined by combining National Cholesterol Education Program Adult Treatment Panel III criteria, Wildman criteria, and homeostatic model assessment criteria for metabolic healthy obesity. The mean serum ferritin level was 103.5 ± 1.2 ng/mL in men and 45.5 ± 0.6 ng/mL in women. The estimated cutoff value of serum ferritin for the prediction of MONW was 127.03 ng/mL in men and 46.87 ng/mL in women. Both men and women who had higher serum ferritin levels than the cutoff value had a higher prevalence of MONW than those individuals who had lower serum ferritin levels than the cutoff value. In the final multivariable adjusted logistic regression model, the odds ratio (95% confidence interval) of MONW in the subjects who had higher serum ferritin levels than the cutoff value was 1.631 (1.312–2.028) in men and 1.298 (1–1.685) in women. In this study, serum ferritin levels were positively associated with MONW, and those subjects who had higher serum ferritin levels than the cutoff value had a higher prevalence and a higher adjusted odds ratio for MONW despite being nonobese.
INTRODUCTION
Serum ferritin is the main protein that regulates iron homeostasis,1 and it is a useful clinical biomarker of the amount of iron that is stored in the body for the evaluation of anemia.2 Serum ferritin is also related to insulin function and systemic inflammation. As an acute phase reactant, serum ferritin levels are increased in an inflammatory environment and are associated with cardiometabolic disease, which is reflected by insulin resistance.3
Elevated serum ferritin levels are related to metabolic syndrome 4 and were found to be relevant to central obesity, hypertension, and dyslipidemia in a cross-sectional study.3,5,6 In a study of white subjects, the prevalence of metabolic syndrome increased as the serum iron levels increased.7 Obesity, inflammation, and metabolic syndrome have been shown to be strongly related to elevated serum ferritin levels in several studies of Western populations,5,8,9 and serum ferritin levels were associated with metabolic syndrome in a recent study of Korean subjects.10
Although metabolic syndrome is strongly associated with obesity, there has been little discussion on serum ferritin level as a predictor of metabolic risk in nonobese subjects. It is important to evaluate the risk of metabolically obese status and to detect metabolic disarrangement in the early phase of insulin resistance before onset of obesity, but stricter criteria are required to evaluate the risk of metabolically obese normal weight (MONW).
In this study, we examined the relationship between serum ferritin levels and MONW and attempted to determine the appropriate cutoff level of serum ferritin for the clinical prediction of MONW in lean Korean adults.
METHODS
Survey and Subjects
The data for this study were from the fourth and fifth Korea National Health Examination and Nutrition Surveys (KNHANES), which were conducted by the Korea Centers for Disease Control and Prevention and the Korean Ministry of Health and Welfare in 2008 and 2010. The data from KNHANES provide comprehensive information on health status, health behavior, nutritional status, and socio-demographics in 600 national districts in Korea.
In total, 21,811 subjects aged >19 years were initially considered for this study. The exclusion criteria included obesity, body mass index (BMI) >25 kg/m2, and cardiovascular disease, including myocardial infarction, angina, renal disease, diabetes mellitus, thyroid disease, cerebral infarction, and malignancy. Subjects who had liver disease, including hepatitis B, hepatitis C, liver cirrhosis, and liver enzyme levels >2 times the normal limit (aspartate aminotransferase [AST] level >80 IU/L and alanine aminotransferase [ALT] level >80 IU/L) were also excluded. In addition, we excluded subjects who had anemia or iron deficiency (serum hemoglobin level <12 g/dL and serum ferritin level <10 μg/L) or who were pregnant. Finally, we excluded subjects who had abnormally high serum ferritin levels (>300 μg/L) because of probable hemochromatosis. After applying all of these exclusion criteria, 9411 subjects were enrolled in this study. All of the participants signed an informed consent form, and the institutional review board of the Korea Centers for Disease Control and Prevention approved the study protocol.
Data Collection
BMI was calculated as weight in kilograms divided by height squared in meters. Waist circumference was measured at the narrowest point between the iliac crest and the lower border of the rib cage. Using a mercury sphygmomanometer (Baumanometer; Baum, Copiague, New York, NY), blood pressure was measured 3 times in the right arm after at least 5 min of rest with the subject in a seated position. Blood samples were obtained after the subjects completed an 8-hour fast. Serum ferritin and insulin levels were measured by an immunoradiometric assay using a 1470 Wizard Gamma Counter (PerkinElmer, Turku, Finland). Serum levels of glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, ALT, and AST were measured enzymatically using a Hitachi Automatic Analyzer 7600 (Tokyo, Japan). Insulin resistance was computed using the homeostasis model assessment of insulin resistance (HOMA-IR) as follows: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405.
Alcohol intake was determined by asking the subjects about their drinking behavior during the month before the interview. Subjects were divided into 3 groups based on alcohol intake: nondrinkers, mild to moderate drinkers (1–30 g of alcohol per day), and heavy drinkers (>30 g of alcohol per day). Subjects were also divided into 3 groups based on their smoking status: nonsmoker, ex-smoker, and current smoker. In addition, there were 3 groups of subjects based on their physical activity level: sedentary (<600 metabolic equivalents [METs]/week), minimally active (600–3000 METs/week), and health-enhancing physical activity (>3000 METs/week). Subjects were divided by their educational level into 2 groups: middle school or less and high school or more. Subjects were also divided into 2 groups based on their monthly income: lowest quartile of income and other quartiles. Total energy and fat intake were separately calculated on the basis of total number of calories consumed and fat percentage.
Definition of Metabolically Obese Normal Weight
By integrating the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III criteria, Wildman criteria, and HOMA criteria,11,12 we created combined diagnostic criteria to determine the metabolic status of non-obese subjects in our study, which we categorized as metabolically healthy normal weight (MHNW) and metabolically obese normal weight (MONW). A subject was considered to have MHNW if 1 or none of the detailed criteria was satisfied, and a subject was considered to have MONW if ≥2 of the criteria were satisfied. The detailed criteria were as follows: systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or use of antihypertensive medication; triglyceride level ≥150 mg/dL or use of lipid-lowering medication; fasting plasma glucose level ≥100 mg/dL or use of an oral hypoglycemic agent or insulin; HDL cholesterol level <40 mg/dL in men and <50 mg/dL in women or use of medication for reduced HDL cholesterol level; and HOMA-IR greater than the 90th percentile in the nondiabetic general Korean population in KNHANES.
Statistical Analysis
All of the statistical analyses were performed using the SAS version 9.2 package (SAS Institute, Cary, NC). The different characteristics among the subgroups were compared using Student t test or χ2 test. A P value of <0.05 was considered to be statistically significant. There was linear regression analysis between serum ferritin levels and cardiometabolic variables, which was adjusted by age and BMI. A receiver operating characteristic (ROC) curve was designed to determine the best cutoff value for serum ferritin to predict MONW. The areas under the ROC curve (AUC) were also investigated for MONW. The sensitivity and specificity for the best prediction of MONW were calculated, and the best possible cutoff value was defined as the highest Youden index ([specificity+sensitivity] – 1). Odds ratios and 95% confidence intervals (CIs) of subgroups according to serum ferritin levels for MONW were compared among models 1, 2, and 3. Model 1 was adjusted for age and BMI; model 2 was adjusted for the same variables as model 1 plus alcohol intake, smoking, and physical activity; and model 3 was adjusted for the same variables as model 2 plus education level, monthly income, and total energy and fat intake per day.
RESULTS
Clinical Characteristics of the Subjects
The mean ages of the men and women were 41.2 ± 0.3 and 42.5 ± 0.3 years, respectively (Table 1). The mean BMI was 22.2 kg/m2 in men and 21.6 kg/m2 in women, and the mean serum ferritin level was 103.5 ± 1.2 ng/mL in men and 45.5 ± 0.6 ng/mL in women. The mean values of BMI, waist circumference, blood pressure, glucose, triglyceride, ALT/AST, and serum ferritin were higher in men than in women. In contrast, the mean values of insulin, LDL cholesterol, and HDL cholesterol were higher in women than in men. In addition, men had higher percentages of smoking and alcohol intake. With regard to physical activity, men had higher percentages of health-enhancing activity, and women had higher percentages of minimal and sedentary activity. Men also had higher total energy and fat intake.
TABLE 1.
Principle Clinical Characteristics of Nonobese Korean Adults in KNHANES, 2008 to 2010
Age- and BMI-Adjusted Linear Regression Between Serum Ferritin Levels and Cardiometabolic Variables
The unstandardized regression coefficients (B) and P values for serum ferritin with respect to each cardiometabolic variable are presented in Table 2, and the variables with statistical significance included triglycerides (B = 0.085, P < 0.001) and glucose (B = 0.995, P < 0.001) in men and triglycerides (B = 0.026, P = 0.034), HDL cholesterol (B = −0.86, P = 0.008) and glucose (B = 0.690, P = 0.001) in women.
TABLE 2.
Age- and BMI-Adjusted Linear Regression Between Serum Ferritin Levels and Cardiometabolic Variables

ROC Analysis to Determine Cutoff Values for Serum Ferritin
The AUC of serum ferritin levels for the prediction of MONW was 0.551 (95% CI, 0.536–0.567; P < 0.001) in men and 0.629 (95% CI, 0.616–0.642; P < 0.001) in women. The best cutoff value of serum ferritin for the prediction of MONW was 127.03 ng/mL in men and 46.87 ng/mL in women, according to the maximum of the Youden index (Fig. 1). The positive predictive values of each cutoff level were 35.1% and 32.6% in men and women, and the negative predictive values were 73.6% and 81.7%.
FIGURE 1.
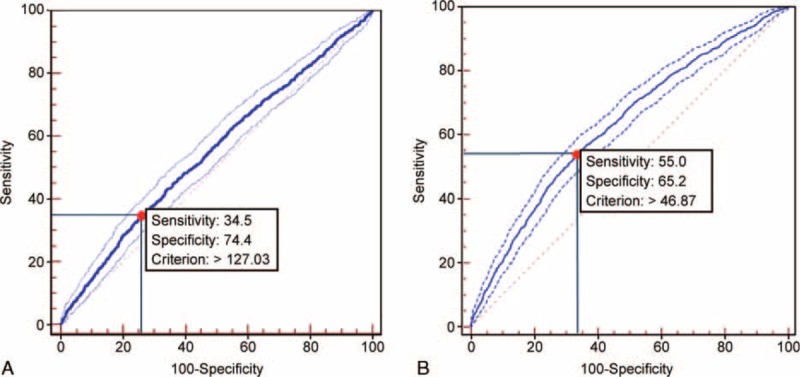
ROC analysis to determine cutoff values for serum ferritin that would predict MONW. A, In men, the cutoff value for MONW was 127.03 ng/mL (AUC = 0.551; 95% CI, 0.536–0.567; P < 0.001, sensitivity 34.5%, specificity 74.4%). B, In women, the cutoff value for MONW was 46.87 ng/mL (AUC = 0.629; 95% CI, 0.616–0.642; P < 0.001, sensitivity 55.0%, specificity 65.2%). MONW = metabolically obese normal weight.
Prevalence of MONW and Its Components According to Serum Ferritin Levels
In men, when comparing group A (subjects with serum ferritin levels lower than the cutoff value [≤ 127.03 ng/mL]) with group B (subjects with serum ferritin levels higher than the cutoff value [>127.03 ng/mL]), the prevalence of MONW in group B (26.5 ± 1%) was higher than that in group A (35.9 ± 1.6%), which was statistically significant (Fig. 2, P < 0.01). In women, when comparing group C (subjects with serum ferritin levels lower than the cutoff value [≤46.87 ng/mL]) with group D (subjects with serum ferritin levels higher than the cutoff value [>46.87 ng/mL]), the prevalence of MONW in group D (41.1 ± 2.3%) was higher than that in group C (21.5 ± 0.7%), which was statistically significant (Fig. 2, P < 0.01).
FIGURE 2.
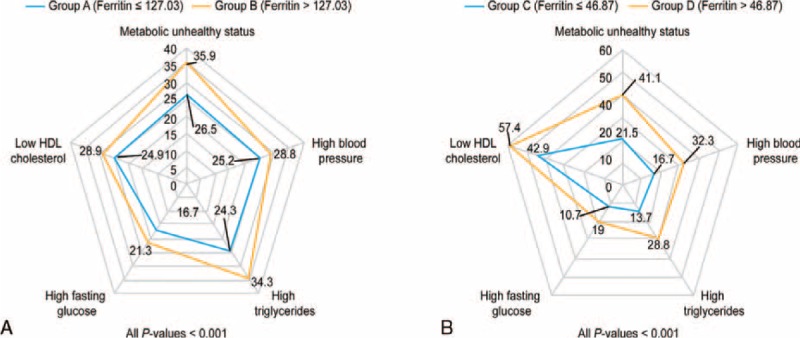
Prevalence of MONW and its components according to serum ferritin levels in KNHANES, 2008 to 2010 in male (a) and female (b), respectively. MONW was defined by the presence of ≥2 of the following criteria: systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or use of antihypertensive medication; triglyceride level ≥150 mg/dL or use of lipid-lowering medication; fasting plasma glucose level ≥100 mg/dL or use of an oral hypoglycemic agent or insulin; HDL cholesterol level <40 mg/dL in men and <50 mg/dL in women or use of medication for reduced HDL cholesterol level; and HOMA-IR greater than the 90th percentile in the nondiabetic general Korean population in KNHANES. The data are presented as a percentage (standard error), and P values were obtained by the χ2 test. HDL = high-density lipoprotein; HOMA-IR = homeostasis model assessment of insulin resistance; MONW = metabolically obese normal weight.
Multivariable Adjusted Logistic Regression for MONW in Subgroups According to Serum Ferritin Levels
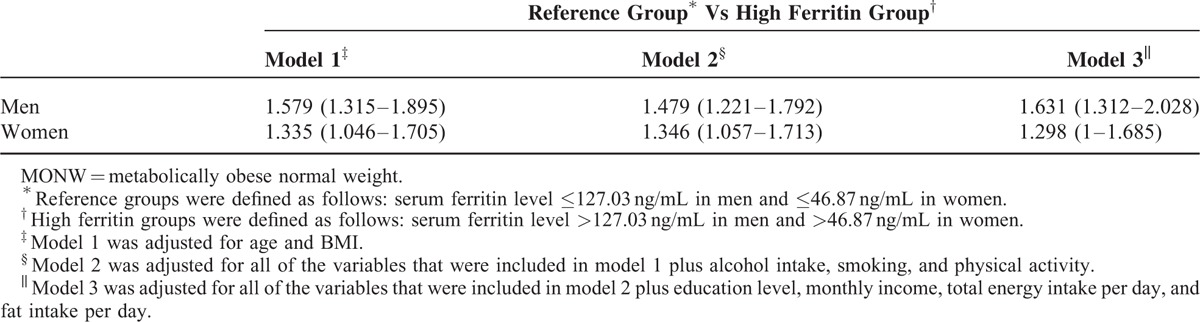
In all of the logistic regression models for both sexes, those with ferritin levels that were higher than the cutoff value had higher odds ratios for MONW than those with ferritin levels that were lower than the cutoff value. In the final multivariable adjusted logistic regression of model 3, the odds ratio for MONW in those with serum ferritin levels that were higher than the cutoff value was 1.631 (1.312–2.028) in men and 1.298 (1–1.685) in women (Table 3).
TABLE 3.
Multivariable Adjusted Odds Ratio and 95% CI of Subgroups According to Serum Ferritin Levels for MONW Among Nonobese Korean Adults in KHANES, 2008 to 2010
Distribution of Serum Ferritin Levels in Accordance With the Number of Components for MONW (Supplemental Figure 1)
As more of the diagnostic components for MONW were satisfied, the mean serum ferritin level increased, and the proportion of subjects with higher serum ferritin levels than the cutoff value also tended to increase.
DISCUSSION
The prevalence of metabolic syndrome is increasing worldwide. According to data from KNHANES, the prevalence of metabolic syndrome in Korea increased from 24.9% in 1998 to 31.3% in 2007.13 This phenomenon is attributed to changing lifestyles, lack of exercise, and a Western diet. As a result, it is important to evaluate the risk of MONW and detect the metabolic disarrangement at an early stage.
As noted above, serum ferritin is the main protein that regulates iron homeostasis in the body, and it is a useful diagnostic marker of iron deficiency anemia or hemochromatosis. Serum ferritin could also be helpful in predicting abnormal glucose metabolism. Serum ferritin appears to be related to hyperinsulinemia in the early phase of insulin resistance, and it inhibits the production of insulin as a more advanced phase is entered.14,15 Iron acts as a catalyst for more reactive hydroxyl radicals and as an oxidative stress at multiple cellular targets.16 In an animal study, insulin secretory capacity was inhibited by increasing β-cell oxidative stress.17 In a study of patients with hemochromatosis, iron accumulation in hepatocytes inhibited the production and metabolism of hepatic insulin.18 Thus, elevated serum ferritin levels are related to type 2 diabetes, obesity, hypertension, dyslipidemia, and metabolic syndrome.19,20
Several recent studies have shown the relationship between serum ferritin levels and metabolic syndrome. In the United States, moderately elevated iron levels are associated with an increased prevalence of metabolic syndrome and could be a marker of insulin resistance.7 Previously, many studies have focused on the relationship between serum ferritin levels and metabolic syndrome mainly in obese populations, but whether this relationship is still available in nonobese populations is uncertain. The results of our study suggest that lean individuals with higher serum ferritin levels need to be screened for MONW, even though they are not obese and are apparently healthy.
In this study, the concept of MONW in nonobese individuals was derived from the idea of “metabolically healthy obesity (MHO).”11,12 Although a close relationship between obesity and metabolic syndrome has been proven in many previous studies, a subgroup of obese individuals does not show an increase in cardiometabolic risk despite high adiposity.21 These individuals appear to have a favorable metabolic profile without obesity-related metabolic derangement and are referred to as the MHO group.22–25 Paradoxically, a subgroup of lean individuals has been reported to have an increase in cardiometabolic risk.21 This unique subgroup of lean individuals is referred to as MONW. However, a standard for the diagnosis of MONW has not yet been established, and therefore, in this study, the definition of MONW has been modified from the most common criteria of MHO, such as the NCEP ATP III criteria, Wildman criteria, Karelis criteria, and HOMA criteria.12 Our criteria do not include hsCRP due to lack of data, and the waist circumference component is also not considered because this study focused on the nonobese general population. The standardization for clinical relevance of these criteria has not yet been achieved, which could be considered to be a major limitation.
This study suggested cutoff values of serum ferritin, which were 127.03 ng/mL in men and 46.87 ng/mL in women, for the prediction of MONW. Subjects with serum ferritin levels that were higher than these cutoff values had a higher prevalence of MONW and a higher adjusted odds ratio. Although ferritin was, in fact, not an excellent discriminator of MONW on the ROC analysis, we performed the ROC curve analysis to ensure that the serum ferritin level could be a potential clinical marker of MONW even in lean individuals and to define proper cutoff levels for the logistic regression analysis.
This study also had other limitations. First, this study was a cross-sectional study, and causation and putative mechanisms cannot be asserted in the association between serum ferritin levels and cardiometabolic variables. Second, we could not specifically exclude subjects who were taking oral agents such as vitamins or iron supplements that affect serum ferritin levels. Third, because serum ferritin levels reflect inflammatory conditions, we should have excluded all of the subjects with inflammation but could not because of a lack of data on inflammation such as C-reactive protein levels. Finally, the subjects in this study were restricted to nonobese Korean adults. It is widely known that obesity is closely related to the risk of type 2 diabetes, hypertension, and metabolic syndrome, and thus, additional studies of obese subjects are needed to define the relationship between serum ferritin levels and metabolically unhealthy status. Despite these limitations, this study is meaningful because it suggests the possibility that the serum ferritin levels could be a clinical marker to reflect the risk and prevalence of MONW in nonobese Korean adults. It could be a clinically meaningful process to assess subjects who appear to be healthy and lean but have MONW. Therefore, additional studies of both lean subjects who have MONW and obese subjects who are metabolically healthy are needed to assess and manage their metabolically unhealthy status in a delicate manner.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUC = the areas under the ROC curve, BMI = body mass index, CIs = confidence intervals, HDL = high-density lipoprotein, HOMA-IR = the homeostasis model assessment of insulin resistance, KNHANES = Korea National Health Examination and Nutrition Surveys, LDL = low-density lipoprotein, METs = metabolic equivalents, MHNW = metabolically healthy normal weight, MHO = metabolically healthy obesity, MONW = metabolically obese normal weight, NCEP ATP = the National Cholesterol Education Program Adult Treatment Panel, ROC = a receiver operating characteristic curve.
DHK and YKR contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Heeney MM, Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin North Am 2004; 18:1379–1403. [DOI] [PubMed] [Google Scholar]
- 2.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003; 101:3359–3364. [DOI] [PubMed] [Google Scholar]
- 3.Williams MJ, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 2002; 165:179–184. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Real JM, Ricart-Engel W, Arroyo E, et al. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 1998; 21:62–68. [DOI] [PubMed] [Google Scholar]
- 5.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men: the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 2001; 25:639–645. [DOI] [PubMed] [Google Scholar]
- 6.Piperno A, Trombini P, Gelosa M, et al. Increased serum ferritin is common in men with essential hypertension. J Hypertens 2002; 20:1513–1518. [DOI] [PubMed] [Google Scholar]
- 7.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004; 27:2422–2428. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340:448–454. [DOI] [PubMed] [Google Scholar]
- 9.Vari IS, Balkau B, Kettaneh A, et al. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2007; 30:1795–1801. [DOI] [PubMed] [Google Scholar]
- 10.Ryoo JH, Kim MG, Lee DW, et al. The relationship between serum ferritin and metabolic syndrome in healthy Korean men. Diabetes Metab Res Rev 2011; 27:597–603. [DOI] [PubMed] [Google Scholar]
- 11.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010; 21:38–43. [DOI] [PubMed] [Google Scholar]
- 12.Yoo HK, Choi EY, Park EW, et al. Comparison of metabolic characteristics of metabolically healthy but obese (MHO) middle-aged men according to different criteria. Korean J Fam Med 2013; 34:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Shin H, Song JH, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care 2011; 34:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer AI, Cheron RG, Dluhy R, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med 1981; 304:319–324. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JG, Lindquist JH, Grambow SC, et al. Potential role of increased iron stores in diabetes. Am J Med Sci 2003; 325:332–339. [DOI] [PubMed] [Google Scholar]
- 16.Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J Mol Cell Cardiol 2009; 47:372–381. [DOI] [PubMed] [Google Scholar]
- 17.Cooksey RC, Jouihan HA, Ajioka RS, et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004; 145:5305–5312. [DOI] [PubMed] [Google Scholar]
- 18.Niederau C, Berger M, Stremmel W, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia 1984; 26:441–444. [DOI] [PubMed] [Google Scholar]
- 19.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005; 112:666–673. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Kim HK, Bae SJ, et al. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011; 60:414–420. [DOI] [PubMed] [Google Scholar]
- 21.Badoud F, Perreault M, Zulyniak MA, et al. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J 2015; 29:748–758. [DOI] [PubMed] [Google Scholar]
- 22.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006; 91:2906–2912. [DOI] [PubMed] [Google Scholar]
- 23.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168:1609–1616. [DOI] [PubMed] [Google Scholar]
- 24.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011; 35:971–981. [DOI] [PubMed] [Google Scholar]
- 25.Yun KE, Chang Y, Jung HS, et al. Impact of body mass index on the risk of colorectal adenoma in a metabolically healthy population. Cancer Res 2013; 73:4020–4027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


