Abstract
Individuals in areas of intense malaria transmission exhibit resistance (or tolerance) to levels of parasitemia in their blood that would normally be associated with febrile illness in malaria-naïve subjects. The resulting level of parasitemia associated with illness (the pyrogenic threshold) is highest in childhood and lowest in adulthood. Clinical parallels between malarial and bacterial endotoxin tolerance have led to the supposition that both share common physiological processes, with nitric oxide (NO) proposed as a candidate mediator. The hypotheses that NO mediates tolerance and blood stage parasite killing in vivo were tested by determining its relationship to age and parasitemia cross-sectionally and longitudinally in a population of 195 children and adults from Papua New Guinea encountering intense malaria exposure. Despite pharmacological clearance of asymptomatic parasitemia, NO production and mononuclear cell NO synthase (NOS) activity were remarkably stable within individuals over time, were not influenced by parasitemia, and varied little with age. These results contrast with previous smaller cross-sectional studies. Baseline NO production and NOS activity did not protect against recurrent parasitemia, consistent with previous data suggesting that NO does not have antiparasitic effects against blood stage infection in vivo. The NO indices studied were markedly higher in specimens from study subjects than in samples from Australian controls, and NOS activity was significantly associated with plasma immunoglobulin E levels, consistent with induction of NO by chronic exposure to other infections and/or host genetic factors. These results suggest that NO is unlikely to mediate killing of blood stage parasites in this setting and is unlikely to be the primary mediator in the acquisition or maintenance of malarial tolerance.
The paroxysmal fever of human malaria is thought to result from induction of endogenous pyrogens such as tumor necrosis factor alpha and interleukin-1 by a parasite toxin (or toxins) released at the time of schizont rupture (22). Yet residents of regions where malaria is highly endemic (such as the northern coast of Papua New Guinea [PNG]) experience relatively few episodes of symptomatic malaria (19), despite an estimated inoculation rate of greater than one infective bite per day (13). Chronic asymptomatic parasitization appears to be common in regions where malaria is highly endemic (12), and levels of parasitemia (31, 38, 41) reportedly exceed those associated with fever in malaria-naïve subjects (21). These observations have led to the proposal that a form of antitoxic immunity to malaria exists that has been termed tolerance, a condition that enables the host to resist the pyrogenic effects of a malaria toxin(s) (17, 32). It has been shown that the threshold of peripheral parasitemia associated with febrile illness can be sharply defined in regions of intense malaria transmission (6, 38, 42) and that this threshold has a striking exponentially decaying relationship with increasing age, from a peak at 1 year of age to a plateau in adulthood (19, 38, 41). In contrast, antiparasitic immune responses (thought to be responsible for lowering parasite densities and therefore the risk of clinical malaria) are generally assumed to increase with advancing age.
Malarial tolerance shares interesting parallels with the well-established phenomenon of bacterial endotoxin tolerance, which has led to the proposition that they are mediated by an ultimately common cellular pathway (18). Repeated injections of endotoxin result in diminishing fever responses in humans over a short period of time (45), and it has been shown that even a first malaria infection can induce cross-tolerance to endotoxin (23, 40). The biological mediator nitric oxide (NO) has been proposed to mediate endotoxin tolerance in experimental models (49) and has been shown to down-regulate endotoxin-induced increases in tumor necrosis factor alpha in animal studies (25) and in human monocytes (47). Furthermore, clinical and genetic association studies have suggested that NO production can protect against severe malaria (2, 24). Strikingly high basal production of NO has been described in disease-free individuals from areas where malaria is endemic (3, 9), and NO levels were reportedly higher in children than adults in one uncontrolled PNG study (17). NO levels were positively correlated with parasitemia in two of these studies (3, 9), consistent with data from animal models (26) and the demonstration that the putative malarial toxin glycosylphosphatidylinositol can induce NO synthase (NOS) in macrophage and endothelial cells (44).
Hence, we and others (18) have hypothesized that NO is a key mediator of malarial tolerance in heavily exposed individuals and that NO levels would positively correlate with parasitemia and inversely correlate with age in parallel with the decaying pyrogenic threshold. To test these propositions, we studied NO production and NOS activity in an asymptomatic malaria-exposed population ranging in age from 1 to 60 years, in whom disease-related inflammatory responses would not confound interpretation of the data. NO donors have been shown to kill blood stage malaria parasites in vitro (37), and it has been suggested that NO may have similar antiparasitic effects in vivo (10, 37). We therefore extended our studies to investigate whether NO production would be inversely related to parasitemia (the opposite of our primary hypothesis) and whether NO indices would predict the likelihood of recurrent parasitemia after pharmacological clearance. We also studied the relationship between NO and baseline plasma immunoglobulin E (IgE) levels (a marker of intestinal parasitosis), as it has been suggested that IgE-mediated induction of NOS2 via CD23 may increase NO in populations where malaria is endemic (34).
MATERIALS AND METHODS
Subjects and controls.
The ethics committees of the PNG Institute of Medical Research and the Menzies School of Health Research approved the study. Subjects were recruited from two coastal villages (Haven and Midiba) in Madang Province, PNG, during the high-transmission wet season between February and May 2000 (14). In this region, four species of malaria that are transmittable to humans are present, the inoculation rate is estimated at one infective bite per day (13), there is little seasonal variation in parasitemia rates (16), and malaria is characterized by relatively mild attacks that decrease in frequency with age (19). Controls were non-malaria-exposed asymptomatic adults from Darwin in tropical north Australia with little exposure to intestinal parasites (20).
Nonpregnant adults and children aged ≥1 years were screened by fingerprick blood smear for entry into the study. Subjects were selectively enrolled (at time point T0) to include balanced numbers of microscopically parasitemic and aparasitemic subjects. Exclusion criteria were as follows: fever (axillary temperature, ≥37.5°C) on any of three occasions within 24 h, antimalarial or nonsteroidal anti-inflammatory drug ingestion (including aspirin) within 1 week, history of recent malaria infection (fever, chills, sweats, headache, or myalgia within 1 week), or diarrhea (as gastroenteritis has been associated with increased NO production) (29). Darwin controls were recruited in February 2002 with the same criteria, except axillary temperatures were recorded only twice, 16 h apart, and controls were not followed up. Subjects with Plasmodium falciparum infection alone or in combination with other species received a single dose of 25 mg of sulfadoxine/kg of body weight and 1.25 mg of pyrimethamine (Roche, Basel, Switzerland)/kg of body weight and subjects with Plasmodium vivax, Plasmodium malariae, and/or Plasmodium ovale infection were given three daily doses of 10 mg base of chloroquine phosphate (Pharmamed, Zejtun, Malta)/kg of body weight. Follow-up was done using the same procedures approximately 2 to 4 weeks after enrollment at T1 and again approximately 7 weeks after initial enrollment at T2.
Specimen collection and processing.
Blood smears were stained with 4% Giemsa stain and examined and/or cross-checked by two field microscopists with ≥12 years of experience). Negative smears had no parasites seen in 100 high-power (magnification, ×1,000) oil-immersion fields. Subjects and controls were given a low-nitrate meal of chicken and rice (and low-nitrate water) and then fasted for 12 h overnight to control for the effect of dietary nitrates on NO measurement (1). Adherence to the fasting protocol was confirmed by supervision (PNG) or questionnaire (Darwin). A second-void urine was collected after the fast, analyzed for infection by dipstick and/or microscopy, and then stored with isopropanol (20% final volume). Blood was collected by venipuncture, plasma was separated by centrifugation, and then peripheral blood mononuclear cells (PBMC) were removed by density centrifugation. Manual leukocyte counts were done with whole blood to calculate parasite densities (27), and all samples were stored at −70°C until assays were carried out.
Laboratory assays.
Combined readings from two blood smears, taken 24 h apart at screening or venipuncture, were used to categorize the parasite species present and to account for periodic fluctuation of parasitemia (12). Systemic NO production (nitrate plus nitrite [NOx]) was measured in urine and plasma with Aspergillus nitrate reductase coupled with the Griess reaction (1). Creatinine (Cr) and plasma IgE were measured with an automated analyzer. Urine NOx excretion was expressed as the ratio of NOx/Cr to control for variations in urine concentration. The percentage of urinary fractional excretion of NOx (FENOx) was calculated thus: FENOx = 100 × (urine NOx/plasma NOx)/(urine Cr/plasma Cr). NOS activity in lysates of unstimulated PBMC pellets was measured as the amount of [14C]arginine that was converted to citrulline per milligram of total cellular protein/hour (46). P. falciparum merozoite surface protein 1 and P. vivax apical membrane antigen 1 were detected by PCR (to assess treatment efficacy) in whole blood spotted onto filter paper as previously described (43).
Statistical methods.
Statistical analysis was performed with Stata 7.0 software (StataCorp, College Station, Tex.). Summary statistics are presented in accordance with the distribution of the data (i.e., means with standard deviations or medians with interquartile ranges [IQR]). Pairwise comparisons of nonparametric continuous data were performed with the Mann-Whitney U test. Otherwise, nonparametric data were logarithmically transformed to normality in the case of plasma NOx, urine NOx/urine Cr, and PBMC NOS activity or considered in ordered quintiles in the case of the highly skewed parasite densities and plasma IgE levels. Correlations were performed after transformation by comparing Pearson's r against a value of 0. Multivariate analyses of continuous data were done using linear regression, and logistic regression was used to compute the odds of parasite recurrence in subjects whose parasitemia was initially cleared by treatment. Analysis of variance for repeated measures was used to examine within-subject variation in continuous measures over time, and paired Wilcoxon tests were used to analyze changes in NO production with clearance or recurrence of parasitemia. P values of <0.05 were considered to indicate statistical significance.
RESULTS
Baseline characteristics.
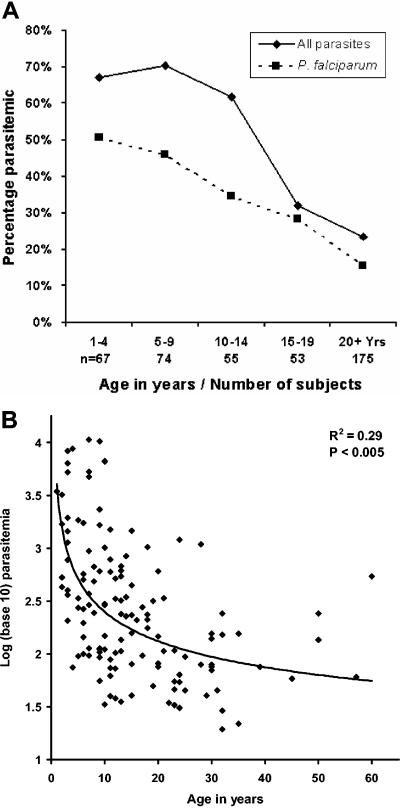
Single blood smears from 424 individuals aged ≥1 year (62% from Midiba; 48% male) were screened by microscopy to enable selection of subjects for enrollment. As a reflection of prevalence at the population level, the proportion of subjects positive for any malaria parasite at screening was highest in the 5- to 9-year-old age group and for P. falciparum in the 1- to 4-year-old age group (Fig. 1A). The age-related prevalence of parasitemia, splenomegaly (83% in subjects aged ≤14 years, with a peak of 92% in children 5 to 9 years old), and stated bed-net use (86% overall; 98% in children 1 to 4 years old) was broadly consistent with previous data reported from this region (16, 19). There were 195 subjects enrolled into the study (median age, 14 years; IQR, 9 to 25; 62% from Midiba) (Table 1). The mean age of the 22 Darwin controls was 35 years (standard deviation, 8), and 41% were male. Venisection failed at T0 in 17 subjects (rain washed out the collection with 16 subjects) and 4 subjects failed to produce a urine sample. Two blood smears taken on consecutive days were available for examination from each of 178 subjects.
FIG. 1.
A. Blood smear results in 424 subjects from Papua New Guinea who were screened for enrollment into the study. Results represent the reading of a single daily smear. (B) Parasite density of P. falciparum in 105 enrolled subjects at baseline. Diamonds signify the higher of two measurements taken 24 h apart.
TABLE 1.
Baseline characteristics of parasitemias in subjects included in the statistical analysisa
| Age group (yr) | n | % Male | Parasitemia
|
Negative (%) | No. (%) of Pfb | Pf/μlc (95% CI) | Parasites/μlc (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf | Pf, Pv | Pf, Pm | Pf, Pv, Pm | Pf, Pv, Po | Pv | Pv, Pm | Pm | Po | |||||||
| 1-4 | 22 | 32 | 11 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 5 (23) | 14 (64) | 1,008 (403-2520) | 1,267 (623-2577) |
| 5-9 | 38 | 53 | 15 | 7 | 3 | 2 | 0 | 4 | 0 | 1 | 0 | 6 (16) | 27 (57) | 429 (219-839) | 453 (267-780) |
| 10-14 | 39 | 51 | 11 | 6 | 4 | 1 | 1 | 4 | 2 | 3 | 0 | 7 (18) | 23 (33) | 323 (176-592) | 259 (156-430) |
| 15-19 | 23 | 39 | 11 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 7 (30) | 16 (70) | 163 (90-295) | 191 (111-327) |
| 20 + | 73 | 45 | 21 | 3 | 0 | 1 | 0 | 9 | 2 | 1 | 1 | 35 (48) | 25 (34) | 107 (68-168) | 95 (69-132) |
| Total | 195 | 46 | 69 | 21 | 8 | 5 | 2 | 20 | 4 | 5 | 1 | 60 (31) | 105 (54) | 280 (207-379) | 262 (202-339) |
Number of subjects with each species (or combined species) of parasite on examination of two consecutive daily blood smears. Pf, P. falciparum: Pv, P. vivax; Pm, P. malariae; Po, P. ovale; Negative, negative for parasites. Shown are results for 195 subjects who did not meet the exclusion criteria at T0, so that the parasitemias given are reflective of those found in asymptomatic individuals.
Number and percentage of subjects with P. falciparum parasitemia either as the sole infecting parasite or in combination with other parasites.
Geometric mean of the highest-density P. falciparum parasitemia and the highest densities of combined species ("Parasites/μl") measured from two consecutive daily blood smears. Note that there is some overlap between the last two columns, which are not mutually exclusive.
Additional parasite species were found in the second smear for 20 (28%) of 71 initially aparasitemic subjects and in 25 (24%) of 106 initially parasitemic subjects. As anticipated, the relationship between level of parasitemia and age was best modeled as an inverse exponential function, both for all parasites (R = 0.54; P < 0.0005) and for parasitemia with P. falciparum (R = 0.46; P < 0.0005) (Fig. 1B). Axillary temperature was recorded on all three occasions within 24 h in 77, 71, and 73% of subjects at T0, T1, and T2, respectively, with all subjects having at least one temperature recording at each time point. All five of the subjects who were febrile (axillary temperature ≥ 37.5°C) at T1 or T2 also reported concurrent symptoms of illness; therefore, subjects with less than three readings who were not febrile on those occasions and who were well were not excluded from the analysis. Eleven of the enrolled subjects met exclusion criteria at T1, and 7 met the criteria at T2 (2 subjects met the criteria at both time points); these subjects were excluded from statistical analyses involving data collected at those times.
Plasma Cr was normally distributed in subjects who were ≤18 years, and none of these children or adolescents had a plasma Cr above the midpoint of their age-standardized reference range (as used by the Royal Darwin Hospital) (data not shown). These low values are likely due to low lean body mass, since 95% of these subjects' weight was less than their median age-standardized weight (28) (data not shown). Plasma Cr was also normally distributed in subjects of ≥19 years, and only two men had a plasma Cr above the normal range of 60 to 120 μmol/liter (Cr was 125 μmol/liter in both men). The median FENOx was 34% in children and adolescents, 34% in adults, and 33% in Darwin controls.
Pharmacological clearance of parasitemia and follow-up.
Follow-up at T1 was successful for 174 (89%) of the 195 enrolled subjects after a median of 14 days (range, 12 to 32 days). Of these subjects, 132 (76%) were studied on the 14th day and 22 (13%) were studied on the 28th day. Those leaving the study did not significantly differ in age, sex, or village of origin from the remaining subjects. Follow-up at T2 was not attempted with all subjects for logistical reasons: follow-up was successful in 94 (92%) of 102 intended cases after a median of 56 days from T0 (range 40 to 77). Antimalarial treatment was given to all but 3 of 135 initially parasitemic eligible subjects at T0. It was successful in 106 (86%) of the 123 subjects (with any parasite) who attended follow-up at T1 and in 82 (86%) of the 95 subjects whose baseline parasitemia included P. falciparum.
PCR was performed with specimens from the 100 nonexcluded subjects at T1 who had been judged aparasitemic by microscopic examination of samples and who had been given treatment to eradicate parasitemia at T0. In samples from 99 subjects with PCR results, P. falciparum infection was evident in only 3 (3%) and P. vivax in 1 (1%). We studied 65 subjects at T2 who had been given treatment to eradicate parasitemia at T0 and who were aparasitemic by microscopic sample examination at T1. Parasitemia recurred in 29 (48%) of these subjects after a median of 42 days (IQR, 30 to 44) from T1, with P. falciparum present in 14 (48%) and P. vivax in 18 (63%) of subjects. As expected, the odds of recurrence of parasitemia at T2 after confirmed clearance at T1 was inversely related to age (odds ratio = 0.5 for successive age group; 95% confidence interval [CI] = 0.3 to 0.7; P < 0.001) and positively related to the time elapsing between T1 and T2 (odds ratio = 1.5 for each successive week; 95% CI = 1.2 −2.0; P = 0.001). There were 51 subjects who had P. falciparum infection at T0 in whom parasitemia was cleared with treatment at T1; P. falciparum was present in 10 (20%) of these subjects at T2. Thus, the efficacy of parasite clearance and the nature of its subsequent recurrence in some subjects were deemed to be appropriate for the purpose of relating measures of NO to parasitemia in this study.
Basal NO production at T0 and its relationship to age.
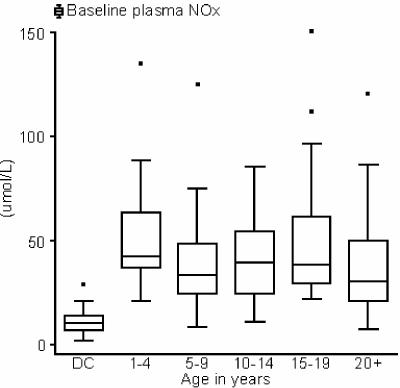
The median plasma NOx was 37 μmol/liter (IQR, 25 to 55) in 178 subjects for whom plasma was available and was significantly higher in each age group than in Darwin controls (Fig. 2). Age only explained 2.5% of the overall variation in plasma NOx in PNG subjects (P = 0.033), and plasma NOx was predicted to fall by 3.5% every 5 years, from a peak of 41 μmol/liter at age 2 to 31 μmol/liter at age 40. Basal PBMC NOS activity in 158 subjects for whom a sample was available (median, 1,030 pmol/mg; IQR, 670 to 1540; 20 samples were lost during transport) varied even less by age group than did plasma NOx. NOS activity was not significantly related to age or sex but was significantly higher in PNG subjects than in Darwin controls (median, 613 pmol/mg; IQR, 495 to 899; P = 0.003). PBMC NOS activity and plasma NOx were not significantly correlated. Urine NOx/urine Cr ratios appeared to decrease significantly across successive age groups from a peak in early childhood to a low in adulthood (r2 = 0.35; P < 0.001). However, this result was almost certainly due to a progressive growth-related increase in the denominator (urine Cr) used to calculate this ratio (5). When 24-h NOx excretion was estimated by adjusting urine NOx/urine Cr ratio in children and adolescents by weight-standardized 24-h urine Cr excretion values (36), this apparent significant decrease disappeared (data not shown).
FIG. 2.
Plasma NOx levels in Darwin controls (DC) and PNG subjects not excluded at T0. The median plasma NOx level in Darwin controls (11 μmol/liter; IQR, 7 to 24) was significantly lower than for each age group of PNG subjects (P < 0.001 for each pairwise comparison; Mann-Whitney U test). In the PNG group, age only explained 2.5% of the overall variation in plasma NOx levels (P = 0.033), with the predicted NOx level falling 3.5% for every 5 years of increasing age. The horizontal lines in the middle of the boxes represents the median, and the boxes extend from the 25th to the 75th percentile. Whiskers extend no further than the most distant data point or 1.5 times the IQR. Outlying values are represented by dots.
Relationship between indices of NO and parasitemia.
There was no significant difference in plasma NOx or PBMC NOS activity between subjects with malaria parasitemia (including all species) and aparasitemic subjects at T0 in univariate analyses or after adjustment for age group and/or sex. Likewise, there was no difference in any measure of NO production between subjects with P. falciparum parasitemia on blood smear and those without (i.e., including aparasitemic subjects and those with parasites other than P. falciparum) or those with P. falciparum parasitemia and aparasitemic subjects. There was no relationship between the highest recorded density of parasitemia over 2 consecutive days (all parasites) or density of P. falciparum parasitemia and any measure of NO production cross-sectionally at T0. Similarly, there was no significant relationship between the level of parasitemia on the day of venisection and any measure of NO production.
There was a high level of consistency in the central tendencies and spread of results between T0, T1, and T2 for all measured indices of NO production. Within individuals, there was no significant change between results taken at two or more time points for nonexcluded subjects in plasma NOx, urine NOx/urine Cr, or PBMC NOS activity (P > 0.20 in each analysis of variance for repeated measures). This longitudinal stability in NO indices was also evident upon clearance or recurrence of parasitemia following successful initial treatment (Table 2). Basal plasma NOx and PBMC NOS activity at T0 were not significantly predictive of recurrent parasitemia, either in a univariate analysis or after controlling for the significant effects of age and elapsed time (data not shown).
TABLE 2.
Longitudinal analysis of effect of clearance or recurrence of parasitemiaa
| Parameter | Value | Median change (IQR)b | Pc |
|---|---|---|---|
| Clearance of malaria parasitemia (all species) | |||
| Plasma NOx (mmol/liter) | 90 | 1.2 (−9.4 to 13.6) | 0.37 |
| PBMC NOS activity (pmol/mg) | 80 | −9.9 (−55.9 to 56.6) | 0.94 |
| Clearance of P. falciparum specifically | |||
| Plasma NOx (mmol/liter) | 70 | −0.03 (−11.1 to 11.9) | 0.98 |
| PBMC NOS activity (pmol/mg) | 61 | −14 (−56.7 to 51.8) | 0.55 |
| Recurrence of malaria parasitemia (all species) | |||
| Plasma NOx (mmol/liter) | 26 | 1.1 (−16.1 to 20.4) | 0.77 |
| PBMC NOS activity (pmol/mg) | 25 | −37.1 (−66.8 to 57.6) | 0.65 |
| Recurrence of P. falciparum specifically | |||
| Plasma NOx (mmol/liter) | 11 | 6.2 (−13.6 to 29.5) | 0.37 |
| PBMC NOS activity (pmol/mg) | 11 | −53.1 (−271 to 57.1) | 0.42 |
Subjects were given treatment to eradicate parasitemia at study point T0 and who were aparasitemic at T1.
Median change (IQR) in parameters between T0 and T1 for clearance of parasitemia and T1 and T2 for recurrence.
Significance of paired Wilcoxon test.
Relationship between NO production and IgE.
Baseline plasma IgE ranged from 35 to 18,400 international units (kU)/liter and was highly skewed (mean, 3,606; median, 2,200; IQR, 1,000 to 4,800). IgE levels increased with age to a peak in the 10- to 14-year-old group before decreasing and leveling out in subjects aged 15 years and older. IgE was significantly predictive of PBMC NOS activity but explained only 4% of the overall variance (P = 0.014). Predicted NOS activity was 9% higher in successive quintiles of IgE (95% CI = 2 to 17) and not effected by age or sex. There was no association between IgE levels and the measures of systemic NO metabolites. IgE levels were not associated with the presence or density of malaria parasitemia in general or of P. falciparum in particular.
DISCUSSION
We studied NO production and NOS activity in a population of children and adults exposed to intense malaria transmission and demonstrated no relationship with parasitemia and little change with advancing age. This pattern contrasts sharply with that of the exponentially decaying age relationship of the pyrogenic threshold and argues against NO being a major mediator of malarial tolerance. All indices of NO measurement were remarkably stable within individuals over time, regardless of the elimination or recurrence of malaria parasitemia. Nevertheless, NO production was much higher in the study subjects from rural PNG than in urban adult controls from northern Australia. Few subject specimens showed evidence of parasitemia on PCR after drug treatment, which minimizes the likelihood that widespread drug resistance, combined with a (low) parasitemic threshold effect for NO production, confounded the results. There was no evidence that differences in renal handling of NO metabolites affected results, since subjects were healthy, had normal renal function, and had FENOx levels similar to those of controls.
The reasons for increased basal NO production in malaria-exposed populations are unclear but may include tropical enteropathy (29) and/or intestinal parasitosis. We observed a modest association between extraordinarily high levels of IgE (as a surrogate marker of parasitic infection) and PBMC NOS activity, in accordance with previous studies using NO metabolites (34, 35). That this relationship was not stronger might reflect a requirement for complexed IgE to optimally activate the CD23 pathway (33) or a contribution of other pathways linking intestinal parasitosis and tropical enteropathy to NO production. It appears from the disassociation of plasma NOx and NOS activity in this study and a previous smaller study (9) that cellular sources other than PBMC may be responsible for elevated NO production. These may include liver, spleen, muscle, gut, and the endothelium of other tissues; these have been associated with NOS2 activation in animal and human malaria and bacterial infections (18). It is possible that malaria and other infections and/or influences have contributed to selection for genetic polymorphisms favoring NOS2 activation in individuals living in PNG (24), although we have not detected common NOS2 polymorphisms in these subjects (8).
That malaria parasitemia was not associated with NO production in the present study, but was in previous smaller cross-sectional studies in Tanzanian children (3) and adults from Papua Province, Indonesia (9), is difficult to explain; factors may include statistical chance, classification of parasitemia (only one smear was examined in a previous study) (3), the epidemiology of nonmalarial infections influencing NO, and/or genetic polymorphisms in NOS2 or other regulatory cytokine genes. For example, a genetically determined maximal level of NO production may have been reached in Madang residents in response to nonmalarial stimuli, such that augmentation by malaria infection was not evident. The longitudinal stability of NOS activity and NO production and lack of protection against recurrent parasitemia support the preponderance of murine data (4) and previous human genetic association studies (24) that suggest NO does not mediate killing of blood stage parasites in vivo, at least in the malaria-tolerant state. These data also argue against NO being a likely candidate for the mediator of density-dependent regulation (10), a phenomenon first described in a Madang cohort (11).
Attention should now refocus on alternative biological pathways that may mediate malarial tolerance, given that it seems unlikely that NO or antibodies to the proposed malaria toxin glycosylphosphatidylinositol (7) are primarily involved. Although earlier studies implicated the importance of NO to endotoxin tolerance development (49), recent work using NOS2 knockout mice has shown that NO is dispensable to this process (48). Experimental evidence is converging to suggest that differential activation of toll-like receptor 2 (by malarial toxin) and toll-like receptor 4 (by bacterial endotoxin) can result in cross-tolerance, independent of paracrine mediators such as NO (15, 30, 39). This accords well with the earlier in vivo experimental data from human studies (23, 40). It is important to note that our findings in asymptomatic individuals do not necessarily exclude other potential antitoxic or anticytoadherence roles for NO, particularly in malaria disease states where there is supportive evidence in both animal models (4) and human clinical studies (2, 24). The findings do, however, suggest that investigative efforts should be directed toward other mechanisms if tolerance-like immunity is to be induced as a novel form of therapy or vaccination for malaria.
Acknowledgments
We gratefully acknowledge Moses Lagog, Erwin Ibam, Kerry Lorry, Mary Misukonis, and Wayne Pederick for technical support; John Condon and Joan Cunningham for logistical support; and Robyn Marsh for proofreading. Finally, we gratefully acknowledge and thank those people who volunteered to participate in this study.
Financial support was received from the National Health and Medical Research Council, Australia (C.S.B. and N.M.A.); the Tudor Foundation; the Mark Nicholson and Alice Hill Malaria Research Fund; National Institute of Health grant R01 AI-41764; the VA Research Service; and the Cooperative Research Centre for Aboriginal and Tropical Health, Australia.
None of the authors have any financial or other relationships (or conflicts of interest) that are relevant to the study.
Editor: F. C. Fang
REFERENCES
- 1.Anstey, N. M., C. S. Boutlis, and J. R. Saunders. 2002. Systemic nitric oxide production in human malaria. I. Analysis of NO metabolites in biological fluids. Methods Mol. Med. 72:461-467. [DOI] [PubMed] [Google Scholar]
- 2.Anstey, N. M., J. B. Weinberg, M. Y. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstey, N. M., J. B. Weinberg, Z. Wang, E. D. Mwaikambo, P. E. Duffy, and D. L. Granger. 1999. Effects of age and parasitemia on nitric oxide production/leukocyte nitric oxide synthase type 2 expression in asymptomatic, malaria-exposed children. Am. J. Trop. Med. Hyg. 61:253-258. [DOI] [PubMed] [Google Scholar]
- 4.Anstey, N., J. Weinberg, and D. Granger. 1999. Nitric oxide and malaria, p. 311-341. In F. C. Fang (ed.), Nitric oxide and infection. Plenum Publishing Corp, New York, N.Y.
- 5.Applegarth, D. A., and P. M. Ross. 1975. The unsuitability of creatinine excretion as a basis for assessing the excretion of other metabolites by infants and children. Clin. Chim. Acta 64:83-85. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong Schellenberg, J. R. M., T. Smith, P. L. Alonso, and R. J. Hayes. 1994. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol. Today 10:439-442. [DOI] [PubMed] [Google Scholar]
- 7.Boutlis, C. S., D. C. Gowda, R. S. Naik, G. P. Maguire, C. S. Mgone, M. J. Bockarie, M. Lagog, E. Ibam, K. Lorry, and N. M. Anstey. 2002. Antibodies to Plasmodium falciparum glycosylphosphatidylinositols: inverse association with tolerance of parasitemia in Papua New Guinean children and adults. Infect. Immun. 70:5052-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutlis, C. S., M. R. Hobbs, R. L. Marsh, M. A. Misukonis, A. N. Tkachuk, M. Lagog, J. Booth, D. L. Granger, M. J. Bockarie, C. S. Mgone, M. C. Levesque, J. B. Weinberg, and N. M. Anstey. 2003. Inducible nitric oxide synthase (NOS2) promoter CCTTT repeat polymorphism: relationship to in vivo nitric oxide production/NOS activity in an asymptomatic malaria-endemic population. Am. J. Trop. Med. Hyg. 69:569-573. [PubMed] [Google Scholar]
- 9.Boutlis, C. S., E. Tjitra, H. Maniboey, M. A. Misukonis, J. R. Saunders, S. Suprianto, J. B. Weinberg, and N. M. Anstey. 2003. Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant Papuan adults. Infect. Immun. 71:3682-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce, M. C., and K. P. Day. 2002. Cross-species regulation of malaria parasitaemia in the human host. Curr. Opin. Microbiol. 5:431-437. [DOI] [PubMed] [Google Scholar]
- 11.Bruce, M. C., C. A. Donnelly, M. P. Alpers, M. R. Galinski, J. W. Barnwell, D. Walliker, and K. P. Day. 2000. Cross-species interactions between malaria parasites in humans. Science 287:845-848. [DOI] [PubMed] [Google Scholar]
- 12.Bruce, M. C., C. A. Donnelly, M. Packer, M. Lagog, N. Gibson, A. Narara, D. Walliker, M. P. Alpers, and K. P. Day. 2000. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology 121:247-256. [DOI] [PubMed] [Google Scholar]
- 13.Burkot, T. R., P. M. Graves, J. A. Cattani, R. A. Wirtz, and F. D. Gibson. 1987. The efficiency of sporozoite transmission in the human malarias, Plasmodium falciparum and P. vivax. Bull. W. H. O. 65:375-380. [PMC free article] [PubMed] [Google Scholar]
- 14.Burkot, T. R., P. M. Graves, R. Paru, R. A. Wirtz, and P. F. Heywood. 1988. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am. J. Trop. Med. Hyg. 39:135-144. [DOI] [PubMed] [Google Scholar]
- 15.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 16.Cattani, J. A., J. L. Tulloch, H. Vrbova, D. Jolley, F. D. Gibson, J. S. Moir, P. F. Heywood, M. P. Alpers, A. Stevenson, and R. Clancy. 1986. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am. J. Trop. Med. Hyg. 35:3-15. [DOI] [PubMed] [Google Scholar]
- 17.Clark, I. A., F. M. al Yaman, W. B. Cowden, and K. A. Rockett. 1996. Does malarial tolerance, through nitric oxide, explain the low incidence of autoimmune disease in tropical Africa? Lancet 348:1492-1494. [DOI] [PubMed] [Google Scholar]
- 18.Clark, I. A., and W. B. Cowden. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221-260. [DOI] [PubMed] [Google Scholar]
- 19.Cox, M. J., D. E. Kum, L. Tavul, A. Narara, A. Raiko, M. Baisor, M. P. Alpers, G. F. Medley, and K. P. Day. 1994. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 88:191-197. [DOI] [PubMed] [Google Scholar]
- 20.Fisher, D., F. McCarry, and B. Currie. 1993. Strongyloidiasis in the Northern Territory. Under-recognised and under-treated? Med. J. Aust. 159:88-90. [PubMed] [Google Scholar]
- 21.Gatton, M. L., and Q. Cheng. 2002. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am. J. Trop Med Hyg. 66:467-473. [DOI] [PubMed] [Google Scholar]
- 22.Hensmann, M., and D. Kwiatkowski. 2001. Cellular basis of early cytokine response to Plasmodium falciparum. Infect. Immun. 69:2364-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyman, A., and P. B. Beeson. 1949. Influence of various disease states upon the febrile response to intravenous injection of typhoid bacterial pyrogen: with particular reference to malaria and cirrhosis of the liver. J. Lab. Clin. Med. 34:1400-1403. [PubMed] [Google Scholar]
- 24.Hobbs, M. R., V. Udhayakumar, M. C. Levesque, J. Booth, J. M. Roberts, A. N. Tkachuk, A. Pole, H. Coon, S. Kariuki, B. L. Nahlen, E. D. Mwaikambo, A. L. Lal, D. L. Granger, N. M. Anstey, and J. B. Weinberg. 2002. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360:1468-1475. [DOI] [PubMed] [Google Scholar]
- 25.Iuvone, T., F. D'Acquisto, R. Carnuccio, and M. Di Rosa. 1996. Nitric oxide inhibits LPS-induced tumor necrosis factor synthesis in vitro and in vivo. Life Sci. 59:L207-L211. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1995. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J. Immunol. 155:5306-5313. [PubMed] [Google Scholar]
- 27.King, M. 1973. A medical laboratory for developing countries. Oxford University Press, London, England.
- 28.Kuczmarski, R. J., C. L. Ogden, S. S. Guo, L. M. Grummer-Strawn, K. M. Flegal, Z. Mei, R. Wei, L. R. Curtin, A. F. Roche, and C. L. Johnson. 2002. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 11:1-190. [PubMed] [Google Scholar]
- 29.Kukuruzovic, R., D. R. Brewster, E. Gray, and N. M. Anstey. 2003. Increased nitric oxide production in acute diarrhoea is associated with abnormal gut permeability, hypokalaemia and malnutrition in tropical Australian aboriginal children. Trans. R. Soc. Trop. Med. Hyg. 97:115-120. [DOI] [PubMed] [Google Scholar]
- 30.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 31.McGregor, I. A., H. M. Gilles, J. H. Walters, A. H. Davies, and F. A. Pearson. 1956. Effects of heavy and repeated malarial infections on Gambian infants and children: effects of erythrocytic parasitization. Br. Med. J. 4994:686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, M. J. 1958. Observations of the natural history of malaria in the semi-resistant west African. Trans. R. Soc. Trop. Med. Hyg. 52:152-168. [DOI] [PubMed] [Google Scholar]
- 33.Nacher, M. 2002. Worms and malaria: noisy nuisances and silent benefits. Parasite Immunol. 24:391-393. [DOI] [PubMed] [Google Scholar]
- 34.Nacher, M., P. Singhasivanon, J. Kaewkungwal, U. Silachamroon, S. Treeprasertsuk, T. Tosukhowong, S. Vannaphan, and S. Looareesuwan. 2002. Relationship between reactive nitrogen intermediates and total immunoglobulin E, soluble CD21 and soluble CD23: comparison between cerebral malaria and nonsevere malaria. Parasite Immunol. 24:395-399. [DOI] [PubMed] [Google Scholar]
- 35.Nacher, M., P. Singhasivanon, B. Traore, S. Vannaphan, F. Gay, D. Chindanond, J. F. Franetich, D. Mazier, and S. Looareesuwan. 2002. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am. J. Trop. Med. Hyg. 66:304-309. [DOI] [PubMed] [Google Scholar]
- 36.Remer, T., A. Neubert, and C. Maser-Gluth. 2002. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am. J. Clin. Nutr. 75:561-569. [DOI] [PubMed] [Google Scholar]
- 37.Rockett, K. A., M. M. Awburn, W. B. Cowden, and I. A. Clark. 1991. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect. Immun. 59:3280-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogier, C., D. Commenges, and J. F. Trape. 1996. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am. J. Trop. Med. Hyg. 54:613-619. [DOI] [PubMed] [Google Scholar]
- 39.Ropert, C., L. R. Ferreira, M. A. Campos, D. O. Procopio, L. R. Travassos, M. A. Ferguson, L. F. Reis, M. M. Teixeira, I. C. Almeida, and R. T. Gazzinelli. 2002. Macrophage signaling by glycosylphosphatidylinositol-anchored mucin-like glycoproteins derived from Trypanosoma cruzi trypomastigotes. Microbes. Infect. 4:1015-1025. [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein, M., J. Mulholland, G. Jeffery, and S. Wolff. 1965. Malaria induced endotoxin tolerance. Proc. Soc. Exp. Biol. Med. 118:283-287. [DOI] [PubMed] [Google Scholar]
- 41.Smith, T., B. Genton, K. Baea, N. Gibson, J. Taime, A. Narara, F. Al Yaman, H. P. Beck, J. Hii, and M. Alpers. 1994. Relationships between Plasmodium falciparum infection and morbidity in a highly endemic area. Parasitology 109:539-549. [DOI] [PubMed] [Google Scholar]
- 42.Smith, T., J. A. Schellenberg, and R. Hayes. 1994. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat. Med. 13:2345-2358. [DOI] [PubMed] [Google Scholar]
- 43.Snounou, G., S. Viriyakosol, W. Jarra, S. Thaithong, and K. N. Brown. 1993. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283-292. [DOI] [PubMed] [Google Scholar]
- 44.Tachado, S. D., P. Gerold, M. J. McConville, T. Baldwin, D. Quilici, R. T. Schwarz, and L. Schofield. 1996. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J. Immunol. 156:1897-1907. [PubMed] [Google Scholar]
- 45.van der Poll, T., and S. J. H. van Deventer. 1999. Endotoxemia in healthy subjects as a human model of inflammation, p. 335-357. In J. C. Marshall and J. Cohen (ed.), Update in intensive care and emergency medicine, 1st ed. Springer-Verlag, New York, N.Y.
- 46.Weinberg, J. B., M. A. Misukonis, P. J. Shami, S. N. Mason, D. L. Sauls, W. A. Dittman, E. R. Wood, G. K. Smith, B. McDonald, and K. E. Bachus. 1995. Hum. mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86:1184-1195. [PubMed] [Google Scholar]
- 47.Zinetti, M., G. Fantuzzi, R. Delgado, E. Di Santo, P. Ghezzi, and M. Fratelli. 1995. Endogenous nitric oxide production by human monocytic cells regulates LPS-induced TNF production. Eur. Cytokine Netw. 6:45-48. [PubMed] [Google Scholar]
- 48.Zingarelli, B., P. W. Hake, and J. A. Cook. 2002. Inducible nitric oxide synthase is not required in the development of endotoxin tolerance in mice. Shock 17:478-484. [DOI] [PubMed] [Google Scholar]
- 49.Zingarelli, B., P. V. Halushka, A. P. Caputi, and J. A. Cook. 1995. Increased nitric oxide synthesis during the development of endotoxin tolerance. Shock 3:102-108. [PubMed] [Google Scholar]