Abstract
It remains unclear whether preexisting cerebral microbleeds (CMBs) increase the risks of worse functional outcome after thrombolytic therapy. We performed a systematic review and meta-analysis to assess the risk of unfavorable outcome in patients with acute ischemic stroke and CMBs.
We searched EMBASE, PubMed, and Web of Science for relevant studies assessing functional outcome in the patients with CMBs following thrombolytic therapy. Fixed-effects and random-effects models were performed.
Five eligible studies including 1974 patients were pooled in meta-analysis. The prevalence of CMBs was 24.3%. The pooled analysis demonstrates odds ratio for preexisting CMBs and the achievement of favorable outcome to be 0.69 (95% CI 0.56–0.86; P = 0.001) with no evidence of statistical heterogeneity (I2 = 46.7%, P = 0.112).
Our meta-analysis of available published data demonstrates an increased risk of worse functional outcome after thrombolytic therapy for acute ischemic stroke in patients with pre-existing CMBs. Future studies are needed to determine whether the risk outweigh the expected benefit of reperfusion therapies.
INTRODUCTION
Cerebral microbleeds (CMBs) defined as small, rounded or circular, hypointense lesions on paramagnetic-sensitive MR sequences, such as T2∗-weighted gradient-recalled echo (GRE) or susceptibility-weighted imaging,1 and are commonly detected in elderly individuals and patients with cerebrovascular diseases.2,3 Histopathological analysis shows that CMBs consist of hemosiderin accumulations from red blood cells that presumably have leaked out of small vessels.4 A recent updated pooled meta-analysis demonstrates an increased risk of symptomatic intracerebral hemorrhage (sICH) after thrombolysis for acute ischemic stroke in patients with CMBs (8.5% vs 3.9%).5 However, whether preexisting CMBs increase the risks of worse functional outcome after thrombolytic therapy still remains uncertain.
Despite the increased postthrombolysis bleeding risk, CMB itself could also have direct effects on cognition, neurologic function, and disability.6,7 On the other hand, the long-term benefit of thrombolytic therapy may outweigh its harms even in some patients with sICH. Most previous studies have investigated the potential association between CMBs and postthrombolysis sICH,8–19 whereas only a few of them reported the relationship between CMBs and functional outcome,15–19 with conflicting results.
We thus performed a meta-analysis to assess whether the presence of CMBs on prethrombolysis MRI resulted in a decreased frequency of favorable outcome in acute ischemic stroke patients receiving thrombolytic therapy.
SUBJECTS AND METHODS
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement.20,21
ETHICS STATEMENT
Ethical approval was not necessary for the current study. Personal data of our previous study were used for this meta-analysis.19 The protocols of MRI-guided IVT have been approved by the human ethics committee of the second Affiliated Hospital of Zhejiang University, School of Medicine, which was decribed in detail elsewhere.19
Search Strategy and Eligibility Criteria
We searched appropriate articles by systematic queries of NCBI (PubMed), ISI Web of Science, and EMBASE databases on the 10th of September 2015, using the following search terms: “micro(-)bleed(s)” or “micro(-)h(a)emorrhage(s)” in association with “thromboly∗” or “fibrinoly∗” or “tissue plasminogen activator” or “rt(-)PA” or “t(-)PA” or “alteplase.” Articles not published in English were translated and case reports were excluded. The references of all identified publications were reviewed for any additional studies not indexed. We contacted authors when there were questions regarding their studies. Two authors (JC and SY) identified potentially relevant studies, resolving any uncertainties with a 3rd author (CL).
Randomized controlled trials or controlled observational studies (retrospective or prospective) were eligible for inclusion if they had defined and assessed functional outcome in patients with acute ischemic stroke treated with thrombolytic therapy, and quantified the odds ratio (OR) in relation to the presence of CMBs on prethrombolysis MRI.
Study Selection and Data Extraction
Two authors (JC and HH) considered all titles and abstracts for eligibility in a systematic manner and went through all articles selected as relevant and extracted data independently. We extracted information on study design, MRI parameters for CMBs detection, methodology of thrombolytic therapy, number and demographics of participants (including age and sex), clinical stroke severity (assessed by National Institutes of Health Stroke Scale), number of participants with preexisting CMBs, number of participants with different functional outcome, and the characteristics of CMBs (burden, location, and pathogenesis) by using a unified data form. Discrepancies were resolved by consensus. Authors of the included articles were contacted for data needing clarification.
Data Analysis
We used a fixed effects model (Mantel and Haenszel method) to calculate the pooled ORs and corresponding 95% CIs, with weights calculated using the inverse variance method, because of the relatively small number of included studies and outcome events. Subgroup analysis was performed to isolate patients treated only with intravenous (IV) tissue plasminogen activator (tPA). Statistical heterogeneity was assessed using I2 statistics with inspection of the forest plot. Publication bias was evaluated with Egger test, Begg test, and the funnel plot. We repeated all analyses using random-effects models. All statistical analysis was performed with Stata 11.2 (StataCorp LP, TX).
RESULTS
We identified 87 articles of PubMed, 99 of EMBASE, and 116 of Web of Science in our initial search. Finally, 5 studies (all published) met our predetermined criteria and were pooled in a meta-analysis (Fig. 1).15–19 The characteristics of included studies are summarized in Table 1 .
FIGURE 1.
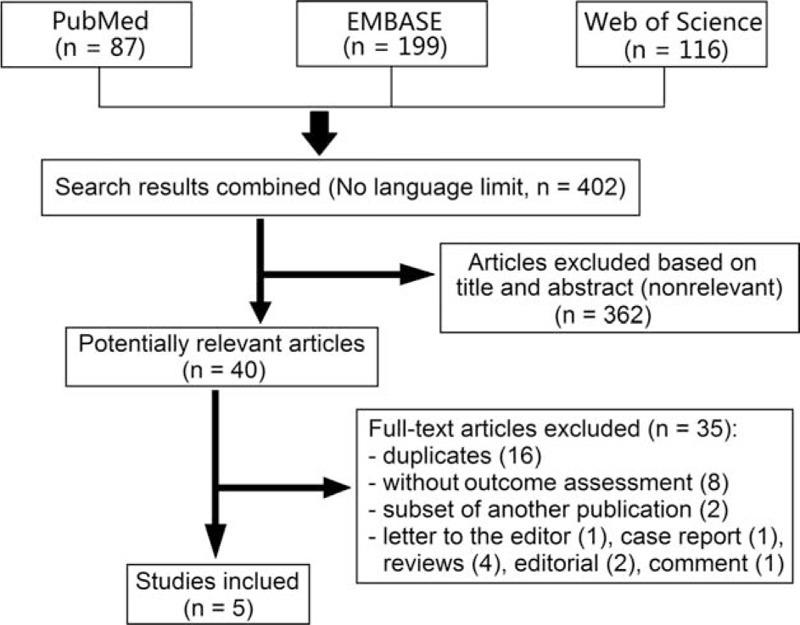
Flow diagram of literature search and study selection.
TABLE 1.
Characteristics of Included Studies
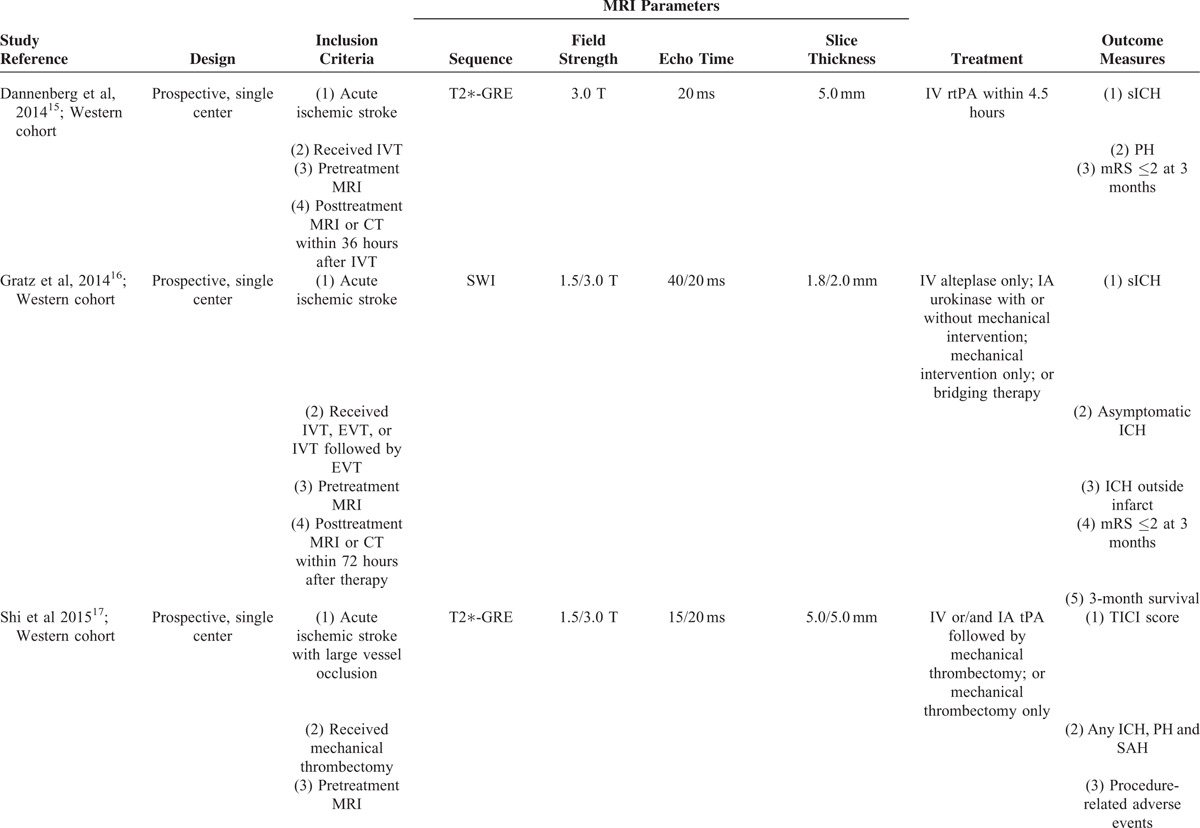
CMBs were identified according to a field guide or/and a neuroimaging standard for CMBs detection.1,22 The maximum diameter of a CMB was defined as 10 mm, except that in Gratz et al's study, was 5 mm.16 Study demographics are summarized in Table 2. Collectively, these studies were composed of 1974 patients (study sample size range: 206–717), 480 (24.3%) of which had CMBs on pretreatment MRI scans. From inspection of each of the studies, patients with preexisting CMBs were older,15–19 had a more severe degree of leukoaraiosis,15,17,19 higher rate of diabetes mellitus,17,19 and higher rate of hypertension (or higher systolic blood pressure),16,18,19 whereas other baseline characteristics were not significantly different. The characteristics of CMBs are summarized in Table 3. About half of the patients with CMBs had exactly 1 CMB, whereas only about one fifth of them had ≥5 CMBs. Interestingly, about one third of the patients with CMBs had strictly lobar CMBs, most of which were considered as presumed pathogenesis of cerebral amyloid angiopathy.
TABLE 1 (Continued).
Characteristics of Included Studies
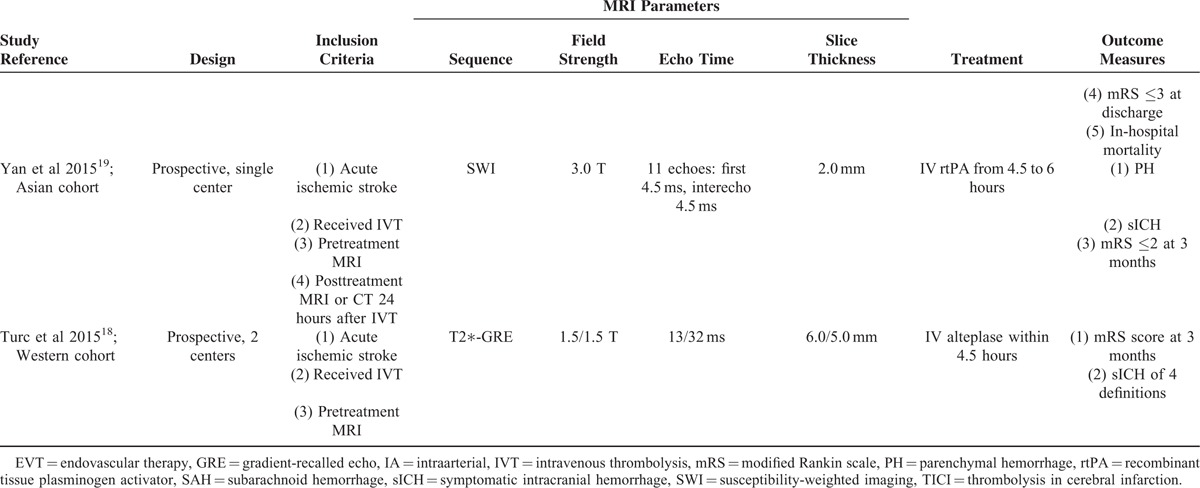
TABLE 2.
Study Demographics and Outcomes
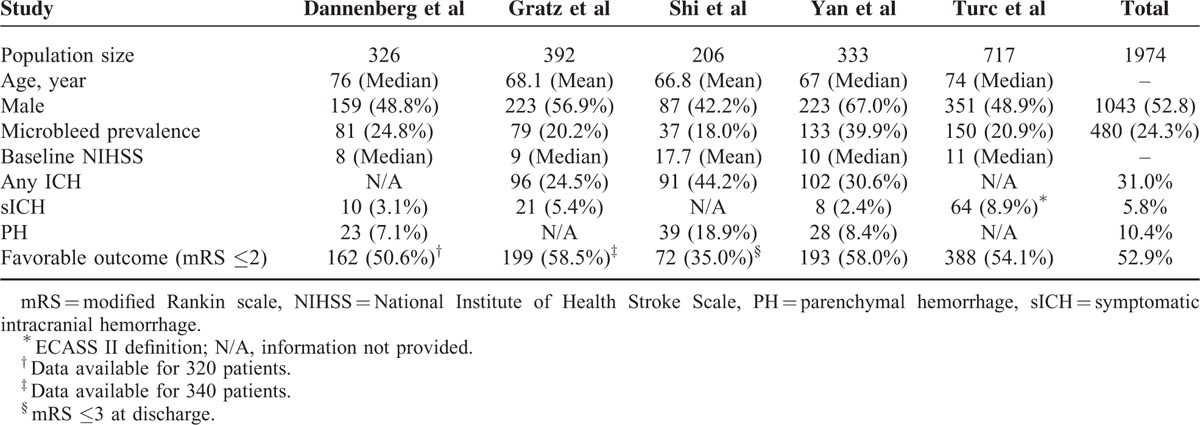
TABLE 3.
Characteristics of CMBs
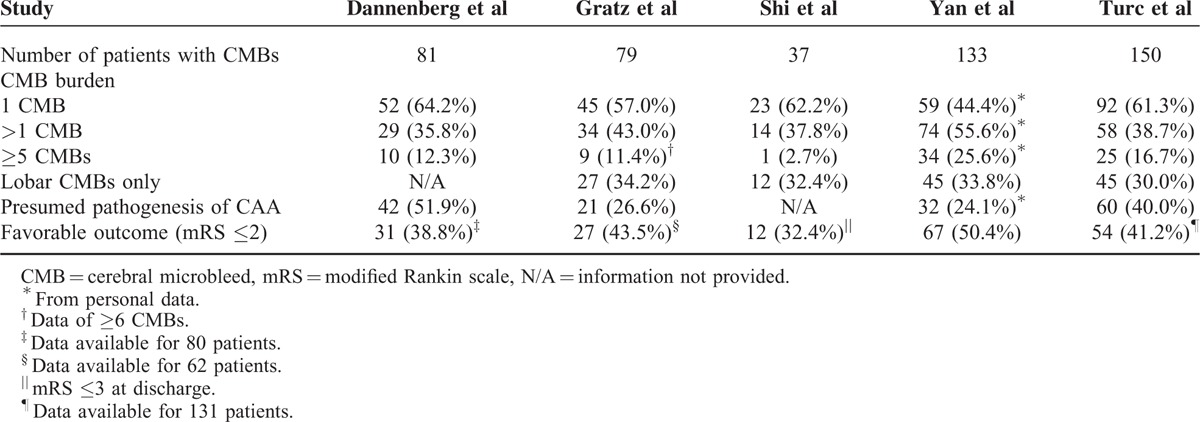
A modified Rankin scale (mRS) score of ≤2 at 3 months was defined as favorable functional outcome in four included studies,15,16,18,19 whereas neurologic status was quantified by mRS score at discharge in Shi et al's study.17 Favorable functional outcome occurred in 52.9% (range: 35.0%–58.5%) of the entire population. Notably, data on functional outcome were not available for 6 patients in Dannenberg et al's study,15 and for 52 patients in Gratz et al's study,16 and 62 patients with pre-stroke mRS score of >2 in Turc et al's study were also excluded from this meta-analysis.18 Among patients with preexisting CMBs, 191 of 443 (43.1%) achieved favorable outcome after thrombolytic therapy compared with 702 of 1411 patients (49.8%) without CMBs. Pooled analysis demonstrated OR for the presence of preexisting CMBs and the achievement of favorable outcome to be 0.69 (95% CI 0.56–0.86; P = 0.001) with no evidence of statistical heterogeneity (I2 = 46.7%, P = 0.112) (Fig. 2). There was no evidence of a publication bias either from the result of Egger test (P = 0.812) or Begg test (P = 1.000), and the shape of the funnel plot seemed symmetrical (Fig. 3).
FIGURE 2.
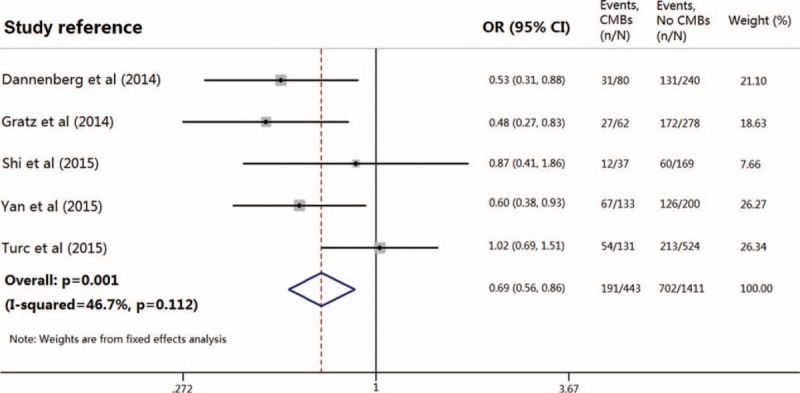
Meta-analysis of the association between favorable functional outcome in patients with acute ischemic stroke treated with thrombolytic therapy, in relation to the presence of preexisting cerebral microbleeds.
FIGURE 3.
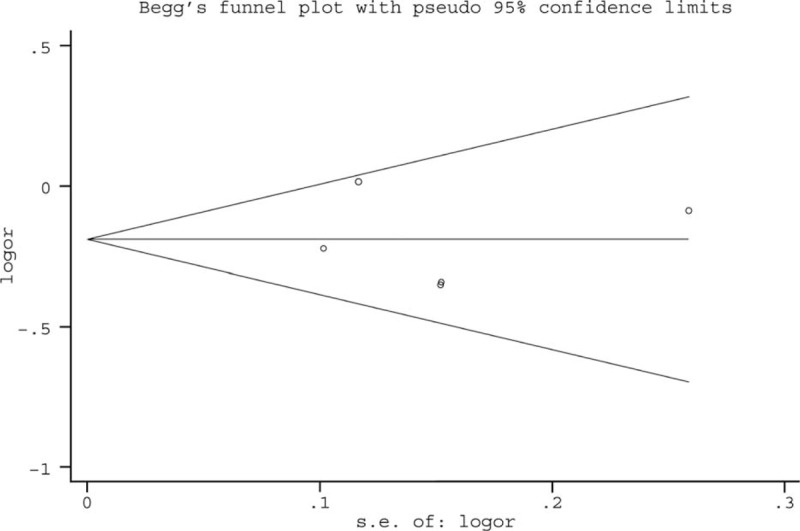
Publication bias from studies about the association between favorable functional outcome and the presence of preexisting cerebral microbleeds.
Four studies, including 1199 patients (n = 312 with CMBs), provided data on patients treated with IV tPA only.15,16,18,19 Pooled analysis of these studies demonstrated OR for the presence of preexisting CMBs and the achievement of favorable outcome to be 0.83 (95% CI 0.74–0.95; P = 0.004) with no evidence of statistical heterogeneity (I2 = 35.1%, P = 0.202) (Fig. 4). All analyses were consistent using a random-effects model.
FIGURE 4.
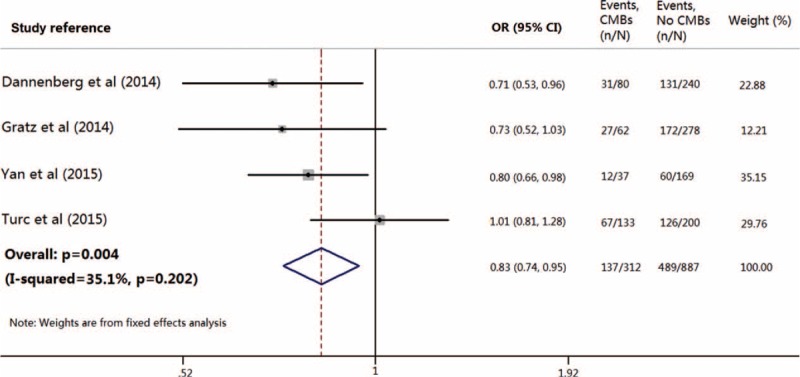
Meta-analysis of the association between favorable functional outcome in patients with acute ischemic stroke treated only with intravenous thrombolysis, in relation to the presence of preexisting cerebral microbleeds.
DISCUSSION
Our meta-analysis in nearly 2000 patients with acute ischemic stroke shows that the presence of preexisting CMBs is associated with a statistically significant decreased rate of favorable functional outcome following thrombolytic therapy. This remains consistent in a subgroup pooled analysis including patients treated with IV tPA only.
The prevalence of CMBs was reported from 4.7% to 15.3% in normal individuals23–26 and might be much higher among patients with ischemic or hemorrhagic strokes because old age and hypertension are risk factors for both conditions. A total of 12.2% to 39.9% of acute ischemic stroke patients receiving thrombolytic therapy were noted to have CMBs on prethrombolysis MRI.8–19 The MRI parameters used varied among previous studies, which was likely to affect the prevalence of CMBs. It has been demonstrated that longer echo time, higher spatial resolution (3D Fourier transform technique), and increased field strength can increase the sensitivity of CMBs detection.1 CMBs might be missed due to a large slice thickness or/and a large interslice gap of MRI scans.27 Moreover, Asian cohort might have a higher proportion of patients with at least one CMB (39.9%) and an important CMB burden (742 CMBs in 133 patients),19 which needs further investigation.
The presence of CMBs on prethrombolysis MRI is not an exclusion criterion for thrombolytic therapy in current guidelines. Most previous studies have been focused on whether the presence, location, or burden, of preexisting CMBs predicts the risk of postthrombolysis sICH,8–19 whereas only a few of them investigated the relationship between CMBs and functional outcome.15–19 Among which, 2 studies found that the presence of CMBs was not associated with 3-month outcome or in-hospital mortality,16,17 while 2 studies observed a significant association between CMB burden and unfavorable outcome in univariate analysis, which lost significance after adjustment for confounding factors.15,18 The rest 1 observed a significant association between extensive (≥3) CMBs and poor outcome in multivariable analysis.19 In above-mentioned studies, patients with preexisting CMBs were older,15–19 had a more severe degree of leukoaraiosis,15,17,19 higher rate of diabetes mellitus,17,19 and higher rate of hypertension (or higher systolic blood pressure).16,18,19 Since leukoaraiosis is highly correlated with CMBs,28 it is unclear if the presence of CMBs is the independent predictor or rather severity of small vessel disease overall. Only a few studies adjusted the severity of leukoaraiosis in multivariate regression analyses.15,17,19 The severity of concomitant leukoaraiosis should be took into consideration in future investigations.
This meta-analysis showed that the presence of pre-existing CMBs was associated with worse functional outcome following thrombolytic therapy. On one hand, patients with CMBs on prethrombolysis MRI developed more sICH than those without (8.5% vs 3.9%),5 meanwhile the presence of sICH independently increased the risk of worse outcome. On the other hand, histopathologic analysis of CMBs generally found these lesions were associated with some degree of surrounding tissue damage,1 and the number of CMBs was found to be independently associated with increased mRS.7 Therefore, pre-existing CMBs might have an additional effect on functional outcome, besides the increased sICH risk.19
Based on current evidence, patients with CMBs on pretreatment MRI do have higher frequencies of sICH and poor functional outcome after thrombolytic therapy. However, the presence of preexisting CMBs should not yet be considered as a contraindication to thrombolysis in otherwise eligible patients. On one hand, these results might be confused by several potential confounders, for example, age and severity of leukoaraiosis, and should thus be treated with caution. On the other hand, since no control groups without thrombolytic therapy were designed in previous studies, it was still questionable whether CMBs-related sICH was likely to exceed the benefits of thrombolysis. Nevertheless, it might be reasonable to take CMBs into account as 1 factor to help guide risk-benefit assessment in difficult decisions. Clinicians should target interventions to reduce sICH in patients with CMBs, such as lowering pretreatment blood pressure targets, in future randomized controlled studies. Our study also provides additional support for more widespread use of MRI in hyperacute stroke treatment.
Our study had several limitations. First, our analysis had inherent biases associated with the use of observational studies. All studies are subject to selection bias since not all acute ischemic stroke patients undergo MRI, and such patients were excluded. However, there was no randomized controlled trial evaluating the risks and benefits of thrombolytic therapy in the patients with preexisting CMBs so far. Second, the use of unadjusted data rendered our analysis vulnerable to confounding variables. Some studies did not provide full information of baseline characteristics likely to be associated with CMBs. Age and the severity of leukoaraiosis are most important. Third, the MRI parameters used varied among included studies. Echo time, field strength, slice thickness, and interslice gap can affect the sensitivity of CMBs detection.
In conclusion, our analysis showed that the presence of preexisting CMBs significantly decreased the rate of favorable functional outcome following thrombolytic therapy. Future large multicenter studies and individual patient data meta-analysis are needed to determine whether the risk outweigh the expected benefit of reperfusion therapies. In addition, current data are limited on whether CMBs are related to the risk of unfavorable functional outcome following endovascular treatment in acute ischemic stroke, which needs further investigations.
Footnotes
Abbreviations: CMB = cerebral microbleed, GRE = gradient-recalled echo, mRS = modified Rankin scale, sICH = symptomatic intracranial hemorrhage, tPA = tissue plasminogen activator.
JC, JF, and SY contributed equally to this work.
This work was supported by the Science Technology Department of Zhejiang Province (2014C33186).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008; 70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 3.Werring DJ, Coward LJ, Losseff NA, et al. Cerebral microbleeds are common in ischemic stroke but rare in TIA. Neurology 2005; 65:1914–1918. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2∗-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999; 20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 5.Charidimou A, Shoamanesh A, Wilson D, et al. Cerebral microbleeds and postthrombolysis intracerebral hemorrhage risk Updated meta-analysis. Neurology 2015; 85:927–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2∗-weighted gradient-echo MRI. Brain 2004; 127 (Pt 10):2265–2275. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan A, Guichard JP, Gschwendtner A, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain 2006; 129 (Pt 9):2375–2383. [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002; 33:95–98. [DOI] [PubMed] [Google Scholar]
- 9.Derex L, Nighoghossian N, Hermier M, et al. Thrombolysis for ischemic stroke in patients with old microbleeds on pretreatment MRI. Cerebrovasc Dis 2004; 17:238–241. [DOI] [PubMed] [Google Scholar]
- 10.Kakuda W, Thijs VN, Lansberg MG, et al. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology 2005; 65:1175–1178. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Lee DH, Ryu CW, et al. Multiple cerebral microbleeds in hyperacute ischemic stroke: impact on prevalence and severity of early hemorrhagic transformation after thrombolytic treatment. AJR Am J Roentgenol 2006; 186:1443–1449. [DOI] [PubMed] [Google Scholar]
- 12.Fiehler J, Albers GW, Boulanger JM, et al. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2∗-weighted magnetic resonance imaging data from 570 patients. Stroke 2007; 38:2738–2744. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Aoki J, Shibazaki K, et al. New appearance of extraischemic microbleeds on T2∗-weighted magnetic resonance imaging 24 hours after tissue-type plasminogen activator administration. Stroke 2013; 44:2776–2781. [DOI] [PubMed] [Google Scholar]
- 14.Moriya Y, Takahashi W, Kijima C, et al. Predictors for hemorrhagic transformation with intravenous tissue plasminogen activator in acute ischemic stroke. Tokai J Exp Clin Med 2013; 38:24–27. [PubMed] [Google Scholar]
- 15.Dannenberg S, Scheitz JF, Rozanski M, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 2014; 45:2900–2905. [DOI] [PubMed] [Google Scholar]
- 16.Gratz PP, El-Koussy M, Hsieh K, et al. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke 2014; 45:1684–1688. [DOI] [PubMed] [Google Scholar]
- 17.Shi ZS, Duckwiler GR, Jahan R, et al. Mechanical thrombectomy for acute ischemic stroke with cerebral microbleeds. J Neurointerv Surg 2015. [DOI] [PubMed] [Google Scholar]
- 18.Turc G, Sallem A, Moulin S, et al. Microbleed status and 3-month outcome after intravenous thrombolysis in 717 patients with acute ischemic stroke. Stroke 2015; 46:2458–2463. [DOI] [PubMed] [Google Scholar]
- 19.Yan S, Jin X, Zhang X, et al. Extensive cerebral microbleeds predict parenchymal haemorrhage and poor outcome after intravenous thrombolysis. J Neurol Neurosurg Psychiatry 2015; 86:1267–1272. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roob G, Schmidt R, Kapeller P, et al. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology 1999; 52:991–994. [DOI] [PubMed] [Google Scholar]
- 24.Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 2004; 35:1831–1835. [DOI] [PubMed] [Google Scholar]
- 25.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008; 79:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010; 41 (10 Suppl):S103–S106. [DOI] [PubMed] [Google Scholar]
- 27.Yan S, Chen Y, Zhang X, et al. New microbleeds after thrombolysis: contiguous thin-slice 3T MRI. Medicine (Baltimore) 2014; 93:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan S, Wan J, Zhang X, et al. Increased visibility of deep medullary veins in leukoaraiosis: a 3-T MRI study. Front Aging Neurosci 2014; 6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]


