Abstract
Studies on the association between inflammatory bowel disease (IBD) and peripheral arterial disease (PAD) are scant. This nationwide population-based cohort study assessed the relationship between IBD and further risk of PAD.
This nationwide population-based cohort study was based on data obtained from the Taiwan National Health Insurance Database from 2000 to 2010, with a follow-up period extending to the end of 2011. We identified inpatients with newly diagnosed IBD by using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. In addition, we selected a comparison cohort from the inpatient claims that was randomly frequency-matched according to age, sex, and index year. We analyzed the risks of PAD by using Cox proportional hazards regression models, including sex, age, and comorbidities.
A total of 11,067 IBD patients and 43,765 controls were enrolled in this study. The risk of developing PAD was 1.29-fold in the patients with IBD compared with the comparison cohort, after age, sex, and comorbidities were adjusted. The patients with IBD who required 2 or more hospitalizations per year were nearly 27.5-fold more likely to have PAD compared with the comparison cohort.
This nationwide population-based cohort study demonstrated that PAD risks are higher in patients with IBD compared with those inpatients without IBD. Careful follow-up observation and aggressive effective treatment should be sought for patients with IBD to reduce the risk of PAD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of inflammatory intestinal disorders mainly comprising 2 types of disease: Crohn's disease (CD) and ulcerative colitis (UC).1 The incidence and prevalence rates of IBD vary among countries and regions. Traditionally, the incidence of this disease is relatively stable in Western countries; however, the IBD incidence has been increasing in Asian countries in the past few years.2–6
Peripheral arterial disease (PAD) is the narrowing of arteries other than those that supply blood to brain or heart, causing a considerable burden on health care systems worldwide.7 The prevalence of peripheral vascular disease in the general population is 12% to 14%, affecting up to 20% of people >70 years.8 According to the Taiwan National Health Insurance (NHI) data analysis, the incidence of invasive PAD treatment is increasing.9 Traditional risk factors for PAD are an older age, male sex, hypertension, diabetes, hyperlipidemia, obesity, smoking, and family history of vascular disease.10–12
Patients with IBD are vulnerable to vascular complications.13 Chung et al observed that IBD patients exhibited a high risk of developing deep vein thrombosis and pulmonary embolism.14 In addition, recent studies have revealed an association between IBD and arterial thromboembolic events.15–17 PAD is a type of arterial disease, but epidemiological studies on the relationship between IBD and the development of PAD are scant. Therefore, we conducted a nationwide population-based cohort study to investigate whether IBD increases the risk of developing PAD.
METHODS
Data Source
The National Health Insurance program was implemented in Taiwan on March 1, 1995 and covered >99% of the 25 million residents in Taiwan. In this study, data were accessed from the National Health Insurance Research Database (NHIRD), which is managed and released for research purposes by the National Health Research Institutes. The Bureau of National Health Insurance (BNHI) scrambled the identification of insurants in the NHIRD to protect their personal information before releasing the data to researchers. A committee of the BNHI is responsible for randomly selecting and verifying the accuracy of claims. The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) was used to code diagnoses. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2-115). The IRB also specifically waived the consent requirement.
Study Participants
Patients in the inpatient claims with newly diagnosed IBD, including Crohn's disease (ICD-9-CM codes 555) and ulcerative colitis (ICD-9-CM codes 556) from 2000 to 2010 were selected as the IBD cohort. The IBD diagnosis is made based on clinical history, physical examination, and objective findings from endoscopic, radiological, laboratory, and histological studies. The index date for patients with IBD was the date of first hospitalization. The comparison cohort was randomly selected from inpatient claims without the history of IBD. For each IBD case, ∼4 comparisons were identified, frequency matched by sex, age (within 5 years), index year of patients in the IBD cohort, and comorbidities including diabetes, hypertension, hyperlipidemia, COPD, heart failure, CAD, stroke, obesity, chronic kidney disease, autoimmune disease, surgery of the intestine, laparotomy, and colorectal cancer. The control-to-case ratio was set at ∼4:1 to enhance the power of statistical tests. In both cohorts, the patients who were diagnosed with PAD (ICD-9-CM codes 440.2, 440.3, 440.8, 440.9, 443, 444.22, 444.8, 447.8, and 447.9) and deep vein thrombosis (ICD-9-CM codes 453.8) before the index date, histories of coagulopathies (ICD-9-CM codes 286, 289.8), sickle-cell disease (ICD-9-CM codes 282.6), age <20 years, or missing information, were excluded.
Outcome and Comorbidities
The main outcome was hospitalization with a new diagnosis of PAD during follow-up. Person-years of the follow-up were calculated for each patient until hospitalization for PAD or censored for loss to follow-up, withdrawal from the insurance program, or the end of 2011.
Comorbidities were identified according to hospital admissions prior to the index date as potential confounding factors. These comorbidities included diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401- 405), hyperlipidemia (ICD-9-CM code 272), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 491, 492, and 496), heart failure (ICD-9-CM code 428), coronary artery disease (CAD) (ICD-9-CM codes 410–414), stroke (ICD-9-CM codes 430–438), obesity (ICD-9-CM code 278.0), chronic kidney disease (ICD-9-CM codes 580–589), autoimmune disease (ICD-9-CM codes 710.0, 710.1, 710.2, 714), surgery of the intestine (ICD-9-CM procedure codes 45.0–45.9, 46.0–46.9, 48.0–48.9), laparotomy (ICD-9-CM procedure codes 54.11, 54.19), and colorectal cancer (ICD-9-CM codes 153–154).
Statistical Analysis
Chi-squared tests were used to examine the differences in the distribution of sex, age, and comorbidities between the IBD and comparison cohorts. Student's t test was used to examine the mean ages and mean follow-up period between both cohorts. The sex-, age-, and comorbidity-specific incidence density rates of PAD per 10,000 person-years of follow-up were calculated for each cohort. Univariable and multivariable Cox proportional hazard models were applied to measure the hazard ratios (HRs) and 95% confidence intervals (CIs) for PAD in the IBD cohort compared with those in the comparison cohort. The multivariable Cox models were simultaneously adjusted for sex, age, and comorbidities of diabetes, hypertension, hyperlipidemia, COPD, heart failure, CAD, stroke, obesity, chronic kidney disease, autoimmune disease, surgery of the intestine, laparotomy, and colorecal cancer. Further analysis was performed to assess the dose–response effect of IBD on the risk of PAD according to the average frequency of hospitalizations per year for IBD. The cumulative incidence curves of PAD were determined using the Kaplan–Meier method between the IBD cohort and the comparison cohort. Their differences were estimated using the log-rank test. All analyses were performed using SAS, Version 9.3, computer software (SAS Institute Inc, Cary, NC). A 2-tailed P < 0.05 was statistically significant.
RESULTS
Our data set included 54,832 participants including both the IBD patient and comparison cohorts. The distributions of gender, age, and comorbidity were similar in the both cohorts, except colorectal cancer. Both cohorts comprised more men than women (54.2% vs 45.8%). The mean age of the comparison cohort was 53 years and that of the IBD cohort was 53.1 years, with ∼37% of the patients in the age group of 40 to 64 years (Table 1). As shown in Figure 1, the cumulative incidence of PAD was higher in the IBD cohort than in the comparison cohort by the end of follow-up. Overall, the incidence of PAD was 1.24-fold higher in the IBD cohort compared with the comparison cohort (23 vs 18.6 per 10,000 person-years). After age, sex, and comorbidities were adjusted, the adjusted HR for developing PAD was 1.29-fold higher (95% CI = 1.22–1.37) for the IBD patients compared with the comparison cohort. The sex-specific adjusted HRs for PAD in the IBD cohort were statistically significant for both the women (HR: 1.25, 95% CI = 1.14–1.36) and men (HR: 1.35, 95% CI = 1.24–1.46) relative to those of the comparison cohort. The age-specific incidence of PAD increased with age in both cohorts. The age-specific adjusted HRs for PAD in the IBD cohort were significantly higher for all age groups, relative to those of the comparison cohort. A comorbidity-specific analysis revealed a high incidence of PAD for patients with comorbidities in both cohorts (Table 2).
TABLE 1.
Comparison of Demographics and Comorbidity Between Inflammatory Bowel Disease Patients and Controls
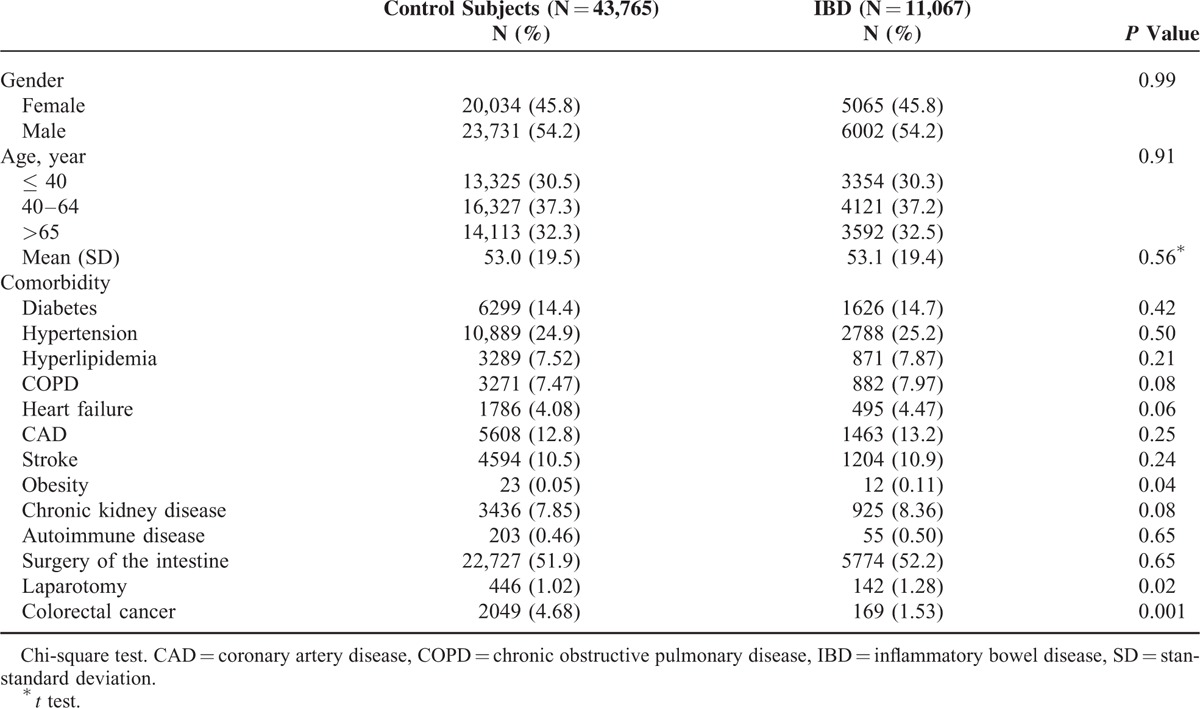
FIGURE 1.
Cumulative incidence of peripheral arterial disease for patients with (dashed line) or without (solid line) inflammatory bowel disease.
TABLE 2.
Incidence and Adjusted Hazard Ratio of Peripheral Arterial Disease Stratified by Sex and Age Compared Between IBD Cohort and Comparison Cohort
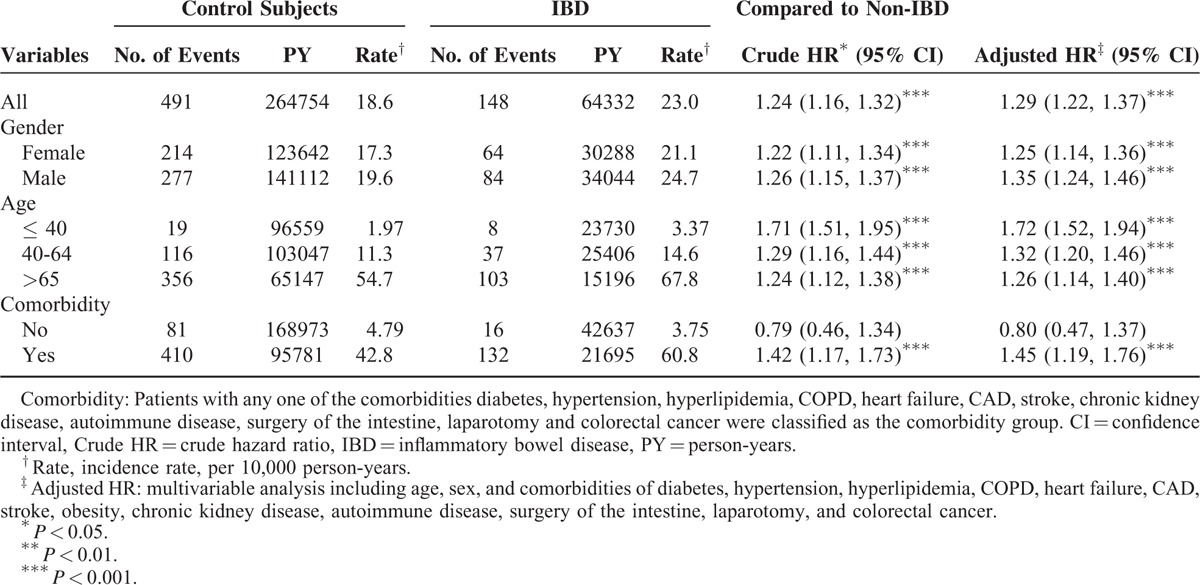
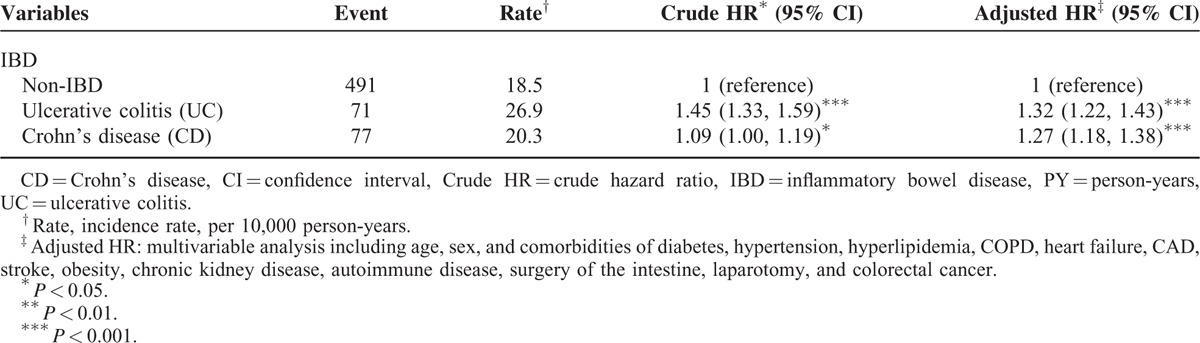
Table 3 presents the risk of PAD according to annual mean hospitalizations for the IBD patients, compared with the comparison cohort. The IBD patients presenting with 2 or more hospitalizations per year exhibited a 27.5-fold increased risk of PAD development compared with the comparison cohort (95% CI = 18.7–40.4). Table 4 shows the incidence and HRs for PAD associated with ulcerative colitis and Crohn's disease in the IBD patients. The patients with UC exhibited a 32% higher risk of PAD compared with the comparison cohort (adjusted HR = 1.32, 95% CI = 1.22–1.43). The CD patients also showed a 27% higher risk of PAD compared with the comparison cohort (adjusted HR = 1.27, 95% CI = 1.18–1.38), which was significant.
TABLE 3.
The Risk of Peripheral Arterial Disease Related to Annual Times of Hospitalization in Patient With IBD and Comparison Cohort Using the Cox Proportional Hazard Regression
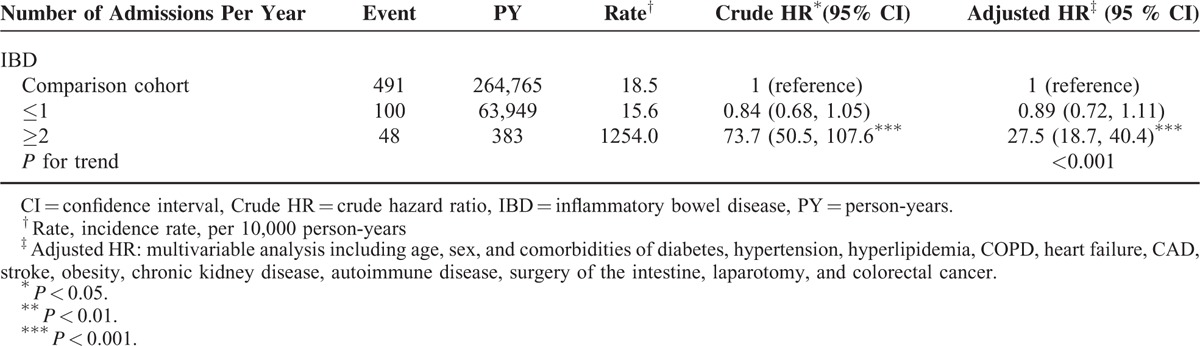
TABLE 4.
Incidence and Adjusted Hazard Ratio of Peripheral Arterial Disease Between Different Entities IBD
DISCUSSION
This is the first study to investigate the long-term risk of PAD in patients with IBD based on nationwide data. We adjusted for several classical PAD risk factors in this population-based cohort study and determined that IBD is an independent risk factor for PAD. We observed that adults with IBD had 1.29-fold risks of developing PAD compared with the comparison cohort. In addition, by using the frequency of hospitalization as a surrogate index of IBD activity, we determined that IBD severity was significantly associated with the risk of PAD (Table 3).
Although the patients with IBD in this study exhibited a higher prevalence of comorbidities and coexistent conditions associated with the development of PAD than the comparison cohort, IBD remained an independent risk factor for developing PAD after covariates were adjusted. The exact mechanisms predisposing patients with IBD to PAD are multifactorial. Long-term inflammation is known to predispose patients to vascular endothelial dysfunction and atherosclerosis.18,19 IBD is characterized by an unusual chronic inflammation of the colon and small intestine. Similarities between the pathophysiological processes in the colonic wall of IBD patients and the process in the arterial wall exist during the progression of atherosclerosis, subsequently resulting in atherosclerotic plaque rupture and thrombotic vascular events. Several reports have indicated that patients with IBD tend to exhibit lipid profile derangement, which promotes atherosclerosis.20–23 The findings of these studies are consistent with our epidemiological results.
Most of the IBD patients in this study were men, and PAD risks increased in both sexes with IBD. The age-stratified effect of IBD on PAD development was the highest in the patients <40 years. However, the incidence of PAD increased with age in the patients with IBD and those in the non-IBD cohorts, which is consistent with previous studies.8 As people age, they become less active, and the function of the cardiopulmonary system deteriorates. Recent studies have shown that frailty, which is commonly associated with aging, can cause subclinical peripheral vascular disease to develop.24 In our study, study cohort with comorbidities were shown to further increase the risk of PAD compared with the comparison group (adjusted HR = 1.45). Among the comorbidities, there were several well-known traditional risk factors for PAD such as hypertension, diabetes, and hyperlipidemia. Recent studies have shown that inflammatory diseases might increase risk of vascular complications.25–26
This study revealed a strong association between an increased number of annual IBD-related medical admissions and the risk of developing PAD. IBD patients with high admission rates may represent increasing IBD flare-ups and severity of the active periods. The results showed that the systemic inflammatory burden with IBD patients may be a key determinant of atherosclerosis risk. Thus, aggressively controlling the inflammatory activity in IBD patients may reduce the vascular complications.
This study had potential limitations. First, the insurance data do not contain detailed information on smoking habits, body mass index, or family history of PAD, all of which might be confounding factors in this study. Consequently, as with previous publications, we adjusted for several comorbidities of smoking-related diseases (including CAD, COPD, and stroke) to make the potential confounding effect of smoking on our study results minimally. These adjust models had been utilized in previous studies. In addition, to adjust for the influence of body mass index, we included several traditional metabolic syndrome comorbidities such as hypertension, diabetes, and hyperlipidemia. Second, the evidence derived from a retrospective cohort study is generally lower in statistical quality than that from randomized trials because of potential biases related to adjustments for confounding variables. Despite our meticulous study design and control measures for confounding factors, bias resulting from unknown confounders might have affected our results.
In conclusion, patients hospitalized for IBD exhibited an increased risk of PAD compared with the comparison cohort. We recommend that physicians carefully monitor vascular complication when caring for patients with IBD.
Footnotes
Abbreviations: CAD = coronary artery disease, CD = Crohn's disease, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, IBD = inflammatory bowel disease, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IRR = incidence rate ratio, NHI = Taiwan's National Health Insurance, NHIRD = National Health Insurance Research Database, PAD = peripheral arterial disease, UC = ulcerative colitis.
Author contributions: all authors have contributed significantly and are in agreement with the content of the manuscript; conception/design: T-YL, Y-GC, C-HK; provision of study materials: C-HK; collection and/or assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
Funding: this study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010; 28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel disease with time, based on systematic review. Gastroenterology 2012; 142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140:1784–1794. [DOI] [PubMed] [Google Scholar]
- 4.Chuang CH, Lin SH, Chen CY, et al. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998–2010. Inflamm Bowel Dis 2013; 19:2815–2819. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology 2013; 145:158–165.e152. [DOI] [PubMed] [Google Scholar]
- 6.Thia KT, Loftus EV, Jr, Sandnorn WJ, et al. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 2008; 103:3167–3182. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT, Hartman L, Town RJ, et al. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med 2008; 13:209–215. [DOI] [PubMed] [Google Scholar]
- 8.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag 2007; 3:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang NT, Chan CL, Lu YT, et al. Invasively-treated incidence of lower extremity peripheral arterial disease and associated factors in Taiwan: 2000–2011 nationwide hospitalized data analysis. BMC Public Health 2013; 13:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012; 308:1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states results from the national health and nutrition examination survey, 1999–2000. Circulation 2004; 110:738–743. [DOI] [PubMed] [Google Scholar]
- 12.Wassel CL, Loomba R, Ix JH, et al. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: the San Diego population study. J Am Coll Cardiol 2011; 58:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarur AJ, Deshpande AR, Pechman DM, et al. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroentero 1; 106:741–747. [DOI] [PubMed] [Google Scholar]
- 14.Chung WS, Lin CL, Hsu WH, et al. Inflammatory bowel disease increases the risks of deep vein thrmobosis and pulmonary embolism in the hospitalized patients: a nationwide cohort study. Thromb Res 2015; 135:492–496. [DOI] [PubMed] [Google Scholar]
- 15.Ha C, Magowan S, Accortt NA, et al. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol 2009; 104:1445–1451. [DOI] [PubMed] [Google Scholar]
- 16.Huang WS, Tseng CH, Chen PC, et al. Inflammatory bowel diseases increase future ischemic stroke: a Taiwanese population-based retrospective cohort study. Eur J Intern Med 2014; 25:561–565. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MS, Lin CL, Chen HP, et al. Long-term risk of acute coronary syndrome in patients with inflammatory bowel disease: a 13-year nationwide cohort study in an Asian population. Inflamm Bowel Dis 2014; 20:502–507. [DOI] [PubMed] [Google Scholar]
- 18.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 2009; 136:585–595.e5. [DOI] [PubMed] [Google Scholar]
- 19.Vainer B, Nielsen OH. Changed colonic profile of P-selectin, platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3 in inflammatory bowel disease. Clin Exp Immunol 2000; 121:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gresele P, Momi S, Migliacci R, et al. venous thromboembolism and ischaemic cardiovascular events. Thromb Haemost 2010; 103:56–61. [DOI] [PubMed] [Google Scholar]
- 21.Van Leuven SI, Hezemans R, Levels JH, et al. Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn's disease. J Lipid Res 2007; 48:2640–2646. [DOI] [PubMed] [Google Scholar]
- 22.Roifman I, Sun YC, Fedwick JP, et al. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2009; 7:175–182. [DOI] [PubMed] [Google Scholar]
- 23.Dagli N, Poyrazoglu OK, Dagli AF, et al. Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology 2010; 61:198–204. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Chou CY, Liu CS, et al. Association between frailty and subclinical peripheral vascular disease in a community-dwelling geriatric population: Taichung Community Health Study for Elders. Geriatr Gerontol Int 2015; 15:261–267. [DOI] [PubMed] [Google Scholar]
- 25.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005; 112:3337–3347. [DOI] [PubMed] [Google Scholar]
- 26.Chung WS, Peng CL, Lin CL, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis 2014; 73:1774–1780. [DOI] [PubMed] [Google Scholar]


